Abstract
The global, randomized NAPOLI‐1 phase 3 trial reported a survival benefit with liposomal irinotecan (nal‐IRI) plus 5‐fluorouracil/leucovorin (nal‐IRI+5‐FU/LV) in patients with metastatic pancreatic ductal adenocarcinoma (mPDAC) after previous gemcitabine‐based therapy. Median overall survival (OS) with nal‐IRI+5‐FU/LV was 6.1 vs 4.2 months with 5‐FU/LV alone (unstratified hazard ratio [HR] = 0.67, P = .012). Herein, we report efficacy and safety results from a post‐hoc subgroup analysis of Asian patients treated at Asian centers. Primary study endpoint was OS; secondary endpoints included progression‐free survival (PFS), objective response rate (ORR), and safety. Patients receiving nal‐IRI+5‐FU/LV (n = 34) had significantly longer median OS versus 5‐FU/LV (n = 35) (8.9 vs 3.7 months; unstratified HR = 0.51, P = .025). Patients had significantly increased median PFS with nal‐IRI+5‐FU/LV versus 5‐FU/LV (4.0 vs 1.4; unstratified HR = 0.48, P = .011), and increased ORR (8.8% vs 0; P = .114). nal‐IRI monotherapy (n = 50) numerically improved efficacy endpoints versus 5‐FU/LV (n = 48): median OS was 5.8 versus 4.3 months (HR = 0.83, P = .423) and median PFS was 2.8 versus 1.4 months (HR = 0.69, P = .155). Grade ≥3 neutropenia was reported more frequently with nal‐IRI+5‐FU/LV versus 5‐FU/LV (54.5% vs 3.4%), and incidence of grade ≥3 diarrhea was comparable between the two arms (3.0% vs 6.9%). This subgroup analysis confirms nal‐IRI+5‐FU/LV as an efficacious treatment option that improves survival in Asian patients with mPDAC that progressed after gemcitabine‐based therapy, with a safety profile agreeing with previous findings. The nal‐IRI+5‐FU/LV regimen should represent a new standard of care for these patients in Asia. (Clinicaltrials.gov: NCT01494506)
Keywords: Asian subgroup, clinical trial, phase 3, liposomal irinotecan, metastatic pancreatic cancer, NAPOLI‐1
The global NAPOLI‐1 phase 3 trial (NCT01494506) evaluated the safety and efficacy of liposomal irinotecan (nal‐IRI) and 5‐fluorouracil/leucovorin (5‐FU/LV) compared with 5‐FU/LV in patients with metastatic pancreatic adenocarcinoma that progressed following gemcitabine‐based therapy. We report data from a post‐hoc subgroup analysis focusing on Asian patients at centers in South Korea and Taiwan. Our findings are consistent with those from the primary analysis and show that, despite association with higher incidence of manageable grade 3‐4 neutropenia, the nal‐IRI+5‐FU/LV regimen is an effective treatment option in Asian patients with gemcitabine‐failed metastatic pancreatic cancer.

1. INTRODUCTION
Pancreatic cancer continues to have a bleak prognosis,1 with an estimated 4.6‐months median overall survival (OS; all stages from diagnosis2) and only limited improvement in 5‐year and 1‐year survival rates. The incidence of pancreatic cancer is projected to rise over the next 10‐15 years, and it is predicted to become the second leading cause of cancer‐related death in the USA by 2030.3 According to 2018 GLOBOCAN estimates, the age‐standardized incidence rate (ASR) for pancreatic cancer in Asia is 3.9/100 000 persons and that of death is 3.6, compared with 7.7 and 7.0 for the USA and Europe combined.4 A recent study including 40 European countries reported estimated ASR of 11.5/100 000 for incidence and 10.9 for death.5
Chemotherapy for metastatic pancreatic cancer is guided by patient performance status (PS). Accordingly, in patients with good PS, first‐line chemotherapy of metastatic pancreatic adenocarcinoma (mPAC) usually comprises FOLFIRINOX (5‐fluorouracil and leucovorin [5‐FU/LV] + irinotecan + oxaliplatin) or gemcitabine in combination with nab‐paclitaxel.2, 6, 7, 8, 9 In those with worse PS, treatment may be limited to gemcitabine monotherapy, oral 5‐FU agents such as S‐1, infusional 5‐FU or best supportive care.2, 7, 8, 10 For patients with mPAC that progressed following gemcitabine‐based therapy, combination treatment with liposomal irinotecan (nal‐IRI) and 5‐FU/LV has recently been approved by regulatory agencies in numerous countries. These approvals followed positive results from the global phase 3 NAPOLI‐1 trial (NCT01494506).11 In the intention‐to‐treat (ITT) population, nal‐IRI+5‐FU/LV combination significantly increased median OS versus 5‐FU/LV alone (6.1 vs 4.2 months; unstratified hazard ratio [HR] = 0.67, P = .012).
The new formulation of the topoisomerase I inhibitor nal‐IRI comprises irinotecan sucrosofate salt encapsulated in pegylated liposomes. The formulation protects the drug from premature conversion and activation in the liver. As a consequence, circulation in the plasma in patients is extended.12, 13, 14 The higher vascular permeability of tumor tissues may promote diffusion of nal‐IRI from the circulation, with subsequent tumor‐associated macrophage uptake and activation of the drug leading to an increase in local SN‐38 concentrations.13, 15, 16, 17 Time over the exposure threshold of the tumors to the active irinotecan metabolite, SN‐38, has also been shown to increase in preclinical tumor models, and in plasma compared with tumor tissue, thereby increasing preclinical activity at lower levels.12, 13, 14
In NAPOLI‐1, ethnic origin was included as one of the stratification criteria (Caucasian vs East Asian vs all others).11 Asian patients furthermore represent a large and diverse population with possible differences in pancreatic cancer incidence, mortality, and treatment options among Asian countries as compared with data available for Western populations.1, 4, 5, 18, 19, 20, 21 Regional differences in treatment outcomes have been observed in pancreatic cancer, and other diseases (eg, advanced gastric cancer).22, 23, 24 Previous work has also indicated differences in drug metabolism affecting the plasma concentration of SN‐38 and irinotecan after nal‐IRI treatment between patients of East Asian ethnicity and Caucasian ethnicity.14, 25 It was reported that Asian patients had a significantly higher mean maximum plasma concentration (Cmax) of unencapsulated SN‐38 and a lower Cmax of total irinotecan after dosing with nal‐IRI compared with Caucasian patients, which was associated with increased grade 3 or 4 neutropenia and decreased grade 3 or 4 diarrhea in Asian versus Caucasian patients.25 Therefore, we carried out a post‐hoc subgroup analysis of the NAPOLI‐1 study assessing the efficacy and safety of nal‐IRI+5‐FU/LV in Asian patients treated at Asian centers, the results of which are presented here.
2. METHODS
2.1. Study overview
Methodology and design of the NAPOLI‐1 study have been described in detail and published elsewhere.11 In summary, NAPOLI‐1 was a global, randomized phase 3 trial evaluating the safety and efficacy of nal‐IRI+5‐FU/LV (80 mg/m2 irinotecan hydrochloride trihydrate salt equivalent to 70 mg/m2 irinotecan free base, followed by 400 mg/m2 LV prior to 2400 mg/m2 5‐FU, every 2 weeks) in adult patients with mPAC that had progressed after gemcitabine‐based therapy compared with 5‐FU/LV alone (200 mg/m2 LV before 2000 mg/m2 5‐FU weekly for the first 4 weeks of each 6‐week chemotherapy cycle). A third arm comprised nal‐IRI monotherapy (120 mg/m2 irinotecan hydrochloride trihydrate salt, equivalent to 100 mg/m2 irinotecan free base every 3 weeks). Although initially designed to compare nal‐IRI monotherapy with 5‐FU/LV alone, the NAPOLI‐1 trial was amended to add the nal‐IRI+5‐FU/LV arm when safety data on the combination became available.26 Patients were randomized 1:1:1. All patients received best supportive care according to local institutional standards as part of their participation in the study. Prophylactic use of granulocyte‐colony stimulating factor (G‐CSF) was permitted only in patients who experienced ≥1 episode of grade 3/4 neutropenia or neutropenic fever while on study treatment, or with documented grade 3/4 neutropenia or neutropenic fever while receiving prior antineoplastic therapy.
Prior to randomization, patients were stratified by ethnicity (Caucasian vs East Asian vs all others), baseline albumin levels (≥40 g/L vs <40 g/L), and Karnofsky Performance Status (KPS) 70‐80 vs ≥90. This post‐hoc analysis included only those patients treated at centers in the Asia region, including the Republic of Korea and Taiwan. Data from the overall ITT population for the nal‐IRI+5‐FU/LV, 5‐FU/LV control, and nal‐IRI monotherapy arms are included to facilitate comparisons and have been partially published with the primary analysis.11
2.2. Inclusion and exclusion criteria
Adult patients (aged 18 years and older, and with KPS ≥70) with histologically or cytologically confirmed pancreatic ductal adenocarcinoma (PDAC) and documented metastatic disease were eligible for inclusion in this study. Measurable or non‐measurable lesions were graded according to RECIST guidelines version 1.1. Patients must have experienced disease progression after previous gemcitabine‐based therapy that had been given in a neoadjuvant, adjuvant (only if distant metastases occurred within 6 months of completing adjuvant therapy), locally advanced, or metastatic disease setting. Adequate renal and hepatic function was required (including normal serum total bilirubin and albumin levels ≥30 g/L), as well as a neutrophil count >1.5 × 109 cells/L. Patients with active central nervous system metastasis, clinically significant gastrointestinal disorders and severe arterial thromboembolic events <6 months before enrolment were excluded.
2.3. Outcomes
Primary efficacy endpoint of NAPOLI‐1 was OS, with secondary endpoints including progression‐free survival (PFS), time to treatment failure (TTF), and overall response rate (ORR). Safety and tolerability of study regimens were also evaluated.
