Supplemental digital content is available in the text.
KEY WORDS: Thromboelastography, TEG 5000, TEG 6s, viscoelastic testing, trauma-induced coagulopathy
Abstract
BACKGROUND
Trauma-induced coagulopathy is a major driver of mortality following severe injury. Viscoelastic goal-directed resuscitation can reduce mortality after injury. The TEG 5000 system is widely used for viscoelastic testing. However, the TEG 6s system incorporates newer technology, with encouraging results in cardiovascular interventions. The purpose of this study was to validate the TEG 6s system for use in trauma patients.
METHODS
Multicenter noninvasive observational study for method comparison conducted at 12 US Levels I and II trauma centers. Agreement between the TEG 6s and TEG 5000 systems was examined using citrated kaolin reaction time (CK.R), citrated functional fibrinogen maximum amplitude (CFF.MA), citrated kaolin percent clot lysis at 30 minutes (CK.LY30), citrated RapidTEG maximum amplitude (CRT.MA), and citrated kaolin maximum amplitude (CK.MA) parameters in adults meeting full or limited trauma team criteria. Blood was drawn ≤1 hour after admission. Assays were repeated in duplicate. Reliability (TEG 5000 vs. TEG 6s analyzers) and repeatability (interdevice comparison) was quantified. Linear regression was used to define the relationship between TEG 6s and TEG 5000 devices.
RESULTS
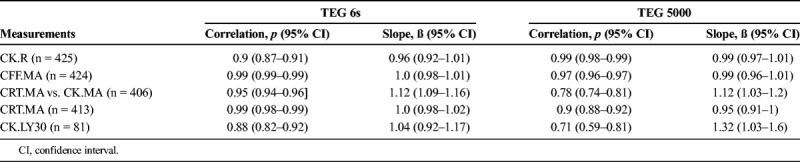
A total of 475 patients were enrolled. The cohort was predominantly male (68.6%) with a median age of 49 years. Regression line slope estimates (ß) and linear correlation estimates (p) were as follows: CK.R (ß = 1.05, ρ = 0.9), CFF.MA (ß = 0.99, ρ = 0.95), CK.LY30 (ß = 1.01, ρ = 0.91), CRT.MA (TEG 6s) versus CK.MA (TEG 5000) (ß = 1.06, ρ = 0.86) as well as versus CRT.MA (TEG 5000) (ß = 0.93, ρ = 0.93), indicating strong reliability between the devices. Overall, within-device repeatability was better for TEG 6s versus TEG 5000, particularly for CFF.MA and CK.LY30.
CONCLUSION
The TEG 6s device appears to be highly reliable for use in trauma patients, with close correlation to the TEG 5000 device and equivalent/improved within-device reliability. Given the potential advantages of using the TEG 6s device at the site of care, confirmation of agreement between the devices represents an important advance in diagnostic testing.
LEVEL OF EVIDENCE
Diagnostic test, level II.
