Supplemental Digital Content is Available in the Text.
Keywords: acute, chronic, persistence, inception cohort, low back pain, two-stage sampling
Abstract
Introduction:
The neurobiological mechanisms underlying recovery from or persistence of low back pain (LBP) remain misunderstood, limiting progress toward effective management. We have developed an innovative two-tier design to study the transition from acute to chronic LBP. The objective of the first tier is to create a provincial web-based infrastructure to recruit and monitor the trajectory of individuals with acute LBP. The objective of the second tier is to fuel hypothesis-driven satellite data collection centers with specialized expertise to study the role of biomechanical, epigenetic, genetic, neuroanatomical, ontological, physiological, psychological, and socioeconomic factors in LBP chronicity.
Methods:
This article describes the first tier of the protocol: establishment of the Core Dataset and Cohort. Adults with acute LBP will be recruited through networks, media, and health care settings. A web-based interface will be used to collect self-reported variables at baseline and at 3, 6, 12, and 24 months. Acute LBP will be defined according to the Dionne 2008 consensus. Measurements will include the Canadian minimum data set for chronic LBP research, DN4 for neuropathic pain, comorbidities, EQ-5D-5L for quality of life, and linkage with provincial medico-administrative databases. The primary outcome will be the transition to chronic LBP, as defined by Deyo 2014. Secondary outcomes include health care resource utilization, disability, sick leave, mood, and quality of life.
Perspective:
This study brings together diverse research expertise to investigate the transition from acute to chronic LBP, characterize the progression to recovery or chronicity, and identify patterns associated with that progression.
1. Introduction
The estimated worldwide 1-month prevalence of low back pain (LBP) is as high as 23%, suggesting that about a quarter of the global population experiences LBP in any given month.25,32 According to a recent systematic review, the lifetime prevalence of LBP ranges from 2% in urban adults aged ≥15 years in Pakistan to 86% among adults (≥18 years) in Germany.25 Low back pain is also the leading cause of global years-lived-with-disability.44 The high prevalence of LBP has significant economic consequences, with direct medical cost estimates ranging from 12.2 to 90 billion US dollars annually.10 Low back pain is the sixth most costly disease, behind ischemic heart disease, motor vehicle accidents, acute respiratory infections, arthropathies, and hypertension.13
Most LBP cases cannot be linked conclusively to a specific nociceptive source or pathoanatomical cause,31 and factors associated with LBP chronicity remain poorly understood.2,21,27 Although some risk factors have been extensively studied (individual/socioeconomic, occupational, pain characteristics, and psychological),5,18,30,33,34 others have not (biomechanical, epigenetic, genetic, and neuroanatomical). A better understanding of the vulnerabilities underlying recovery or persistence of LBP will allow for early intervention and prevention of the progression to a chronic state.
Many regions or countries are developing acute LBP cohorts including Australia, United States, and Denmark.9,22,28,35 Although comprehensive longitudinal data can be derived from these cohorts, limitations exist. For example, recruitment of participants from a limited number of clinicians located in a restricted geographical region excludes individuals who have not seen a professional, who have seen a professional not participating in the cohort study, or who live outside the designated region. A number of additional large8,16 and smaller14,15 prospective cohorts have initiated recruitment without a clear focus on any particular stage of LBP. Finally, other large cohorts have been built to identify the occupational as well as the sociodemographic, general health, and psychological risk factors for the occurrence of chronic LBP without a clear strategy for the integration of these factors with biomechanical, epigenetic, genetic, and neuroanatomical characteristics.19,26,39 A unifying project, focused on the transition from acute to chronic LBP that brings together basic, clinical, and epidemiological methods/expertise, is needed to understand the complex nature of these factors and their interactions.
