Supplemental Digital Content is available in the text.
Keywords: Brain tumors, Cohort study, Ultrafine particles
Background:
Ambient ultrafine particles (UFPs, <0.1 µm) can reach the human brain, but to our knowledge, epidemiologic studies have yet to evaluate the relation between UFPs and incident brain tumors.
Methods:
We conducted a cohort study of within-city spatial variations in ambient UFPs across Montreal and Toronto, Canada, among 1.9 million adults included in multiple cycles of the Canadian Census Health and Environment Cohorts (1991, 1996, 2001, and 2006). UFP exposures (3-year moving averages) were assigned to residential locations using land-use regression models with exposures updated to account for residential mobility within and between cities. We followed cohort members for malignant brain tumors (ICD-10 codes C71.0–C71.9) between 2001 and 2016; Cox proportional hazards models (stratified by age, sex, immigration status, and census cycle) were used to estimate hazard ratios (HRs) adjusting for fine particle mass concentrations (PM2.5), nitrogen dioxide (NO2), and various sociodemographic factors.
Results:
In total, we identified 1,400 incident brain tumors during the follow-up period. Each 10,000/cm3 increase in UFPs was positively associated with brain tumor incidence (HR = 1.112, 95% CI = 1.042, 1.188) after adjusting for PM2.5, NO2, and sociodemographic factors. Applying an indirect adjustment for cigarette smoking and body mass index strengthened this relation (HR = 1.133, 95% CI = 1.032, 1.245). PM2.5 and NO2 were not associated with an increased incidence of brain tumors.
Conclusions:
Ambient UFPs may represent a previously unrecognized risk factor for incident brain tumors in adults. Future studies should aim to replicate these results given the high prevalence of UFP exposures in urban areas.
Outdoor air pollution and diesel exhaust are each classified as known human carcinogens based primarily on evidence related to lung cancer.1,2 In urban areas, diesel exhaust and other combustion processes are important sources of ambient ultrafine particles (<0.1 µm, UFPs)3,4 but little is known about the long-term health effects of these pollutants. To date, only a small number of studies have examined the relation between UFPs and cancer including studies of lung,5 prostate,6 and postmenopausal breast cancer.7 In general, evidence from these studies is inconclusive and remains limited with respect to the impact of ambient UFP exposures on cancer incidence in exposed populations.
Over the past several years, numerous studies have documented the ability of small inhaled particles to reach the brain.8 In particular, animal evidence suggests that a fraction of inhaled nanoparticles translocate to the brain when deposited in either the nasal epithelium (via the olfactory nerve) or the alveolar epithelium by entering into the systemic circulation and eventually crossing the blood-brain barrier.8–12 The rate of particle translocation generally depends on particle size and surface properties (e.g., charge, protein/lipid coatings) with smaller size favoring more rapid translocation.8,11 Moreover, the detrimental impacts of particle translocation may occur through particle retention at the target site (i.e., through the generation of reactive oxygen species in the brain12) or indirectly through inflammatory mediators produced in response to the particles.8 In general, while the rate of translocation of particles to the brain is slow, elimination rates are also low and thus particles may accumulate over time with long-term exposures.8
Importantly, the translocation of airborne particles to the brain is also supported by human evidence as Maher et al.13 recently reported the presence of combustion-related nanoparticles in the brains of subjects who lived in Mexico and the United Kingdom. In general, however, epidemiologic evidence related to outdoor air pollution and brain tumors is limited and somewhat inconsistent with four studies reporting positive associations14–17 and two others reporting no relation.18,19 In the most recent study of European cohorts,17 PM2.5 absorbance (a marker for traffic-related air pollution) was most strongly associated with malignant brain tumors suggesting that traffic pollution may be an important source of exposure for these types of cancer. However, there have been no studies to date examining the relation between brain tumors and ambient UFPs and these pollutants are of particular interest as they are known to reach the human brain. Here we address this important knowledge gap in a study of ambient UFPs and brain tumor incidence across Canada’s two largest cities.
METHODS
Study Population
Our study population was based on four cycles (1991, 1996, 2001, and 2006) of the Canadian Census Health and Environment Cohort (CanCHEC). These datasets were formed by linking Census long-form questionnaires (which collect data on approximately 20% of Canadian households every 5 years) to postal code histories and mortality and cancer incidence records through the Statistics Canada Social Linkage Data Environment.20–22 The in-scope study population included noninstitutionalized respondents under the age of 90 who lived in Toronto or Montreal for at least 1 year between 1998 and the end of follow-up in 2016. We used residential postal code histories (from annual income tax filings) to estimate time-varying UFP exposures to account for residential mobility both within and between cities. The CanCHEC dataset was created under the authority of the Statistics Act and approved by the Executive Management Board (reference number: 045-2015) at Statistics Canada. This approval is equivalent to that of standard research ethics boards.