Uncontrolled noncompressible bleeding is the leading cause of preventable deaths in both civilian and military scenarios.1–3 Trauma-induced coagulopathy (TIC) is a major driver of mortality following severe injury, and the management of TIC is focused on early, goal-directed therapy to achieve hemostasis.4,5 Viscoelastic goal-directed resuscitation has been shown to reduce mortality after injury,6 and viscoelastic testing plays a major role in guiding blood product transfusion in critically injured patients.7,8 The American College of Surgeons Trauma Quality Improvement Program recommends the use of thromboelastography, if available, in its guidelines for patients at risk for massive transfusion following trauma.9
There are multiple platforms for viscoelastic testing, one of which is the TEG 5000 Hemostasis Analyzer (Haemonetics Corp., Braintree, MA). The TEG system is proven to be superior to conventional coagulation testing in trauma patients10 as well as in patients undergoing cardiac surgery11 and liver transplantation.12 Limitations of the TEG 5000 Hemostasis Analyzer include the technical requirements of pipetting the blood sample, and vulnerability to vibration. The TEG 6s Hemostasis Analyzer (Haemonetics Corp.) was designed to eliminate these issues, to provide more reliable measurements at the site of care. The TEG 6s Hemostasis Analyzer uses resonance-frequency viscoelasticity measurements and a disposable multichannel microfluidic cartridge that eliminates pipetting and enhances portability, which potentially decreases vulnerability to vibration and reduces user-error. It also has the potential for assay-specific multichannel testing. The TEG 6s system has been utilized to assess coagulation in cardiovascular surgery and cardiovascular interventions11 and has been shown to have a greater ease of use and precision when compared with the TEG 5000 system in patients undergoing coronary revascularization.13,14
Rapid and reliable assessment of coagulopathy is critical to point-of-care resuscitation after injury. Although there are substantial benefits to viscoelastic testing in trauma, and multiple platforms exist for this testing, the adoption and integration of this technology is still an ongoing process. The potential reduction in user-error and increased portability of the TEG 6s system represent needed advances in point-of-care technology available in the emergency department, operating room, and surgical intensive care unit. Recent data suggest that use of the TEG 6s system during ground and air transport is feasible,15–17 providing the potential for viscoelastic testing in the prehospital setting for the early identification of coagulopathy. However, to date, the comparison of the TEG 6s Hemostasis Analyzer to the existing TEG 5000 system technology for use in trauma has not been investigated. The purpose of the present study was to underscore the utility and reliability of the TEG 6s system for use in trauma patients via comparison to TEG 5000, as well as to assess the internal consistency of the TEG 6s device.
METHODS
Study Design
This trial was a multicenter, noninvasive, observational study for method comparison purposes with minimal risk to the patient. This method comparison was conducted according to the Clinical and Laboratory Standards Institute Guideline EP09-A2 Method Comparison and Bias Estimation Using Patient Samples18 to support the equivalence for assay measurements of citrated kaolin reaction time (CK.R), citrated kaolin percent clot lysis at 30 minutes (CK.LY30), citrated RapidTEG maximum amplitude (CRT.MA), and citrated functional fibrinogen maximum amplitude (CFF.MA). These four measurements were selected because they are included in major decisions regarding blood component administration and antifibrinolytics in severely injured patients. The study was conducted at 12 American College of Surgeons verified or state designated Levels I and II Trauma Centers. Each center obtained institutional review board (IRB) approval prior to initiation. The study was performed under waiver of consent, and four of the IRBs requested that study subjects (or legally authorized representatives) be provided with a Study Information Sheet and be provided the opportunity to withdraw from the study. The trial data were used for a comparative analysis of the established TEG 5000 system and the new TEG 6s Hemostasis Analyzer in injured patients. The study examined the agreement between TEG 5000 and TEG 6s systems coagulation analysis results. There was no study intervention to the patient other than the collection of blood sample(s) in three additional 4.5-mL, or five 2.7-mL vacutainers (3.2% sodium citrate, blue top). These samples were obtained, when feasible, with the standard of care (SOC) blood draws. Blood samples from each subject were analyzed in duplicate using the TEG 6s and TEG 5000 systems by experienced research personnel. Investigators were blinded to the TEG 6s analyzer results. No viscoelastic testing included as part of this protocol was used to guide clinical care. Samples were for research purposes only, and the decision to order viscoelastic testing as part of clinical care was at the discretion of the clinician and SOC for each institution. Samples were obtained as soon as possible upon emergency department arrival but no later than 1 hour after admission. Assessment of the agreement between the TEG 6s and the TEG 5000 assay measurements was performed by analyzing the relationship between the first replicate of the TEG 6s analyzer and the mean of two replicates of the TEG 5000 system.
Each assay and measurement is provided in an assay-measurement format. So as an example, for CK.R, the assay is CK and the measurement is R.
Study Oversight
Sites were required to complete instrument validation and proficiency testing for all involved research personnel. Predefined validation testing was completed for both of the TEG systems prior to analysis of patient samples and data collection. Site proficiency was assessed using predefined proficiency assessments for the correct use of the TEG 5000 and TEG 6s Hemostasis Analyzers. Central Statistical Risk Based Monitoring was conducted on a periodic basis for all TEG device data for early detection of data integrity and study center compliance issues, enrollment monitoring, optimization of the site audit and retraining schedule, and overall increase of the study final data quality.
