Abstract
Introduction:
Multidisciplinary care is recommended for disabling persistent low back pain (pLBP) nonresponsive to primary care. Cognitive functional therapy (CFT) is a physiotherapy-led individualised intervention targeting psychological, physical, and lifestyle barriers to recovery, to self-manage pLBP.
Objectives:
This pilot study investigated clinical outcomes and pain thresholds after a 12-week CFT pathway in patients with severe pLBP referred to a University Pain Center. Exploratory analyses compared changes in clinical outcomes, opioid consumption, and costs after CFT with changes after a multidisciplinary pain management (MPM) pathway.
Methods:
In total, 47 consecutively referred pLBP patients consented to the CFT pathway. At baseline, 3 and 6 months, clinical outcomes and PPTs were assessed. Control patients (n = 99) who had completed an MPM pathway in the last 3 years were matched from the clinical pain registry used in the Pain Center in a 3:1 ratio based on propensity scores derived from relevant baseline variables of the CFT cases.
Results:
Most clinical outcomes and low back pressure pain threshold were improved at 3 and 6 months after the CFT pathway. Compared with MPM, CFT patients had significantly larger reductions in disability and improved quality of life after the interventions at a lower cost (−3688€ [confidence interval: −3063 to −4314€]). Reduction in pain intensity and proportion of patients withdrawing from opioids (18.2% vs 27.8%) were similar between CFT and MPM groups.
Conclusion:
Improvements in clinical and experimental pain were found after the CFT pathway. Fully powered randomized controlled trials comparing CFT with an MPM program in patients with disabling pLBP are warranted to control for the current limitations.
Keywords: Low back pain, Persistent pain, Chronic pain, Opioids, Cognitive functional therapy, Multidisciplinary pain rehabilitation
1. Introduction
Low back pain (LBP) is the leading cause of disability.57 Despite increasing resources being spent on managing this condition, a significant proportion does not fully recover within a year.11 Failure to recover often results in a trajectory of seeking pain specialist's second opinions, surgical evaluations,58 and high use of opioid medication.21 Evidence suggests that persistent low back pain (pLBP) is a multidimensional biopsychosocial problem38,39 with various contributing factors, such as negative pain cognitions, pain-related fear and emotional distress,27,31,33,55,59 avoidant and protective movement behaviors,10 and unhelpful lifestyle factors such as activity avoidance and sleep problems.2
Current guidelines recommend that patients with pLBP who do not benefit from primary care treatment should be referred to multidisciplinary pain rehabilitation in secondary care settings.6,37 However, such treatments are often expensive, not easily accessible, and have small effects.16,49 Therefore, less expensive and more accessible management strategies targeting these multidimensional barriers to recovery may facilitate earlier improvements.
Cognitive functional therapy (CFT)35,36 is a physiotherapy-led individualised intervention that targets physical, lifestyle, and psychological barriers to recovery, to coach people to self-manage pLBP. Cognitive functional therapy has shown promising results in patients with pLBP in primary care with low to moderate disability compared with exercise and manual therapy at 12-month54 and 36-month19 follow-ups. A recent RCT demonstrated CFT to be more effective in reducing disability levels at 6 and 12 months, than group education and exercise in people with moderate disabling pLBP.34 To date, the effectiveness of CFT has not been investigated in patients with severe pLBP and high levels of disability who have not benefited from primary care treatments, and it has not been compared with a multidisciplinary pain management program (MPM). Although cognitive and emotional factors, such as levels of fear and distress, have been observed to reduce after CFT,35,54 no study has yet investigated whether clinical improvements after CFT are associated with changes in pain sensitivity, which would add important knowledge regarding the possible underlying mechanisms of change associated with CFT.
As a precursor to a larger-scale randomized controlled trial comparing CFT with MPM, this pilot study investigated clinical outcomes and pressure pain thresholds (PPTs) after a 12-week CFT treatment pathway in patients with severe pLBP referred to a University Hospital Pain Center. In addition, exploratory analyses comparing changes in clinical outcomes, opioid consumption, and treatment costs in patients who took the CFT pathway with a matched cohort who took the MPM pathway were performed on clinical outcomes, opioid consumption, and treatment costs.
It was hypothesized that (1) CFT would result in improvement in clinical outcomes and pain sensitivity, and (2) improvements in clinical pain would be associated with improvements in pain sensitivity.
2. Materials and methods
The CONSORT-NPT was used as a guideline for reporting this study,3 which was conducted in accordance with the Helsinki Declaration, registered at the Danish Data Protection Agency (17/23847), approved by the local ethics committee (S-20170029), and all patients provided written informed consent.
2.1. Cognitive functional therapy participants
All patients referred to the Odense University Pain Center between June 2017 and February 2018, who reported pain (>6 months) in the lower back as their primary pain complaint, were invited to participate in the study. Patients between 18 and 75 years of age and adept in Danish were eligible. Patients were excluded if any of these criteria were present: pregnancy, former/present addictive behavior, neurological, or cardiovascular diseases. Patients included in the CFT pathway were requested not to receive other treatments while participating in the study but were allowed to continue analgesics.
2.2. Case–control patients
The Pain Center care group was retrospectively selected from 250 eligible patients in the PainData Registry used in the Pain Center, who had LBP, had completed the end of treatment questionnaire, had received the multidisciplinary intervention of interest, and had given electronic consent that data could be used for research. For referral, patients must have had pain >6 months and report significant disability and psychological distress affecting daily life. Patients in this setting have moderate to severe pain intensity, high disability, and psychological distress, and most report pain in more than one body area.50,52
2.3. Case–control matching
Each person in the CFT group was matched with 3 people in the Pain Center care group, to account for the potential diversity of treatment exposure in the control group. Case/control matching used propensity scores based on the following baseline variables: age, sex, body mass index, LBP intensity, LBP duration, number of pain areas, Pain Disability Index (PDI) score, anxiety, depression, pain catastrophization, fear of movement, health-related quality of life (QOL), and use of analgesics or opioids. After matching (STATA psmatch2), the distributions of the baseline variables used in the propensity scoring were not statistically different (STATA pstest) between the cases and controls (t tests P = 0.295–0.957, likelihood-ratio test P = 0.49, Rubin R = 1.28), indicating that no selection bias was present on these measured variables.