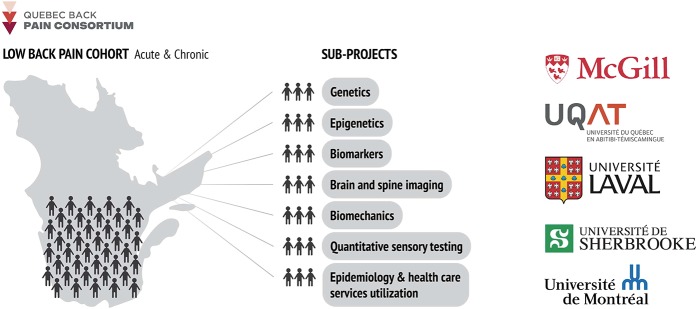
The Quebec Low Back Pain Study (QLBPS), a strategic initiative of the Quebec Back Pain Consortium (QBPC; https://backpainconsortium.ca) and the Quebec Pain Research Network (QPRN; https://qprn.ca/en), was created to address these limitations. The proposed design enables the integration of information across multiple domains, thus providing a comprehensive view of risk factors for LBP chronicity. The scientific committee of the QBPC is composed of experts from diverse disciplines including physical therapy and biomechanics, epigenetics, genetics, neuroanatomy, medicine, epidemiology, and health ontology and psychology, in addition to patients' perspective representatives. The long-term goal of our consortium is to establish a province-wide online database for longitudinal studies of individuals with LBP. This will provide a comprehensive view of the risk factors for the development of chronic LBP that will break disciplinary silos.
2. Methods
This multidisciplinary, longitudinal cohort protocol has been prepared according to the SPIRIT 2013 guidelines for completeness and quality of trial protocols7 and complies with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.42,43
2.1. Context
The QLBPS was developed by the QBPC, a strategic initiative of the QPRN funded by the Fonds de Recherche du Québec—Santé, a provincial government funding agency. The scientific committee has been meeting regularly since 2015 to develop a shared vision and to design and implement the study described here. Figure 1 illustrates the vision and participating institutions.
Figure 1.
Quebec Back Pain Consortium vision and participating members.
2.2. Study design
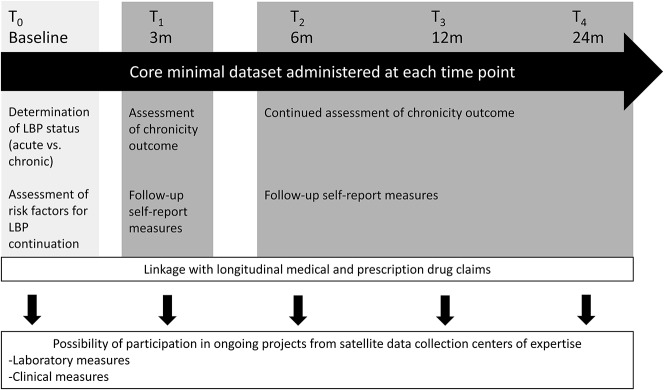
We will use a 2-stage approach (Fig. 2). The first stage, described here, will create the QLBPS Core Dataset and Cohort. Self-reported variables will be assessed in this provincial sample of patients suffering from LBP with follow-ups at 3, 6, 12, and 24 months after baseline. The Cohort will then “fuel” smaller studies in the second stage where patients will be invited to visit specific research laboratories for more extensive measurements (eg, biomechanics, epigenetics, genetics, neuroimaging, and sensory testing). All data collected from the first stage will be available for the satellite projects conducted in the second stage.
Figure 2.
The Quebec Low Back Pain Study methodology. T0 to T4 represent the different data collection time point for all patients enrolled. Everyone will complete the core minimal data set 5 times within the period of 2 years. The baseline questionnaires will be used to categorize patients into acute or chronic LBP and identify risk factors that predict the maintenance of the LBP. At 3 months and for all subsequent time points, LBP status will be re-evaluated along with secondary outcomes. When patients consent, the provincial health plan number (RAMQ, Régie de l'assurance maladie du Québec) will be collected to merge with their medical and prescription drug claims. Finally, at any point during their active participation in the study, patients could be solicited to participate in one or more of the ongoing satellite projects. LBP, low back pain.
2.3. Inclusion and exclusion criteria
Potential participants will be 18 years and older, have internet access, be fluent in either French or English, and have self-reported LBP. No specific exclusion criteria will be applied.
