Abstract
The intracellular parasite Toxoplasma gondii resides inside a vacuole, which shields it from the host’s intracellular defense mechanisms. The cytokine interferon gamma (IFNγ) upregulates host cell effector pathways that are able to destroy the vacuole, restrict parasite growth and induce host cell death. Interferon-inducible GTPases such as the Guanylate Binding Proteins (GBPs), autophagy proteins and ubiquitin-driven mechanisms play important roles in Toxoplasma control in mice and partly also in humans. The host inflammasome is regulated by GBPs in response to bacterial infection in murine cells and may also respond to Toxoplasma infection. Elucidation of murine Toxoplasma defense mechanisms are guiding studies on human cells, while inevitably leading to the discovery of human-specific pathways that often function in a cell type-dependent manner.
Introduction
Toxoplasma gondii is an important pathogen of animals and humans with ~30% of the world’s population chronically infected. While immunocompetent people generally control the infection, Toxoplasma infection can lead to congenital abnormalities, ocular disease and health problems in the immunocompromised. Although Toxoplasma can infect any warm-blooded animal, mice are considered important intermediate hosts as they are natural prey of cats, the definitive host, which is likely why many Toxoplasma secreted effectors target murine restriction mechanisms.
Toxoplasma can invade any nucleated cell and resides inside the cell in a parasitophorous vacuole (PV). The PV membrane (PVM) shields the parasite from intracellular cytoplasmic defense mechanisms that have evolved to detect cytoplasmic pathogens. However, in both mice and humans, the immune system ultimately controls the initial acute phase of the infection and the parasite transitions into a cyst form that characterizes the chronic state of the infection.
Interferon gamma (IFNγ) is the central cytokine in eliciting anti-Toxoplasma effector mechanisms. These mechanisms involve either the direct destruction of the PVM, the acidification of the intravacuolar environment, the starvation of the parasite inside the vacuole or the activation of host cell death upon infection. The parasite restricting strategies ultimately employed depend on the host organism and the cell type (reviewed in [1,2]).
Different Toxoplasma strains (e.g. types I, II and III are the classical North American and European strains) vary in their genomes, resulting in divergent resistance to these host defense mechanisms. For example, the polymorphic virulence factors ROP5/ROP18 specifically counteract murine and not human defense mechanisms (reviewed in [3,4,5••,6••] and see Table 1).
Table 1.
Summary of IRG/GBP mediated control of Toxoplasma in different murine and human cell lines. For references see main text.
| Mouse (MEF, macrophages, astrocytes) |
Human epithelial A549 | Human HAP1 (haploid fibroblastlike leukemia cell) | |||
|---|---|---|---|---|---|
| IRGs | GBPs | Toxoplasma virulence factors | GBPs | GBPs | |
| Recruited to the PV or Toxoplasma | Irgm2, Irgm3, Irga6, Irgb6, Irgd recruited to the PV | •Gbp1, 2, 3, 5, 7 recruited to PV •Gbp2 recruited to Toxoplasma |
Combating GTPase recruitment: ROP18 (type I/II) ROP5 (type I/III) |
GBP1 not recruited to PV | GBP1–5 recruited to 8% PVs |
| Ability to control Toxoplasma | Irgm1, Irgm3, Irga6, Irgd able to control Toxoplasma replication | •Gbps on chromosome 3 in bulk (1, 2, 3, 5, 7) control Toxoplasma replication •Gbp1 controls Toxoplasma replication |
ROP16 (type I/III) ROP17 GRA7 |
GBP1 controls on of type II, but not type I | GBP1–5 do not control replication of type II |
| Enhancing GTPase recruitment: GRA15 (type II) |
|||||
By now we have a more detailed picture of how IFNγ-activated mechanisms combat Toxoplasma in murine cells, yet we are only at the beginning of understanding human Toxoplasma control. At the center of murine Toxoplasma control are IFNγ-inducible GTPases, the Immunity Regulated GTPases (IRGs) and Guanylate Binding Proteins (GBPs), regulated by autophagy proteins and interconnecting with ubiquitin-driven pathogen control. Here, we focus on how GBPs, autophagy and ubiquitin restrict the parasite and on how GBPs are linked to host cell death pathways to control infection in general. We elucidate lessons learnt from mouse studies and the current and future roles this knowledge will bring to human studies.
Divergent cell-autonomous GBP-mediated control of Toxoplasma in mice and humans
IFNγ upregulates expression of host IRGs and GBPs. Mice possess 23 IRGs, while humans have only one truncated ubiquitously expressed IRG that is not IFNγ-inducible (IRGM) [7]. IRGM is a risk locus for tuberculosis [8], with a currently unclear molecular function on other intravacuolar pathogens [9]. On the contrary, humans have 7 GBPs, while mice have 11 active GBP family members [7]. IRGs and GBPs collaborate in their function in mice [10,11,12••]. Family members of both IRGs and GBPs control Toxoplasma through different mechanisms depending on host species and cell type and different Toxoplasma strains differ in their susceptibility to IRG/GBP-mediated restriction because of strain differences in effectors that counteract the IRGs/GBPs (see below and Table 1).
How do GBPs restrict Toxoplasma in mice?