Patient Population
Adult patients (males or females 18 years and older) who met the full or limited trauma team criteria of the American College of Surgeons or similar criteria established per institutional guidelines were included. A small percentage of the study population was expected to have CK.LY30 values in the upper analytical measurement range (AMR) of the assay. If the number of subjects with CK.LY30 levels in the upper and mid AMR was below 10%, the inclusion criteria could be modified to enrich for subjects with the desired CK.LY30 range. Enrichment criteria included: systolic blood pressure < 90 mm Hg, base excess of < −6 mEq/L, Glasgow Coma Scale (GCS) <6, or pH < 7.2 upon arrival to the trauma center. Adverse events related to venipuncture and medical device reports were recorded.
Patients were excluded from study enrollment if they were deemed unfit for participation in the study by the principal investigator, or if they were participating in another clinical trial that would not be scientifically or medically compatible with this study. Four of the IRBs requested that study subjects (or legally authorized representatives) be provided with a Study Information Sheet and be provided the opportunity to withdraw from the study.
Testing and Data Processing
The citrated patient blood samples were tested concurrently in the TEG 6s system (using the Citrated Multichannel Cartridge) and in the TEG 5000 system (using the following reagents: citrated RapidTEG, citrated kaolin, citrated kaolin in heparinase, and citrated functional fibrinogen).
Data for all thromboelastography measurements were recorded. Medications that could affect the coagulation status, and any type of blood product and intravenous fluid administered up to the point of blood draw for TEG testing were captured in the electronic case report form. Information collected in the electronic case report form also included: demographic information, location/mechanism of injury, estimated time of injury, hospital arrival time, TEG test blood sample collection time, patient disposition at the TEG test blood draw, vital signs, conventional coagulation tests including platelet count (if available), prothrombin time/international normalized ratio (PT/INR), activated partial thromboplastin time (aPTT), and fibrinogen levels, trauma assessments (Injury Severity Score [ISS], GCS), admission status, all-cause mortality (through 24 hours), and if available, the SOC hematocrit, SOC hemoglobin, and SOC blood gas analysis results. Patients were not followed-up after the blood sample for analysis had been obtained, except for assessment of all-cause mortality at 24 hours, and number of units of red blood cells administered in 24 hours, medical device reporting events, and adverse events due to blood draws, if not part of SOC.
Agreement Analysis
The analysis focuses on the agreement between the TEG 6s and TEG 5000 parameters, as well as on the internal replicability of the two TEG 6s and two TEG 5000 measurements. The primary analysis of the method comparison was based on agreement between the first replicate of TEG 6s measurements and the mean of the two TEG 5000 replicates. As the precision of the TEG 5000 measurement depends on the operator, the mean of two tests provides a more robust baseline for comparison, while the TEG 6s is an automated, cartridge-based test and therefore the first sample was prospectively determined to be used for the comparison, as consistent with FDA standards for method-comparison studies. The main criterion for assessment of the agreement between the two devices was based on the assessment of the predicted bias and its acceptability at the medical decision points. The medical decision points were defined as the cutoff points of the normal reference ranges. The predicted bias, defined as the difference between the expectation of the test result and the results of the reference test, was estimated using a regression modeling framework describing the relationship between the TEG 6s and TEG 5000 measurements. Deming regression was the default method and Passing-Bablock regression was used in the event of nonnormality of bias distribution.
The secondary assessment of the agreement between the devices included bias estimation at the AMR, estimates of the coefficients defining the linear relationship between the devices, Pearson correlation coefficient estimates, visual assessment of the agreement suggested by agreement plots (Fig. 1), and error analysis including Bland-Altman Plots (Supplemental Digital Content 1, Fig. 1, http://links.lww.com/TA/B499).
Figure 1.