Brain Tumor Case Identification
We identified incident brain tumor cases (Malignant Neoplasms of the Brain: ICD-10 codes C71.0–C71.9) between 2001 and 2016 using the Canadian Cancer Registry. The follow-up period (i.e., the at-risk period) was restricted to years between 2001 and 2016 to avoid extrapolating UFP exposures many years in the past (i.e., for cohort members enrolled in 1991 and 1996). As a sensitivity analysis, outcome definition was limited to ICD-10 code C71.9 (malignant neoplasms of the brain, unspecified site) which captures gliomas (the most common type of malignant brain tumors in adults) and other brain tumor types with site unspecified within the brain. Other outcomes (including other types of cancer) were not examined.
Air Pollution Exposure Assessment
UFP exposures were assigned to residential locations (i.e., six-digit postal codes, about the size of city block face or large apartment complex) using land-use regression models for Montreal (2011–2012)4 and Toronto (2010–2011).23 These models were developed using mobile monitoring data collected during the summer and winter months and explained the majority of spatial variations in ambient UFPs across each city (Montreal: RCV2 = 0.60; Toronto: RCV2 = 0.50). All UFP exposures were assigned as 3-year moving averages with a 1-year lag to account for residential mobility within or between cities. At the start of follow-up, each participant was assigned an exposure based on their residential location (from the land-use regression models) for the years 1998, 1999, and 2000. Each year, we updated this exposure to account for residential mobility. For example, exposure estimates for 2005 were based on the average value for residential locations in 2002, 2003, and 2004. We did not project spatial variations in outdoor UFP concentrations back in time owing to the absence of long-term monitoring data for UFPs and a low correlation with other monitored pollutants.
We assigned long-term estimates (i.e., 3-year moving averages with 1-year lag) of ambient fine particulate air pollution (PM2.5) and nitrogen dioxide (NO2) concentrations to cohort members as previously described.24,25 Annual average concentrations were available for PM2.5 exposures assigned during follow-up and long-term estimates of spatial variations in NO2 concentrations reflected the year 2006. Briefly, the PM2.5 exposure model was based on a combination of aerosol optical depth, chemical transport model, and land-use information and estimated concentrations at a 1-km2 spatial resolution.24 Ambient NO2 concentrations were estimated using a national land-use regression model including parameters such as satellite-derived NO2 and distances to major roads and highways. The primary purpose of including long-term estimates of ambient PM2.5 and NO2 concentrations was to evaluate the sensitivity of UFP/brain tumor associations to spatial variations in other ambient air pollutants.
Statistical Analysis
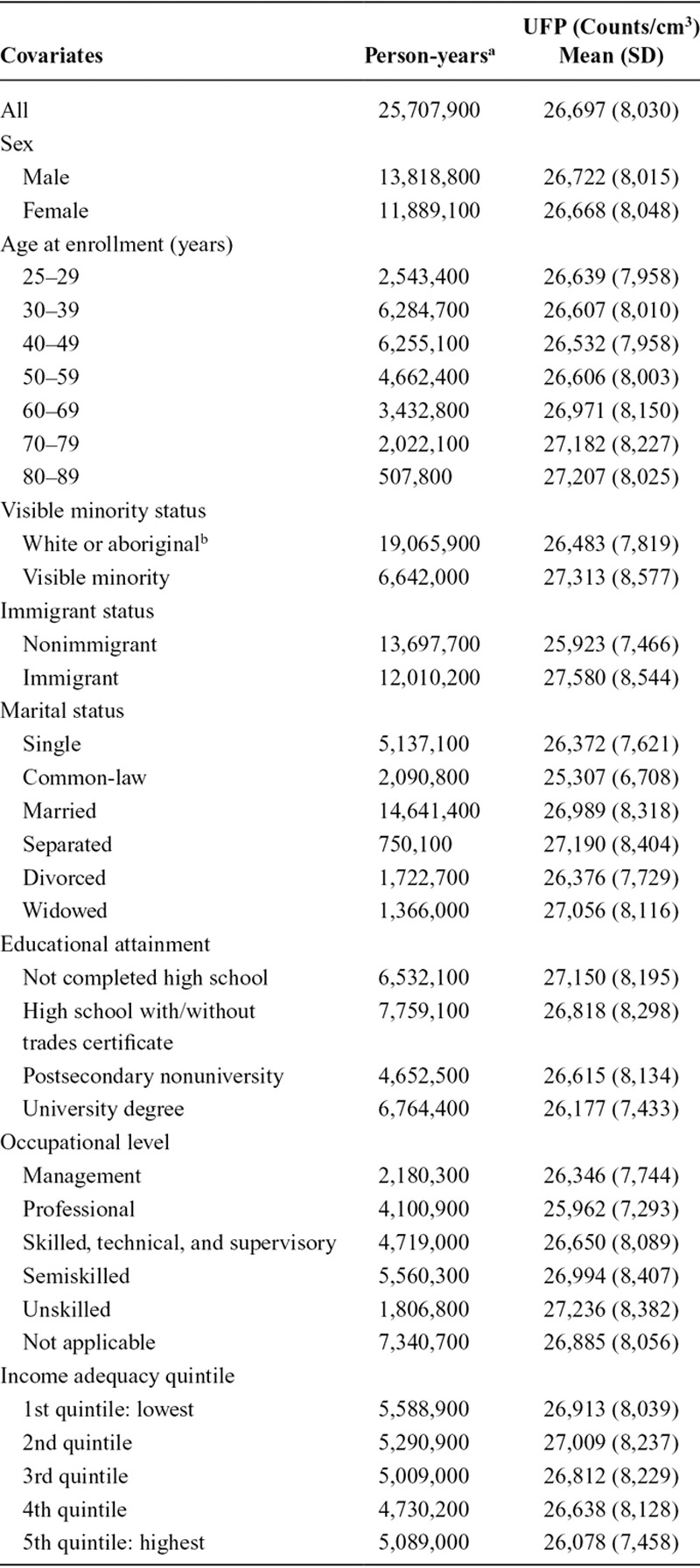
