Abstract
Programmed death‐ligand 1 (PD‐L1) is an immune modulator that promotes immunosuppression by binding to programmed death‐1 of T‐lymphocytes. Although tumor cell PD‐L1 expression has been shown to be associated with the clinical response to anti–PD‐L1 antibodies, its concise regulatory mechanisms remain elusive. In this study, we evaluated the associations of tumor PD‐L1 expression and immune cell infiltrating patterns in 146 cases of early lung adenocarcinoma (AC) to investigate the possible extrinsic regulation of tumor PD‐L1 by immune cells. Using immunohistochemistry, cell surface PD‐L1 expression in tumor cells was observed in 18.5% of stage 0‐IA lung AC patients. Tumor PD‐L1 positivity was significantly associated with stromal invasion, which was accompanied by increased tumor‐associated macrophages (TAM), CD8+ cytotoxic T cells and FoxP3+ regulatory T cells. Among these immune cells, TAM and CD8+ T cells significantly accumulated in PD‐L1‐positive carcinoma cell areas, which showed a tumor cell nest‐infiltrating pattern. Although CD8+ T cells are known to induce tumor PD‐L1 expression via interferon‐ɣ production, the increased TAM within tumors were also associated with tumor cell PD‐L1 positivity, independently of CD8+ T cell infiltration. Our in vitro experiments revealed that PD‐L1 expression in lung cancer cell lines was significantly upregulated by co–culture with M2‐differentiated macrophages; expression of PD‐L1 was reduced to baseline levels following treatment with a transforming growth factor‐β inhibitor. These results demonstrated that tumor‐infiltrating TAM are extrinsic regulators of tumor PD‐L1 expression, indicating that combination therapy targeting both tumor PD‐L1 and stromal TAM might be a possible strategy for effective treatment of lung cancer.
Keywords: B7‐H1 antigen, macrophages, non–small‐cell lung carcinoma, transforming growth factor beta, tumor microenvironment
In this study, we showed that tumor‐associated macrophage (TAM) infiltration was an additional factor related to tumor programmed death‐ligand 1 (PD‐L1) expression in early lung adenocarcinoma. Our in vitro experiments demonstrated that M2‐differentiated macrophages facilitated tumor PD‐L1 expression through transforming growth factor‐β. These results suggested that TAM were extrinsic regulators of tumor PD‐L1 expression and could serve as potential therapeutic targets.
1. INTRODUCTION
Lung cancer, particularly non–small cell lung cancer (NSCLC), is the leading cause of cancer death worldwide, accounting for more than 1.7 million deaths every year.1 During the past decade, developments in molecular‐targeted therapies have led to major breakthroughs in the understanding, diagnosis and management of NSCLC, and treatment for advanced NSCLC is becoming increasingly personalized. Numerous molecular mutations and fusions in NSCLC patients have been discovered, including epidermal growth factor receptor (EGFR), BRAF, anaplastic lymphoma kinase (ALK) and ROS1. In addition to these therapeutic strategies, immunotherapy is emerging as a major modality in NSCLC treatment, and development of immune checkpoint inhibitors has recently shown efficacy in prolonging survival of NSCLC patients.2
Immune checkpoint pathways play a critical role in suppressing the anti–tumor T cell‐mediated immune response in the tumor microenvironment.3, 4 Programmed death‐ligand 1 (PD‐L1), also known as B7‐H1 or CD274, a type I transmembrane protein, is a major immune checkpoint molecule and is expressed on the surfaces of various carcinoma cells such as lung, colon, melanoma and leukemic cells.5, 6 PD‐L1 inhibits activation, expansion and acquisition of effector functions of activated cytotoxic CD8+ T cells through its interaction with programmed death‐1 (PD‐1; also known as PDCD1) of T cells, leading to immune escape. Therapeutic antibodies targeting the PD‐L1/PD‐1 axis have been developed for clinical application, and have shown remarkable clinical responses in a variety of advanced cancers.7 Importantly, tumor PD‐L1 expression has been shown to be associated with the clinical response to anti–PD‐1/PD‐L1 antibodies, and its immunohistochemical detection has been established as a biomarker for the selection of NSCLC patients for anti–PD1/PD‐L1 immunotherapy. To predict the efficacy and to optimize anti–PD‐L1/PD‐1 therapies, alone or in combination, it has become increasingly important to understand the mechanisms regulating tumor PD‐L1 expression.