2.4. Recruitment
Recruitment was initiated in November 2018 and is ongoing; 46 acute LBP patients completed the baseline questionnaires in the 7-month period between November 2018 and June 2019. In consultation with marketing experts, we then optimized of our recruitment strategy, resulting in 7 additional acute LBP patients weekly between June 2019 and August 2019. The recruitment strategy includes the following target populations: (1) general population in the province of Quebec (through advertisement in newspapers, public means of transportation, and social media such as Facebook); (2) individuals or institutions by email list servers; (3) populations of individuals at risk of LBP (through advertisement with patient advocacy organizations or professional societies [ie, Association québécoise de la douleur chronique, QPRN, Canadian Pain Society]); (4) unions of workers at risk for LBP (including but not limited to construction workers, warehouse workers, public service workers [ie, police and firefighters], nurses, other allied health professionals, and bus drivers); and (5) medical populations (through advertisements in emergency units, medical, physiotherapy, chiropractic, radiology clinics, and pharmacies).
In parallel to the acute-phase recruitment, individuals with chronic LBP who self-register for the study will be included in the Core Database. Their longitudinal data will be valuable for comparative purposes, for additional trajectory analyses, and for recruitment of chronic LBP participants in affiliated satellite projects.
2.5. Data collection methods
The recruitment strategies listed above will all include an invitation to a web platform (https://backpainconsortium.ca/), and/or aliases mybackhurts.ca or malaudos.ca. On this platform, interested participants will be asked to provide their postal code, sex, age, pain location, and intensity. Potential participants will confirm their interest by providing their email and full name and will complete a CAPTCHA (Completely Automated Public Turing Test To Tell Computers and Humans Apart) verification. Only those providing all the required information and that self-identify with LBP will receive an email inviting them to access the baseline online survey. This email will also include a link to the informed consent form, will encourage potential participants to read the document carefully, and will inform them that the study coordinator is available for any questions. Participants will be enrolled after they electronically provide their consent to participate. They will also be asked whether they are willing to be contacted again by other researchers and/or if they are willing to donate a blood sample. Once enrolled, they will be automatically redirected to the web-based baseline self-administered questionnaire. Baseline and follow-up questionnaires are hosted on the data collection platform Research Electronic Data Capture (REDCap) for electronic collection and management of research data. Completion of the baseline questionnaire takes approximately 20 minutes to complete. At 3, 6, 12, and 24 months after completing the baseline questionnaire, participants will receive an email with a unique link for completion of follow-up web-based questionnaires that take approximately 20 minutes to complete.
Evidenced-based retention strategies1,20,36 will be applied. At each time point, participants who do not complete the above-mentioned questionnaires will be contacted by the study coordinator by phone and/or e-mail no more than 3 times. Other retention strategies include sending a quarterly newsletter to recruited participants to inform them of study progress, to share relevant information and resources about LBP, and to “humanize” the research by bridging the gap between researchers' goals and participants using photographs and biographies, for example. Participants will have the potential to share their experience with other participants through the newsletter and will receive tokens of appreciation as they complete steps in the study such as a pen, a fridge magnet, or a badge. Finally, all participants will be invited to enter into drawings for prepaid VISA cards.
2.6. Study variables and validated measurements
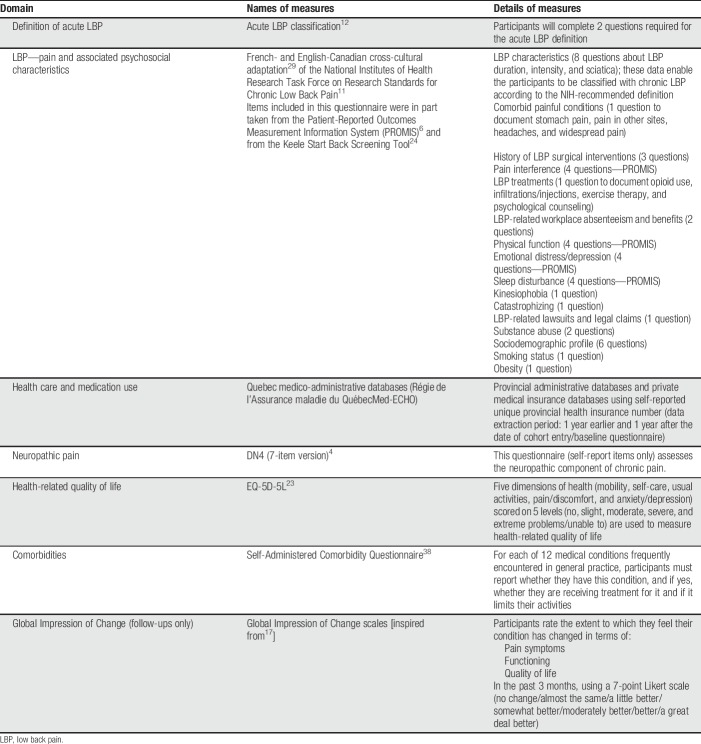
Multiple meetings between the authors and a thorough review of the existing literature on LBP and psychometric measurements were conducted to select the core data set. Criteria for inclusion in the core data set was based on recommendations from expert research groups, including the National Institutes for Health Research (NIH) Task Force on Research Standards for Chronic Low Back Pain11 and the IMMPACT consensus recommendations.41 Measures included in the core data set are presented in Table 1. A trick question (please select number 4 in the options below) has been inserted to gauge the quality of participants' responses. Permission has been obtained to use the NIH, DN4, and EQ-5D-5L questionnaires for research purposes. The complete set of questions is included in Supplementary Appendix 1 (available as supplemental digital content at http://links.lww.com/PR9/A59). The psychometric properties of the scales are included in Supplementary Appendix 2 (available as supplemental digital content at http://links.lww.com/PR9/A59).