Cox proportional hazard models26 were used to estimate the association between ambient UFP concentrations and brain tumor incidence in Montreal and Toronto. A directed acyclic graph (DAG) outlining the conceptual relation between UFPs and brain tumors (and potential confounding factors) is provided in eFigure 1; http://links.lww.com/EDE/B613. We examined several models to explore this relation. First, we developed a base model including UFP concentrations and strata for age (5-year groups), sex, immigration status, and census year (i.e., 1991, 1996, 2001, and 2006). We used strata variables to allow for different baseline hazard functions across age, sex, immigrant status, and census year. Next, we examined the impact of adding covariates for SES variables (described in Table 1; visible minority status, occupational level, educational attainment, marital status, and income quintile), PM2.5, and NO2 both individually and combined. In constructing the visible minority variable, we grouped white and aboriginal cohort members together as aboriginal people made up a small portion of overall participants (11,300 of 1.9 million, or 0.6%) and person-years (116,100 of 25,707,900 person-years, or 0.45%). All UFP hazard ratios are scaled per 10,000 particles/cm3. All descriptive statistics were rounded to the nearest hundred for confidentiality (as required by Statistics Canada).
TABLE 1.
Descriptive Statistics for CanCHEC Cohort Members (1991, 1996, 2001, 2006)
Indirect Adjustment for Smoking and Body Mass Index
As sensitivity analyses, we applied a method of indirect adjustment for smoking and body mass index as these unmeasured parameters have been associated with brain tumors in previous studies.27,28 As indicated in the DAG presented in eFigure 1; http://links.lww.com/EDE/B613, smoking and body mass index are not causes of long-term outdoor UFP concentrations (i.e., intervening on individual-level smoking or BMI is not expected to change long-term outdoor UFP concentrations) and thus are not represented in the DAG as confounders in the typical sense. Nevertheless, chance associations could confound the relation between outdoor UFP concentrations and brain tumor incidence, and the indirect adjustment method was applied to address this possibility. Likewise, SES variables (i.e., income, education) could be related to smoking/BMI and this relation could in turn bias the observed relation between outdoor UFP concentrations and brain tumors. Applying the indirect adjustment method for smoking and BMI also addresses this possibility.
A detailed description of the indirect adjustment method is available elsewhere.29 Briefly, this method requires information on the correlation between observed and missing risk factors from an ancillary data source (e.g., national health surveys) as well as estimates of the relation between the missing risk factors and brain tumor incidence. For our analyses, information related to the correlation between observed and missing risk factors was obtained from multiple cycles of Canadian Community Health Survey (CCHS; 2001, 2003, 2005, 2007, 2009, 2011, 2013); the CCHS is a biannual national health survey that collects information on the health status and behaviors (i.e., smoking and BMI) of noninstitutionalized Canadians age 12 years and older. Risk estimates for the relation between brain tumors and smoking and body mass index were obtained from the published literature.27,28 The CCHS and CanCHEC databases were compared with respect to UFP concentrations across age, sex, and SES variables; median differences were small in magnitude (typically less than 1,000/cm3) supporting the use of the CCHS database in estimating correlations between observed and missing risk factors (eTable 1; http://links.lww.com/EDE/B613).