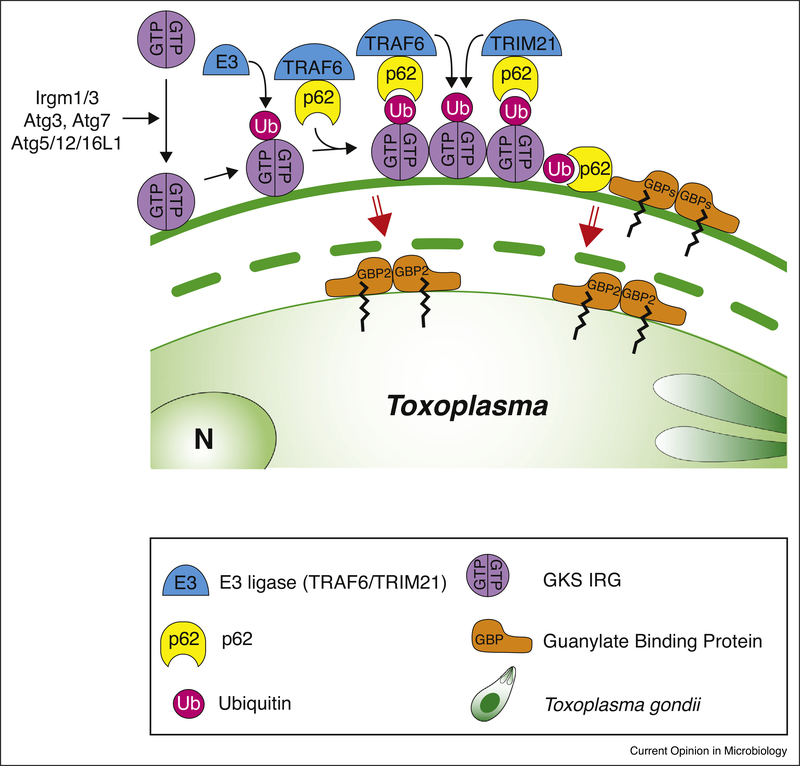
Three regulatory IRGs are of the ‘GMS’ motif type and keep the effector ‘GKS’ motif type IRGs at endomembranes in an inactive state [13,14]. Upon release, the ‘GKS’ IRGs target pathogen vacuolar membranes devoid of GMS IRGs, enabling a cascade of host defense molecules to accumulate (Figure 1). ROP17/ROP18 in cooperation with ROP5 and GRA7 virulence factors of type I parasites target ‘GKS’ IRGs for phosphorylation keeping the IRGs off the PV (reviewed in [15]), a mechanism that also effects the recruitment of GBPs [16]. Central to the host defense cascade is also ubiquitin, which is deposited onto the vacuole of Toxoplasma and Chlamydia in a like fashion by a yet unidentified E3 ubiquitin ligase [12••]. The scaffold protein p62 binds to ubiquitin and deposits the E3 ubiquitin ligase TNF-receptor associated factor (TRAF)6, which engages in a feedback loop to recruit more ubiquitin [12••]. Tripartite Motif Containing (TRIM)21 is another recruited E3 ubiquitin ligase that additionally is critical to Toxoplasma defense in vivo [17•]. Ubiquitin and p62 both enable further recruitment of GBPs to the vacuoles, a pathway that is active in mouse embryonic fibroblasts (MEFs) and murine macrophages [10,11,16,18]. Rab GDP-dissociation Inhibitor (GDI)α acts as a negative regulator of GBP deposition around the vacuole [19]. Both IRGs and GBPs at the PV are essential to disrupt the vacuolar membrane by vesiculation exposing the pathogen inside [10,20]. The exact mechanism of vacuolar disruption is still unknown.
Figure 1.
GBP-mediated restriction of Toxoplasma in murine cells.
In murine cells, type II and III Toxoplasma vacuoles are attacked by a range of host proteins leading to the disruption of the vacuolar membrane. The ‘GMS’ IRGs (not shown) block ‘GKS’ IRG activation. Once activated, ‘GKS’ IRGs accumulate on the vacuole and recruit an unknown seeding E3 ubiquitin ligase, as well as the p62-interacting E3 ubiquitin ligases TRAF6 and TRIM21. GBPs target to the vacuole via p62-dependent and independent mechanisms. Ubiquitination of the vacuole is of the K48 and K63 linkage type on substrate proteins that potentially include IRGs and GBPs themselves. Rupture of the vacuole is dependent on IRGs, GBPs and p62.
The IRG/GBP GTPases seemingly destroy the vacuoles of only a limited number of vacuolar pathogens: Toxoplasma gondii, Chlamydia trachomatis, and Encephalitozoon cuniculi. Toxoplasma and E. cuniculi do not enter the host cell through a phagocytic mechanism but have a unique ‘active’ invasion mechanism that excludes most host membrane proteins from the vacuolar membrane [21,22]. However, there are no regulatory IRGs on the host plasma membrane and it is therefore unclear if the specific mode of invasion (e.g. through phagocytosis vs. active invasion) determines why the vacuoles of only some pathogens are targeted by the IRGs [52]. Murine Chlamydia species adapted to its specific host avoid IRG/ GBP recognition, but when the human adapted Chlamydia trachomatis is for example studied in murine cells it gets targeted by IRGs/GBPs, strongly suggesting putative bacterial inhibitory effectors regulating IRGs [23•]. Once effector IRGs bind to the vacuolar membrane and destroy it, the pathogen is exposed and its outer membrane can be targeted for GBP-mediated destruction [24••]. Interestingly, the apicomplexan Plasmodium berghei in the liver does not get recognized by any member of the IRG family and GKS IRG knockout mice (Irga6) have the same parasite load as infected wild-type mice [25]. Recently it was shown that also the replication complex (RC) of +RNA viruses, a vacuole-like structure these viruses use for their replication, can be targeted by IRGs and GBPs in murine cells and by GBPs in human cells. This IRG/GBP targeting to the RC was necessary for full IFNγ-mediated inhibition of these viruses. Because the membrane of the RC is derived from host endomembranes it is unclear why the regulatory IRGs would not prevent the activation of effector IRGs on the RC. It was proposed that there might be a common unknown PAMP between pathogens targeted by the IRGs/GBPs [26].
How do GBPs restrict Toxoplasma in human cells?