Table 1.
Details of measures and variables included in the minimal core data set.
2.7. Characterization of low back pain status
The criteria for acute LBP will be based on the consensus statement of Dionne and colleagues.12 According to their recommendations, patients who answer “yes” to the 2 following questions are suffering from acute LBP: (1) In the past 4 weeks, have you had pain in your lower back (in the area shown on the diagram), and (2) if yes, was this pain bad enough to limit your usual activities or change your daily routine for more than one day?
2.8. Primary outcome
The primary outcome of the Core Dataset is whether participants who reported acute LBP at baseline will transition to chronic LBP. Chronic LBP will be defined based on the NIH task force recommendations that define chronic LBP as an ongoing problem for at least 3 months and that has resulted in a problem on at least half of the days in the past 6 months.11
2.9. Secondary outcomes
Secondary outcomes include the duration of the LBP episode and/or the presence (and number) of recurrent LBP episodes,40 work status, use of health care resources and direct medical costs incurred (including economic burden data), participants' functional limitations and sick leave, mood, as well as the longitudinal trajectory of health-related quality of life.
2.10. Data analysis (Core Dataset)
All study variables will be screened for normality as well as univariate and multivariate outliers. Missing data will be handled with multiple imputation when indicated.37 Mean values, medians, and SDs will be computed for continuous variables while percentages and frequencies will be computed for categorical variables. Depending on the distribution of variables, longitudinal changes in pain severity and their predictors will be examined using various multivariate approaches, including latent class analysis or hierarchical cluster informed by linear regression. A P-value ≤0.05 will be considered statistically significant.
The QLBPS Core Cohort is a continuous recruitment platform; thus, no minimum or maximum number of participants has been set.
2.11. Ethical approval and confidentiality
The QLBPS Core Dataset (first tier) has received ethical approval from McGill University (A06-M22-18A) and will be conducted in conformity with the ethical principles set forth by the Regulatory Framework in Health Research at the McGill University Health Centre in accordance with the second edition of the Tri-council Policy Statement. Participants will be automatically assigned a study ID by REDCap ensuring that only the principal investigator and study coordinators will have access to participants' ID. Nominal data and study ID numbers are kept in a separate Excel file protected by a password. All other study investigators and staff will have access to denominalized data. Any important protocol modifications will be subject to approval from the scientific committee of the Quebec Back Pain Consortium and the Ethics Committee of McGill University.
2.12. Second-stage sampling
In the baseline online survey, participants will be asked whether they are willing to be contacted for participation in additional studies, where they live and whether they would consider donating blood. The answers will be recorded in the Core Database and will be used to identify participants who may be contacted by other QPRN-approved investigators for satellite data collection centers. If participants are interested in donating blood, they will receive an email from the QLBPS Biobank Team. The biobank is covered under a separate sister protocol at the McGill Ethics Committee entitled “Quebec Low Back Pain Biobank” (A08-M23-18A).