2.4. Statistical analyses
Data described in this subgroup analysis are based on the NAPOLI‐1 trial primary analysis data cut‐off date of February 14, 2014. Median OS, OS rate at 12 months, and PFS were all estimated using the Kaplan‐Meier method. Treatment group comparisons were done for patients receiving the nal‐IRI+5‐FU/LV combination compared with those receiving 5‐FU/LV combination control who were included after the protocol amendment. Hazard ratios (HR) were derived using the Cox proportional hazards model, with treatment as the independent variable. Overall survival comparisons were done using unstratified log‐rank tests; PFS and TTF were analyzed using the log‐rank method; ORR using Fisher’s exact test. P‐values are descriptive and significance is defined at P < .05. ORR was defined as the percentage of patients with best overall response (complete response [CR] or partial response [PR]) in the population. Best overall response was graded according to RECIST v1.1 criteria.
3. RESULTS
Of the 417 patients included in the NAOPLI‐1 study, 132 patients enrolled at 13 participating centers in Asia (five in the Republic of Korea and eight in Taiwan) were included in the current post‐hoc analysis (Figure 1). These patients were randomly assigned to receive nal‐IRI+5‐FU/LV (n = 34), nal‐IRI monotherapy (n = 50), or 5‐FU/LV (n = 48). Of the 48 patients assigned to receive 5‐FU/LV, 35 enrolled after the protocol amendment adding the nal‐IRI+5‐FU/LV combination arm, and served as comparator for the combination treatment arm.
Figure 1.
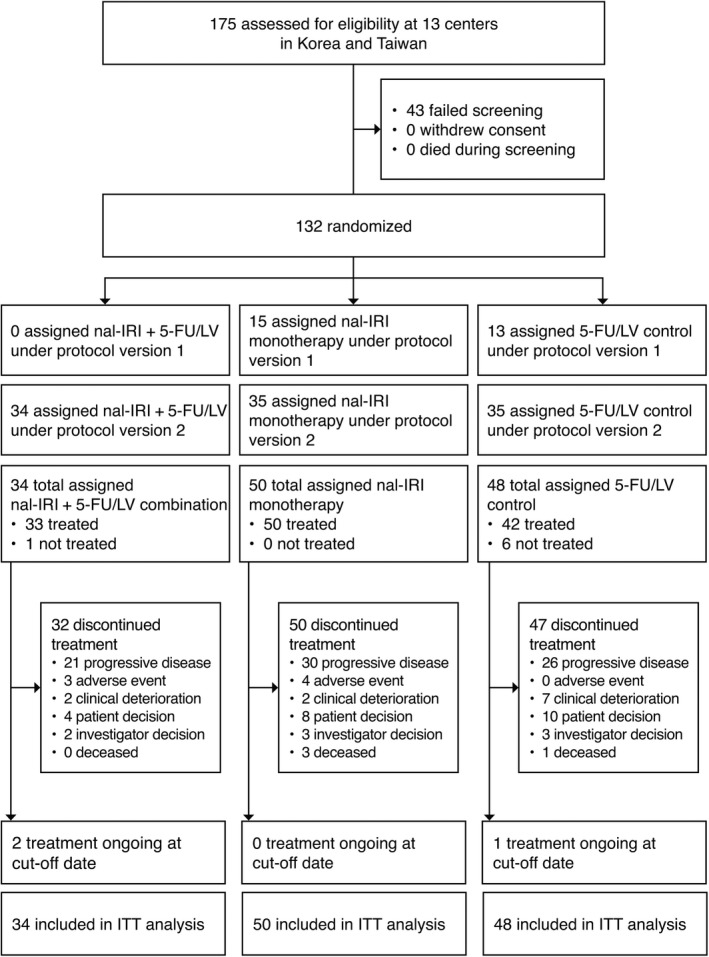
CONSORT diagram explaining the patient population included in this subgroup analysis (ITT population). 5‐FU, 5‐fluorouracil; LV, leucovorin (folinic acid); nal‐IRI, liposomal irinotecan; ITT, intention‐to‐treat
This analysis focuses on comparison of the nal‐IRI+5‐FU/LV combination with 5‐FU/LV control (enrolled after the protocol amendment) in patients from the Asia region. In the NAPOLI‐1 study, nal‐IRI monotherapy showed clinical activity versus 5‐FU/LV: ORR was 6.0% versus 0.7% (P = .020), and median PFS was 2.7 versus 1.6 months (HR = 0.81, P = .100). Although median OS was 4.9 months with nal‐IRI monotherapy versus 4.2 months with 5‐FU/LV (HR = 0.99; P = .942), this did not reach statistical significance. Therefore, data for nal‐IRI monotherapy are included in the present analysis for completeness (Tables 1, 2, 3, 4, 5 and Figure 2).
Table 1.
Baseline demographics and clinical characteristics of the Asia region population and overall population for the nal‐IRI+5‐FU/LV and 5‐FU/LV treatment groups (ITT population)
| Asia region population | Overall population | |||||||
|---|---|---|---|---|---|---|---|---|
| Combination therapy | Monotherapy | Combination therapy | Monotherapy | |||||
|
nal‐IRI+ 5‐FU/LV n = 34 |
5‐FU/LV n = 35 |
nal‐IRI n = 50 |
5‐FU/LV n = 48 |
nal‐IRI+ 5‐FU/LV n = 117 |
5‐FU/LV n = 119 |
nal‐IRI n = 151 |
5‐FU/LV n = 149 |
|
| Gender, n (%) | ||||||||
| Female | 16 (47.1) | 13 (37.1) | 23 (46.0) | 18 (37.5) | 48 (41.0) | 52 (43.7) | 64 (42.4) | 68 (45.6) |
| Male | 18 (52.9) | 22 (62.9) | 27 (54.0) | 30 (62.5) | 69 (59.0) | 67 (56.3) | 87 (57.6) | 81 (54.4) |
| Age, y | ||||||||
| Median | 60.0 | 58.0 | 62.5 | 60.0 | 63.0 | 62.0 | 65.0 | 63.0 |
| Min, Max | 43, 81 | 34, 73 | 39, 87 | 34, 78 | 41, 81 | 34, 80 | 31, 87 | 34, 83 |
| Ethnicity, n (%) | ||||||||
| Asian | 34 (100.0) | 35 (100.0) | 50 (100.0) | 48 (100.0) | 34 (29.1) | 36 (30.3) | 52 (34.4) | 50 (33.6) |
| Caucasian | 0 | 0 | 0 | 0 | 72 (61.5) | 76 (63.9) | 89 (58.9) | 92 (61.7) |
| Other | 0 | 0 | 0 | 0 | 11 (9.4) | 7 (5.9) | 10 (6.7) | 7 (4.7) |
| Baseline KPS, n (%)a | ||||||||
| 90‐100 | 22 (64.7) | 22 (62.9) | 31 (62.0) | 29 (60.4) | 69 (59.0) | 57 (47.9) | 86 (57.0) | 76 (51.0) |
| 70‐80 | 12 (35.3) | 13 (37.1) | 19 (38.0) | 19 (39.6) | 45 (38.5) | 61 (51.3) | 65 (43.0) | 72 (48.3) |
| 50‐60 | 0 | 0 | 0 | 0 | 3 (2.6) | 0 | 0 | 0 |
| Missing | 0 | 0 | 0 | 0 | 0 | 1 (0.8) | 0 | 1 (0.7) |
| Baseline CA19‐9, n (%)b | ||||||||
| Median (U/mL) | 475.5 | 4945.0 | 1888.0 | 4101.5 | 1278.0 | 1292.0 | 2189.0 | 1019.0 |
| <40 U/mL | 7/32 (21.9) | 3 (8.6) | 9 (18.0) | 6 (12.5) | 22/114 (19.3) | 23/114 (20.2) | 21/146 (14.4) | 28/144 (19.4) |
| ≥40 U/mL | 25/32 (78.1) | 32 (91.4) | 41 (82.0) | 42 (87.5) | 92/114 (80.7) | 91/114 (79.8) | 125/146 (85.6) | 116/144 (80.6) |
| Missing | 2 (5.9) | 0 | 0 | 0 | 3 (2.6) | 5 (4.2) | 5 (3.3) | 5 (3.4) |
| Baseline albumin, n (%)a | ||||||||
| <4.0 g/dL | 18 (52.9) | 18 (51.4) | 25 (50.0) | 26 (54.2) | 64 (54.7) | 65 (54.6) | 88 (58.3) | 83 (55.7) |
| ≥4.0 g/dL | 16 (47.1) | 17 (48.6) | 25 (50.0) | 22 (45.8) | 53 (45.3) | 54 (45.4) | 63 (41.7) | 66 (44.3) |
| Median (g/dL) | 4.0 | 3.9 | 4.1 | 3.8 | 4.1 | 4.0 | 4.0 | 4.0 |
| Measurable metastatic lesions at baseline, n (%) | ||||||||
| 1 | 9 (26.5) | 8 (22.9) | 11 (22.0) | 8 (16.7) | 19 (16.2) | 22 (18.5) | 36 (23.8) | 26 (17.4) |
| 2 | 13 (38.2) | 15 (42.9) | 23 (46.0) | 22 (45.8) | 49 (41.9) | 58 (48.7) | 63 (41.7) | 72 (48.3) |
| 3 | 3 (8.8) | 6 (17.1) | 7 (14.0) | 9 (18.8) | 22 (18.8) | 15 (12.6) | 22 (14.6) | 21 (14.1) |
| >3 | 1 (2.9) | 2 (5.7) | 0 | 3 (6.3) | 7 (6.0) | 8 (6.7) | 7 (4.6) | 10 (6.7) |