2.4. Cognitive functional therapy pathway
The CFT pathway included treatment performed by 1 of 3 CFT-trained physiotherapists at a private physiotherapy clinic while patients were on the Pain Center waiting list (approximately 8 months from referral). Each patient received up to 8 consultations over a period of 3 months. The intervention comprised 3 components: (1) making sense of pain: context-based patient education focusing on the multidimensional nature of pain and disability, while reducing the threat of structural damage and correcting unhelpful beliefs; (2) exposure with control: graded exposure to painful, feared, or avoided activities with body relaxation and extinguishing protective behaviours; and (3) lifestyle changes: patients were encouraged to perform 20 to 30 minutes of physical activity daily based on their preference and taught strategies to manage stress and poor sleep.36
2.5. Control pathway
Treatment at the Pain Center can be diverse, and control patients were only eligible if they had received a multidisciplinary intervention consisting of a combination of (1) medical treatment with a specialist pain consultant (ie, individual adjustment of analgesics to improve effect and reduce side effects) AND (2) one or more of the following: individual consultations with a pain psychologist or social worker with cognitive-behavioral therapy training or participation in a group session with relaxation therapy or mindfulness, as these represent the most comprehensive pathway. The Pain Center pathways are based on elements from cognitive-behavioral therapy, acceptance and commitment therapy, and mindfulness-based stress reduction programs (http://www.ouh.dk/wm164091), which have shown moderate effects in this population and thus have similarities with some of the CFT components. For this pathway, there was only 1 outcome time point, which was the end of their individualized treatment. Because this was individualized, the outcome time point was variable, with a median of 9 months (interquartile range 3–19).
2.6. Outcomes
All outcomes were blinded to study participants, physiotherapists, and researchers until after the last follow-up.
2.6.1. Pain intensity
Pain intensity was measured using Numerical Pain Rating Scale (NRS)7 showing good test–retest reliability in patients with chronic pain.18 Peak and average pain during the past 24 hours were rated on 2 NRSs ranging from 0 = “no pain” to 10 = “worst pain imaginable.”
2.6.2. Pain-related disability
Pain-related disability was assessed with the PDI and the Oswestry Disability Questionnaire (ODI). The PDI40 is a generic pain-related disability scale that assesses the degree to which chronic pain interferes with daily activities. This is constructed using 11-item NRSs in which 0 = “no disability” and 10 = “worst disability.” This study used only the 5 voluntary activities items, which yielded a 0 to 50 pain-related disability score as previous psychometric analyses indicated that the obligatory activation subscale has inadequate internal reliability.45
The ODI14,15 showing good validity and reliability in patients with low back pain53 is a back pain–specific self-report measure comprising statements for the patient to select that reflect the patient's ability to manage their everyday life despite their pain. Each item is scored between 0 and 5 with a total score between 0 and 50, and a percentage score is calculated based on the patients' total score divided by the total possible score.
2.6.3. Pain-related cognitions and emotions
Catastrophic thinking related to pain was assessed using the Pain Catastrophizing Scale47 showing acceptable test–retest reliability and internal consistency in patients with low back pain.17 The Pain Catastrophizing Scale instructions ask participants to indicate the degree to which they experienced each of 13 thoughts or feelings when experiencing pain, on a 5-point Likert scale with 0 = not at all and 4 = all the time. The score is 0 to 52 with a higher score indicating a high level of pain catastrophizing.
Fear of movement was assessed with the 17-item Tampa Scale of Kinesiophobia questionnaire25 showing good reliability and acceptable concurrent validity in patients with low back pain.48 Each item is rated on a 4-point Likert scale with 1 = “strongly disagree” and 4 = “strongly agree” with a higher score indicating higher levels of fear of movement/kinesiophobia (scale 17–68).
Depression and anxiety was assessed with the Patient Health Questionnaire-9 (PHQ-9)26 and the Generalized Anxiety Disorder-7 (GAD-7) questionnaire46 showing good validity and reliability29,44 and has been widely used to measure depressive and anxiety symptoms in chronic pain populations.1 Questions are assessed on a 4-point Likert scale, ranging from 0 = “not at all” to 3 = “nearly every day” with a higher score indicating higher depression (scale 0–27) or anxiety (scale 0–21) severity.
Health-related QOL was measured on the 0 to 100 Visual Analog Scale from the EuroQol 5-D questionnaire with 100 indicating the best QOL.13 Assessment of QOL with the 0 to 100 Visual Analog Scale has shown good correlations with SF-36, degree of independence, and lower depression scores in patients with chronic conditions.43
2.6.4. Treatment costs
Treatment costs were calculated accounting for the different number of sessions for each individual. Calculation of costs for the CFT intervention was based on the payment rates detailed in the collective agreement between the Danish Physiotherapy Association and the Danish regions. Calculation of costs for the Pain Center care group was based on rates for diagnostic-related groups (DRG rates) itemized in the collective agreement between the Danish Government and the Danish regions.
2.6.5. Outcomes available for comparison of the 2 treatment pathways
In addition to the estimates of treatment costs, the following pain-related variables were suitable for outcome comparison between CFT and the Pain Center care pathways: duration of pain, use of analgesics and opioids, intensity of average clinical pain, pain-related disability (PDI), body chart (pain drawing) body areas, and health-related QOL, sex, age, height, and weight.