Concentration–Response Relation
The shape of the concentration–response relation between outdoor UFP concentrations and brain tumor incidence was examined using the Shape Constrained Health Impact Function (SCHIF) method developed by Nasari et al.30 Specifically, this method utilizes flexible concentration transformations (i.e., linear, sublinear, supralinear, and sigmodal) within a Cox model and produces biologically plausible concentration–response relations that increase monotonically with concentration. The final concentration–response curve was based on weights from the three best-fitting models identified by the SCHIF method.
RESULTS
Cohort characteristics are presented in Table 1. In total, we followed 1,938,100 adults (25,707,900 person-years) in Montreal and Toronto between 2001 and 2016 and 1,400 incident cases of brain tumors were included in the analyses. We excluded approximately 10 million person-years based on our inclusion criteria (i.e., less than 90 years of age at start of follow-up and resided in Toronto or Montreal for at least 1 year between 1996 and 2016). Incident brain tumors were more common among women (n = 800) than among men (n = 600) and were primarily diagnosed between age 40 and 69 years (eTable 2; http://links.lww.com/EDE/B613).
Mean UFP values assigned to all in-scope person-years (i.e., 3-year moving averages with a 1-year lag) were 26,697/cm3 (standard deviation [SD] = 8,030/cm3) and ranged from 6,697/cm3 to 97,158/cm3. Spatial variations in ambient UFPs were weakly correlated with PM2.5 (r = 0.14) and NO2 (r = 0.20) whereas PM2.5 and NO2 were more strongly correlated (r = 0.66). The low correlation between UFPs and other air pollutants is commonly observed in Canada31; this relates in part to the fact that the prevalence of diesel vehicles (a major source of UFPs) is low and thus it is possible to have areas with elevated NO2 concentrations (from gasoline vehicles) without major elevations in UFPs. Moreover, PM2.5 tends to be a regional pollutant without major spatial variations within-cities. The mean PM2.5 concentration in this study was 8.68 μg/m3 (SD = 2.42 μg/m3) and the mean NO2 concentrations was 20.2 ppb (SD = 8.43 ppb).
Hazard ratios describing the relation between ambient UFPs and incident brain tumors are listed in Table 2. UFPs were consistently associated with increased brain tumor incidence in all models examined and the hazard ratio remained elevated after adjustment for sociodemographic factors as well as PM2.5 and NO2 (HR = 1.112, 95% CI = 1.042, 1.188). A slightly stronger association was observed in the fully adjusted model (i.e., all SES variables, PM2.5, and NO2) when the outcome was limited to ICD-10 code C71.9 (HR = 1.170, 95% CI = 1.000, 1.372); however, this estimate was less precise owing to a smaller number of cases (n = 200 for ICD-10 code C71.9).
TABLE 2.
Hazard Ratios (95% CI) for UFP Concentrations (per 10,000/cm3) and Incident Brain Tumors (n = 1,400) in Montreal and Toronto, Canada (2001–2016)
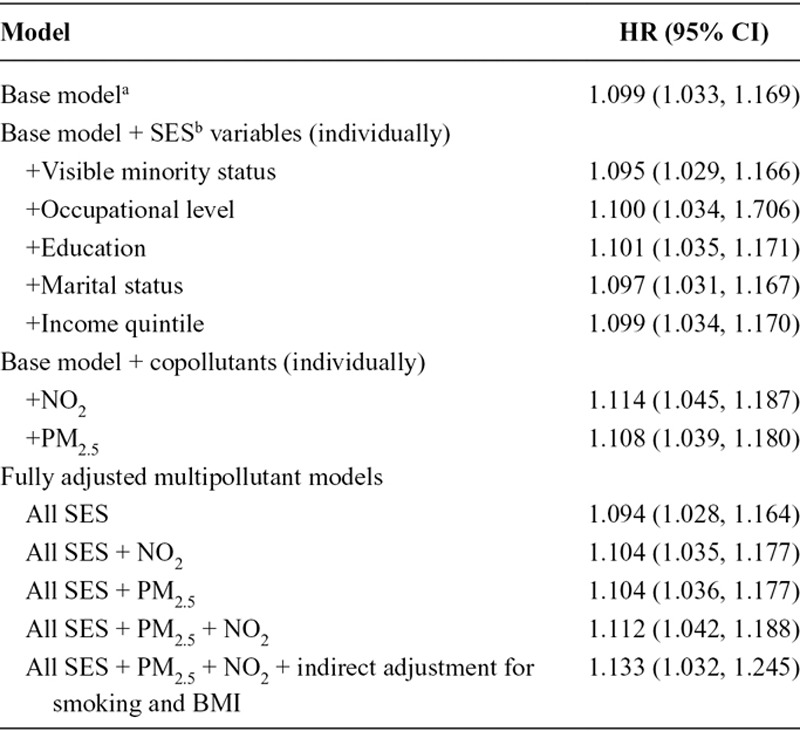
Applying the indirect adjustment for cigarette smoking and body mass index (with adjustment for all SES variables, PM2.5, and NO2) slightly increased the strength of the observed association between UFPs and brain tumors (HR = 1.133, 95% CI = 1.032, 1.245). This association was stronger in Toronto (HR = 1.171, 95% CI = 1.021, 1.343) than Montreal (HR = 1.022, 95% CI = 0.814, 1.283) but city-specific risk estimates were less precise and more than half (n = 850) of the cases were from Toronto. The concentration–response curve for ambient UFP concentrations and incident brain tumors is shown in the Figure. This relation was s-shaped with a smaller slope at lower concentrations.