| Anatomical location of lesions at baseline, n (%)c | ||||||||
| Liver | 18 (52.9) | 24 (68.6) | 30 (60.0) | 34 (70.8) | 75 (64.1) | 84 (71.8) | 101 (66.9) | 108 (72.5) |
| Homozygous for UGT1A1*28, n (%) | 1 (2.9) | 0 | 0 | 0 | 7 (6.0) | 9 (7.6) | 7 (4.6) | 13 (8.7) |
| Disease stage at diagnosis, n (%) | ||||||||
| IA | 1 (2.9) | 0 | 0 | 0 | 1 (0.9) | 2 (1.7) | 1 (0.7) | 2 (1.3) |
| IB | 0 | 0 | 2 (4.0) | 0 | 1 (0.9) | 3 (2.5) | 4 (2.6) | 3 (2.0) |
| IIA | 2 (5.9) | 3 (8.6) | 11 (22.0) | 4 (8.3) | 6 (5.1) | 9 (7.6) | 19 (12.6) | 11 (7.4) |
| IIB | 11 (32.4) | 7 (20.0) | 7 (14.0) | 7 (14.6) | 26 (22.2) | 22 (18.5) | 26 (17.2) | 25 (16.8) |
| III | 6 (17.6) | 3 (8.6) | 8 (16.0) | 6 (12.5) | 21 (17.9) | 19 (16.0) | 30 (19.9) | 24 (16.1) |
| IV | 14 (41.2) | 22 (62.9) | 22 (44.0) | 31 (64.6) | 61 (52.1) | 62 (52.1) | 70 (46.4) | 82 (55.0) |
| Primary tumor location, n (%) | ||||||||
| Head only | 23 (67.6) | 18 (51.4) | 30 (60.0) | 23 (47.9) | 70 (59.8) | 65 (54.6) | 92 (60.9) | 77 (51.7) |
| Body only | 3 (8.8) | 8 (22.9) | 6 (12.0) | 11 (22.9) | 12 (10.3) | 19 (16.0) | 16 (10.6) | 26 (17.4) |
| Tail only | 4 (11.8) | 6 (17.1) | 7 (14.0) | 9 (18.8) | 14 (12.0) | 19 (16.0) | 24 (15.9) | 24 (16.1) |
| Multi‐locations incl. head | 0 | 0 | 1 (2.0) | 0 | 6 (5.1) | 4 (3.4) | 7 (4.6) | 4 (2.7) |
| Multi‐locations excl. head | 3 (8.8) | 3 (8.6) | 3 (6.0) | 4 (8.3) | 9 (7.7) | 10 (8.4) | 7 (4.6) | 14 (9.4) |
| Unknown | 0 | 0 | 2 (4.0) | 1 (2.1) | 6 (5.1) | 2 (1.7) | 5 (3.3) | 4 (2.7) |
| Previous lines of metastatic therapy, n (%) | ||||||||
| 0 | 5 (14.7) | 6 (17.1) | 4 (8.0) | 8 (16.7) | 15 (12.8) | 15 (12.6) | 17 (11.3) | 19 (12.8) |
| 1 | 20 (58.8) | 21 (60.0) | 25 (50.0) | 28 (58.3) | 62 (53.0) | 67 (56.3) | 86 (57.0) | 86 (57.7) |
| ≥2 | 9 (26.5) | 8 (22.9) | 21 (42.0) | 12 (25.0) | 40 (34.2) | 37 (31.1) | 48 (31.8) | 44 (29.5) |
| Prior anticancer therapy, n (%)d | ||||||||
| Gemcitabine alone | 13 (38.2) | 12 (34.3) | 13 (26.0) | 33 (68.8) | 53 (45.3) | 55 (46.2) | 67 (44.4) | 66 (44.3) |
| Gemcitabine combination | 21 (61.8) | 23 (65.7) | 37 (74.0) | 33 (68.8) | 64 (54.7) | 64 (53.8) | 84 (55.6) | 83 (55.7) |
| Fluorouracil‐containing | 13 (38.2) | 16 (45.7) | 27 (54.0) | 22 (45.8) | 50 (42.7) | 52 (43.7) | 70 (46.4) | 63 (42.3) |
| Irinotecan‐containing | 1 (2.9) | 3 (8.6) | 3 (6.0) | 3 (6.3) | 12 (10.3) | 17 (14.3) | 17 (11.3) | 17 (11.4) |
| Platinum‐containing | 9 (26.5) | 14 (40.0) | 24 (48.0) | 18 (37.5) | 38 (32.5) | 41 (34.5) | 54 (35.8) | 45 (30.2) |
| Post‐study anticancer therapy, n (%)d | ||||||||
| Received post‐study anticancer therapy | 17 (50.0) | 18 (51.4) | 24 (48.0) | 24 (50.0) | 36 (30.8) | 45 (37.8) | 54 (35.8) | 55 (36.9) |
| Gemcitabine combination | 7 (20.6) | 6 (17.1) | 7 (14.0) | 9 (18.8) | 11 (9.4) | 12 (10.1) | 16 (10.6) | 17 (11.4) |
| Fluorouracil‐containing | 11 (32.4) | 14 (40.0) | 20 (40.0) | 18 (37.5) | 22 (18.8) | 30 (25.2) | 39 (25.8) | 37 (24.8) |
| Irinotecan‐containing | 4 (11.8) | 7 (20.0) | 6 (12.0) | 9 (18.8) | 8 (6.8) | 9 (7.6) | 7 (4.6) | 11 (7.4) |
| Platinum‐containing | 11 (32.4) | 8 (22.9) | 14 (28.0) | 13 (27.1) | 19 (16.2) | 22 (18.5) | 25 (16.6) | 27 (18.1) |
| Other non‐investigational agents | 5 (14.7) | 5 (14.3) | 7 (14.0) | 5 (10.4) | 13 (11.1) | 9 (7.6) | 15 (9.9) | 11 (7.4) |
| Investigational agents | 1 (2.9) | 0 | 0 | 0 | 3 (2.6) | 4 (3.4) | 5 (3.3) | 5 (3.4) |
| Not recorded | 17 (50.0) | 17 (48.6) | 26 (52.0) | 24 (50.0) | 81 (69.2) | 74 (62.2) | 97 (64.2) | 94 (63.1) |
| Median time since last prior anticancer therapy, months (1st and 3rd quartiles) | 1.1 (0.8, 1.4) | 1.0 (0.6, 1.3) | 1.1 (0.7, 1.5) | 1.0 (0.6, 1.4) | 1.4 (0.9, 2.1) | 1.1 (0.9, 2.2) | 1.3 (0.9, 1.9) | 1.2 (0.9, 2.0) |
| Median time since initial diagnosis, months (1st and 3rd quartiles) | 10.2 (5.1, 19.9) | 7.3 (4.8, 10.5) | 12.4 (6.6, 18.2) | 6.9 (3.9, 11.5) | 10.3 (6.2, 17.1) | 10.3 (6.2, 15.1) | 10.4 (6.1, 17.2) | 9.5 (5.7, 14.4) |
| Median time from last study drug exposure to first post‐study anticancer therapy, weeks (1st and 3rd quartiles) | 2.9 (2.4, 3.9) | 2.9 (1.9, 3.8) | 3.7 (3.0, 5.9) | 3.1 (2.9, 4.0) | 3.1 (2.6, 5.5) | 3.6 (2.9, 4.9) | 4.4 (3.1, 5.7) | 3.7 (2.9, 4.9) |
Baseline is defined as the last measurement obtained prior to study drug administration.
Abbreviations: 5‐FU, 5‐fluorouracil; CA19‐9, carbohydrate antigen 19‐9; ITT, intention‐to‐treat; KPS, Karnofsky performance status; LV, leucovorin (folinic acid); nal‐IRI, liposomal irinotecan.
KPS and albumin summaries are based on classification per randomization.
Includes only patients with a measured CA19‐9 level prior to treatment. In the overall intent‐to‐treat population, data were missing for three patients in the nal‐IRI+5‐FU/LV group and in five patients in the 5‐FU/LV group (enrolled under protocol version 2).
Based on lesion locations according to RECIST guidelines v1.1. Includes all measurable and non‐measurable lesions, and all metastatic and non‐metastatic lesions.
Columns add up to ≥100% as some patients received more than one prior line of therapy or more than one post‐study treatment anticancer therapy and may therefore be included in more than one category.
Table 2.