2.6.6. Assessment of pressure pain sensitivity
Pressure pain thresholds that have shown good within- and between-session reliability22,51 were assessed by HBV who was not involved in the CFT pathway. Pressure pain thresholds were assessed locally at the right erector spinae muscle (3 cm from the fourth lumbar spinous process) and at the left upper trapezius muscle (10 cm horizontally from the acromion in direct line with the seventh cervical spinous process) using a handheld pressure algometer (Somedic Sales AB, Norra Mellby, Sweden) with a stimulation area of 1 cm2, and a pressure rate of 30 kPa/s. Patients were instructed to press a button when the pressure was perceived as the first sensation of minimal pain. Two PPT assessments with 20-second intervals between assessments were completed for each site and the average used for analysis.
2.7. Statistical analyses
Two stages of analysis were performed, one to describe the change in the CFT pathway and the second for the comparison of outcomes between the 2 different pathways.
2.7.1. Analysis 1
Analysis 1 involved, first, describing the number of dropouts from the CFT pathway and comparing their baseline characteristics with those that completed the CFT pathway. For continuous scores, P-values were calculated using independent t tests for normally distributed variables and Mann–Whitney U tests for non-normally distributed variables. For categorical scores, P-values were calculated using χ2 tests. Second, the magnitude of change from baseline to the 3- and 6-month time points was estimated for those that completed CFT pathway, using paired t tests for continuous outcomes (Wilcoxon rank-sum tests if not normally distributed), and the calculation of proportions and odds ratios for dichotomous outcomes. Finally, the change in PPTs between baseline and 3 months, and baseline and 6 months were calculated, and as these data were not normally distributed, Spearman rho correlations were used to describe the association between those changes and pain-related disability or pain intensity at the same outcome time points.
2.7.2. Analysis 2
Analysis 2 estimated the CFT pathway effect by comparing the CFT group outcome after treatment with that of Pain Center care pathway. Between-pathway comparisons in outcomes were analyzed using linear regression, with adjustment for baseline scores of the dependent variable. Bootstrapped standard errors were estimated to adjust for slight departures from normality due to skew, and 95% confidence intervals were constructed from those standard errors.
For all analyses of continuous data, when that data were normally distributed, effects sizes were calculated. For within-pathway change scores, those effect sizes were standardized mean changes (SMCs) using a bias-corrected bootstrap method of 1000 samples with replacement.12 For between-pathway differences, those effect sizes were standardized mean differences (SMDs or Cohen's D) also using a bias-corrected bootstrap method of 1000 samples with replacement.12 The strength of effect sizes was categorized using Cohen's criteria (greater than 0.8 as large, 0.5 as moderate, and smaller than 0.2 as small).8 The same bootstrap method was applied for dichotomous data using the Yang and Dalton method.60
There were few missing data. Across the estimates in analysis 1, the missingness was 0.8% at baseline, 0.8% at 3 months, and 4.0% at 6 months. Across the estimates in analysis 2, the missingness for the CFT group was 0.0% at baseline, 0.6% at 3 months, and 2.5% at 6 months, and for the Pain Center group was 0.0% at baseline and 3.0% after treatment. Because of this, and that the analyses were made with statistical techniques that are unbiased in the presence of data missing at random, no data were imputed. All statistical analyses were performed using STATA version 15.1 (StataCorp, College Station, TX) with a P-value of <0.05 as the threshold for statistical significance.
3. Results
3.1. Cognitive functional therapy cases
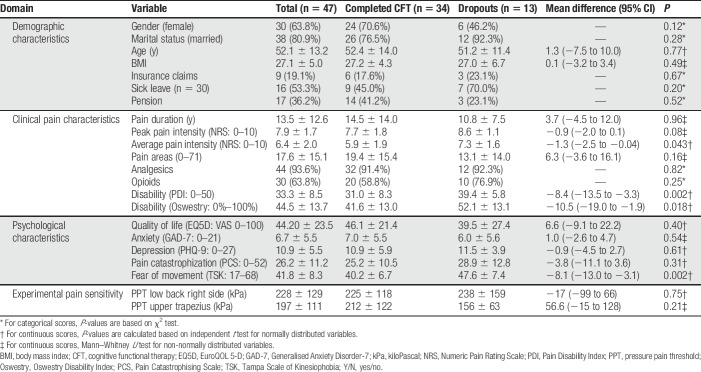
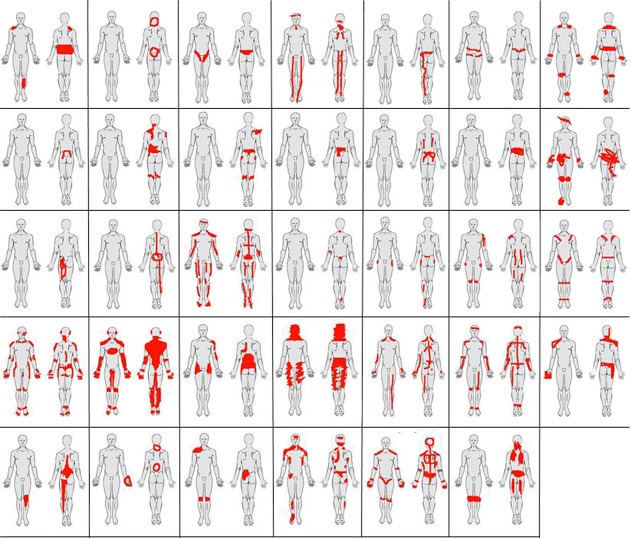
Forty-seven patients consented to participate in the study (Fig. 1 and Table 1). Six patients were excluded after the first session of CFT, and 7 dropped out within the first 3 CFT sessions due to lack of motivation or due to other health-related issues being investigated. Thirty-four patients completed the CFT pathway; receiving a mean of 6.6 ± 1.3 treatment sessions over 12 weeks and were included in analysis 1. Most of the patients reported pain in several areas of the body (Fig. 2). At baseline, the 72.3% of patients who completed CFT had lower scores on average pain intensity, pain-related disability, and kinesiophobia compared with patients who did not complete (Table 1).
Figure 1.