Tumor PD‐L1 expression is known to be triggered by extrinsic as well as intrinsic factors such as gene aberrations and oncogenic drivers. Previous studies have reported that tumor‐infiltrating lymphocytes upregulate PD‐L1 expression on primary or metastatic tumor cells through release of interferon (INF)‐ɣ in an adaptive immune‐resistance fashion, suggesting that altered tumor stroma is a crucial extrinsic regulator of tumor PD‐L1 expression. However, despite the diversity of stromal immune cells, information regarding the association between tumor PD‐L1 expression and immune cell infiltration patterns is still limited. In this study, we analyzed the relationship between tumor PD‐L1 expression and infiltrating immune cell patterns in early lung adenocarcinoma (AC) patients, and further investigated the possible extrinsic regulation of tumor PD‐L1 by tumor‐associated macrophages (TAM).
2. MATERIALS AND METHODS
2.1. Patients
On the basis of the availability of tumor PD‐L1 expression status and clinicopathological data, we enrolled a series of 146 Japanese patients with Stage 0‐IA lung AC surgically resected between 2010 and 2014 at Keio University Hospital, Tokyo, Japan. All patients were staged according to the 8th edition of the TNM Classification for lung cancer.8 Pathologic diagnosis was made by expert pulmonary pathologists (KE and YH) essentially on the basis of the 2015 World Health Organization Classification of Tumors of the Lung.9 Smoking histories were obtained through a rigorous interview of each patient by experienced thoracic surgeons. All patients included in this study provided informed consent for research and the study plan was approved by the institutional review board of the Keio University School of Medicine (Nos. 20170351 and 20170352).
2.2. Immunohistochemistry and staining assessment
Paraffin sections were subjected to immunohistochemistry for rabbit anti–PD‐L1 (1:200; E1L3N; Cell Signaling Technology), mouse anti–CD3 (1:200; LN10; Leica), CD8 (1:100; Nichirei; C8/144B), mouse anti–FoxP3 (1:400; 236A/E7; Abcam), mouse anti–CD68 (1:100; PGM1; DAKO), mouse anti–CD163 (1:200; 10D6; Leica) or mouse anti–CD204 (1:125; SRA‐E5; Transgenic). They were followed by reactions with ImmPRESS polymer HRP‐conjugated anti–rabbit or anti–mouse antibodies (Vector Laboratories). Color was developed with 3, 3′‐diaminobenzidine tetrahydrochloride (Sigma‐Aldrich). After immunohistochemistry, the sections were counterstained with hematoxylin. For assessment of tumor cell PD‐L1 expression, the percentages of tumor cells with membranous PD‐L1 positive staining were recorded. A score of 5% or more was categorized as “PD‐L1‐positive” and a score of <5% as “PD‐L1‐negative” based on recent studies of tumor PD‐L1 expression.10, 11, 12, 13 For assessment of inflammatory cell infiltration in the tumor, numbers of CD3−, CD8−, FoxP3−, CD68−, CD163− or CD204‐immunostained cells were counted by observing five different fields which include both cancer cell nests and stromal areas at ×200 magnification without knowledge of the samples examined. In contrast, the severity of infiltration of immune cells into cancer cell nests but not tumor stromal areas was evaluated using a “tumor‐infiltrating immune cell score,” which was modified from “tumor‐infiltrating lymphocytes score” originally defined in the previous literature.14 Briefly, we evaluated CD163+ or CD204+ TAM, CD8+ T cells or FoxP3+ T cells on top of cancer cell nests as 0 (absent), 1+ (mild), 2+ (moderate) or 3+ (marked) by using CD163−, CD204−, CD8− or FoxP3‐stained sections, respectively. For the heterogenous study, we investigated PD‐L1+ cases (27 cases), and assessment of inflammatory cell densities and tumor‐infiltrating immune cell score in PD‐L1+ and PD‐L1− areas was performed by observing five different fields at ×200 magnification in each area.