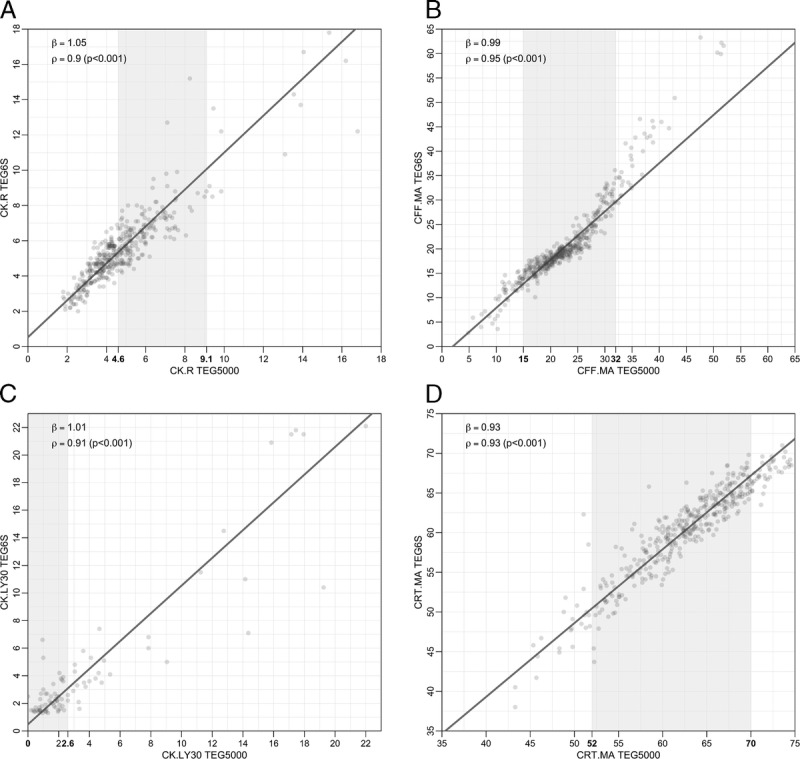
Comparison of TEG 6s vs. TEG 5000 Replicates for CK.R (panel A), CFF.MA (panel B), CK.LY30 (panel C), and CRT.MA (panel D). Axes numbers in bold indicate reference range; the overall range of graph is in line with the AMR.
Analysis of within device agreement (or replicability) using the two replicates of the same measurement included correlation analysis, agreement of the replicates assessed by means linear regression, visual analysis of the replicates and regression line estimates, predicted bias analysis (second replicate vs. first replicate taken as a reference measurement) at medical decision points and AMRs, and error analysis with Bland-Altman plots (Supplemental Digital Content 2, Figs. 2 and 3, http://links.lww.com/TA/B500).
The target enrollment sample size was approximately 500 subjects allowing up to a 20% drop out rate. The sample size was estimated using historic variability of the TEG parameters under consideration. In areas where there were insufficient clinical samples, data from donor samples that were modified in vitro were used to supplement patient samples, in accordance with the protocol and within the prespecified limits. This supplementation was limited to an addition of up to 10% of the samples, in line with generally accepted best practice.
RESULTS
Patient Cohort
A total of 475 patients were enrolled across all centers. Demographics for the study population are presented in Table 1. The cohort was predominantly male (68.6%) with a median (interquartile range) age of 49 years (18–95 years). Most injuries were due to a blunt mechanism (78.1%) and the overall mortality at 24 hours was 3.4%. A total of 43.3% of patients required ICU admission. The mean (standard deviation [SD]) GCS score was 12 (4) and the mean (SD) ISS was 14 (13) with 20.0% of patients having an ISS greater than 25. There was one study-related adverse event in this study. A subject was reported to have a ruptured vein and severity was assessed as mild. There were no medical device and incident reports recorded in the study.
TABLE 1.