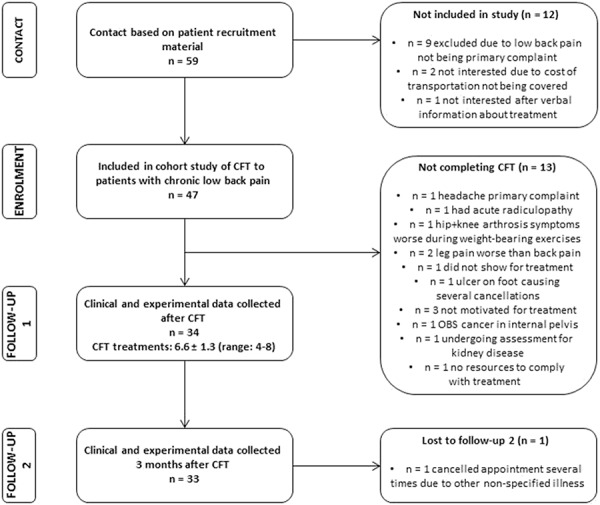
Flow chart.
Table 1.
Baseline characteristics of the patients consenting to participate in the CFT pathway.
Figure 2.
Pain drawings from the 34 patients who completed the cognitive functional therapy pathway.
3.2. Change in clinical outcomes and pressure pain thresholds after cognitive functional therapy
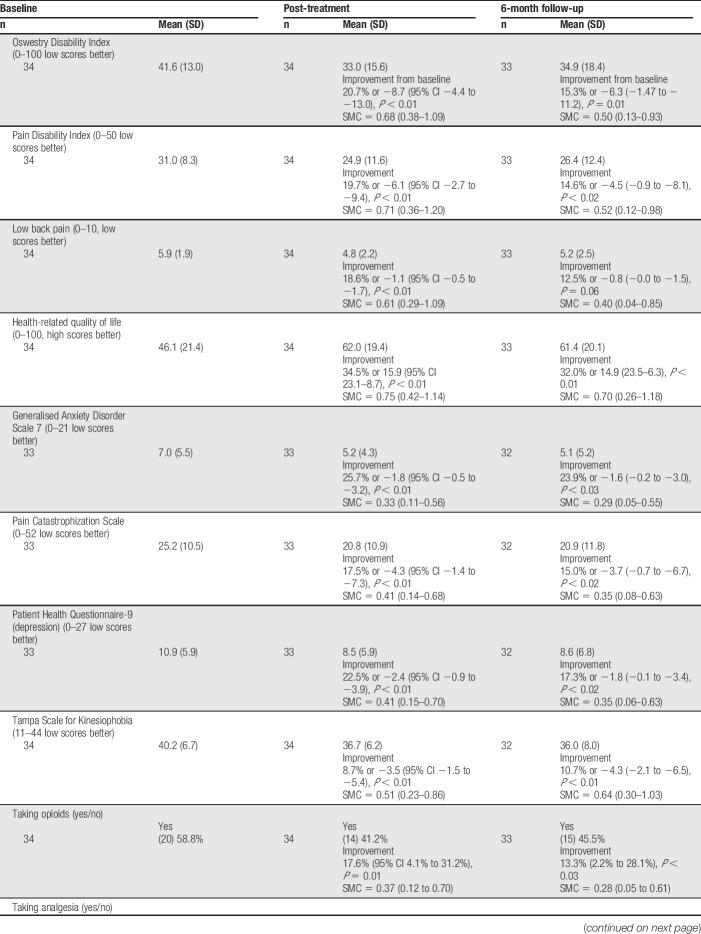
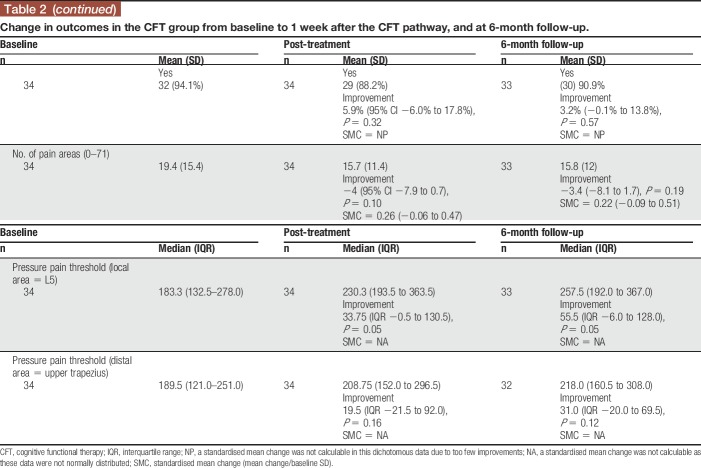
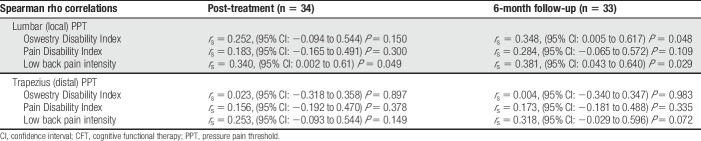
In the CFT group, improvements were seen from baseline to end of treatment in disability, pain intensity, kinesiophobia, pain catastrophization, anxiety, depression, health-related QOL, and a reduction in the proportion taking opioids (%). Those effects were moderate in size, with the point estimates of SMCs ranging from 0.33 to 0.75. Also, there was an increase in lumbar PPT (Table 2). At the end of the 3-month treatment period, there was a moderate-sized association between changes in lumbar PPT and changes in pain intensity (rs = 0.340, P = 0.049; Table 3).
Table 2.
Change in outcomes in the CFT group from baseline to 1 week after the CFT pathway, and at 6-month follow-up.
Table 3.
Correlations between change in pressure pain thresholds and change in pain intensity or pain-related disability in the CFT group.