2.3. Macrophage culture
As previously described,15 human peripheral blood mononuclear cells (PBMC) were isolated from transfusion blood provided by the Japanese Red Cross Society and cultured for 8 days in Iscove's modified Dulbecco's medium (Thermo Fisher Scientific) containing 10% (v/v) human AB serum (Cosmo Bio) and M‐CSF (50 ng/mL) at a density of 3.5 × 106 cells/1.5 mL/well in 12‐well cluster plates (Corning). They were then polarized to M2a macrophages through addition of IL‐4 (20 ng/mL) and IL‐13 (25 ng/mL) or to M2c macrophages through addition of IL‐10 (25 ng/mL) and dexamethasone (7 nmol/L), or left untreated for the duration of the culture (M0), following previous studies.16
2.4. Direct co–culture experiment and immunofluorescent staining for programmed death‐ligand 1
A549ffLuc‐cp156 cells, which express the fusion protein of firefly luciferase and circularly permuted variant of Venus (ffLuc‐cp156; a variant of green fluorescent protein), were established and characterized previously.17 A549ffLuc‐cp156 cells were co–cultured with confluent M2a or M2c macrophages on the Lab‐Tek chamber slides (Nalge Nunc International) for 2 days. Co–cultured cells were fixed with methanol/acetone/formaldehyde,18 and double‐immunostained with rabbit anti–PD‐L1 antibody, followed by reactions with anti–rabbit IgG conjugated to Alexa‐Flour 594 (Invitrogen). The cells were counterstained with DAPI (Invitrogen) and mounted in Vectashield (Vector). As for controls, cells were reacted with rabbit non–immune IgG (data not shown). These samples were observed by confocal microscope (LSM 510; Zeiss).
2.5. Immunoblotting
Cultured cells were homogenized in lysis buffer containing 50 mmol/L Tris‐HCl, pH 7.4, 150 mmol/L NaCl, 10 mmol/L CaCl2, 1% NP‐40, complete proteinase inhibitors (Roche Diagnostics) and homogenate supernatants were used for sodium dodecyl sulfate‐polyacrylamide gel electrophoresis.18 Proteins were transferred to polyvinylidene difluoride membranes, and the membranes were incubated with rabbit anti–PD‐L1 antibody (E1L3N; Cell Signaling Technology) or rabbit anti–β‐actin antibody conjugated with HRP (13E5; Cell Signaling Technology). For anti–PD‐L1 antibody, the membranes were subsequently incubated with secondary antibodies. They were further visualized by chemiluminescence.
2.6. Stimulation of cancer cells with M2 macrophage‐producing cytokines
Serum‐starved human NSCLC cell lines A549 and H1975 were treated with human transforming growth factor (TGF)‐β1 (2 ng/mL; R&D Systems) or human IL‐10 (10 ng/mL; R&D Systems), human tumor necrosis factor (TNF)‐α (10 ng/mL; BioLegend), human IL‐6 (10 ng/mL; BioLegend), human IL‐1α (10 ng/mL; R&D Systems) or human IL‐1β (10 ng/mL; R&D Systems) in serum‐free RPMI1640 containing 0.2% lactalbumin hydrolysate for 12 or 24 hours. Cells were also treated with vehicle alone as controls. The expression of PD‐L1 under treatment with these cytokines was analyzed by quantitative PCR.