Summary of efficacy for Asia region patients and the overall population (ITT population)
| Asia region population | Overall ITT population | |||||||
|---|---|---|---|---|---|---|---|---|
| Combination therapy | Monotherapy | Combination therapy | Monotherapy | |||||
|
nal‐IRI+5‐FU/LV n=34 |
5‐FU/LV n=35 |
nal‐IRI n=50 |
5‐FU/LV n=48 |
nal‐IRI+5‐FU/LV n=117 |
5‐F/LV n=119 |
nal‐IRI n=151 |
5‐FU/LV n=149 |
|
| Overall survival | ||||||||
| Median OS time, mo | 8.9 | 3.7 | 5.8 | 4.3 | 6.1 | 4.2 | 4.9 | 4.2 |
| 95% CI | 4.4–10.4 | 2.7–6.4 | 4.8–7.4 | 3.1–5.7 | 4.8–8.9 | 3.3–5.3 | 4.2–5.6 | 3.6–4.9 |
| HRa | 0.51 | 0.83 | 0.67 | 0.99 | ||||
| 95% CI | 0.28–0.93 | 0.53–1.31 | 0.49–0.92 | 0.77–1.28 | ||||
| P‐valueb | .025 | .423 | .012 | .942 | ||||
| Progression‐free survival | ||||||||
| Median PFS time, mo | 4.0 | 1.4 | 2.8 | 1.4 | 3.1 | 1.5 | 2.7 | 1.6 |
| 95% CI | 1.5–5.7 | 1.3–2.0 | 1.5–4.1 | 1.3–1.9 | 2.7–4.2 | 1.4–1.8 | 2.1–2.9 | 1.4–1.8 |
| HRa | 0.48 | 0.69 | 0.56 | 0.81 | ||||
| 95% CI | 0.27–0.85 | 0.44–1.07 | 0.41–0.75 | 0.63–1.04 | ||||
| P‐valueb | .011 | .155 | <.001 | .100 | ||||
| Best overall response, n (%) | ||||||||
| ORR | 8.8 | 0 | 10.0 | 0 | 16.2 | 0.8 | 6.0 | 0.7 |
| P‐value | .114 | .056 | <.001 | .020 | ||||
| PR | 3 (8.8) | 0 | 5 (10.0) | 0 | 19 (16.2) | 1 (0.8) | 9 (6.0) | 1 (0.7) |
| SD | 15 (44.1) | 5 (14.3) | 18 (36.0) | 8 (16.7) | 39 (33.3) | 26 (21.8) | 54 (35.8) | 35 (23.5) |
| Non‐CR/non‐PD | 0 | 0 | 2 (4.0) | 0 | 3 (2.6) | 2 (1.7) | 3 (2.0) | 2 (1.3) |
| PD | 11 (32.4) | 18 (51.4) | 20 (40.0) | 24 (50.0) | 34 (29.1) | 56 (47.1) | 51 (33.8) | 71 (47.7) |
| NE | 5 (14.7) | 12 (34.3) | 5 (10.0) | 16 (33.3) | 22 (18.8) | 34 (28.6) | 34 (22.5) | 40 (26.8) |
| CBR | 18 (52.9) | 5 (14.3) | 23 (46.0) | 8 (16.7) | 58 (49.5) | 27 (22.6) | 63 (41.8) | 36 (24.2) |
| Tumor marker (CA19‐9) response | ||||||||
| CA19‐9 response rate, n/N (%) | 8/25 (32.0) | 2/26 (7.7) | 12/41 (29.3) | 4/36 (11.1) | 28/97 (28.9) | 7/81 (8.6) | 29/123 (23.6) | 12/105 (11.4) |
| P‐valuec | <.001 | .024 | <.001 | .024 | ||||
Best overall response is based on RECIST criteria v1.1.
Abbreviations: 5‐FU, 5‐fluorouracil; CBR, clinical benefit response (PR + SD); CI, confidence interval; CR, complete response; HR, hazard ratio; ITT, intention‐to‐treat; LV, leucovorin (folinic acid); mo, months; nal‐IRI, liposomal irinotecan; NE, not evaluable; ORR, objective response rate; PR, partial response; SD, stable disease.
Unstratified hazard ratios were derived using Cox’s proportional hazards model, with treatment as the independent variable.
Two‐sided P‐values from log‐rank test.
Two‐sided P‐values from pairwise comparisons of tumor marker response rates using Fisher’s exact test.
Table 3.
Treatment duration and exposure in the nal‐IRI+5‐FU/LV and 5‐FU/LV treatment groups (safety population)
| Asia region safety population | Overall safety population | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Combination therapy | Monotherapy | Combination therapy | Monotherapy | ||||||
|
nal‐IRI+ 5‐FU/LV n = 33 |
5‐FU/LV n = 29 |
nal‐IRI n = 50 |
5‐FU/LV n = 42 |
nal‐IRI+ 5‐FU/LV n = 117 |
5‐FU/LV n = 105 |
nal‐IRI n = 147 |
5‐FU/LV n = 134 |
||
| No. of treatment cycles received | |||||||||
| Mean (SD) | 5.9 (5.9) | 1.8 (1.5) | 4.0 (3.0) | 1.8 (1.6) | 6.6 (6.3) | 2.0 (2.0) | 3.7 (2.7) | 2.0 (2.1) | |
| Median (1st and 3rd quartiles) | 3.0 (2, 8) | 1.0 (1, 2) | 3.0 (2, 6) | 1.0 (1, 2) | 3.0 (2, 9) | 1.0 (2, 9) | 2.5 (2, 5) | 1.0 (1, 2) | |
| Minimum time on treatment, n (%) | |||||||||
| ≥6 wk | 22 (66.7) | 20 (69.0) | 40 (80.0) | 30 (71.4) | 82 (72.6) | 77 (72.6) | 119 (80.4) | 101 (74.8) | |
| ≥12 wk | 13 (39.4) | 7 (24.1) | 21 (42.0) | 10 (23.8) | 47 (40.9) | 31 (29.2) | 58 (39.2) | 40 (29.6) | |
| ≥18 wk | 11 (33.3) | 4 (13.8) | 13 (26.0) | 6 (14.3) | 40 (34.8) | 17 (16.0) | 33 (22.3) | 22 (16.3) | |
| Relative dose intensity (%), mean (SD)a | |||||||||
| nal‐IRI | 74.9 (20.1) | n/a | 90.2 (11.5) | n/a | 83.2 (17.7) | n/a | 90.2 (11.8) | 95.1% (−)b | |
| 5‐FU | 75.5 (20.6) | 94.9 (13.3) | n/a | 95.1 (12.3) | 83.9 (18.1) | 95.7 (11.2) | n/a | 95.6 (11.1) | |
| Duration of exposure (weeks), mean (SD)c | |||||||||
| nal‐IRI | 14.7 (13.8) | n/a | 12.8 (9.7) | n/a | 15.0 (13.7) | n/a | 11.9 (9.0) |
n/a |
|
| 5‐FU | 14.7 (13.8) | 9.4 (9.3) | n/a | 9.7 (9.9) | 15.0 (13.7) | 10.0 (10.8) | n/a | 10.4 (11.3)b | |
Cycle lengths differ for each regimen: nal‐IRI+5‐FU/LV, 2 wk; nal‐IRI monotherapy, 3 wk; 5‐FU/LV, 6 wk.
Abbreviations: 5‐FU, 5‐fluorouracil; LV, leucovorin (folinic acid); nal‐IRI, liposomal irinotecan; n/a, not applicable; SD, standard deviation.
Relative dose intensity is a function of both amount of study drug received and the time frame over which it was received expressed as a percentage of that planned study drug in the protocol‐defined schedule.
One patient randomized to the 5‐FU/LV arm of the overall population erroneously received 6 wk of nal‐IRI+5‐FU/LV.
Duration of exposure is the time from (the date of the last dose of study administration + projected days to next dose of study drug administration – date first study drug administration)/7.
Table 4.
Grade ≥3 TEAE reported for ≥5% of patients in any treatment groupa
| n (%) | Asia region safety population | Overall safety population | ||||||
|---|---|---|---|---|---|---|---|---|
| Combination therapy | Monotherapy | Combination therapy | Monotherapy | |||||
|
nal‐IRI+ 5‐FU/LV n = 33 |
5‐FU/LV n = 29 |
nal‐IRI n = 50 |
5‐FU/LV n = 42 |
nal‐IRI+ 5‐FU/LV n = 117 |
5‐FU/LV n = 105 |
nal‐IRI n = 147 |
5‐FU/LV n = 134 |
|
| Any grade ≥3 TEAE | 29 (87.9) | 17 (58.6) | 34 (68.0) | 23 (54.8) | 90 (76.9) | 60 (57.1) | 112 (76.2) | 75 (56.0) |
| Neutropeniab | 18 (54.5) | 1 (3.4) | 17 (34.0) | 1 (2.4) | 32 (27.4) | 2 (1.9) | 22 (15.0) | 2 (1.5) |
| Anemia | 7 (21.2) | 2 (6.9) | 12 (24.0) | 6 (14.3) | 11 (9.4) | 5 (4.8) | 16 (10.9) | 9 (6.7) |
| WBC count decreased | 7 (21.2) | 0 | 4 (8.0) | 0 | 9 (7.7) | 0 | 4 (2.7) | 0 |
| Asthenia | 4 (12.1) | 3 (10.3) | 3 (6.0) | 5 (11.9) | 9 (7.7) | 6 (5.7) | 10 (6.8) | 9 (6.7) |
| Biliary tract infection | 3 (9.1) | 0 | 1 (2.0) | 1 (2.4) | 3 (2.6) | 1 (1.0) | 1 (0.7) | 2 (1.5) |
| Abdominal pain | 2 (6.1) | 0 | 5 (10.0) | 1 (2.4) | 8 (6.8) | 6 (5.7) | 12 (8.2) | 8 (6.0) |
| Decreased appetite | 2 (6.1) | 1 (3.4) | 6 (12.0) | 2 (4.8) | 5 (4.3) | 2 (1.9) | 13 (8.8) | 3 (2.2) |
| Nausea | 2 (6.1) | 0 | 3 (6.0) | 1 (2.4) | 9 (7.7) | 2 (1.9) | 8 (5.4) | 4 (3.0) |
| Sepsis | 2 (6.1) | 0 | 1 (2.0) | 0 | 4 (3.4) | 1 (1.0) | 3 (2.0) | 1 (0.7) |
| Vomiting | 2 (6.1) | 0 | 6 (12.0) | 0 | 13 (11.1) | 3 (2.9) | 20 (13.6) | 4 (3.0) |
| Diarrhea | 1 (3.0) | 2 (6.9) | 8 (16.0) | 2 (4.8) | 15 (12.8) | 6 (5.7) | 31 (21.1) | 6 (4.5) |
| Febrile neutropenia | 1 (3.0) | 0 | 5 (10.0) | 0 | 2 (1.7) | 0 | 6 (4.1) | 0 |
| Hyponatremia | 1 (3.0) | 0 | 3 (6.0) | 0 | 3 (2.6) | 2 (1.9) | 9 (6.1) | 2 (1.5) |
| Fatigue | 0 | 0 | 3 (6.0) | 1 (2.4) | 16 (13.7) | 4 (3.8) | 9 (6.1) | 5 (3.7) |
| Hydronephrosis | 0 | 2 (6.9) | 0 | 2 (4.8) | 0 | 2 (1.9) | 0 | 2 (1.5) |
| Hyperglycemia | 0 | 0 | 4 (8.0) | 1 (2.4) | 1 (0.9) | 2 (1.9) | 8 (5.4) | 3 (2.2) |
| Hypokalemia | 0 | 1 (3.4) | 7 (14.0) | 2 (4.8) | 4 (3.4) | 2 (1.9) | 17 (11.6) | 3 (2.2) |
| Leukopenia | 0 | 0 | 3 (6.0) | 0 | 1 (0.9) | 0 | 4 (2.7) | 0 |
Abbreviations: 5‐FU, 5‐fluorouracil; LV, leucovorin (folinic acid); nal‐IRI, liposomal irinotecan; n/a, not applicable; SD, standard deviation; WBC, white blood cell.