2.13. Satellite projects
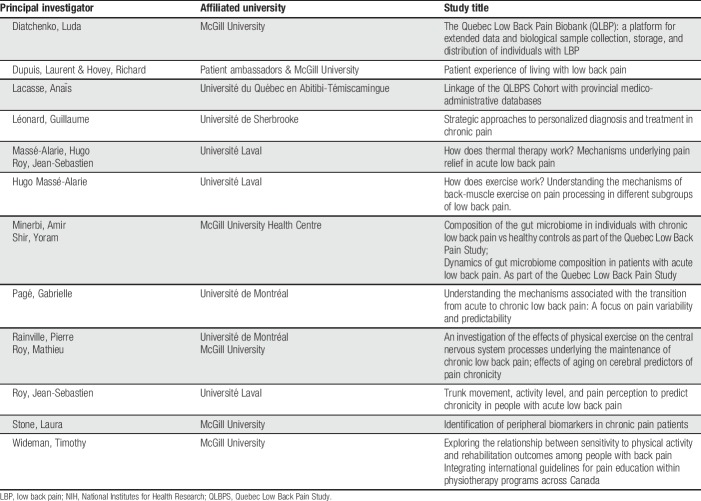
Researchers with ethical approval at their home institutions and from the scientific committee of the QLBPS will be provided participants' study ID, name, and contact information. This is the only time that investigators will have access to nominalized data and will be for recruitment purposes only. All other internal and external researchers will have access to the deidentified QLBPS Core Dataset upon request. A current list of scientific committee-approved satellite projects is shown in Table 2.
Table 2.
Satellite projects affiliated with the Quebec Low Back Pain Study.
2.14. Assignation and allocation rules for the satellite projects
Participants who agreed to be contacted to participate in additional studies will be assigned to ongoing satellite projects associated with the QLBPS based on each project's inclusion and exclusion criteria. As part of the consortium, researchers of ongoing satellite projects will have a shared script and recruitment efforts will be coordinated by the consortium study coordinator. This shared workflow will optimize the utility of the cohort and minimize burden to patients. Potential bias to the dispatch process of participants into satellite projects include geographic location of participants, which may limit the projects they can enroll in, and participants' willingness to undergo procedures such as blood donation or magnetic resonance imaging. Bias in the assignment to satellite studies that are in competition for subjects will be mitigated by the shared scripts and managed by the coordinator. More specifically, participants meeting the criteria for more than one satellite project will be assigned to projects in turn; priority will alternate between each project, taking into account the targeted sample size.
2.15. Dissemination
Results from the Core Dataset as well as those from satellite projects will be presented at national and international conferences and published in peer-reviewed journals in the fields of pain and musculoskeletal diseases, as well as discipline-specific journals relevant to the specific questions and methodologies of the satellite projects.
3. Discussion
A multidisciplinary approach investigating the factors contributing to the recovery from LBP, the recurrence of LBP episodes, and the factors influencing the transition to chronic LBP will be facilitated by the pragmatic two-stage sampling approach where patients with acute LBP are continuously enrolled in a core longitudinal cohort and subsequently recruited for satellite projects tackling specific questions. Given the high global incidence and costs of LBP,10,25,32 improved understanding of the mechanisms driving LBP chronicity is desperately needed.21,27
This study design confers multiple advantages. First, the existence of the Core Dataset and the composition of its scientific committee will allow for the examination of the chronicity of LBP from a large array of research perspectives including biomechanical, epigenetic, genetic, neuroanatomical, ontological, psychological, physiological, and socioeconomical mechanisms. This approach will make it possible to examine simultaneously the multifactorial determinants of LBP by providing adequate statistical power and measurement. Second, a common set of carefully selected measures assessed from the acute phase (baseline) up to 2-year follow-up will be available to all researchers. These measures are based on the minimum data set developed by the NIH Task Force on Research Standards for Chronic Low Back Pain11 and on our Canadian adaptation of those recommendations.29 Third, the Core Dataset will include participants from the entire province of Quebec, ensuring representation from urban and rural areas and remote regions. Given its web-based format, the design also allows for expansion nationally and internationally. Fourth, for participants who provide their unique provincial health insurance number, health care use, including medication, can be studied to assess important aspects of the economic burden of LBP. Finally, the design provides a practical solution for deep phenotyping of a subset of participants while maintaining the sample sizes needed for epidemiological studies.