2.7. Statistical analysis
Association between PD‐L1 expression and a categorical variable was tested with Pearson's χ2 test or Fisher's exact test, and association between the PD‐L1 expression and a continuous variable was tested with the Mann‐Whitney U test or Student's t test as appropriate. We performed univariate and multivariable logistic regression analyses to assess the immune cell predictors of tumor PD‐L1 positivity and estimated the odds ratio (OR) and its 95% confidence interval (95% CI). A receiver operating characteristic (ROC) curve was used to determine high and low immune cells. Briefly, based on ROC curves, we determined the cut‐off value of 273.3 cells/mm2, 292.5 cells/mm2 and 68.1 cells/mm2 for the cell density of CD204+ TAM, CD8+ T cells and FoxP3+ T cells, respectively. Factors with P < 0.05 in univariate analyses were entered into the multivariable regression. The statistical significance level for all tests was two‐sided 0.05. All statistical analyses were conducted using GraphPad Prism 7 or SPSS 24.0 software (IBM).
Protocols for all other procedures are provided in Data S1.
3. RESULTS
3.1. Tumor programmed death‐ligand 1 expression is associated with stromal invasion accompanied by increased immune cells in early lung adenocarcinoma
Among 146 early lung AC, we observed PD‐L1 positivity (defined as 5% or more of tumor cells with membranous staining) in 27 tumors (18.5%) by immunohistochemistry (Figure 1A). PD‐L1 was expressed on both cancer and immune cells, and these cells could be distinguished by morphological features. In PD‐L1‐positive tumors, PD‐L1 expression in tumor cells showed a heterogeneous staining pattern. For example, 5%‐24%, 25%‐49% and more than 50% of tumor cells were positive for PD‐L1 in 16, 6 and 5 of 27 cases, respectively (Figure 1B). Analyses of the correlations between tumor PD‐L1 status and clinicopathological and molecular characteristics showed that tumor PD‐L1 positivity was significantly associated with a higher rate of smoking history (P < 0.001), stromal invasion (P < 0.001), tumor size (P < 0.001), EGFR wild‐type status (P = 0.003) and vascular invasion (P = 0.039; Table 1). Among them, stromal invasion is known to affect stromal immune responses in the tumor microenvironment.19 Immunohistochemical analyses consistently revealed that invasive AC contained significantly increased CD68+, CD163+ or CD204+ macrophages, CD8+ cytotoxic T cells, and FoxP3+ regulatory T cells compared to noninvasive AC in situ (Figure 1C).
Figure 1.
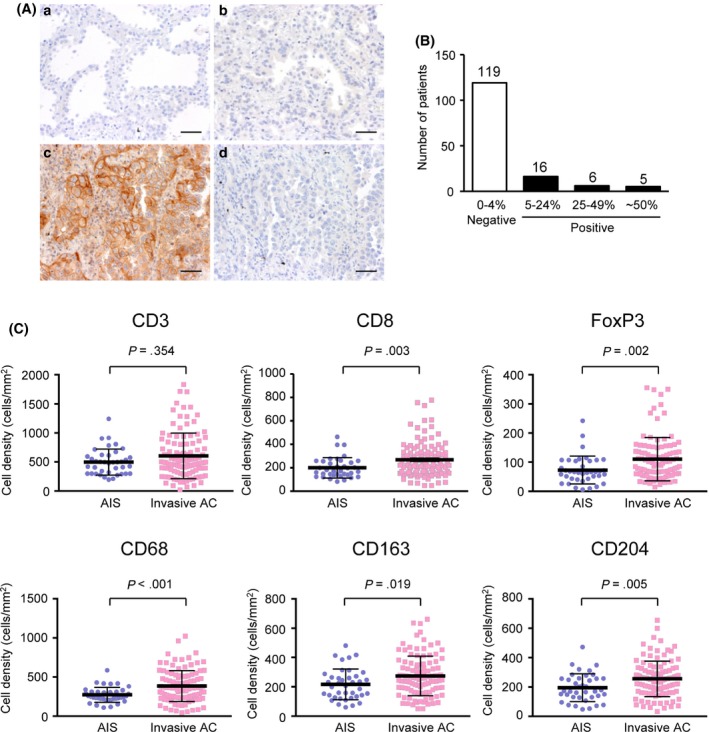
Immunohistochemical analysis of tumor programmed death‐ligand 1 (PD‐L1) membranous expression. A, Representative images of immunohistochemical staining for PD‐L1 of adenocarcinoma in situ (AIS) (a), PD‐L1‐negative invasive adenocarcinoma (AC) (b) and PD‐L1‐positive invasive AC (c). (d) shows PD‐L1‐positive invasive AC immunostained with non–immune IgG. Scale bars, 50 μm. B, Number of patients in groups classified according to the tumor PD‐L1 positivity. C, Relationship between stromal invasion (AIS vs invasive AC) and CD3−, CD8−, FoxP3−, CD68−, CD163− or CD204− immunostained immune cell densities (AIS, n = 39; Invasive AC, n = 107). A Mann‐Whitney U test was performed
Table 1.