Patients with multiple occurrences are counted only once in any category. Safety population included patients who received at least one dose of study drug. Adverse events coded using MedDRA version 14.1. Treatment emergent adverse events (TEAE) are events that occurred or worsened on or after the day of first dose of the study drug and within 30 d after last administration of study drug.
Based on summary term comprising agranulocytosis, decreased neutrophil count, febrile neutropenia, granulocytopenia, neutropenia, neutropenic sepsis, and pancytopenia.
Table 5.
TEAE (any grade) resulting in dose delay or dose reduction (in ≥5% of patients in any arm), and treatment discontinuation (in ≥2% of patients in any arm)a
| Asia region safety population | Overall safety population | |||||||
|---|---|---|---|---|---|---|---|---|
| Combination therapy | Monotherapy | Combination therapy | Monotherapy | |||||
|
nal‐IRI+ 5‐FU/LV n = 33 |
5‐FU/LV n = 29 |
nal‐IRI n = 50 |
5‐FU/LV n = 42 |
nal‐IRI+ 5‐FU/LV n = 117 |
5‐FU/LV n = 105 |
nal‐IRI n = 147 |
5‐FU/LV n = 134 |
|
| Patients with TEAE leading to any dose modification, n (%) | 28 (84.8) | 8 (27.6) | 30 (60.0) | 12 (28.6) | 83 (70.9) | 37 (35.2) | 81 (55.1) | 48 (35.8) |
| Dose delayb | 28 (84.8) | 8 (27.6) | 21 (42.0) | 12 (28.6) | 72 (61.5) | 33 (31.4) | 49 (33.3) | 43 (32.1) |
| Dose reductionc | 16 (48.5) | 1 (3.4) | 21 (42.0) | 2 (4.8) | 39 (33.3) | 4 (3.8) | 46 (31.3) | 5 (3.7) |
| Treatment discontinuation | 4 (12.1) | 0 | 5 (10.0) | 0 | 13 (11.1) | 7 (6.7) | 17 (11.6) | 10 (7.5) |
| Dose delay, n (%) | ||||||||
| Neutropenia | 16 (48.5)d | 1 (3.4) | 9 (18.0)d | 1 (2.4)d | 30 (25.6)e | 5 (4.8) | 13 (8.8)e | 5 (3.7)e |
| WBC count decreased | 11 (33.3) | 0 | 1 (2.0) | 0 | 14 (12.0) | 1 (1.0) | 1 (0.7) | 1 (0.7) |
| Asthenia | 3 (9.1) | 2 (6.9) | 2 (4.0) | 3 (7.1) | 5 (4.3) | 2 (1.9) | 4 (2.7) | 3 (2.2) |
| Leukopenia | 3 (9.1) | 0 | 1 (2.0) | 0 | 7 (6.0) | 1 (1.0) | 1 (0.7) | 1 (0.7) |
| Febrile neutropenia | 2 (6.1) | 0 | 1 (2.0) | 0 | 3 (2.6) | 0 | 2 (1.4) | 0 |
| Platelet count decreased | 2 (6.1) | 0 | 0 | 0 | 6 (5.1) | 0 | 1 (0.7) | 1 (0.7) |
| Vomiting | 2 (6.1) | 0 | 0 | 1 (2.4) | 7 (6.0) | 2 (1.9) | 4 (2.7) | 3 (2.2) |
| Diarrhea | 1 (3.0) | 1 (3.4) | 1 (2.0) | 1 (2.4) | 9 (7.7) | 4 (3.8) | 8 (5.4) | 4 (3.0) |
| Fatigue | 0 | 0 | 1 (2.0) | 1 (2.4) | 8 (6.8) | 0 | 3 (2.0) | 1 (0.7) |
| Tumor‐associated fever | 0 | 2 (6.9) | 0 | 2 (4.8) | 0 | 2 (1.9) | 0 | 2 (1.5) |
| Dose reduction, n (%) | ||||||||
| Neutropenia | 12 (36.4)d | 0 | 12 (24.0)d | 0d | 21 (17.9)e | 0 | 14 (9.5)e | 0e |
| WBC count decreased | 5 (15.2) | 0 | 3 (6.0) | 0 | 6 (5.1) | 0 | 3 (2.0) | 0 |
| Anemia | 4 (12.1) | 0 | 4 (8.0) | 0 | 4 (3.4) | 0 | 6 (4.1) | 0 |
| Vomiting | 1 (3.0) | 0 | 4 (8.0) | 0 | 2 (1.7) | 0 | 9 (6.1) | 0 |
| Diarrhea | 0 | 0 | 3 (6.0) | 0 | 7 (6.0) | 0 | 17 (11.6) | 0 |
| Febrile neutropenia | 0 | 0 | 4 (8.0) | 0 | 1 (0.9) | 0 | 5 (3.4) | 0 |
| Treatment discontinuation, n (%) | ||||||||
| Ascites | 1 (3.0) | 0 | 0 | 0 | 2 (1.7) | 0 | 0 | 0 |
| Biliary tract infection | 1 (3.0) | 0 | 0 | 0 | 1 (0.9) | 0 | 0 | 0 |
| Neutropenia | 1 (3.0)f | 0 | 1 (2.0)f | 0f | 2 (1.7)e | 1 (1.0)e | 2 (1.4)e | 1 (0.7)e |
| Pneumonia | 1 (3.0) | 0 | 0 | 0 | 1 (0.9) | 0 | 0 | 0 |
| Sepsis | 1 (3.0) | 0 | 0 | 0 | 2 (1.7) | 1 (1.0) | 1 (0.7) | 1 (0.7) |
| Septic shock | 1 (3.0) | 0 | 0 | 0 | 1 (0.9) | 0 | 0 | 0 |
| Cholangitis suppurative | 0 | 0 | 1 (2.0) | 0 | 0 | 0 | 1 (0.7) | 0 |
| Diarrhea | 0 | 0 | 0 | 0 | 2 (1.7) | 0 | 3 (2.0) | 0 |
| Enterocolitis infectious | 0 | 0 | 1 (2.0) | 0 | 0 | 0 | 1 (0.7) | 0 |
| Jaundice cholestatic | 0 | 0 | 1 (2.0) | 0 | 0 | 0 | 1 (0.7) | 0 |
| Leukopenia | 0 | 0 | 1 (2.0) | 0 | 1 (0.9) | 0 | 1 (0.7) | 0 |
| Respiratory failure | 0 | 0 | 1 (2.0) | 0 | 0 | 0 | 1 (0.7) | 0 |
| Vomiting | 0 | 0 | 0 | 0 | 2 (1.7) | 0 | 3 (2.0) | 1 (0.7) |
| Patients with any TEAE related to study drug leading to death (all causes), n (%) | 1 (3.0)g | 0 | 2 (4.0)h | 0 | 1 (0.9)g | 0 | 4 (2.7)h | 0 |
Abbreviations: 5‐FU, 5‐fluorouracil; LV, leucovorin (folinic acid); nal‐IRI, liposomal irinotecan; WBC, white blood cell.
Patients with multiple occurrences are counted only once in any category. Safety population included patients who received at least one dose of study drug. Adverse events coded using MedDRA version 14.1. Treatment emergent adverse events (TEAE) are events that occurred or worsened on or after the day of first dose of the study drug and within 30 d after last administration of study drug.
TEAE with action taken as: dose not given or infusion interrupted.
TEAE with action taken as: dose decreased or slowing infusion rate.
Summary term comprising neutropenia, neutrophil count decreased, and febrile neutropenia.
Summary term comprising agranulocytosis, decreased neutrophil count, febrile neutropenia, granulocytopenia, neutropenia, neutropenic sepsis, and pancytopenia.
Summary term comprising neutrophil count decreased and neutropenia.
Causes of death: Septic shock on study day 11, n = 1.
Causes of death: Disseminated intravascular coagulation and pulmonary embolism on study day 12 (n = 1), gastrointestinal syndrome on study day 30 (n = 1), septic shock on study day 101 (n = 1), and infectious colitis on study day 206 (n = 1).
Figure 2.