Considerable human cell type variation exists with regard to GBP-mediated restriction of Toxoplasma. In HAP1 cells (haploid fibroblast-like leukemia cells) 6% of PVs recruited hGBP1–5 and a total hGBP deletion showed no defect in IFNγ-mediated Toxoplasma control [27]. In contrast, hGBP1 is recruited to the PVs of both type I and II Toxoplasma and restricts their growth in mesenchymal stromal cells, while hGBP2 and 5 have no functional effect [28]. In epithelial A549 cells, hGBP1 specifically restricts type II Toxoplasma replication without targeting the PV [29•]. Thus, hGBP recruitment does not necessarily predict its putative defense function in human cells. It remains unstudied whether the PV remained intact in these human cell types and by which mechanism the hGBPs restrict Toxoplasma. Of note, hGBP1, 2 and 5 harbor a CAAX box for isoprenylation at their C-terminus, potentially enabling targeting to various membranous compartments, as shown for hGBP1 and the Golgi [30,31]. Furthermore, yet unidentified parasite virulence factors may only interfere with the hGBP system in select human cell types. Currently, no data is available on the GBP-mediated restriction of Toxoplasma in human macrophages.
The role of autophagy in restricting Toxoplasma in mice and humans
Autophagy is a catabolic pathway generally used by a cell to clear cytoplasmic material, but it can be extended to the destruction of pathogens [32] (see also the review by I. Coppens in this issue). Importantly, autophagy is tightly regulated by nutrient sensing pathways [33].
Murine autophagy pathways targeting Toxoplasma
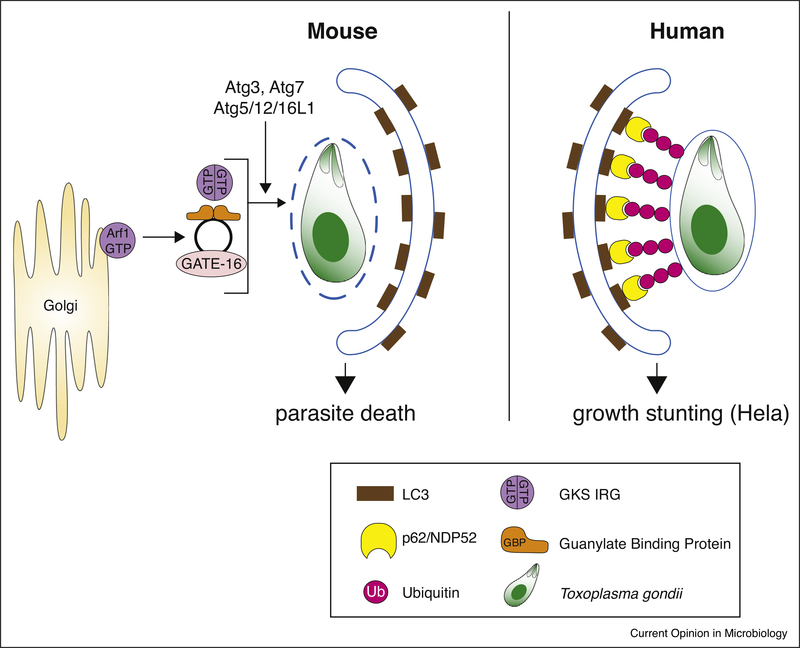
The autophagy-related (Atg) proteins Atg7, Atg3 and the Atg12-Atg5-Atg16L1 complex, which are involved in delivery and conjugation of the ubiquitin-like protein Microtubule-associated protein 1A/1B-light chain 3 (LC3) to the autophagosomal membrane, are necessary to target the IRGs and GBPs to the PVM [27,34–39] (Figure 2). All murine LC3 homologs as well as Gamma-aminobutyric acid receptor-associated proteins (GABARAPs) are also targeted to the PV [35,38]. Park et al. found all LC3 homologues to control IFNγ-driven Toxoplasma restriction, while using a different method of analysis for in vitro parasite replication, Sasai et al. deemed only GABARAPL2 (GATE-16) to be essential [38,40••]. Regardless, in vivo, only the GABARAPL2 (GATE-16) deficiency rendered mice as highly susceptible to Toxoplasma infection as IFNγR−/− mice [40••]. This property is by virtue of an ADP-ribosylation factor 1 (Arf1)-binding motif found in GABARAPs, which upon binding to Arf1 possibly activates this Golgi-localized membrane trafficking regulator [40••]. Atg3, 5, 7 and 16L1 are additionally needed to deposit ubiquitin and the ubiquitin adaptor protein p62 around the PV, whereby p62 is thought to facilitate MHC class I presentation of vacuolar antigens to CD8 T cells in infected IFNγ-stimulated MEF and DCs [39]. In slight contrast, p62 has been found to directly control Toxosplasma in MEFs, via its participation of GBP and TRAF6 recruitment, leading to increased ubiquitin deposition around the PV [37].
Figure 2.
Autophagy-mediated restriction of Toxoplasma in human and murine cells.
In mice, the Atg proteins Atg7, Atg3 and the Atg12-Atg5-Atg16L1 complex, all involved in delivery and conjugation of LC3 to the autophagosomal membrane, are necessary to target the IRGs and GBPs to the Toxoplasma PVM. GATE-16 is the only LC3-like protein essential for controlling Toxoplasma infection in vivo, by activating the Golgi-localized membrane trafficking regulator Arf1 and keeping GBPs in a non-aggregated form in the cytoplasm of cells. GBPs and IRGs disrupt the PVM and LC3-driven autophagosomes either clear the parasite itself or the membrane remnants that remain. In humans, Atg7/16L1 (not pictured) target ubiquitin to the Toxoplasma PVM. This leads to the recruitment of p62 and NDP52 and subsequently LC3, without acidification of the PV and disruption of the PVM. The parasite is eventually enveloped in the autophagic double membrane where it fails to grow and replicate further.
It was thought that once the parasite vacuole is destroyed, in a second step, autophagy membranes form around the denuded parasite, clearing it by classical acidification. This process is dependent on Irgm3 [20]. More recently, the notion was put forward that acidic clearance of material involves only the remnant membranes rather than the parasite itself and that canonical degradative autophagy is not required to restrict Toxoplasma [27,35,36]. The simple question remains as to what happens to the arguably dying parasite and the material it leaves behind? Possibilities are antigen presentation to CD8 T cells of vacuolar content (reviewed in [41]) and stimulation of host cell death by exposed pathogen material (see below).