Clinicopathological and molecular characteristics of lung adenocarcinoma according to tumor programmed death‐ligand 1 (PD‐L1) expression status (negative vs positive)
| Characteristic | PD‐L1 negative (n = 119) | PD‐L1 positive (n = 27) | P‐value |
|---|---|---|---|
| Sex | |||
| Male | 54 (45.4%) | 17 (63.0%) | .099 |
| Female | 65 (54.6%) | 10 (37.0%) | |
| Age at surgery, year | 69 [33‐84] | 66 [34‐79] | .325 |
| Smoking | |||
| Never | 88 (73.9%) | 10 (37.0%) | <.001 |
| Former | 31 (26.1%) | 17 (63.0%) | |
| CEA | 2.1 [0.3‐63.8) | 2.85 [0.7‐7.6] | .828 |
| Histological type | |||
| AIS | 39 (32.8%) | 0 (0%) | <.001 |
| Invasive AC | 80 (67.2%) | 27 (100%) | |
| MIA | 15 | 0 | |
| Lepidic | 23 | 3 | |
| Acinar | 0 | 2 | |
| Papillary | 35 | 19 | |
| Solid | 1 | 3 | |
| IMA | 6 | 0 | |
| Diameter (mm) | 0.6 [0.5‐3.0] | 1.5 [0.6‐3.0] | <.001 |
| EGFR mutation | |||
| Present | 64 (53.8%) | 6 (22.2%) | .003 |
| Absent | 55 (46.2%) | 21 (77.8%) | |
| Lymphatic permeation | |||
| Present | 3 (2.5%) | 3 (11.1%) | .077 |
| Absent | 116 (97.5%) | 24 (88.9%) | |
| Vascular invasion | |||
| Present | 4 (3.4%) | 4 (14.8%) | .039 |
| Absent | 115 (96.6%) | 23 (85.2%) | |
AC, adenocarcinoma; AIS, adenocarcinoma in situ; CEA, carcinoembryonic antigen; EGFR, epidermal growth factor receptor; IMA, invasive mucinous adenocarcinoma; MIA, minimally invasive adenocarcinoma.
3.2. Tumor‐associated macrophages and CD8+ T cells accumulated in programmed death‐ligand 1‐positive carcinoma cell nests, and showed an infiltrating pattern
We next examined the association between tumor PD‐L1 positivity and various immune cell infiltration patterns in invasive AC by immunohistochemistry. Increased immune cells during stromal invasion, including CD163+ or CD204+ macrophages known as TAM, CD8+ cytotoxic T cells and FoxP3+ regulatory T cells, also accumulated to a significantly greater extent in PD‐L1+ compared with PD‐L1− invasive AC (Figure 2). To further investigate the relationship between the heterogeneity of tumor PD‐L1 expression and immune cell infiltration patterns within tumors, areas of the PD‐L1+ invasive AC tumors were further divided into those that showed tumor PD‐L1 expression (PD‐L1+ areas) and those that showed little or no PD‐L1 expression (PD‐L1− areas) for each patient. Comparing the numbers of immune cells in the PD‐L1+ and PD‐L1− areas, we found that there were significantly higher numbers of CD163+ or CD204+ TAM, CD8+ T cells and FoxP3+ T cells in the PD‐L1+ areas than in the PD‐L1− areas (Figure 3A,B). Moreover, tumor‐infiltrating immune cell scores evaluating immune cells on top of cancer cell nests revealed that CD163+ or CD204+ TAM and CD8+ T cells tended to infiltrate into cancer cell nests in PD‐L1+ areas compared to PD‐L1− areas, whereas FoxP3+ T cells were mainly observed in the tumor stroma even in PD‐L1+ areas (Figure 3C,D). Immune cells infiltrating into cancer cell nests can export growth factors, cytokines and other molecules to neighboring tumor cells in a direct fashion, suggesting a strong association between the infiltration of TAM and CD8+ T cells and tumor PD‐L1 expression.