In the CFT group, there were also improvements from baseline to the 6-month follow-up in disability, health-related QOL, anxiety, pain catastrophization, depression, kinesiophobia, and a reduction in the proportion taking opioids. Again, the point estimates of the SMCs were moderate size, and there continued to be an increase in lumbar PPT. At 6 months, there were moderate-sized associations between changes in lumbar PPT and changes in pain-related disability (ODI) scores (rs = 0.348, P = 0.048) and pain intensity (rs = 0.381, P = 0.029).
3.3. Comparison between cognitive functional therapy cases and pain Center care
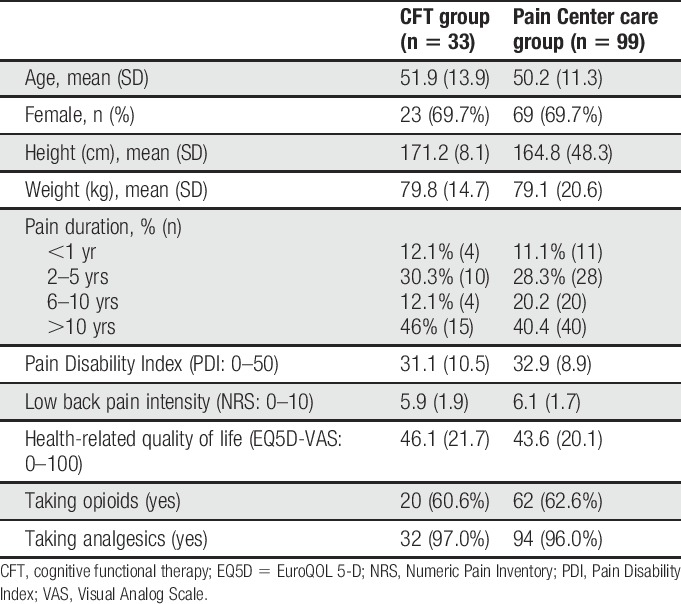
One of the 34 patients who completed CFT could not be matched in the propensity score matching. Consequently, 33 CFT cases and 99 matched control cases were included in analysis 2 (Fig. 2 and Table 4). Pain Center care patients received a mean of 16.4 ± 7.4 treatment sessions over a median of 9 months. The treatment period in the Pain Center care pathway was approximately 3 times longer and 14.7 times the cost per patient.
Table 4.
Baseline characteristics in 33 CFT cases and 99 matched control patients.
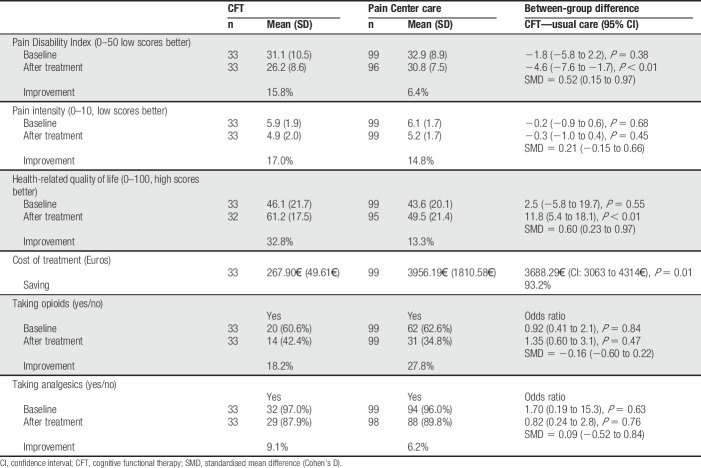
Greater reductions in PDI and greater improvements in health-related QOL were observed for the CFT group, but there were no differences on all other outcomes (Table 5).
Table 5.
Comparisons of outcomes in the CFT and Pain Center care pathways.
4. Discussion
4.1. Summary of results
This is the first study comparing a CFT pathway to an MPM pathway for patients with severe and disabling pLBP. While acknowledging the limitations of the study design of this pilot case–control study without randomization, the main findings of this pilot study was significant improvements in pain intensity, pain-related disability, pain-related cognitions, emotions, QOL, use of opioids, and back PPT in patients with severe pLBP after the CFT pathway. In addition, the CFT group had larger reductions in pain-related disability and improved QOL at a markedly lower cost at the end of the treatment period compared with the MPM group.
These findings are potentially important, and somewhat surprising, given that these patients were nonresponsive to primary care, and many were taking opioids and on sick leave. This patient profile is known to be very resistant to change. Furthermore, they were provided with a relatively small treatment dose by a single physiotherapist and had no additional booster sessions outside of the 3-month treatment period. However, randomized studies with larger samples are required to reduce the imprecision in many of the estimates of observed effects and control for the limitations of the study design.
The observed changes for pain-related disability and psychological factors are in line with the aim of CFT which directly targets personally relevant psychological, physical, and lifestyle barriers to recovery in an individualized manner, to return people to valued activities. These findings are consistent with previous CFT clinical studies19,35,54 reporting reductions in negative pain cognitions and emotional distress and improvements in pain coping and pain self-efficacy.5,35,54 This model of care is aligned to recent clinical guidelines advocating physical and psychological interventions in people with high levels of psychological distress. Typically, this is delivered in a multidisciplinary care environment, while CFT targets this in an integrated manner delivered by a single physiotherapist.
In general, the MPM group reported small to modest improvements in pain intensity, disability, and QOL of a similar magnitude as recently reported by multidisciplinary pain centers in Canada38 and in a large meta-analysis.24 However, the likely heterogeneity in outcome make these results somewhat challenging from a cost-effectiveness perspective, as it is difficult to predict which LBP patients will benefit from an MPM program.