FIGURE.
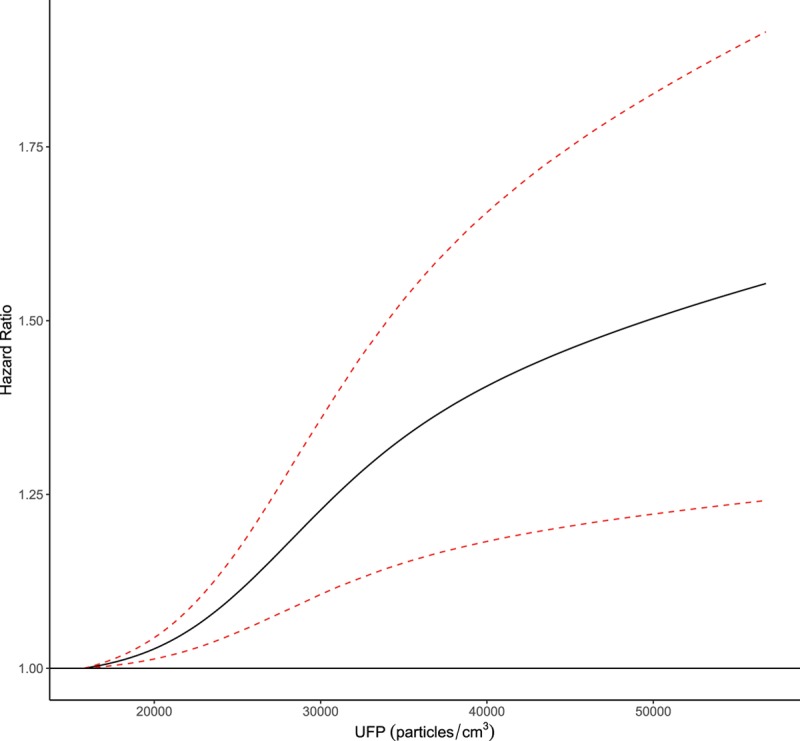
Concentration–response relation between ambient UFP concentrations and brain tumor incidence in Montreal and Toronto, Canada (2001–2016). The model is stratified by 5-year age groups, sex, census cycle (1991, 1996, 2001, 2006), and immigrant status and adjusted for visible minority status, occupational level, education, marital status, and income quintile. Dashed red lines indicated the 95% confidence interval. Hazard ratios (the solid black line) for the concentration–response curve are calculated in reference to the first percentile of UFP concentrations in the cohort (15,767/cm3) up to the 99th percentile (56,767/cm3).
Spatial variations in PM2.5 and NO2 were not positively associated with brain tumor incidence (eTables 3, 4; http://links.lww.com/EDE/B613). Specifically, hazard ratios for interquartile changes in PM2.5 (per 3 µg/m3) and NO2 (per 10 ppb) in fully adjusted models including all SES variables and UFPs were 0.907 (95% CI = 0.762, 1.079) and 0.950 (95% CI = 0.851, 1.061), respectively.