Some limitations must nonetheless be noted. First, no formal medical validation of participants' LBP or its potential drivers will be included for the Core Dataset as all measures will be self-reported. However, medical validation will be possible in satellite projects. Second, there will be geographical barriers to participation in satellite projects such as the physical distance from research laboratories with specialized equipment or expertise (eg, MRI facilities specialized in brain and spinal imaging). Third, to keep the questionnaire short, not all domains are fully explored. For example, although the NIH section includes several questions on employment and legal status, job satisfaction is not assessed. Finally, the Core Dataset is available online through a web-based platform; participation may therefore be difficult for individuals with limited computer skills. This concern is mitigated by (1) availability of our staff to guide participants by phone and (2) data showing that 88% of individuals in Canada have access to Internet for personal use.3
Ultimately, the Core Dataset will provide: (1) a pool of potential LBP patients for other research studies, (2) a database allowing for investigation s into acute to chronic LBP, and (3) analysis of the relationships between parameters collected as part of the Core and affiliated studies, such as pain trajectories and blood-based genetic or epigenetic factors.
In summary, the QLBPS Core Cohort and Dataset, with its innovative two-stage sampling approach, will constitute a valuable platform for the continuous enrolment of LBP patients to facilitate the integrated investigation of factors (eg, biomechanical, epigenetic, genetic, neuroanatomical, ontological, physiological, psychological, and socioeconomic) contributing to the transition from acute to chronic LBP, how these factors might change with time as pain progresses, and what are the health care utilization and medication patterns associated with that progression. The integration of data gathered from the QLBPS Core Dataset, and the satellite data collection centers of expertise will support the development of comprehensive profiles of patients at risk of LBP chronicity.
Disclosures
L. Diatchenko and F. Montagna report grant SCA145102 from the Strategy for Patient Oriented Research (SPOR) during the conduct of the study. L.S. Stone reports grants and nonfinancial support from Quebec Pain Research Network during the conduct of the study. The remaining authors have no conflicts of interest to declare.
Previous presentations: Quebec Pain Research Network Annual General Meeting November 2017—Project update, Quebec Pain Research Network Annual General Meeting January 2019—Project update, Canadian Pain Network Biomarkers Meeting—January 2019—Project overview.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A59.
Acknowledgements
The authors want to thank Nabiha Benyamina Douma, who contributed to the literature review on chronic low back pain risk factors, Dr Geneviève Lavigne for contributions to the drafting of this manuscript, the Alan Edwards Pain Management Unit for infrastructure support, Ms Iulia Tufa for technical assistance, the Quebec Pain Research Network (QPRN) for funding and support, and Mr Louis Coupal for bioinformatics services.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
G.M. Pagé, A. Lacasse contributed equally to the manuscript.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
References
- [1].Abshire M, Dinglas VD, Cajita MI, Eakin MN, Needham DM, Himmelfarb CD. Participant retention practices in longitudinal clinical research studies with high retention rates. BMC Med Res Methodol 2017;17:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ardakani EM, Leboeuf-Yde C, Walker BF. Can we trust the literature on risk factors and triggers for low back pain? A systematic review of a sample of contemporary literature. Pain Res Manag 2019;2019:6959631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bernier M. L'utilisation d'Internet chez les Québécois. Science, technologie et innovation en bref. Montreal: Institut de la statistique du Québec, 2017. p. 9. [Google Scholar]
- [4].Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, Cunin G, Fermanian J, Ginies P, Grun-Overdyking A, Jafari-Schluep H, Lanteri-Minet M, Laurent B, Mick G, Serrie A, Valade D, Vicaut E. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). PAIN 2005;114:29–36. [DOI] [PubMed] [Google Scholar]