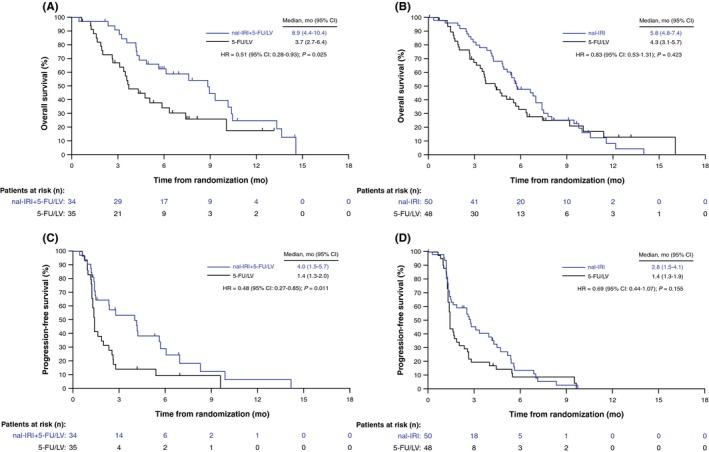
Kaplan‐Meier survival analyses in patients at centers in the Asia region. A, Overall survival (OS) with nal‐IRI+5‐FU/LV combination. B, OS with nal‐IRI monotherapy. C, Progression‐free survival (PFS) with nal‐IRI+5‐FU/LV combination. D, PFS with nal‐IRI monotherapy, all vs 5‐FU/LV controls (intention‐to‐treat [ITT] population). 5‐FU, 5‐fluorouracil; CI, confidence interval; HR, unstratified hazard ratio; LV, leucovorin (folinic acid); mo, months; nal‐IRI, liposomal irinotecan. Vertical bars indicate censoring points
3.1. Patient baseline characteristics and demographics
Patient demographics and baseline clinical characteristics were generally balanced across the nal‐IRI+5‐FU/LV and 5‐FU/LV treatment arms (Table 1). Some differences between the Asia region compared with the overall ITT population became apparent: all patients treated at Asian centers were of Asian ethnicity compared with approximately one‐third in the overall population. A higher proportion of Asian patients had a baseline KPS of 90‐100 versus the overall population for nal‐IRI+5‐FU/LV and 5‐FU/LV, respectively, and fewer Asian patients in the nal‐IRI+5‐FU/LV arm had stage IV disease at initial diagnosis compared to the overall population (Table 1). In the nal‐IRI+5‐FU/LV and 5‐FU/LV arms, fewer Asian patients had received ≥2 lines of prior metastatic therapy compared with the overall population. Correspondingly, the proportion of patients who received one line of prior metastatic therapy was increased among Asian patients (Table 1). Only a single Asian patient in the nal‐IRI+5‐FU/LV arm was homozygous for the UGT1A1*28 genotype (Table 1). Fewer Asian patients had previously received gemcitabine monotherapy, whereas gemcitabine‐containing combinations had been used more frequently compared with the overall population. A minority of Asian patients had previously received treatment containing non‐liposomal irinotecan. A greater proportion of patients in the present analysis received post‐study anticancer therapy compared with the overall population, with fluorouracil‐containing therapies being the most commonly used in both populations (Table 1).
3.2. Efficacy
3.2.1. Overall survival
Asian patients who received nal‐IRI+5‐FU/LV experienced a longer median OS (8.9 months [95% confidence interval (CI): 4.4‐10.4]) compared with those who received 5‐FU/LV (3.7 months [2.7‐6.4]) (unstratified HR = 0.51, P = .025) (Table 2, Figure 2A). Median OS in patients who received nal‐IRI monotherapy was 5.8 months (4.8‐7.4) versus 4.3 months (3.1‐5.7) with 5‐FU/LV alone (unstratified HR = 0.83, P = .423) (Figure 2B). Six‐month OS rate of patients in the nal‐IRI+5‐FU/LV arm was 62% (95% CI: 0.4‐0.8) versus 34% (0.2‐0.5) with 5‐FU/LV, whereas the 12‐month OS rates in the two treatment arms were 25% (0.1‐0.4) and 17% (0.0‐0.4), respectively.
3.2.2. Progression‐free survival
Median PFS of 4.0 months (95% CI: 1.5‐5.7) in patients receiving nal‐IRI+5‐FU/LV was significantly longer compared with 1.4 months (1.3‐2.0) in patients treated with 5‐FU/LV alone (unstratified HR = 0.48, P = .011) (Table 2, Figure 2C). Median PFS with nal‐IRI monotherapy was 2.8 months (1.5‐4.1) versus 1.4 months (1.3‐1.9) with 5‐FU/LV alone (unstratified HR = 0.69, P = .155) (Figure 2D).
3.2.3. Objective response rate
A partial response was observed in three patients with nal‐IRI+5‐FU/LV and none in those treated with 5‐FU/LV alone, with an ORR of 8.8% and 0%, respectively (P = .114) (Table 2). Stable disease (SD) was reported in 15 patients (44.1%) with nal‐IRI+5‐FU/LV and five patients (14.3%) with 5‐FU/LV. In total, 11 patients (32.4%) in the nal‐IRI+5‐FU/LV group versus 18 patients (51.4%) in the 5‐FU/LV group had progressive disease as best recorded response. Five patients (14.7%) in the nal‐IRI+5‐FU/LV group and 12 patients (34.3%) in the control group were not evaluable for ORR. An increase in ORR was observed in patients treated with nal‐IRI monotherapy compared with patients who received the monotherapy control (10.0% vs 0), but this did not reach statistical significance (P = .056). Partial response was observed in five patients in the nal‐IRI monotherapy group, with 18 patients having SD.
3.2.4. CA19‐9 response
The proportion of patients who achieved a tumor marker response (≥50% decrease from abnormal baseline value) was comparable between the global ITT population and Asian patients (Table 2). In the Asia patient population, 32.0% and 7.7% (P < .001) achieved a CA19‐9 response compared with 28.9% and 8.6% (P < .001) in the overall ITT population (nal‐IRI+5‐FU/LV and 5‐FU/LV groups, respectively). A CA19‐9 response was observed in 29.3% and 11.1% (P = .024) of Asian patients receiving nal‐IRI monotherapy and 5‐FU/LV, which was comparable to these treatment groups in the overall ITT population (23.6% and 11.4%, P = .024).
3.2.5. Time to treatment failure
Patients treated with nal‐IRI+5‐FU/LV had a longer median TTF (2.2 months [95% CI: 1.4‐4.0]) than patients treated with 5‐FU/LV (1.3 months [1.0‐1.4]; P = .006) in the Asia patient population. Median TTF observed in patients receiving nal‐IRI monotherapy versus 5‐FU/LV was comparable to the combination arms (2.5 [1.4‐2.8] vs 1.3 [1.1‐1.4] months; P = .085). These results were similar to those seen in the overall population, where patients treated with nal‐IRI+5‐FU/LV had a median TTF of 2.3 months (95% CI: 1.6‐2.8) compared with 1.4 months (1.3‐1.4; P < .001) for patients treated with 5‐FU/LV.
3.2.6. Treatment duration and dose intensity
Patients treated with nal‐IRI+5‐FU/LV received an increased median number of treatment cycles compared with those receiving 5‐FU/LV (3.0 vs 1.0), reflecting findings of the primary analysis (Table 3). In the present analysis, a larger proportion of patients in the combination arm spent ≥12 and ≥18 weeks on treatment compared with the control arm, whereas a similar percentage of patients in both arms were treated for ≥6 weeks, with 66.7% in the combination arm and 69.0% in the control arm (Table 3). Mean relative dose intensities and duration of exposure in treatment arms of the present analysis, including the nal‐IRI monotherapy arm, were generally similar to the overall safety population (Table 3). We observed that mean relative dose intensities for nal‐IRI and 5‐FU were somewhat lower in Asian patients receiving nal‐IRI+5‐FU/LV compared with the overall safety population (74.9% and 75.5% vs 83.2% and 83.9%).
3.2.7. Safety and tolerability
The Asia region combination safety population comprised 62 patients, of whom 33 received nal‐IRI+5‐FU/LV, and 29 received the 5‐FU/LV combination control (Table 4). Incidence of grade ≥3 treatment‐emergent adverse events (TEAE) between the two groups was 87.9% and 58.6%. The most common grade ≥3 TEAE reported in the nal‐IRI+5‐FU/LV arm compared with the 5‐F/LV arm were neutropenia (54.5% vs 3.4%), anemia (21.2% vs 6.9%), and decreased white blood cell (WBC) count (21.2% vs 0) (Table 4). In the 5‐FU/LV control group, the most common grade ≥3 TEAE were asthenia (10.3%), anemia, diarrhea, and hydronephrosis (all 6.9%). The incidence of complications associated with neutropenia was low and comparable to the overall study population; for example, grade ≥3 febrile neutropenia occurred in one patient (3.0%) receiving nal‐IRI+5‐FU/LV versus none in the 5‐FU/LV control group (Table 4). Grade ≥3 diarrhea affected one patient (3.0%) in the nal‐IRI+5‐FU/LV arm versus two patients (6.9%) in the 5‐FU/LV arm.
A greater proportion of patients receiving nal‐IRI+5‐FU/LV versus 5‐FU/LV experienced TEAE of any grade resulting in dose delay (84.8% vs 27.6%), reduction (48.5% vs 3.4%), or treatment discontinuation (12.1% vs 0) (Table 5). The main TEAE of any grade necessitating dose delays in ≥5% of patients receiving nal‐IRI+5‐FU/LV were neutropenia (48.5%), and decreased WBC count (33.3%), whereas asthenia and tumor‐associated fever (each 6.9%) were the most prevalent TEAE of any grade in those treated with 5‐FU/LV. Neutropenia‐associated events were the most common causes reported for dose reductions in ≥5% of patients in the nal‐IRI+5‐FU/LV arm, and no TEAE of any grade in the control group reached the threshold. A drug‐related TEAE leading to death occurred in one patient (3.0%, septic shock) receiving nal‐IRI+5‐FU/LV. No drug‐related TEAE leading to death were reported among patients receiving 5‐FU/LV. Nine patients (27.3%) in the nal‐IRI+5‐FU/LV group and 13 patients (26.0%) in the nal‐IRI monotherapy group received G‐CSF during the study, whereas no patients treated in either 5‐FU/LV group required this treatment.