DISCUSSION
We conducted a population-based study of within-city spatial variations in ambient UFP concentrations and incident brain tumors in Canada’s two largest cities. In general, our findings suggest that ambient UFPs are positively associated with brain tumor incidence and these pollutants may represent a previously unrecognized risk factor for brain tumors. Moreover, the relation between UFPs and brain tumors was not explained by other air pollutants such as PM2.5 mass concentrations or NO2 which are more frequently monitored in urban environments. On an absolute scale, the magnitude of the observed association suggests that each 10,000/cm3 increase in 3-year average ambient UFP concentration contributes to approximately 1 new case per 100,000 population (assuming a baseline age-standardized incidence rate of 8 per 100,000).32
Existing evidence related to outdoor air pollution and brain tumors is inconsistent. For example, McKean-Cowdin et al.18 examined the relation between PM2.5 and NO2 and brain tumor mortality in the Cancer Prevention Study-II cohort (1982–2000) and reported small inverse associations similar in magnitude to those observed in the present study. Raaschou-Nielsen et al.15 reported a positive association between NOx (a marker for traffic pollution) and incident brain tumors in the Danish Diet, Cancer, and Health cohort in 2011 but a weaker association was observed in a follow-up case–control study including a much larger number of cases.19 Similarly, weak positive associations were observed between brain tumors and PM2.5, NO2, and NOx in the Danish Nurse Cohort with stronger associations observed among obese subjects and those with lower levels of physical activity.16 Finally, the most recent study of air pollution and brain tumors reported weak positive associations between PM2.5 and NO2 and brain tumors across multiple European cohorts with the strongest association observed for PM2.5 absorbance (a marker of traffic pollution).17 Collectively, existing evidence suggests that traffic-related air pollution may be associated with a small increased risk of brain tumor incidence/mortality but few studies have examined this relation to date. Moving forward, particular attention should be paid to UFPs as they are known to reach the brain8–13 and our findings suggest a positive association with brain tumors independent of PM2.5 or NO2 (which were not positively associated with brain tumor incidence). Moreover, most brain tumors are rapidly fatal and few known risk factors have been identified for these types of cancer; therefore, it is important to identify potential environmental causes of brain tumors to inform future policy interventions.
This study had a number of important advantages including high-resolution estimates of within-city spatial variations in ambient UFP concentrations, updated exposure information (i.e., UFPs, NO2, and PM2.5) for subjects moving both within or between cities, a large study population, incident cases, and detailed individual-level information on a number of important socioeconomic factors. However, it is also important to note several limitations. First, the exposure models used to estimate spatial variations in outdoor UFP concentrations in Montreal (2011–2012) and Toronto (2010–2011) were based on data collected toward the end of the follow-up period (2001–2016) and exposure measurement error almost certainly impacted our results. This error likely has a temporal component (impacted by changes in emissions over time) and a spatial component (impacted by local changes in infrastructure that could influence the movement of traffic sources through space). While this error likely contributed to imprecision in our results, a systematic difference in the magnitude of exposure error by case status seems unlikely and the overall impact of exposure measurement error was likely a bias toward the null. Another possible concern is that trends in brain cancer incidence may influence our results (e.g., if incidence was higher/lower closer to the time of land-use regression model development, this could contribute to differential exposure measurement error); however, data compiled by the Canadian Cancer Society indicate that age-standardized incidence rates for brain/CNS tumors were been stable between 2001 and 2010.33 Nevertheless, because outdoor UFP concentrations have likely decreased over time (owing to improved vehicle efficiency), our data may not be appropriate in identifying absolute threshold concentrations for UFP impacts on brain tumors if overall exposure levels were elevated toward the beginning of the follow-up period (data are not currently available to verify or refute this hypothesis). Furthermore, as for UFPs, exposure measurement error likely also impacted our estimates of PM2.5 and NO2 and this may have resulted in residual confounding by these factors. However, including PM2.5 and/or NO2 in the models tended to increase hazard ratios for UFPs and brain tumors and thus more precise estimates would be expected to further strengthen this relation. As in all studies, we also cannot rule out residual confounding of the UFP–brain tumor relation by some unmeasured factors; however, it is unclear what these factors may be as there are few known risk factors for brain tumors and to explain our results these factors would also have to be related to spatial variations in outdoor UFPs.
A second limitation was the absence of individual-level smoking and body mass index data for CanCHEC cohort members. As outlined in the DAG presented in the eFigure 1; http://links.lww.com/EDE/B613, individual-level smoking and BMI are not likely causes of long-term exposures to outdoor UFPs (i.e., changing individual-level smoking status or BMI is not expected to change long-term outdoor UFP levels) and thus these parameters are not identified in the DAG as confounders in the typical sense. However, chance associations between smoking or BMI and UFPs could still confound the analysis; thus, we conducted an indirect adjustment for these unmeasured risk factors to evaluate the sensitivity of our findings to this potential source of bias. The results of the indirect adjustment for smoking and BMI suggested a stronger association between UFPs and brain tumors, thus suggesting that confounding by smoking or BMI is not a likely explanation for the observed relation between UFPs and brain tumors.