Study Cohort Demographics

TEG 5000 versus TEG 6s Device Comparison
Linear regression analysis demonstrated strong reliability between the first replicate of the TEG 6s system and the mean of two replicates of the TEG 5000 system with regression line slope estimates (ß) and linear correlation estimates (ρ) as follows: CK.R (ß = 1.05, ρ = 0.9), CFF.MA (ß = 0.99, ρ = 0.95), CK.LY30 (ß = 1.01, ρ = 0.91), CRT.MA (TEG 6s) versus CK.MA (TEG 5000) (ß = 1.06, ρ = 0.86) as well as versus CRT.MA (TEG® 5000) (ß =0.93 and ρ = 0.93) (Fig. 1 and Supplemental Digital Content 3, Figure 4, http://links.lww.com/TA/B501). The quantitative analysis presented for CK.LY30 represents 86 total samples where the LY30 was greater than 1.25%. Measurements below 1.25% are classified as “low CK.LY30 values” for the purpose of this method comparison study and are treated in the framework of a qualitative agreement analysis due to increased variability below this measurement. Measurements above 1.25% are treated in the same framework as the other parameters' analyses. There were 301 samples with TEG 6s CK.LY30 values below the 1.25% cutoff point. Of these 301 samples, 255 (85%) had TEG 5000 CK.LY30 values (in replicate 1) that were also below 1.25%. Therefore, there was agreement in 85% of the samples in the qualitative analysis. Among the remaining 46 samples (15% of 301) that were below 1.25%, 34 were within the normal reference range (0–2.6) and only 12 (4%) of the samples were above the 2.6% cutoff point value for normal reference range. Therefore, in 96% of samples, the TEG 6s as well as the TEG 5000 CK.LY30 values were in agreement with each other with respect to falling within the normal reference range. The predicted bias at the medical decision points for all the parameters met the predefined bias acceptance criteria. The summary of method comparison analysis, analysis of within device variability, and bias estimates are shown in Supplemental Digital Content 4, Tables 1–3, http://links.lww.com/TA/B502.
Overall within-device repeatability (as judged by regression line slope estimate, Pearson correlation analysis, and predictive bias at the medical decision limits) was excellent for all measurements (Table 2). However, the analysis of the within device agreement (or replicability), based on the agreement of the two replicates of the same device, suggests that the TEG 6s analyzer appears to be generally more consistent than TEG 5000 analyzer. The visual analysis of the two device replicates, presented in Supplemental Digital Content 5, Figures 5 and 6, (http://links.lww.com/TA/B503) shows that replicates of all TEG 5000 parameters, except CK.R, apparently have a higher variability (or spread) compared with TEG 6s parameters. The higher consistency of the TEG 6s can also be supported by the correlation analysis presented in Table 2. This analysis shows that the two replicates of TEG 6s parameters (except CK.R) not only have better estimates of the Pearson correlation coefficient but also the variance of the estimates is much smaller as implied by the length of the confidence intervals associated with the correlation coefficients. The analysis of the tracings (data not shown) of the TEG 5000 and TEG 6s replicates revealed that device- or operator-related tracing abnormalities are much more frequent in the TEG 5000 system compared with the TEG 6s system, with the latter having only a few failures.
TABLE 2.
Analysis of Within Device Variability for the TEG 6s and TEG 5000 Hemostasis Analyzers
DISCUSSION
In this method comparison study, the TEG 6s device was found to have excellent reliability when compared to the TEG 5000 device in a representative cohort of injured patients. Within device repeatability for CFF.MA and CK.LY30 appeared to be better (as judged by the regression line slope estimate and the predictive bias at the medical decision limits) for the TEG 6s device versus the TEG 5000 device, while the CK.R repeatability was slightly better for the TEG 5000 device versus TEG 6s device. Within device repeatability, comparing CRT.MA versus CK.MA was very similar for both devices. Pearson linear correlation coefficient estimates were above 0.9 for all measurements compared with their counterparts of the same assays (CK.LY30 vs. CK.LY30, CK.R vs. CK.R, CFF.MA vs. CFF.MA, and CRT.MA vs. CRT.MA).
In this study, subjects were chosen who met the full or limited trauma team activation criteria and were treated in Level I or II Trauma Centers. These eligibility criteria were intended to result in a quantitatively adequate representation of the general trauma patient population, including more severely injured patients who would potentially benefit from coagulation analysis with the TEG system. The trauma study population for this study provided adequate representation of the target ISS distribution. In general, the patient population with reported ISSs in this study showed a slightly more even distribution among the different severity categories than the distribution reported in the National Trauma Database 2016 report.19 Severely and very severely injured cohorts were well represented with observed percentages higher than in the historic trauma database19 as would be expected based on our eligibility criteria of full or limited trauma team activation.