Overall, the safety findings for patients receiving nal‐IRI+5‐FU/LV at Asian centers were comparable with those treated with nal‐IRI+5‐FU/LV in the overall safety population (Table 4). There was an increased incidence of grade ≥3 neutropenia (54.5% vs 27.4%) and decreased WBC count (21.2% vs 7.7%) compared with the overall nal‐IRI+5‐FU/LV safety population. Conversely, there was a decreased incidence of diarrhea (3.0% vs 12.8%) compared with the overall nal‐IRI+5‐FU/LV safety population. Neutropenia‐related TEAE more frequently led to dose delays and dose reductions in Asian patients receiving nal‐IRI+5‐FU/LV versus those in the overall safety population, whereas the incidence of treatment discontinuation was similar (Table 5). Two out of seven patients with the homozygous UGT1A1*28 genotype developed grade ≥3 neutropenia in the overall nal‐IRI+5‐FU/LV arm. One of the two patients was in the Asian subgroup. This patient received an initial dose of 60 mg/m2 nal‐IRI as recommended by the study protocol and developed treatment‐related grade 3 neutropenia during the first two treatment cycles, but no diarrhea. The neutropenia was managed initially with interruption of treatment, followed by reduction of nal‐IRI dose to 50 mg/m2 at the third treatment cycle. Grade 3 neutropenia recurred and the dose of nal‐IRI was further reduced to 40 mg/m2 at the fourth treatment cycle. A total of seven treatment cycles were given before treatment discontinuation as a result of disease progression.
4. DISCUSSION
The global NAPOLI‐1 study showed that nal‐IRI in combination with 5‐FU/LV was efficacious and well tolerated in patients with mPDAC. This post‐hoc subgroup analysis of the study was carried out to ascertain whether there were any differences in response to treatment between the overall NAPOLI‐1 ITT population and the Asian subpopulation. Our findings show that the clinical outcomes in Asian patients were numerically improved compared with that of the overall study population. An important finding of this analysis was that nal‐IRI+5‐FU/LV increased OS in patients treated at centers in the Asia region versus the 5‐FU/LV control group. The increase in median OS was more pronounced in this population than that observed in the overall NAPOLI‐1 ITT population. Median OS among Asian patients in this analysis was 8.9 months with nal‐IRI+5‐FU/LV versus 3.7 months with 5‐FU/LV (HR = 0.51, P = .025) compared with 6.1 months versus 4.2 months (HR = 0.67, P = .012) in the NAPOLI‐1 ITT population. Similarly, median PFS was increased in Asian patients with nal‐IRI+5‐FU/LV versus 5‐FU/LV (4.0 vs 1.4 months; HR = 0.48, P = .011) compared with the NAPOLI‐1 study ITT population, where median PFS was 3.1 months with nal‐IRI+5‐FU/LV versus 1.5 months with 5‐FU/LV (unstratified HR = 0.56, P < .001). nal‐IRI monotherapy also showed some signs of activity, although this did not translate into any significant OS benefits.
In general, with the exception of ORR, patients at Asian centers had better outcomes than ITT patients. The ORR among patients at Asian centers treated with nal‐IRI+5‐FU/LV or 5‐FU/LV was 8.8% and 0%, respectively, compared with 16% and 1% of ITT patients. This apparent difference should be interpreted cautiously due to the relatively small number of patients in the analysis. It is notable that 25% of patients at Asian centers treated with nal‐IRI+5‐FU/LV were alive at 12 months. This is an encouraging finding in this patient population suffering from an advanced‐stage disease characterized by a dire prognosis and short survival, particularly following failure of previous gemcitabine‐based therapy,2, 10, 27 and suggests that this treatment combination could be a valuable option for second‐line treatment of Asian patients with mPDAC.
The nal‐IRI+5‐FU/LV regimen was generally well tolerated among Asian patients, with a tolerability profile comparable to that seen in the NAPOLI‐1 overall safety population. Differences in adverse events between the present subgroup analysis versus the NAPOLI‐1 overall safety population in patients receiving nal‐IRI+5‐FU/LV include a limited increase in the incidence of grade ≥3 TEAE (87.9% vs 76.9%), likely driven by increases in neutropenia (54.5% vs 27.4%). Conversely, grade ≥3 diarrhea occurred less frequently in Asian patients compared with patients in the overall safety population (3.0% vs 12.8%). It has been previously shown that Asian race is a significant predictive factor for neutropenia among Asian patients receiving nal‐IRI.25 Although the incidence of grade ≥3 neutropenia was increased with nal‐IRI+5‐FU/LV treatment in the Asian population, only one case of treatment discontinuation was reportedly as a result of neutropenia. The incidence of neutropenia‐associated complications in these patients was low and similar to the overall safety population. Given the prevalence of neutropenia among TEAE of any grade leading to dose reductions or delays, this suggests that the majority of neutropenia events in these patients were manageable with established protocols.
In a population PK study of nal‐IRI, the incidence of grade 3‐4 neutropenia and diarrhea was significantly associated with plasma Cmax of unencapsulated SN‐38 (uSN‐38) and total irinotecan (tIRI), respectively.25 Interestingly, Asian patients were found to have significantly higher uSN‐38 Cmax and lower tIRI levels than the Caucasian population.25 Although the findings provide a PK‐based explanation for the ethnic differences in the incidence of neutropenia and diarrhea following nal‐IRI treatment, the underlying molecular mechanisms behind these differences remain unclear.
In a previous randomized study of nal‐IRI versus irinotecan versus docetaxel in gastric cancer showed that, for patients treated with nal‐IRI, grade 3‐4 neutropenia occurred more frequently for patients heterozygous for UGT1A1*6 (40% [2/5] versus the wild type genotype (3% [1/30]; P = .022).14, 28 As the UGT1A1*6 allele occurs more frequently in Asian versus Caucasian populations,28 could the increased incidence of neutropenia among the Asian patients in the NAPOLI‐1 study versus the overall population be influenced by the presence of the UGT1A1*6 genotype in the Asian population? Unfortunately, this genotype was not investigated in the NAPOLI‐1 study population so we cannot draw any conclusions regarding this. In contrast, the UGT1A1*28 genotype was not a predictor for uSN‐38 clearance in the population PK study.25 The finding that the single Asian patient homozygous for UGT1A1*28 was one of only two patients who suffered from grade 3 neutropenia out of the seven patients with this genotype in the nal‐IRI+5‐FU/LV arm suggests that other ethnic factors may render Asian patients more susceptible to nal‐IRI‐related neutropenia. Further investigation of these factors is warranted, as the low number of UGT1A1*28‐homozygous patients in this study does not allow any firm conclusions to be drawn. The observed lower dose intensities in Asian patients receiving nal‐IRI+5‐FU/LV compared with the overall combination arm likely reflect the increased number of dose reductions and delays among these patients.
The present analyses have not been corrected for potential imbalances, which should therefore be borne in mind when interpreting the data. A possible explanation for both the efficacy and tolerability differences between patients at Asian centers and the overall NAPOLI‐1 ITT population may relate to different pharmacokinetic (PK) profiles of nal‐IRI and its active metabolite SN‐38 in Asian and Caucasian patient populations. In a recent population pharmacokinetics analysis including patients in the nal‐IRI+5‐FU/LV arm of NAPOLI‐1, longer OS and PFS were associated with a longer SN‐38 exposure time above threshold and higher average concentration of total irinotecan, total SN‐38, and unencapsulated SN‐38, with the highest association observed for SN‐38 time above threshold.25 Furthermore, the study found that ethnicity (Caucasian vs East Asian) was a highly predictive baseline factor for total irinotecan and SN‐38 concentrations in plasma, and that patients of East Asian ethnicity had lower total irinotecan and higher SN‐38 levels in plasma compared with their Caucasian counterparts. The differences in plasma concentrations between the two populations may explain the higher frequency of neutropenia and lower incidence of diarrhea in the Asian population. This ethnicity‐pharmacokinetic association and its implication for treatment toxicity has been described in patients receiving non‐liposomal irinotecan, and it has been suggested that ethnicity may influence irinotecan payload release kinetics from nal‐IRI liposomes and ultimately plasma irinotecan levels.25, 29 However, we also note that a higher proportion of patients in the Asia region population had a baseline KPS ≥90, one prior line of metastatic therapy (as opposed to ≥2 prior lines), a lower proportion of stage IV disease at initial diagnosis, and a higher proportion of post‐study anticancer therapy compared with the overall ITT population, which could influence the observed outcomes in light of smaller patient numbers and improved patient physical condition at the onset of study treatment.
This post‐hoc subgroup analysis shows that nal‐IRI+5‐FU/LV is effective in Asian patients with mPDAC that has progressed after gemcitabine‐based therapy and shows a safety profile that is in agreement with earlier findings. The current findings echo those of the primary analysis, providing consistent evidence that the nal‐IRI+5‐FU/LV regimen is a tolerable treatment option in this patient population. This is particularly relevant considering the relative paucity of data on efficacious and approved treatment regimens for Asian patients with gemcitabine‐refractory metastatic pancreatic cancer.11, 19, 21, 30, 31, 32
DISCLOSURE
Yung‐Jue Bang reports a consulting/advisory role for AstraZeneca, Novartis, Genentech/Roche, MSD, Merck Serono, Bayer, BMS, Eli Lilly, Taiho, Daiichi‐Sankyo, Astellas, BeiGene, GreenCross, Samyang Biopharm, Hanmi, Genexine, and Shire; research grants (to the institution for clinical trials) from AstraZeneca, Novartis, Genentech/Roche, MSD, Merck Serono, Bayer, BMS, GSK, Pfizer, Eli Lilly, Boehringer‐Ingelheim, MacroGenics, Boston Biomedical, FivePrime, Curis, Taiho, Takeda, Ono, Daiichi Sankyo, Astellas, BeiGene, Green Cross, CKD Pharma, and Genexine. Chung‐Pin Li reports no disclosures. Kyung‐Hun Lee reports an advisor role for AstraZeneca, Bayer, Eisai, Ono Pharmaceuticals, Roche and Samsung Bioepis; honoraria from AstraZeneca and Roche. Chang‐Fang Chiu reports no disclosures. Joon Oh Park reports an advisor role for Shire. Yan‐Shen Shan reports no disclosures. Jun Suk Kim reports no disclosures. Jen‐Shi Chen reports no disclosures. Hyun‐Jeong Shim reports no disclosures. Kun‐Ming Rau reports no disclosures. Hye Jin Choi reports no disclosures. Do‐Youn Oh reports an advisor/consultant role for AstraZeneca, Novartis, Genentech/Roche, Merck Serono, Bayer, Taiho, ASLAN, Halozyme, Zymework, and Shire; research grants from AstraZeneca, Novartis, Array, Eli Lilly, and Green Cross. Bruce Belanger was employed by Merrimack Pharmaceuticals, Inc., at the time of the study, and is currently an employee at Ipsen Bioscience, Inc. Li‐Tzong Chen reports honoraria from Eli Lilly, Novartis, Bristol‐Myers‐Squibb, Ono Pharmaceutical, TTY Biopharm, PharmaEngine, MSD, Syncore Bio, Five Prime, AstraZeneca, and Ipsen; a consultant or advisor role for Eli Lilly, Novartis, Bristol‐Myers‐Squibb, Ono Pharmaceutical, PharmaEngine, MSD, Five Prime, AstraZeneca; and research funding from Novartis, Merck Serono, TTY Biopharm, SynCoreBio, Polaris, Celgene, and Pfizer.