Human autophagy pathways targeting Toxoplasma
In IFNγ-stimulated HeLa cells, ubiquitin is deposited around type II and III Toxoplasma PVs to mark them for non-canonical autophagy that leads to non-acidic growth stunting [6••]. This pathway employs the ubiquitin adaptor proteins p62 and the human-specific Nuclear Domain 10 Protein (NDP)52 and is dependent on ATG7 and ATG16L1. In contrast, in HAP1 cells, ATG16L1 KO does not have an effect on Toxoplasma type II restriction and only a marginal effect on GBP recruitment [6••] and in human forskin fibroblasts (HFFs) ATG5 knockdown did not impact IFNγ-mediated growth restriction of Toxoplasma type I [57]. In human umbilical endothelial vein cells (HUVEC), an autophagy-independent, ubiquitin and p62-dependent endo-lysosomal acidification and elimination of Toxoplasma type II was observed [5••]. The function of ATG proteins in controlling Toxoplasma in human macrophages has not been tested.
Autophagy is a pathway intimately connected with cellular metabolism. In many human cell types IFNγ-mediated restriction of Toxoplasma is mediated by the upregulation of Indoleamine-2,3-dioxygenase (IDO), which by degrading L-tryptophan, inhibits the growth of the tryptophan auxotrophic Toxoplasma [42]. Surprisingly, GBP versus IDO-mediated restriction of Toxoplasma has not been investigated in the same cell type. It is thus possible that these pathways counter-regulate each other and co-exist. For example, nutrient starvation can upregulate autophagy which might redirect proteins important for both autophagy and GBP function (e.g. LC3 and ubiquitin) to autophagosomal membranes instead of the vacuolar membrane. How exactly Toxoplasma is restricted in a human cell might depend on the phagocytic ability of the cell versus induced GBP and IDO levels.
The role of host cell death in restricting Toxoplasma-potential roles for GBPs?
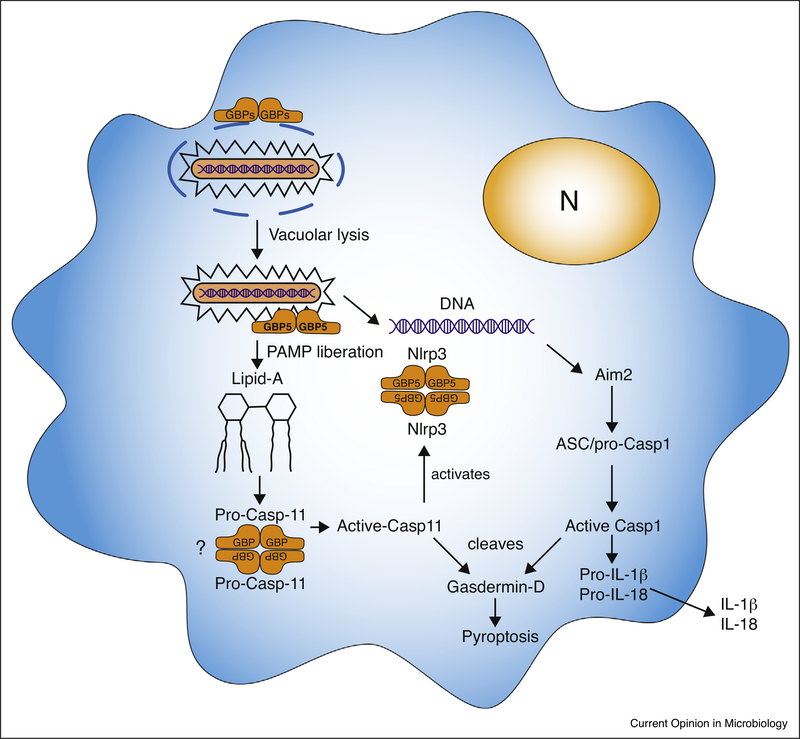
GPBs can also mediate a programmed form of host cell death called pyroptosis, which involves the activation of the inactive zymogen Caspase-1 (Cysteine-aspartic protease). Because intracellular pathogens need host cells for replication, destroying this niche is an effective way of inhibiting pathogen growth. Upon recognition of cytoplasmic PAMPs by cytoplasmic PRRs such as the Neuronal Apoptosis Inhibitor proteins (NAIPs), Nucleotide binding Oligomerization (NOD)-like receptors (NLRs) and AIM2-Like receptors (ALRs), macromolecular complexes containing pro-Casp1 get formed upon which Casp1 is activated by proximity-induced autoproteolysis. Active Casp1 then subsequently cleaves the proinflammatory cytokines pro-IL1β and pro-IL18 upon which active IL1β and IL18 is released. Casp1 also cleaves Gasdermin-D thereby removing the N-terminus-mediated inhibition of the C-terminal pore-forming domain allowing formation of a multimeric pore in the host cell plasma membrane eventually causing host cell death (reviewed in [43]).
GBPs can mediate the exposure of pathogen PAMPs to cytosolic PRR thereby activating the inflammasome and pyroptosis and two different mechanisms have been reported (Figure 3):
Figure 3.
Inflammasome activation driven by GBPs.
GBPs can mediate inflammasome activation by lysing the vacuole of pathogens and/or direct lysing cytosolic bacteria leading to the exposure of PAMPS such as LPS and DNA which can activate Casp11 and AIM2, respectively. Certain GBPs can also tetramerize and bind to Casp11 or Nlrp3 thereby lowering the threshold for their activation. For more details see main text.