Validation of the TEG 6s device for use in trauma represents an important advance in viscoelastic testing and trauma care. Although the TEG 6s and TEG 5000 devices have recently been shown to have strong correlation in a small ICU cohort study,20 this study represents the first analysis of its kind specifically focusing on trauma. A major potential advance for the TEG 6s device in trauma is a move toward more coagulation assessment at the site of care, as has been shown to be feasible in a small cohort study of trauma activations.21 The better performance profile of the TEG 6s device for most measures compared with the TEG 5000 system may be due to the design features which avoid dependence on technical skills in pipetting and susceptibility to vibration. These are particularly important advances toward the use of viscoelastic testing in prehospital care, and the TEG 6s device has recently been tested in simulated environments of aeromedical evacuation,16,17,22 and in preclinical models of ground and air medical transport.15 Taken together with the results of the present analysis, the TEG 6s device may be considered a reliable viscoelastic platform and diagnostic for trauma patients with potential for use at the site of care. The improved platform may allow for broad utilization of the device by health care professionals beyond laboratory technicians.
A recent study of a smaller sample size (n = 67 patients) by authors with clinical experience with both TEG and ROTEM devices compared the TEG 6s and the ROTEM Sigma devices in trauma.23 Strong correlations were observed between the measured parameters of the two devices, except in the case of EXTEM versus the rapid TEG which are different methodologies that do not align as well. Correlation of less strength was also found for angle, a parameter that was not part of this study. Comparison studies have also been carried out between the ROTEM Sigma and its predecessor, the ROTEM delta, which showed good correlation between the two platforms.24 However, this single-center trial did not contain enough patients (n = 30) to validate the reference ranges,25 method comparison for parameters was performed as a bundle across different assays, and critical clinical decision points differed between the two devices. For the present multicenter study at 12 clinical sites, we enrolled 475 patients to ensure the comparison was sufficiently powered, and adhered to the FDA guidance for method comparison studies for diagnostic devices.
This study has important limitations. The correlations between the TEG 6s and TEG 5000 systems were lowest for CK.LY30, although still considered to be very good for both within and between device comparisons. The low number of patients with hyperfibrinolysis (CK.LY30 > 2.6%) limits the assessment of the TEG 6s systems reliability in this cohort. Although the TEG 5000 device has previously been compared with conventional coagulation testing and found to be clinically superior for use in trauma,10 these comparisons remain untested for the TEG 6s device, although the strong correlation between the TEG 6s and TEG 5000 devices suggests that similar results may be expected. Although the study was conducted in multiple centers with a heterogeneous population of trauma patients, each of the centers has experience with the clinical use of TEG 5000 device for trauma, so the applicability of the results to centers with less experience with viscoelastic testing remains untested. Certain parameters that are available on the TEG 5000 analyzer (such as angle, K-time, ACT) were not studied and have not been included in the regulatory filing for clearance in a trauma setting.26
In conclusion, the TEG 6s Hemostasis Analyzer appears to be highly reliable for use in trauma patients, with close correlation to the TEG 5000 device as well as equivalent, if not improved, within-device reliability. Given the potential advantages of the TEG 6s device for use at the site of care, the confirmation of agreement between the TEG 6s and TEG 5000 systems in trauma represents an important advance in diagnostic testing for injured patients.
Supplementary Material
AUTHORSHIP
H.E.A., with support from J.H. and the principal investigators participated in study design. All authors participated in data collection. M.D.N., E.E.M., and J.H. participated in the Initial drafting of article. All authors participated in critical revision of article for intellectual content and all authors participated in approval of the final version for submission.
ACKNOWLEDGMENTS
The authors would like to thank Zorayr Manukyan, PhD, ClinStatDevice LLC for support with statistical considerations for study design, data quality monitoring, analysis methodology and execution, and also to thank Elmar Reinhold Burchardt, MD, University of Witten-Herdecke, Germany for support of clinical trial conception and operations, data review and analysis, article review and editing. We thank Meridian HealthComms, Plumley, UK for providing medical writing support, which was funded by Haemonetics SA, Signy, Switzerland in accordance with Good Publication Practice (GPP3).