Similarly, we did not have individual-level information on other potential risk factors for brain tumors including family history or life-time exposure to ionization radiation. It was not possible to indirectly adjust for these factors and we cannot rule out potential confounding (away from or toward the null) by these variables if by chance they were systematically related to outdoor UFP concentrations across Montreal and Toronto. Finally, given the rarity of brain tumors, our analyses focused primarily on total malignant brain tumors (ICD-10 codes C71.0–C71.9: Malignant Neoplasms of the Brain) to maximize precision and does not provide an in-depth examination of specific tumor subtypes. As a result, if UFPs are only associated with specific types of brain tumors, the hazard ratios presented earlier may underestimate the true impact of UFPs on these specific tumor subtypes.
In conclusion, we conducted to our knowledge the first cohort study of outdoor UFP concentrations and incident brain tumors and noted a consistent positive association. This relation was robust to adjustment for various sociodemographic factors as well as indirect adjustment for smoking and body mass index. Future studies should aim to replicate our findings as UFPs are known to reach the human brain and exposure prevalence is high in urban areas around the world.
Supplementary Material
Footnotes
This study was funded by Health Canada. S.W. also received support from a GRePEC salary award funded by the Cancer Research Society, the Quebec Ministry of Economy, Science and Innovation, and FRQS (Fonds de Recherche du Québec-Santé).
The authors report no conflicts of interest.
All exposure data used in this manuscript are freely available. The CanCHEC cohort is not publicly available but can be accessed through Statistics Canada Research Data Centers conditional on the necessary approval/review procedures.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
REFERENCES
- 1.Benbrahim-Tallaa L, Baan RA, Grosse Y, et al. ; International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of diesel-engine and gasoline-engine exhausts and some nitroarenes. Lancet Oncol. 2012;13:663–664. [DOI] [PubMed] [Google Scholar]
- 2.Loomis D, Grosse Y, Lauby-Secretan B, et al. ; International Agency for Research on Cancer Monograph Working Group IARC. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013;14:1262–1263. [DOI] [PubMed] [Google Scholar]
- 3.Weichenthal S, Van Ryswyk K, Kulka R, Sun L, Wallace L, Joseph L. In-vehicle exposures to particulate air pollution in Canadian metropolitan areas: the urban transportation exposure study. Environ Sci Technol. 2015;49:597–605. [DOI] [PubMed] [Google Scholar]
- 4.Weichenthal S, Ryswyk KV, Goldstein A, Bagg S, Shekkarizfard M, Hatzopoulou M. A land use regression model for ambient ultrafine particles in Montreal, Canada: a comparison of linear regression and a machine learning approach. Environ Res. 2016;146:65–72. [DOI] [PubMed] [Google Scholar]
- 5.Weichenthal S, Bai L, Hatzopoulou M, et al. Long-term exposure to ambient ultrafine particles and respiratory disease incidence in Toronto, Canada: a cohort study. Environ Health. 2017;16:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weichenthal S, Lavigne E, Valois MF, et al. Spatial variations in ambient ultrafine particle concentrations and the risk of incident prostate cancer: a case-control study. Environ Res. 2017;156:374–380. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg MS, Labrèche F, Weichenthal S, et al. The association between the incidence of postmenopausal breast cancer and concentrations at street-level of nitrogen dioxide and ultrafine particles. Environ Res. 2017;158:7–15. [DOI] [PubMed] [Google Scholar]
- 8.Heusinkveld HJ, Wahle T, Campbell A, et al. Neurodegenerative and neurological disorders by small inhaled particles. Neurotoxicology. 2016;56:94–106. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell R, Maher BA. Evaluation and application of biomagnetic monitoring of traffic-derived particulate pollution. Atmos Environ. 2009;43:2095–2103. [Google Scholar]