GBPs can direct the destruction of the vacuolar membrane of certain gram-negative bacteria thereby releasing Lipopolysaccharide (LPS) to the cytoplasm. The CARD domain of Casp11 (human CASP4/5) can directly bind to Lipid-A in aggregates of LPS resulting in Casp11 activation and Gasdermin-D-mediated host cell death. Casp11 also mediates the secretion of IL-1β and IL-18 via activation of the Nlrp3-Asc-Casp1 inflammasome through an unknown mechanism.
Instead of aiding the lysis of the vacuole, GBPs together with Irgb10 can localize to the bacterial cell membrane (and within the bacteria) of cytosolic bacteria (e.g. Francisella escaped from its vacuole). By vesiculating the bacterial membrane they mediate the release of LPS and DNA, PAMPs that can subsequently activate Casp11 and the AIM2 inflammasome [44,45].
GBP5 (or the GBP cluster on chr3, GBPchr3) is not needed for inflammasome activation upon E. coli LPS transfection, possibly because this results in high cytoplasmic LPS concentrations that might already be able to concentrate Casp11 sufficiently for its activation. In contrast, efficient L. pneumophila LPS detection is dependent on GBPchr3 and Casp11 [46] which might be because L.pneumophila lipid A has longer fatty acid chains compared to E. coli and Salmonella LPS.
It has also been shown that GBP5 is needed for Nlrp3 inflammasome activation of mouse BMDM and a human macrophage cell line stimulated with LPS and triggered by ATP or Nigericin or infection with Salmonella or Listeria [47]. Similarly, rapid inflammasome activation by Chlamydia muridarum was independent of vacuolar lysis by the GBPs, but rather involved GBP(chr3)-mediated Casp1/Casp11 Nlrp3 and AIM2 inflammasome activation [48•]. The GTPase domain of GBP5 was shown to bind to the pyrin domain of Nlrp3 and the multimerization of GBP5 was suggested to increase the local concentration of Casp1 bound to Nlrp3/Asc and thereby its activation [47]. Although other studies did not replicate the influence of GBP5 on the Nlrp3 inflammasome [49,50] it has been noted [7] that the genetic background of the region around the GBP5 deletion was 129 in the study by Shenoy et al. [47], while it was C57BL/6 in the Meunier study. C57BL/6 vs. A/J macrophage differences in Toxoplasmacidal activity maps to a region on Chr3 that contains the GBPs. C57BL/6, and a variety of other mouse strains, do not express GBP1 upon IFNγ induction, while A/J and 129 does [51], possibly explaining these mouse strain differences in Toxoplasmacidal activities [52].
If GBPs mediate inflammasome activation of parasites or viruses is currently unknown. The Nlrp3 and Nlrp1 inflammasome are important for Toxoplasma control in murine [53,54] and rat macrophages [55], respectively. Both Nlrp3 and Nlrp1 are important for in vivo murine control of Toxoplasma [54]. Neither the Toxoplasma molecules or cellular changes recognized by these inflammasomes nor the role of GBPs, if any, have been investigated. Rapid host cell death has been observed upon invasion of mouse and human IFNγ-stimulated fibroblasts by Toxoplasma [56,57] and E. cuniculi [58]. Death of IFNγ-stimulated fibroblasts upon infection with type II and III Toxoplasma strains was dependent on IRG-mediated destruction of the Toxoplasma vacuole membrane and did not resemble apoptosis nor was there cleavage of Caspase-1 or IL-1β [56]. However, pyroptosis can be activated without cleavage of Caspase-1 and therefore further experiments will have to investigate the exact mechanism of cell death.
Conclusions
Much progress has been made toward the understanding of how the cell-autonomous defense to Toxoplasma is organized in IFNγ-stimulated murine cells. IRGs and GBPs as disruptors of the PVM are at the center of Toxoplasma counter-measures with the PV initially tagged by ubiquitin. This process is intimately driven by autophagy proteins. Exposed parasite material likely triggers host cell death, a response generally coordinated by GBPs in bacterial defense. It remains to be seen whether GBPs also mediate murine host cell death upon Toxoplasma infection.
The picture is less clear when considering the human host defense to Toxoplasma. Here, ubiquitin also plays a central role, but, the cell type drives the ultimate fate of the parasite - destruction versus growth restriction. Additionally, it remains to be seen whether the Toxoplasma restricting capacity of GBPs is at the PV or exerted from another location inside human cells. While tryptophan catabolism is an important IFNγ-mediated restriction mechanism in human cells, it is not clear how this pathway interacts with autophagy and GBPs. Most importantly, how Toxoplasma is sensed and subsequently restricted in human macrophages is not well understood. As technology advances, specifically with the advent of genome-wide host and parasite CRISPR screens [59,60] and stem cell technologies to generate non-transformed human cell types, our understanding of the human host defense in physiologically relevant cell systems to Toxoplasma could rapidly improve.
Acknowledgements
The Saeij lab is funded by NIH R01AI080621 and R21EY024593. The Frickel lab is funded by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001076), the UK Medical Research Council (FC001076), and the Wellcome Trust (FC001076). We thank Haley Wood for drawing the figures and Barbara Clough for critical reading of the manuscript.
Footnotes
Conflicts of interest
None
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Clough B, Frickel EM: The Toxoplasma Parasitophorous vacuole: an evolving host-parasite frontier. Trends Parasitol 2017, 33:473–488. [DOI] [PubMed] [Google Scholar]
- 2.Krishnamurthy S, Konstantinou EK, Young LH, Gold DA, Saeij JPJ: The human immune response to Toxoplasma: autophagy versus cell death. PLoS Pathog 2017, 13:e1006176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunter CA, Sibley LD: Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat Rev Microbiol 2012, 10:766–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hakimi M-A, Bougdour A: Toxoplasma’s ways of manipulating the host transcriptome via secreted effectors. Curr Opin Microbiol 2015, 26:24–31. [DOI] [PubMed] [Google Scholar]
- 5.Clough B, Wright JD, Pereira PM, Hirst EM, Johnston AC, Henriques R, Frickel EM: K63-linked ubiquitination targets Toxoplasma gondii for endo-lysosomal destruction in IFNγ-stimulated human cells. PLoS Pathog 2016, 12:e1006027.•• Human primary endothelial cells stimulated with IFNγ destroy ubiquitinated Toxoplasma PVs via lysosomal acidifiction. HeLa cells (this reference and reference 6) on the contrary do not acidify the PV, but growth stunt the parasite. It will be important to test other human cell types in the future to identify the range of IFNγ-dependent host defense mechanisms in humans.