Source of funding: This study was funded by Haemonetics Corporation, Braintree, USA. The data analysis was supported by a third party provider (ClinStatDevice LLC), funded by Haemonetics. Neither Drs. Neal, Moore, nor any of the co-authors received payment from Haemonetics for efforts associated with the article. The manuscript was drafted by Dr Neal, with support from Dr Moore and Dr Hartmann (Haemonetics). Medical writing support was funded by Haemonetics AS, Signy, Switzerland.
DISCLOSURE
MDN receives research support from the NIH and the United States Department of Defense. He receives research support from Janssen Pharmaceuticals, Haemonetics, Accriva Diagnostics, Instrument Laboratories, and Noveome Therapeutics. He has served as a paid scientific advisory board member to Janssen Pharmaceuticals and CSL Behring. E.E.M. received research support from Haemonetics for consumable supplies. M.W. and S.T. received research grants from Haemonetics for equipment, reagents and logistical and statistical support for research. R.A.C. received funding from Haemonetics for this research and receives funding for research from the NIH and DOD. L.Z.K. received funding from Haemonetics for this research and receives research support from the NIH. M.S. received consultancy payments from Haemonetics, Arsenal Medical and Velico Medical to his University. His university received funding from Haemonetics for this research. A.J.S. is on the speakers bureau for BMS, Pfizer, Janssen, and AstraZeneca. L.L. was previously a speaker for Haemonetics. R.D.W. is on the Editorial Board, Journal of Trauma and Acute Care Surgery (board service, no compensation) and the Editorial Board, SURGERY (honorarium for serving as Associate Editor for Social Media). N.S. received funding from Haemonetics for this research. J.H. and H.E.A. are or were employees of Haemonetics Corporation, Braintree, USA. For the remaining authors, no relevant conflicts were declared.
Footnotes
Published online: November 11, 2019.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
REFERENCES
- 1.Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shackelford SA, Del Junco DJ, Powell-Dunford N, Mazuchowski EL, Howard JT, Kotwal RS, Gurney J, Butler FK, Jr., Gross K, Stockinger ZT. Association of prehospital blood product transfusion during medical evacuation of combat casualties in Afghanistan with acute and 30-day survival. JAMA. 2017;318(16):1581–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tisherman SA, Schmicker RH, Brasel KJ, Bulger EM, Kerby JD, Minei JP, Powell JL, Reiff DA, Rizoli SB, Schreiber MA. Detailed description of all deaths in both the shock and traumatic brain injury hypertonic saline trials of the resuscitation outcomes consortium. Ann Surg. 2015;261(3):586–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neal MD, Moore HB, Moore EE, Freeman K, Cohen MJ, Sperry JL, Zuckerbraun BS, Park MS, TACTIC Investigators. Clinical assessment of trauma-induced coagulopathy and its contribution to postinjury mortality: a TACTIC proposal. J Trauma Acute Care Surg. 2015;79(3):490–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore HB, Winfield RD, Aibiki M, Neal MD. Is coagulopathy an appropriate therapeutic target during critical illness such as trauma or sepsis? Shock. 2017;48(2):159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez E, Moore EE, Moore HB, et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263(6):1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez E, Moore EE, Moore HB. Management of trauma-induced coagulopathy with thrombelastography. Crit Care Clin. 2017;33(1):119–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juffermans NP, Wirtz MR, Balvers K, et al. Towards patient-specific management of trauma hemorrhage: the effect of resuscitation therapy on parameters of thromboelastometry. J Thromb Haemost. 2019;17(3):441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ACS TQIP. Massive transfusion in trauma guidelines. https://www.facs.org/-/media/files/quality-programs/trauma/tqip/transfusion_guildelines.ashx?la=en October 2014. Accessed July 18, 2019.