- 10.Kreyling WG. Discovery of unique and ENM- specific pathophysiologic pathways: comparison of the translocation of inhaled iridium nanoparticles from nasal epithelium versus alveolar epithelium towards the brain of rats. Toxicol Appl Pharmacol. 2016;299:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oberdörster G, Elder A, Rinderknecht A. Nanoparticles and the brain: cause for concern? J Nanosci Nanotechnol. 2009;9:4996–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oppenheim HA, Lucero J, Guyot AC, et al. Exposure to vehicle emissions results in altered blood brain barrier permeability and expression of matrix metalloproteinases and tight junction proteins in mice. Part Fibre Toxicol. 2013;10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maher BA, Ahmed IA, Karloukovski V, et al. Magnetite pollution nanoparticles in the human brain. Proc Natl Acad Sci U S A. 2016;113:10797–10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boeglin ML, Wessels D, Henshel D. An investigation of the relationship between air emissions of volatile organic compounds and the incidence of cancer in Indiana counties. Environ Res. 2006;100:242–254. [DOI] [PubMed] [Google Scholar]
- 15.Raaschou-Nielsen O, Andersen ZJ, Hvidberg M, et al. Air pollution from traffic and cancer incidence: a Danish cohort study. Environ Health. 2011;10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jørgensen JT, Johansen MS, Ravnskjær L, et al. Long-term exposure to ambient air pollution and incidence of brain tumours: the Danish nurse cohort. Neurotoxicology. 2016;55:122–130. [DOI] [PubMed] [Google Scholar]
- 17.Andersen ZJ, Pedersen M, Weinmayr G, et al. Long-term exposure to ambient air pollution and incidence of brain tumour: the European Study of Cohorts for Air Pollution Effects (ESCAPE). Neuro Oncol. 2018;20:420–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKean-Cowdin R, Calle EE, Peters JM, et al. Ambient air pollution and brain cancer mortality. Cancer Causes Control. 2009;20:1645–1651. [DOI] [PubMed] [Google Scholar]
- 19.Poulsen AH, Sørensen M, Andersen ZJ, Ketzel M, Raaschou-Nielsen O. Air pollution from traffic and risk for brain tumors: a nationwide study in Denmark. Cancer Causes Control. 2016;27:473–480. [DOI] [PubMed] [Google Scholar]
- 20.Christidis T, Labrecque-Synnott F, Pinault l, Saidi A, Tjepkema M. The 1996 CanCHEC: Canadian census health and environment cohort profile. Analy Studies Methods Ref. 2018;11-633:013. [Google Scholar]
- 21.Pinault L, Fines P, Labrecque-Synnott F, Saidi A, Tjepkema M. The Canadian census-tax-mortality cohort: a 10-year follow-up. Anal Studies Methods Ref. 2016;11-633:003. [Google Scholar]
- 22.Wilkins R, Tjepkema M, Mustard C, Choinière R. The Canadian census mortality follow-up study, 1991 through 2001. Health Rep. 2008;19:25–43. [PubMed] [Google Scholar]
- 23.Weichenthal S, Van Ryswyk K, Goldstein A, Shekarrizfard M, Hatzopoulou M. Characterizing the spatial distribution of ambient ultrafine particles in Toronto, Canada: a land use regression model. Environ Pollut. 2016;208(pt A):241–248. [DOI] [PubMed] [Google Scholar]
- 24.van Donkelaar A, Martin RV, Spurr RJ, Burnett RT. High-resolution satellite-derived PM2.5 from optimal estimation and geographically weighted regression over North America. Environ Sci Technol. 2015;49:10482–10491. [DOI] [PubMed] [Google Scholar]
- 25.Hystad P, Setton E, Cervantes A, et al. Creating national air pollution models for population exposure assessment in Canada. Environ Health Perspect. 2011;119:1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;20:187–220. [Google Scholar]
- 27.Li HX, Peng XX, Zong Q, et al. Cigarette smoking and risk of adult glioma: a meta-analysis of 24 observational studies involving more than 2.3 million individuals. Onco Targets Ther. 2016;9:3511–3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niedermaier T, Behrens G, Schmid D, Schlecht I, Fischer B, Leitzmann MF. Body mass index, physical activity, and risk of adult meningioma and glioma: a meta-analysis. Neurology. 2015;85:1342–1350. [DOI] [PubMed] [Google Scholar]
- 29.Shin HH, Cakmak S, Brion O, et al. Indirect adjustment for multiple missing variables applicable to environmental epidemiology. Environ Res. 2014;134:482–487. [DOI] [PubMed] [Google Scholar]
- 30.Nasari MM, Szyszkowicz M, Chen H, et al. A class of non-linear exposure-response models suitable for health impact assessment applicable to large cohort studies of ambient air pollution. Air Qual Atmos Health. 2016;9:961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weichenthal S, Hatzopoulou M, Goldberg MS. Exposure to traffic-related air pollution during physical activity and acute changes in blood pressure, autonomic and micro-vascular function in women: a cross-over study. Part Fibre Toxicol. 2014;11:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2018. 2018. Toronto, ON: Canadian Cancer Society; Available at: cancer.ca/Canadian-Cancer-Statistics-2018-EN. Accessed 4 April 2019. [Google Scholar]
- 33.Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics 2015. 2015. Toronto, ON: Canadian Cancer Society; Available at: http://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2015-EN.pdf?la=en. Accessed 14 April 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