- 6.Selleck EM, Orchard RC, Lassen KG, Beatty WL, Xavier RJ, Levine B, Virgin HW, Sibley LD: A noncanonical autophagy pathway restricts Toxoplasma gondii growth in a strain-specific manner in IFN-γ-activated human cells. MBio 2015, 6: e01157–e1215.•• Epithelial HeLa cells target ubiquitinated PVs for growth stunting in IFNγ-stimulated cells via non-canonical autophagy. This host defense pathway and the one described in endothelial cells (reference 5) is avoided by type I parasites, suggestive of the presence of a human Toxoplasma virulence factor.
- 7.Pilla-Moffett D, Barber MF, Taylor GA, Coers J: Interferon-inducible GTPases in host resistance, inflammation and disease. J Mol Biol 2016, 428:3495–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parkes M, Barrett JC, Prescott NJ, Tremelling M, Anderson CA, Fisher SA, Roberts RG, Nimmo ER, Cummings FR, Soars D et al. : Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn’s disease susceptibility. Nat Genet 2007, 39:830–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh SB, Davis AS, Taylor GA, Deretic V: Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science 2006, 313:1438–1441. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto M, Okuyama M, Ma JS, Kimura T, Kamiyama N, Saiga H, Ohshima J, Sasai M, Kayama H, Okamoto T et al. : A cluster of interferon-γ-inducible p65 GTPases plays a critical role in host defense against Toxoplasma gondii. Immunity 2012. 10.1016/j.immuni.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Selleck EM, Fentress SJ, Beatty WL, Degrandi D, Pfeffer K, Virgin HW, MacMicking JD, Sibley LD: Guanylate-binding protein 1 (Gbp1) contributes to cell-autonomous immunity against Toxoplasma gondii. PLoS Pathog 2013, 9:e1003320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haldar AK, Foltz C, Finethy R, Piro AS, Feeley EM, Pilla-Moffett DM, Komatsu M, Frickel EM, Coers J: Ubiquitin systems mark pathogen-containing vacuoles as targets for host defense by guanylate binding proteins. Proc Natl Acad Sci USA 2015, 112:E5628–E5637.•• This is the first demonstration that ubiquitin targets Chlamydia and Toxoplasma vacuoles for destruction in IFNγ-stimulated murine cells. GBPs and ubiquitin are intimately linked via the E3 ubiquitin ligase TRAF6 and p62, both responsible for keeping GBPs at the vacuole. Future studies should identify the seeding E3 ubiquitin ligase.
- 13.Hunn JP, Koenen-Waisman S, Papic N, Schroeder N, Pawlowski N, Lange R, Kaiser F, Zerrahn J, Martens S, Howard JC: Regulatory interactions between IRG resistance GTPases in the cellular response to Toxoplasma gondii. EMBO J 2008, 27:2495–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haldar AK, Saka HA, Piro AS, Dunn JD, Henry SC, Taylor GA, Frickel EM, Valdivia RH, Coers J: IRG and GBP host resistance factors target aberrant, “Non-self” vacuoles characterized by the missing of “Self” IRGM proteins. PLoS Pathog 2013, 9: e1003414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller UB, Howard JC: The impact of Toxoplasma gondii on the mammalian genome. Curr Opin Microbiol 2016, 32:19–25. [DOI] [PubMed] [Google Scholar]
- 16.Virreira Winter S, Niedelman W, Jensen KD, Rosowski EE, Julien L, spooner E, Caradonna K, Burleigh BA, Saeij JPJ, Ploegh HL et al. : Determinants of GBP recruitment to Toxoplasma gondii vacuoles and the parasitic factors that control it. PLoS ONE 2011, 6:e24434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foltz C, Napolitano A, Khan R, Clough B, Hirst EM, Frickel EM: TRIM21 is critical for survival of Toxoplasma gondii infection and localises to GBP-positive parasite vacuoles. Sci Rep 2017, 7:5209..• TRIM21 is identified as an essential E3 ubiquitin ligase for in vivo control of Toxoplasma. TRIM21, like ubiquitin and GBPs target the same parasite vacuoles during murine host defense.
- 18.Degrandi D, Konermann C, Beuter-Gunia C, Kresse A, Würthner J, Kurig S, Beer S, Pfeffer K: Extensive characterization of IFNinduced GTPases mGBP1 to mGBP10 involved in host defense. J Immunol 2007, 179:7729–7740. [DOI] [PubMed] [Google Scholar]
- 19.Ohshima J, Sasai M, Liu J, Yamashita K, Ma JS, Lee Y, Bando H, Howard JC, Ebisu S, Hayashi M et al. : RabGDIα is a negative regulator of interferon-γ-inducible GTPase-dependent cellautonomous immunity to Toxoplasma gondii. Proc Natl Acad Sci USA 2015. 10.1073/pnas.1510031112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling YM, Shaw MH, Ayala C, Coppens I, Taylor GA, Ferguson DJP, Yap GS: Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med 2006, 203:2063–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fasshauer V, Gross U, Bohne W: The parasitophorous vacuole membrane of Encephalitozoon cuniculi lacks host cell membrane proteins immediately after invasion. Eukaryot Cell 2005, 4:221–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mordue DG, Desai N, Dustin M, Sibley LD: Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring. J Exp Med 1999, 190:1783–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haldar AK, Piro AS, Finethy R, Espenschied ST, Brown HE, Giebel AM, Frickel EM, Nelson DE, Coers J: Chlamydia trachomatis is resistant to inclusion ubiquitination and associated host defense in gamma interferon-primed human epithelial cells. MBio 2016, 7:e01417–e1516.• Human cells target ubiquitin to inclusions of the rodent-adapted pathogen Chlamydia muridarum, whereas the human-adapted species C. trachomatis is resistant to this host defense. The ubiquitin-marked inclusions also contain p62, LC3 and GBP1 and the trigger the elimination of C. muridarum. C. trachomatis has evolved strategies to interfere with the human-specific ubiquitination machinery.