- [5].Cancelliere C, Donovan J, Stochkendahl MJ, Biscardi M, Ammendolia C, Myburgh C, Cassidy JD. Factors affecting return to work after injury or illness: best evidence synthesis of systematic reviews. Chiropr Man Therap 2016;24:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, Devellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai JS, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays R, Group PC. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol 2010;63:1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, Dickersin K, Hróbjartsson A, Schulz KF, Parulekar WR, Krleza-Jeric K, Laupacis A, Moher D. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen Y, Campbell P, Strauss VY, Foster NE, Jordan KP, Dunn KM. Trajectories and predictors of the long-term course of low back pain: cohort study with 5-year follow-up. PAIN 2018;159:252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Costa LdCM, Maher CG, McAuley JH, Hancock MJ, Herbert RD, Refshauge KM, Henschke N. Prognosis for patients with chronic low back pain: inception cohort study. BMJ 2009;339:b3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J 2008;8:8–20. [DOI] [PubMed] [Google Scholar]
- [11].Deyo RA, Dworkin SF, Amtmann D, Andersson G, Borenstein D, Carragee E, Carrino JA, Chou R, Cook K, DeLitto A, Goertz C, Khalsa P, Loeser J, Mackey S, Panagis J, Rainville J, Tosteson T, Turk D, Von Korff M, Weiner DK. Focus article report of the NIH task force on research standards for chronic low back pain. Clin J Pain 2014;30:701–12. [DOI] [PubMed] [Google Scholar]
- [12].Dionne CE, Dunn KM, Croft PR, Nachemson AL, Buchbinder R, Walker BF, Wyatt M, Cassidy JD, Rossignol M, Leboeuf-Yde C, Hartvigsen J, Leino-Arjas P, Latza U, Reis S, Gil Del Real MT, Kovacs FM, Oberg B, Cedraschi C, Bouter LM, Koes BW, Picavet HS, van Tulder MW, Burton K, Foster NE, Macfarlane GJ, Thomas E, Underwood M, Waddell G, Shekelle P, Volinn E, Von Korff M. A consensus approach toward the standardization of back pain definitions for use in prevalence studies. Spine (Phila Pa 1976) 2008;33:95–103. [DOI] [PubMed] [Google Scholar]
- [13].Druss BG, Marcus SC, Olfson M, Pincus HA. The most expensive medical conditions in America. Health Aff (Millwood) 2002;21:105–11. [DOI] [PubMed] [Google Scholar]
- [14].Dunn KM, Campbell P, Jordan KP. Long-term trajectories of back pain: cohort study with 7-year follow-up. BMJ Open 2013;3:e003838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dunn KM, Croft PR. Classification of low back pain in primary care: using “bothersomeness” to identify the most severe cases. Spine 2005;30:1887–92. [DOI] [PubMed] [Google Scholar]
- [16].Farjam M, Askari A, Hoseinipour A, Homayounfar R, Jamshidi J, Khodabakhshi F, Zakeri H. A cohort study protocol of low back pain in rural area inhabitants: Fasa Low Back Pain Cohort Study (FABPACS). Galen Med J 2016;5:225–9. [Google Scholar]
- [17].Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. PAIN 2001;94:149–58. [DOI] [PubMed] [Google Scholar]
- [18].Foster NE, Thomas E, Bishop A, Dunn KM, Main CJ. Distinctiveness of psychological obstacles to recovery in low back pain patients in primary care. PAIN 2010;148:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Garg A, Hegmann KT, Moore JS, Kapellusch J, Thiese MS, Boda S, Bhoyr P, Bloswick D, Merryweather A, Sesek R, Deckow-Schaefer G, Foster J, Wood E, Sheng X, Holubkov R; BackWorks Study T. Study protocol title: a prospective cohort study of low back pain. BMC Musculoskelet Disord 2013;14:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hanna KM, Scott LL, Schmidt KK. Retention strategies in longitudinal studies with emerging adults. Clin Nurse Spec 2014;28:41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, Hoy D, Karppinen J, Pransky G, Sieper J, Smeets RJ, Underwood M; Lancet Low Back Pain Series Working G. What low back pain is and why we need to pay attention. Lancet 2018;391:2356–67. [DOI] [PubMed] [Google Scholar]
- [22].Henschke N, Maher CG, Refshauge KM, Herbert RD, Cumming RG, Bleasel J, York J, Das A, McAuley JH. Prognosis in patients with recent onset low back pain in Australian primary care: inception cohort study. BMJ 2008;337:a171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, Bonsel G, Badia X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Quality Life Res 2011;20:1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hill JC, Dunn KM, Lewis M, Mullis R, Main CJ, Foster NE, Hay EM. A primary care back pain screening tool: identifying patient subgroups for initial treatment. Arthritis Rheum 2008;59:632–41. [DOI] [PubMed] [Google Scholar]