A novel aspect of this study was the increase in PPT at the back after the CFT intervention. Although lower PPT's have been reported in people with pLBP and emotional distress,41,42 to date, no study has reported an increase in PPT at the lower back after a CFT intervention. The exact underlying mechanism for these changes are unknown; however, it is known that cognitive processes and lifestyle factors affect both pain9,20 and pain sensitivity,32 and it has been hypothesized that reduced fear of pain and pain-related distress may impact tissue sensitivity. Interestingly, remote trapezius PPT did not increase, suggesting that CFT did not cause a generalized change in pain sensitivity, which point to more local or segmental than systemic mechanisms. Whether these local changes reflect factors such as the normalization of spinal movement and reduced protective guarding of the low back, which are key components in the CFT intervention, is not known. The exact contribution of pain sensitivity mechanisms to spinal pain is unclear, and it has even been suggested that pain sensitivity is a poor marker for the subjective experience of pain and disability.23 Nonetheless, these preliminary findings of an increase in PPT at the lower back after CFT intervention and an association with reduced pain intensity and pain-related disability suggest that changes in pain sensitivity should be further investigated in studies of CFT.
4.2. Results compared with previous cognitive functional therapy studies
Compared with previous studies investigating CFT in primary care patients with low to moderate disability,19,35,54 the present results do not display similar large effects on pain, disability, cognitive, and emotional variables. The findings are in line with a recent RCT reported moderate and long-term reductions in disability, but not pain, in people with disabling LBP after CFT compared with group exercise and education.34 Interestingly, CFT has consistently demonstrated significant long-term effect on fear in previous studies,5,19,35,54 and fear has been proposed as a potential mediating effect of disability within the CFT intervention.5 In this study, the changes in fear were relatively low, which is similar to recent findings,34 but larger changes in other emotional constructs, eg, depression and anxiety were seen, indicating possible different mechanism of change in this complex cohort of patients. In a recent RCT, CFT resulted in improvements in pain self-efficacy, risk of chronicity, and pain coping compared with group education and exercise.34
The explanation for the positive results of CFT in this cohort may relate to the nature of the intervention. The CFT intervention targets feared and avoided activities through behavioural experiments that involve exposure to threatening tasks while training relaxation and abolishing protective and safety behaviours. These behavioural experiments are specifically designed to challenge pain-related movement and activity avoidance beliefs and behaviours.4 Although speculative, this re-engagement with valued activities coupled with increased pain self-efficacy may result in positive effects on mood and emotional status. The present findings are in line with previous research on management of LBP involving exposure treatments.28,56 Interestingly, patients who did not complete the CFT intervention also showed higher disability and kinesiophobia than patients who completed the intervention, which may indicate that they could be the individuals who might benefit most from CFT core components. However, not all patients are willing or ready to actively engage in this treatment approach.
4.3. Limitations
This is a small pilot case–control study without randomization, so participants were only balanced on the measured variables. The findings may potentially be influenced by nonspecific effects as patients receiving CFT were enrolled in a clinical trial, while patients in the usual MPM group were not. Not all potential outcome variables were available for control patients, and no evaluation of other health care services and costs outside of the study was performed, which could potentially influence the nondirect treatment-related costs. There was a high dropout rate in the beginning of the CFT pathway suggesting that the timing of the pathway was not optimal for all patients or that it did not suit the expectations of all patients. As participant motivation for choosing CFT pathway or Pain Center care was not assessed in this study, we cannot rule out that potential differences may have inflated the results. Although cases were matched at start of pathways, the long wait time for usual Pain Center care may have negative effects across a range of domains including health-related QOL and psychological well-being.30 Based on the exploratory nature of several of the statistical analyses, we acknowledge the risk of Type I error.
5. Conclusions
Patients receiving CFT showed significant improvements in pain intensity, pain-related disability, pain-related cognitions, emotions, QOL, use of opioids, and PPTs. The CFT pathway produced superior outcomes for pain-related disability and QOL at a much lower cost for patients with severe disabling pLBP compared with an MPM pathway. Although we are cautious not to over interpret this case–control data, fully powered RCTs investigating these results in this setting is warranted, as there is an urgent need to identify alternative, clinically and cost-effective interventions to help people manage disabling pLBP.
Disclosures
P. O'Sullivan, K. Ussing and J.V. Johansen occasionally receive payments for clinical workshops on cognitive functional therapy (CFT). H.B. Vaegter received funding for this study by the Research and Development Fund, and the Danish Physiotherapy Research Fund. The remaining authors have no conflicts of interest to declare.
Previous presentation of research: The content of this manuscript was presented at the 10th International World Congress on Low Back and Pelvic Girdle Pain in October 2019.
Acknowledgements
The authors thank Prof Anne Smith for her statistical and interpretative advice.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Bair MJ, Wu J, Damush TM, Sutherland JM, Kroenke K. Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom Med 2008;70:890–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bjorck-van Dijken C, Fjellman-Wiklund A, Hildingsson C. Low back pain, lifestyle factors and physical activity: a population based-study. J Rehabil Med 2008;40:864–9. [DOI] [PubMed] [Google Scholar]