- 10.Holcomb JB, Minei KM, Scerbo ML, et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Ann Surg. 2012;256(3):476–486. [DOI] [PubMed] [Google Scholar]
- 11.Dias JD, Sauaia A, Achneck HE, Hartmann J, Moore EE. Thromboelastography-guided therapy improves patient blood management and certain clinical outcomes in elective cardiac and liver surgery and emergency resuscitation: a systematic review and analysis. J Thromb Haemost. 2019;17(6):984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallett SV. Clinical utility of viscoelastic tests of coagulation (TEG/ROTEM) in patients with liver disease and during liver transplantation. Semin Thromb Hemost. 2015;41(5):527–537. [DOI] [PubMed] [Google Scholar]
- 13.Gurbel PA, Bliden KP, Tantry US, et al. First report of the point-of-care TEG: a technical validation study of the TEG-6S system. Platelets. 2016;27(7):642–649. [DOI] [PubMed] [Google Scholar]
- 14.Olechowski B, Dalton RT, Khanna V, Ashby A, Vavyla M, Mariathas M, Harris S, Nicholas Z, Mahmoudi M, Curzen N. Detection of individual responses to clopidogrel: validation of a novel, rapid analysis using thrombelastography 6s. Cardiovasc Ther. 2018;36(4):e12433. [DOI] [PubMed] [Google Scholar]
- 15.Roberts TR, Jones JA, Choi JH, et al. Thromboelastography on-the-go: evaluation of the TEG 6s device during ground and high-altitude aeromedical evacuation with extracorporeal life support. J Trauma Acute Care Surg. 2019;87:S119–S127. [DOI] [PubMed] [Google Scholar]
- 16.Scott R, Burns B, Ware S, Oud F, Miller M. The reliability of thromboelastography in a simulated rotary wing environment. Emerg Med J. 2018;35(12):739–742. [DOI] [PubMed] [Google Scholar]
- 17.Meledeo MA, Peltier GC, McIntosh CS, Voelker CR, Bynum JA, Cap AP. Functional stability of the TEG 6s hemostasis analyzer under stress. J Trauma Acute Care Surg. 2018;84(6S Suppl 1):S83–S88. [DOI] [PubMed] [Google Scholar]
- 18.NCCLS. Method Comparison and Bias Estimation Using Patient Samples; Approved Guideline—Second Edition. Pennsylvania, USA: NCCLS; 2002. [Google Scholar]
- 19.National Trauma Data Bank. National Trauma Data Bank 2016 Annual Report. https://www.facs.org/-/media/files/quality-programs/trauma/ntdb/ntdb-annual-report-2016.ashx?la=en 2016, Accessed July 18, 2019.
- 20.Lloyd-Donald P, Churilov L, Zia F, Bellomo R, Hart G, McCall P, Mårtensson J, Glassford N, Weinberg L. Assessment of agreement and interchangeability between the TEG5000 and TEG6S thromboelastography haemostasis analysers: a prospective validation study. BMC Anesthesiology. 2019;16:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morton S, Galea J, Uprichard J, Hudson A. The practicalities and barriers of using TEG6s in code red traumas: an observational study in one London major trauma centre. CJEM. 2019;21(3):361–364. [DOI] [PubMed] [Google Scholar]
- 22.Gill M. The TEG®6s on shaky ground? A novel assessment of the TEG®6s performance under a challenging condition. J Extra Corpor Technol. 2017;49(1):26–29. [PMC free article] [PubMed] [Google Scholar]
- 23.Ziegler B, Voelckel W, Zipperle J, Grottke O, Schöchl H. Comparison between the new fully automated viscoelastic coagulation analysers TEG 6s and ROTEM sigma in trauma patients: a prospective observational study. Eur J Anaesthesiol. 2019;36:834–842. [DOI] [PubMed] [Google Scholar]
- 24.Schenk B, Gorlinger K, Treml B, Tauber H, Fries D, Niederwanger C, Oswald E, Bachler M. A comparison of the new ROTEM® sigma with its predecessor, the ROTEMdelta. Anaesthesia. 2019;74(3):348–356. [DOI] [PubMed] [Google Scholar]
- 25.Field A, Poole T, Bamber JH. ROTEM® sigma reference range validity. Anaesthesia. 2019;74(8):1063. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Food and Drug Administration (FDA). K183160: TEG 6s Hemostasis System. https://www.accessdata.fda.gov/cdrh_docs/pdf18/K183160.pdf May 9, 2019. Accessed August 21, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