- [25].Hoy D, Bain C, Williams G, March L, Brooks P, Blyth F, Woolf A, Vos T, Buchbinder R. A systematic review of the global prevalence of low back pain. Arthritis Rheum 2012;64:2028–37. [DOI] [PubMed] [Google Scholar]
- [26].Jarvik JG, Hollingworth W, Heagerty PJ, Haynor DR, Boyko EJ, Deyo RA. Three-year incidence of low back pain in an initially asymptomatic cohort: clinical and imaging risk factors. Spine 2005;30:1541–8. [DOI] [PubMed] [Google Scholar]
- [27].Kent PM, Keating JL. Can we predict poor recovery from recent-onset nonspecific low back pain? A systematic review. Man Ther 2008;13:12–28. [DOI] [PubMed] [Google Scholar]
- [28].Kent P, Kongsted A, Jensen TS, Albert HB, Schiottz-Christensen B, Manniche C. SpineData - a Danish clinical registry of people with chronic back pain. Clin Epidemiol 2015;7:369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lacasse A, Roy JS, Parent AJ, Noushi N, Odenigbo C, Page G, Beaudet N, Choiniere M, Stone LS, Ware MA; Quebec Pain Research Network's Steering Committee of the Low Back Pain Strategic I. The Canadian minimum dataset for chronic low back pain research: a cross-cultural adaptation of the National Institutes of Health Task Force Research Standards. CMAJ Open 2017;5:E237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lagersted-Olsen J, Bay H, Jorgensen MB, Holtermann A, Sogaard K. Low back pain patterns over one year among 842 workers in the DPhacto study and predictors for chronicity based on repetitive measurements. BMC Musculoskelet Disord 2016;17:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet 2016;389:736–47. [DOI] [PubMed] [Google Scholar]
- [32].Manchikanti L, Singh V, Falco FJ, Benyamin RM, Hirsch JA. Epidemiology of low back pain in adults. Neuromodulation 2014;17:3–10. [DOI] [PubMed] [Google Scholar]
- [33].Manek NJ, Macgregor AJ. Epidemiology of back disorders: prevalence, risk factors, and prognosis. Curr Opin Rheumatol 2005;17:134. [DOI] [PubMed] [Google Scholar]
- [34].Marcuzzi A, Wrigley PJ, Dean CM, Graham PL, Hush JM. From acute to persistent low back pain: a longitudinal investigation of somatosensory changes using quantitative sensory testing—an exploratory study. Pain Rep 2018;3:e641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mehling WE, Gopisetty V, Bartmess E, Acree M, Pressman A, Goldberg H, Hecht FM, Carey T, Avins AL. The prognosis of acute low back pain in primary care in the United States: a 2-year prospective cohort study. Spine 2012;37:678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Robinson KA, Dinglas VD, Sukrithan V, Yalamanchilli R, Mendez-Tellez PA, Dennison-Himmelfarb C, Needham DM. Updated systematic review identifies substantial number of retention strategies: using more strategies retains more study participants. J Clin Epidemiol 2015;68:1481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rubin DB. Multiple imputation for nonresponse in surveys. Hoboken, NJ: Wiley-Interscience, 2004. [Google Scholar]
- [38].Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum 2003;49:156–63. [DOI] [PubMed] [Google Scholar]
- [39].Smedley J, Egger P, Cooper C, Coggon D. Prospective cohort study of predictors of incident low back pain in nurses. BMJ 1997;314:1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Stanton TR, Henschke N, Maher CG, Refshauge KM, Latimer J, McAuley JH. After an episode of acute low back pain, recurrence is unpredictable and not as common as previously thought. Spine (Phila Pa 1976) 2008;33:2923–8. [DOI] [PubMed] [Google Scholar]
- [41].Turk DC, Dworkin RH, Allen RR, Bellamy N, Brandenburg N, Carr DB, Cleeland C, Dionne R, Farrar JT, Galer BS, Hewitt DJ, Jadad AR, Katz NP, Kramer LD, Manning DC, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robinson JP, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Witter J. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. PAIN 2003;106:337–45. [DOI] [PubMed] [Google Scholar]
- [42].von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–9. [DOI] [PubMed] [Google Scholar]
- [43].von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–9.25046131 [Google Scholar]
- [44].Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, Carter A, Casey DC, Charlson FJ, Chen AZ. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A59.