- [3].Boutron I, Altman DG, Moher D, Schulz KF, Ravaud P. CONSORT statement for randomized trials of nonpharmacologic treatments: a 2017 update and a CONSORT extension for nonpharmacologic trial abstracts. Ann Intern Med 2017;167:40–7. [DOI] [PubMed] [Google Scholar]
- [4].Bunzli S, McEvoy S, Dankaerts W, O'Sullivan P, O'Sullivan K. Patient perspectives on participation in cognitive functional therapy for chronic low back pain. Phys Ther 2016;96:1397–407. [DOI] [PubMed] [Google Scholar]
- [5].Caneiro JP, Smith A, Linton SJ, Moseley GL, O'Sullivan P. “How does change unfold?” an evaluation of the process of change in four people with chronic low back pain and high pain-related fear managed with cognitive functional therapy: a replicated single-case experimental design study . Behav Res Ther 2019;117:28–39. [DOI] [PubMed] [Google Scholar]
- [6].Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer M, Fu R, Dana T, Kraegel P, Griffin J, Grusing S, Brodt ED. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 2017;166:493–505. [DOI] [PubMed] [Google Scholar]
- [7].Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23:129–38. [PubMed] [Google Scholar]
- [8].Cohen J. Statistical power analysis for the behavioural sciences. 2nd ed Hillsdale: Erlbaum, 1988. [Google Scholar]
- [9].Crettaz B, Marziniak M, Willeke P, Young P, Hellhammer D, Stumpf A, Burgmer M. Stress-induced allodynia—evidence of increased pain sensitivity in healthy humans and patients with chronic pain after experimentally induced psychosocial stress. PLoS One 2013;8:e69460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dankaerts W, O'Sullivan P, Burnett A, Straker L, Davey P, Gupta R. Discriminating healthy controls and two clinical subgroups of nonspecific chronic low back pain patients using trunk muscle activation and lumbosacral kinematics of postures and movements: a statistical classification model. Spine (Phila Pa 1976) 2009;34:1610–8. [DOI] [PubMed] [Google Scholar]
- [11].Dunn KM, Jordan K, Croft PR. Characterizing the course of low back pain: a latent class analysis. Am J Epidemiol 2006;163:754–61. [DOI] [PubMed] [Google Scholar]
- [12].Efron B, Tibshirani RJ, An introduction to the bootstrap. New York: Chapman and Hall, 1993. [Google Scholar]
- [13].EuroQol. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- [14].Fairbank JC, Couper J, Davies JB, O'Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy 1980;66:271–3. [PubMed] [Google Scholar]
- [15].Fairbank JC, Pynsent PB. The Oswestry Disability Index . Spine (Phila Pa 1976) 2000;25:2940–52; discussion 2952. [DOI] [PubMed] [Google Scholar]
- [16].Fashler SR, Cooper LK, Oosenbrug ED, Burns LC, Razavi S, Goldberg L, Katz J. Systematic review of multidisciplinary chronic pain treatment facilities. Pain Res Manag 2016;2016:5960987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fernandes L, Storheim K, Lochting I, Grotle M. Cross-cultural adaptation and validation of the Norwegian pain catastrophizing scale in patients with low back pain. BMC Musculoskelet Disord 2012;13:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ferraz MB, Quaresma MR, Aquino LR, Atra E, Tugwell P, Goldsmith CH. Reliability of pain scales in the assessment of literate and illiterate patients with rheumatoid arthritis. J Rheumatol 1990;17:1022–4. [PubMed] [Google Scholar]
- [19].Fersum KV, Smith A, Kvale A, Skouen JS, O'Sullivan P. Cognitive functional therapy in patients with non specific chronic low back pain A randomized controlled trial 3-year follow up. Eur J Pain 2019;23:1416–24. [DOI] [PubMed] [Google Scholar]
- [20].Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain 2013;14:1539–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gore M, Sadosky A, Stacey BR, Tai KS, Leslie D. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine (Phila Pa 1976) 2012;37:E668–77. [DOI] [PubMed] [Google Scholar]
- [22].Graven-Nielsen T, Vaegter HB, Finocchietti S, Handberg G, Arendt-Nielsen L. Assessment of musculoskeletal pain sensitivity and temporal summation by cuff pressure algometry: a reliability study. PAIN 2015;156:2193–202. [DOI] [PubMed] [Google Scholar]
- [23].Hubscher M, Moloney N, Leaver A, Rebbeck T, McAuley JH, Refshauge KM. Relationship between quantitative sensory testing and pain or disability in people with spinal pain-a systematic review and meta-analysis. PAIN 2013;154:1497–504. [DOI] [PubMed] [Google Scholar]
- [24].Kamper SJ, Apeldoorn AT, Chiarotto A, Smeets RJ, Ostelo RW, Guzman J, van Tulder MW. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: cochrane systematic review and meta-analysis. BMJ 2015;350:h444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kori SH, Miller RP, Todd DD. Kinisophobia: a new view of chronic pain behavior. Pain Manag 1990;1:35–43. [Google Scholar]
- [26].Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Linton SJ. A review of psychological risk factors in back and neck pain. Spine (Phila Pa 1976) 2000;25:1148–56. [DOI] [PubMed] [Google Scholar]
- [28].Linton SJ, Boersma K, Jansson M, Overmeer T, Lindblom K, Vlaeyen JWS. A randomized controlled trial of exposure in vivo for patients with spinal pain reporting fear of work-related activities. Eur J Pain 2008;12:722–30. [DOI] [PubMed] [Google Scholar]
- [29].Lowe B, Kroenke K, Herzog W, Grafe K. Measuring depression outcome with a brief self-report instrument: sensitivity to change of the Patient Health Questionnaire (PHQ-9). J Affect Disord 2004;81:61–6. [DOI] [PubMed] [Google Scholar]
- [30].Lynch ME, Campbell F, Clark AJ, Dunbar MJ, Goldstein D, Peng P, Stinson J, Tupper H. A systematic review of the effect of waiting for treatment for chronic pain. PAIN 2008;136:97–116. [DOI] [PubMed] [Google Scholar]
- [31].Main CJ, Foster N, Buchbinder R. How important are back pain beliefs and expectations for satisfactory recovery from back pain? Best Pract Res Clin Rheumatol 2010;24:205–17. [DOI] [PubMed] [Google Scholar]