ACKNOWLEDGMENTS
The authors would like to thank the patients, their families, all investigators and support staff for their participation in this study. Rights for nal‐IRI now reside with Ipsen in the USA (April 2017); PharmaEngine, Inc. holds the rights in Taiwan; Servier holds rights in the rest of the world. Bruce Belanger (Merrimack Pharmaceuticals, Inc., at the time of study, now Ipsen) was responsible for statistical analyses of this post‐hoc study. The authors acknowledge Beloo Mirakhur (former employee of Ipsen Bioscience Inc., Basking Ridge, NJ, USA) for her valuable contributions to the development of this manuscript. Medical writing support was provided by Florian Szardenings of Physicians World Europe GmbH (Mannheim, Germany), initially funded by Shire (Zug, Switzerland) and subsequently by Servier Global Medical Affairs (Suresnes, France).
Bang Y‐J, Li C‐P, Lee K‐H, et al. Liposomal irinotecan in metastatic pancreatic adenocarcinoma in Asian patients: Subgroup analysis of the NAPOLI‐1 study. Cancer Sci. 2020;111:513–527. 10.1111/cas.14264
Funding information
The NAPOLI‐1 study (ClinicalTrials.gov identifier: NCT01494506) was sponsored by Merrimack Pharmaceuticals, Inc., Cambridge, MA, USA. This post‐hoc analysis was sponsored by Shire. Although employees of the sponsor were involved in the design, collection, analysis, interpretation, fact‐checking of information, and coordination and collation of comments, the content of this manuscript, the interpretation of the data, and the decision to submit the manuscript for publication in Cancer Science was made by the authors independently.
DATA AVAILABILITY STATEMENT
Individual participant data, including data dictionaries, will not be available for this study. The primary study was published in 201511 and on Clinicaltrials.gov, identifier NCT01494506. Ipsen owns the NAPOLI‐1 study data and Shire carried out this subanalysis.
REFERENCES
- 1. Malvezzi M, Carioli G, Bertuccio P, et al. European cancer mortality predictions for the year 2017, with focus on lung cancer. Ann Oncol. 2017;28:1117‐1123. [DOI] [PubMed] [Google Scholar]
- 2. Carrato A, Falcone A, Ducreux M, et al. A systematic review of the burden of pancreatic cancer in Europe: real‐world impact on survival, quality of life and costs. J Gastrointest Cancer. 2015;46:201‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913‐2921. [DOI] [PubMed] [Google Scholar]
- 4. Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer; 2018. [Google Scholar]
- 5. Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356‐387. [DOI] [PubMed] [Google Scholar]
- 6. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817‐1825. [DOI] [PubMed] [Google Scholar]
- 7. NCCN . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Pancreatic Adenocarcinoma Version 1.2018; 2018:1‐153.
- 8. Sohal DPS, Kennedy EB, Khorana A, et al. Metastatic pancreatic cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2018;36(24):2545‐2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691‐1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ducreux M, Cuhna AS, Caramella C, et al. Appendix 6: Cancer of the pancreas: MCBS eUpdate published online 20 June 2017 (www.esmo.org/Guidelines/Gastrointestinal‐Cancers). Ann Oncol. 2017;28:iv157. [DOI] [PubMed] [Google Scholar]
- 11. Wang‐Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine‐based therapy (NAPOLI‐1): a global, randomised, open‐label, phase 3 trial. Lancet. 2016;387:545‐557. [DOI] [PubMed] [Google Scholar]
- 12. Chiang NJ, Chao TY, Hsieh RK, et al. A phase I dose‐escalation study of PEP02 (irinotecan liposome injection) in combination with 5‐fluorouracil and leucovorin in advanced solid tumors. BMC Cancer. 2016;16:907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kalra AV, Kim J, Klinz SG, et al. Preclinical activity of nanoliposomal irinotecan is governed by tumor deposition and intratumor prodrug conversion. Cancer Res. 2014;74:7003‐7013. [DOI] [PubMed] [Google Scholar]
- 14. Roy AC, Park SR, Cunningham D, et al. A randomized phase II study of PEP02 (MM‐398), irinotecan or docetaxel as a second‐line therapy in patients with locally advanced or metastatic gastric or gastro‐oesophageal junction adenocarcinoma. Ann Oncol. 2013;24:1567‐1573. [DOI] [PubMed] [Google Scholar]
- 15. Drummond DC, Noble CO, Guo Z, Hong K, Park JW, Kirpotin DB. Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer Res. 2006;66:3271‐3277. [DOI] [PubMed] [Google Scholar]
- 16. Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K. Intracellular roles of SN‐38, a metabolite of the camptothecin derivative CPT‐11, in the antitumor effect of CPT‐11. Cancer Res. 1991;51:4187‐4191. [PubMed] [Google Scholar]
- 17. Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751‐760. [DOI] [PubMed] [Google Scholar]
- 18. Pourhoseingholi MA, Vahedi M, Baghestani AR. Burden of gastrointestinal cancer in Asia; an overview. Gastroenterol Hepatol Bed Bench. 2015;8:19‐27. [PMC free article] [PubMed] [Google Scholar]
- 19. Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S‐1, S‐1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31:1640‐1648. [DOI] [PubMed] [Google Scholar]
- 20. Ioka T, Ueno M, Ueno H, et al. TAS‐118 (S‐1 plus leucovorin) versus S‐1 in patients with gemcitabine‐refractory advanced pancreatic cancer: a randomised, open‐label, phase 3 study (GRAPE trial). Eur J Cancer. 2019;106:78‐88. [DOI] [PubMed] [Google Scholar]
- 21. Ueno M, Okusaka T, Omuro Y, et al. A randomized phase II study of S‐1 plus oral leucovorin versus S‐1 monotherapy in patients with gemcitabine‐refractory advanced pancreatic cancer. Ann Oncol. 2016;27:502‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first‐line therapy in advanced gastric cancer: a randomized, double‐blind, placebo‐controlled phase III study. J Clin Oncol. 2011;29:3968‐3976. [DOI] [PubMed] [Google Scholar]
- 23. Sawaki A, Yamada Y, Yamaguchi K, et al. Regional differences in advanced gastric cancer: exploratory analyses of the AVAGAST placebo arm. Gastric Cancer. 2018;21:429‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tabernero J, Kunzmann V, Scheithauer W, et al. nab‐Paclitaxel plus gemcitabine for metastatic pancreatic cancer: a subgroup analysis of the Western European cohort of the MPACT trial. Onco Targets Ther. 2017;10:591‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adiwijaya BS, Kim J, Lang I, et al. Population pharmacokinetics of liposomal irinotecan in patients with cancer. Clin Pharmacol Ther. 2017;102:997‐1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chibaudel B, Maindrault‐Goebel F, Bachet JB, et al. PEPCOL: a GERCOR randomized phase II study of nanoliposomal irinotecan PEP02 (MM‐398) or irinotecan with leucovorin/5‐fluorouracil as second‐line therapy in metastatic colorectal cancer. Cancer Med. 2016;5:676‐683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73‐85. [DOI] [PubMed] [Google Scholar]
- 28. Chiang NJ, Chang JY, Shan YS, Chen LT. Development of nanoliposomal irinotecan (nal‐IRI, MM‐398, PEP02) in the management of metastatic pancreatic cancer. Expert Opin Pharmacother. 2016;17:1413‐1420. [DOI] [PubMed] [Google Scholar]
- 29. de Man FM, Goey AKL, van Schaik RHN, Mathijssen RHJ, Bins S. Individualization of irinotecan treatment: a review of pharmacokinetics, pharmacodynamics, and pharmacogenetics. Clin Pharmacokinet. 2018;57:1229‐1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ioka T, Komatsu Y, Mizuno N, et al. Randomised phase II trial of irinotecan plus S‐1 in patients with gemcitabine‐refractory pancreatic cancer. Br J Cancer. 2017;116:464‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ohkawa S, Okusaka T, Isayama H, et al. Randomised phase II trial of S‐1 plus oxaliplatin vs S‐1 in patients with gemcitabine‐refractory pancreatic cancer. Br J Cancer. 2015;112:1428‐1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yoo C, Hwang JY, Kim JE, et al. A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second‐line therapy in patients with gemcitabine‐refractory advanced pancreatic cancer. Br J Cancer. 2009;101:1658‐1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual participant data, including data dictionaries, will not be available for this study. The primary study was published in 201511 and on Clinicaltrials.gov, identifier NCT01494506. Ipsen owns the NAPOLI‐1 study data and Shire carried out this subanalysis.