- 24.Kravets E, Degrandi D, Ma Q, Peulen T-O, Klümpers V, Felekyan S, Kühnemuth R, Weidtkamp-Peters S, Seidel CA, Pfeffer K: Guanylate binding proteins directly attack Toxoplasma gondii via supramolecular complexes. Elife 2016, 5:e11479.•• Murine GBPs target the Toxoplasma PV from at least two subcellular reservoirs and form orchestrated supramolecular complexes of multimers. Upon PVM rupture mGBP2 attacks the plasma membrane of the parasite. Watch the movies in this publications, they are great!
- 25.Liesenfeld O, Parvanova I, Zerrahn J, Han S-J, Heinrich F, Muñoz M, Kaiser F, Aebischer T, Buch T, Waisman A et al. : The IFN-γ-inducible GTPase, Irga6, protects mice against Toxoplasma gondii but not against Plasmodium berghei and some other intracellular pathogens. PLoS ONE 2011, 6:e20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biering SB, Choi J, Halstrom RA, Brown HM, Beatty WL, Lee S, McCune BT, Dominici E, Williams LE, Orchard RC et al. : Viral replication complexes are targeted by LC3-guided interferon-inducible GTPases. Cell Host Microbe 2017, 22 74–85.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohshima J, Lee Y, Sasai M, Saitoh T, Su Ma J, Kamiyama N, Matsuura Y, Pann-Ghill S, Hayashi M, Ebisu S et al. : Role of mouse and human autophagy proteins in IFN-γ-induced cell-autonomous responses against Toxoplasma gondii. J Immunol 2014, 192:3328–3335. [DOI] [PubMed] [Google Scholar]
- 28.Qin A, Lai D-H, Liu Q, Huang W, Wu Y-P, Chen X, Yan S, Xia H, Hide G, Lun Z-R et al. : Guanylate-binding protein 1 (GBP1) contributes to the immunity of human mesenchymal stromal cells against Toxoplasma gondii. Proc Natl Acad Sci USA 2017, 114:1365–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnston AC, Piro A, Clough B, Siew M, Virreira Winter S, Coers J, Frickel EM: Human GBP1 does not localize to pathogen vacuoles but restricts Toxoplasma gondii. Cellular Microbiol 2016, 18:1056–1064.• Human GBP1 restricts the replication of type II and not type I Toxoplasma in epithelial cells without targeting to the PV. This implies GBPs can operate a yet unidentified type of host defense distinct from the classical vacuolar membrane disruption.
- 30.Modiano N, Lu YE, Cresswell P: Golgi targeting of human guanylate-binding protein-1 requires nucleotide binding, isoprenylation, and an IFN-gamma-inducible cofactor. Proc Natl Acad Sci USA 2005, 102:8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tripal P, Bauer M, Naschberger E, Mörtinger T, Hohenadl C, Cornali E, Thurau M, StüCrzl M: Unique features of different members of the human guanylate-binding protein family. J Interferon Cytokine Res 2007, 27:44–52. [DOI] [PubMed] [Google Scholar]
- 32.Gomes LC, Dikic I: Autophagy in antimicrobial immunity. Mol Cell 2014, 54:224–233. [DOI] [PubMed] [Google Scholar]
- 33.Efeyan A, Comb WC, Sabatini DM: Nutrient-sensing mechanisms and pathways. Nature 2015, 517:302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khaminets A, Hunn JP, Könen-Waisman S, Zhao YO, Preukschat D, Coers J, Boyle JP, Ong Y-C, Boothroyd JC, Reichmann G et al. : Coordinated loading of IRG resistance GTPases on to the Toxoplasma gondii parasitophorous vacuole. Cellular Microbiol 2010, 12:939–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi J, Park S, Biering SB, Selleck E, Liu CY, Zhang X, Fujita N, Saitoh T, Akira S, Yoshimori T et al. : The parasitophorous vacuole membrane of Toxoplasma gondii is targeted for disruption by ubiquitin-like conjugation systems of autophagy. Immunity 2014, 40:924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Z, Fux B, Goodwin M, Dunay IR, Strong D, Miller BC, Cadwell K, Delgado MA, Ponpuak M, Green KG et al. : Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe 2008, 4:458–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haldar AK, Piro AS, Pilla DM, Yamamoto M, Coers J: The E2-like conjugation enzyme Atg3 promotes binding of IRG and Gbp proteins to chlamydia- and toxoplasma-containing vacuoles and host resistance. PLoS ONE 2014, 9:e86684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park S, Choi J, Biering SB, Dominici E, Williams LE, Hwang S: Targeting by AutophaGy proteins (TAG): Targeting of IFNG-inducible GTPases to membranes by the LC3 conjugation system of autophagy. Autophagy 2016, 12:1153–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee Y, Sasai M, Ma JS, Sakaguchi N, Ohshima J, Bando H, Saitoh T, Akira S, Yamamoto M: p62 plays a specific role in interferon-γ-induced presentation of a toxoplasma vacuolar antigen. Cell Rep 2015, 13:223–233. [DOI] [PubMed] [Google Scholar]
- 40.Sasai M, Sakaguchi N, Ma JS, Nakamura S, Kawabata T, Bando H, Lee Y, Saitoh T, Akira S, Iwasaki A et al. : Essential role for GABARAP autophagy proteins in interferon-inducible GTPase-mediated host defense. Nat Immunol 2017, 18:899–910.•• Heroic effort to identify the GABARAP protein GATE-16 as the only LC3 homolog essential for in vivo Toxoplasma control. GATE-16 specifically associates with the golgi-localised small GTPase Arf1 to ensure uniform distribution of IRGs and GBPs.