- [32].Meints SM, Mawla I, Napadow V, Kong J, Gerber J, Chan ST, Wasan AD, Kaptchuk TJ, McDonnell C, Carriere J, Rosen B, Gollub RL, Edwards RR. The relationship between catastrophizing and altered pain sensitivity in patients with chronic low-back pain. PAIN 2019;160:833–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Meyer K, Tschopp A, Sprott H, Mannion AF. Association between catastrophizing and self-rated pain and disability in patients with chronic low back pain. J Rehabil Med 2009;41:620–5. [DOI] [PubMed] [Google Scholar]
- [34].O'Keeffe M, O'Sullivan P, Purtil H, Bargary N, O'Sullivan K. Cognitive functional therapy compared with a group-based exercise and education intervention for chronic low back pain: a multicentre randomised controlled trial (RCT). BJSM 2019. 10.1136/bjsports-2019-100780. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].O'Sullivan K, Dankaerts W, O'Sullivan L, O'Sullivan PB. Cognitive functional therapy for disabling nonspecific chronic low back pain: multiple case-cohort study. Phys Ther 2015;95:1478–88. [DOI] [PubMed] [Google Scholar]
- [36].O'Sullivan PB, Caneiro JP, O'Keeffe M, Smith A, Dankaerts W, Fersum K, O'Sullivan K. Cognitive functional therapy: an integrated behavioral approach for the targeted management of disabling low back pain. Phys Ther 2018;98:408–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Oliveira CB, Maher CG, Pinto RZ, Traeger AC, Lin CC, Chenot JF, van Tulder M, Koes BW. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J 2018;27:2791–803. [DOI] [PubMed] [Google Scholar]
- [38].Page MG, Boyd K, Ware MA. Examination of the course of low back pain intensity based on baseline predictors and health care utilization among patients treated in multidisciplinary pain clinics: a Quebec Pain Registry Study. Pain Med 2019;20:564–73. [DOI] [PubMed] [Google Scholar]
- [39].Pincus T, Kent P, Bronfort G, Loisel P, Pransky G, Hartvigsen J. Twenty-five years with the biopsychosocial model of low back pain-is it time to celebrate? A report from the twelfth international forum for primary care research on low back pain. Spine (Phila Pa 1976) 2013;38:2118–23. [DOI] [PubMed] [Google Scholar]
- [40].Pollard CA. Preliminary validity study of the pain disability index. Percept Mot Skills 1984;59:974. [DOI] [PubMed] [Google Scholar]
- [41].Rabey M, Slater H, O'Sullivan P, Beales D, Smith A. Somatosensory nociceptive characteristics differentiate subgroups in people with chronic low back pain: a cluster analysis. PAIN 2015;156:1874–84. [DOI] [PubMed] [Google Scholar]
- [42].Rabey M, Smith A, Beales D, Slater H, O'Sullivan P. Differing psychologically derived clusters in people with chronic low back pain are associated with different multidimensional profiles. Clin J Pain 2016;32:1015–27. [DOI] [PubMed] [Google Scholar]
- [43].Schrag A, Selai C, Jahanshahi M, Quinn NP. The EQ-5D—a generic quality of life measure-is a useful instrument to measure quality of life in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 2000;69:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Seo JG, Park SP. Validation of the Generalized Anxiety Disorder-7 (GAD-7) and GAD-2 in patients with migraine. J Headache Pain 2015;16:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Soer R, Koke AJ, Vroomen PC, Stegeman P, Smeets RJ, Coppes MH, Reneman MF. Extensive validation of the pain disability index in 3 groups of patients with musculoskeletal pain. Spine (Phila Pa 1976) 2013;38:E562–8. [DOI] [PubMed] [Google Scholar]
- [46].Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–7. [DOI] [PubMed] [Google Scholar]
- [47].Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess 1995;7:524–32. [Google Scholar]
- [48].Swinkels-Meewisse EJ, Swinkels RA, Verbeek AL, Vlaeyen JW, Oostendorp RA. Psychometric properties of the Tampa Scale for kinesiophobia and the fear-avoidance beliefs questionnaire in acute low back pain. Man Ther 2003;8:29–36. [DOI] [PubMed] [Google Scholar]
- [49].Thomsen AB, Sorensen J, Sjogren P, Eriksen J. Economic evaluation of multidisciplinary pain management in chronic pain patients: a qualitative systematic review. J Pain Symptom Manage 2001;22:688–98. [DOI] [PubMed] [Google Scholar]
- [50].Vaegter HB, Graven-Nielsen T. Pain modulatory phenotypes differentiate subgroups with different clinical and experimental pain sensitivity. PAIN 2016;157:1480–8. [DOI] [PubMed] [Google Scholar]
- [51].Vaegter HB, Handberg G, Graven-Nielsen T, Edwards R. Hypoalgesia after exericse and cold pressor test are reduced in chronic musculuskeletal pain patients with high pain sensitivity. Clin J Pain 2015;32:58–69. [DOI] [PubMed] [Google Scholar]
- [52].Vaegter HB, Handberg G, Kent P. Brief psychological screening questions can be useful for ruling out psychological conditions in patients with chronic pain. Clin J Pain 2018;34:113–21. [DOI] [PubMed] [Google Scholar]
- [53].Vianin M. Psychometric properties and clinical usefulness of the Oswestry Disability Index. J Chiropr Med 2008;7:161–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Vibe Fersum K, O'Sullivan P, Skouen JS, Smith A, Kvale A. Efficacy of classification-based cognitive functional therapy in patients with non-specific chronic low back pain: a randomized controlled trial. Eur J Pain 2013;17:916–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Vlaeyen JW, Crombez G. Fear of movement/(re)injury, avoidance and pain disability in chronic low back pain patients. Man Ther 1999;4:187–95. [DOI] [PubMed] [Google Scholar]
- [56].Vlaeyen JW, de Jong J, Geilen M, Heuts PH, van Breukelen G. Graded exposure in vivo in the treatment of pain-related fear: a replicated single-case experimental design in four patients with chronic low back pain. Behav Res Ther 2001;39:151–66. [DOI] [PubMed] [Google Scholar]
- [57].Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, III, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Walker J, Holloway I, Sofaer B. In the system: the lived experience of chronic back pain from the perspectives of those seeking help from pain clinics. PAIN 1999;80:621–8. [DOI] [PubMed] [Google Scholar]
- [59].Wertli MM, Rasmussen-Barr E, Held U, Weiser S, Bachmann LM, Brunner F. Fear-avoidance beliefs-a moderator of treatment efficacy in patients with low back pain: a systematic review. Spine J 2014;14:2658–78. [DOI] [PubMed] [Google Scholar]
- [60].Yang DS, Dalton JE. A unified approach to measuring the effect size between two groups using SAS. Available at: https://www.lerner.ccf.org/qhs/software/lib/stddiff.pdf. Accessed March 1, 2019.