- 41.Jensen KDC: Antigen presentation of vacuolated apicomplexans — two gateways to a vaccine antigen. Trends Parasitol 2016, 32:88–90. [DOI] [PubMed] [Google Scholar]
- 42.Gupta SL, Carlin JM, Pyati P, Dai W, Pfefferkorn ER, Murphy MJ: Antiparasitic and antiproliferative effects of indoleamine 2,3-dioxygenase enzyme expression in human fibroblasts. Infection Immunity 1994, 62:2277–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Man SM, Karki R, Kanneganti T-D: Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 2017, 277:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Man SM, Karki R, Sasai M, Place DE, Kesavardhana S, Temirov J, Frase S, Zhu Q, Malireddi RKS, Kuriakose T et al. : IRGB10 liberates bacterial ligands for sensing by the AIM2 and Caspase-11-NLRP3 inflammasomes. Cell 2016, 167 382–396. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meunier E, Wallet P, Dreier RF, Costanzo S, Anton L, Rühl S, Dussurgey S, Dick MS, Kistner A, Rigard M et al. : Guanylatebinding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat Immunol 2015. 10.1038/ni.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pilla DM, Hagar JA, Haldar AK, Mason AK, Degrandi D, Pfeffer K, Ernst RK, Yamamoto M, Miao EA, Coers J: Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc Natl Acad Sci USA 2014. 10.1073/pnas.1321700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shenoy AR, Wellington DA, Kumar P, Kassa H, Booth CJ, Cresswell P, MacMicking JD: GBP5 Promotes NLRP3 inflammasome assembly and immunity in mammals. Science 2012. 10.1126/science.1217141. [DOI] [PubMed] [Google Scholar]
- 48.Finethy R, Jorgensen I, Haldar AK, de Zoete MR, Strowig T, Flavell RA, Yamamoto M, Nagarajan UM, Miao EA, Coers J: Guanylate binding proteins enable rapid activation of canonical and noncanonical inflammasomes in Chlamydia-infected macrophages. Infection Immunity 2015. 10.1128/IAI.00856-15.• Murine GBPs do not only disrupt inclusions of intracellular pathogens, but are independently able to promote canonical and non-canonical inflammasome activation. The study shows that GBPs control the kinetics of inflammasome activation in macrophages in response to Chlamydia infection.
- 49.Man SM, Karki R, Malireddi RKS, Neale G, Vogel P, Yamamoto M, Lamkanfi M, Kanneganti T-D: The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat Immunol 2015. 10.1038/ni.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meunier E, Dick MS, Dreier RF, Schürmann N, Broz DK, Warming S, Roose-Girma M, Bumann D, Kayagaki N, Takeda K et al. : Caspase-11 activation requires lysis of pathogencontaining vacuoles by IFN-induced GTPases. Nature 2014. 10.1038/nature13157. [DOI] [PubMed] [Google Scholar]
- 51.Staeheli P, prochazka M, Steigmeier PA, Haller O: Genetic control of interferon action: mouse strain distribution and inheritance of an induced protein with guanylate-binding property. Virology 1984, 137:135–142. [DOI] [PubMed] [Google Scholar]
- 52.Hassan MA, Jensen KD, Butty V, Hu K, Boedec E, Prins P, Saeij JPJ: Transcriptional and linkage analyses identify loci that mediate the differential macrophage response to inflammatory stimuli and infection. PLoS Genet 2015, 11: e1005619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ewald SE, Chavarria-Smith J, Boothroyd JC: NLRP1 is an inflammasome sensor for Toxoplasma gondii. Infection Immunity 2014, 82:460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gorfu G, Cirelli KM, Melo MB, Mayer-Barber K, Crown D, Koller BH, Masters S, Sher A, Leppla SH, Moayeri M et al. : Dual role for inflammasome sensors NLRP1 and NLRP3 in murine resistance to Toxoplasma gondii. MBio 2014:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cirelli KM, Gorfu G, Hassan MA, Printz M, Crown D, Leppla SH, Grigg ME, Saeij JPJ, Moayeri M: Inflammasome sensor NLRP1 controls rat macrophage susceptibility to Toxoplasma gondii. PLoS Pathog 2014, 10:e1003927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao YO, Khaminets A, Hunn JP, Howard JC: Disruption of the Toxoplasma gondii parasitophorous vacuole by IFNgamma-inducible immunity-related GTPases (IRG proteins) triggers necrotic cell death. PLoS Pathog 2009, 5:e1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niedelman W, Sprokholt JK, Clough B, Frickel EM, Saeij JPJ: Cell death of gamma interferon-stimulated human fibroblasts upon Toxoplasma gondii infection induces early parasite egress and limits parasite replication. Infection Immunity 2013, 81:4341–4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.da Fonseca Ferreira-da-Silva M, Springer-Frauenhoff HM, Bohne W, Howard JC: Identification of the microsporidian Encephalitozoon cuniculi as a new target of the IFNγ-inducible IRG resistance system. PLoS Pathog 2014, 10: e1004449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sidik SM, Huet D, Ganesan SM, Huynh M-H, Wang T, Nasamu AS, Thiru P, Saeij JPJ, Carruthers VB, Niles JC et al. : A genome-wide CRISPR screen in Toxoplasma identifies essential apicomplexan genes. Cell 2016, 166 1423–1435.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park RJ, Wang T, Koundakjian D, Hultquist JF, Lamothe-Molina P, Monel B, Schumann K, Yu H, Krupzcak KM, Garcia-Beltran W et al. : A genome-wide CRISPR screen identifies a restricted set of HIV host dependency factors. Nat Genet 2017, 49:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]