Figure 2.
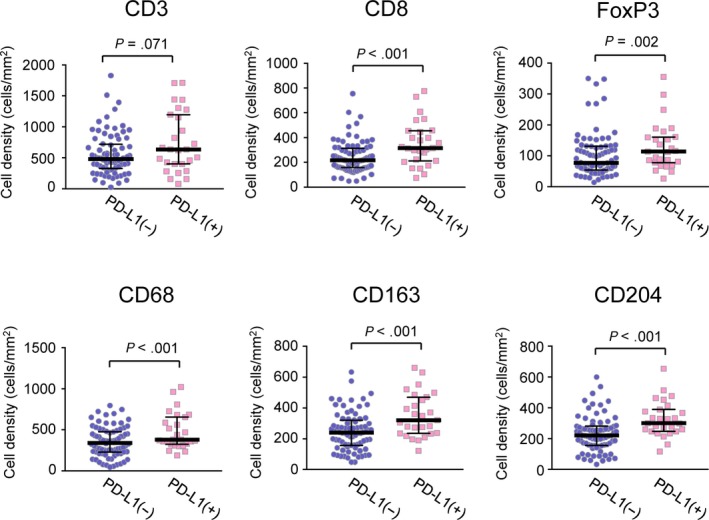
Relationship between tumor programmed death‐ligand 1 (PD‐L1) expression and CD3−, CD8−, FoxP3−, CD68−, CD163− or CD204− immunostained immune cell densities in invasive adenocarcinomas (AC). A Mann‐Whitney U test was performed (PD‐L1‐negative invasive AC, n = 80; PD‐L1‐positive invasive AC, n = 27)
Figure 3.
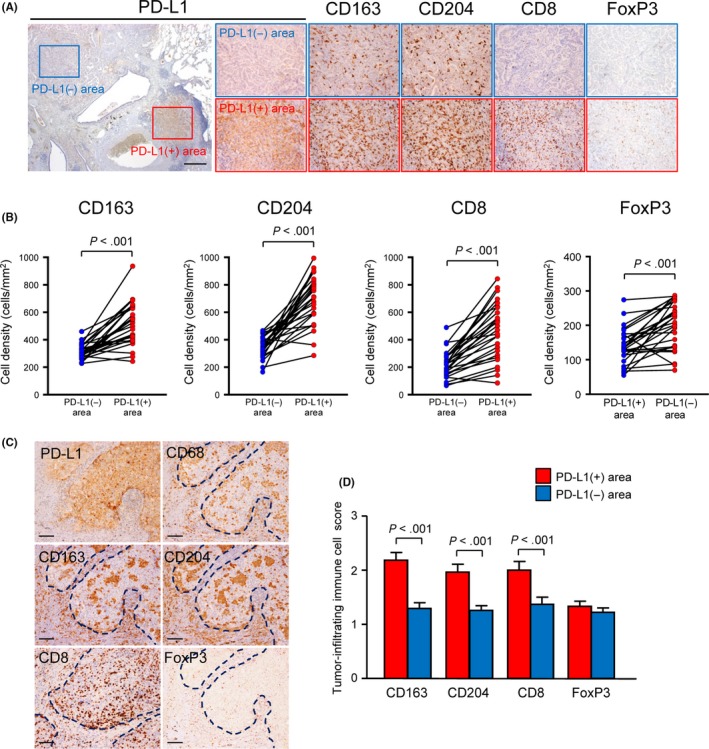
Relationship between heterogeneity of tumor programmed death‐ligand 1 (PD‐L1) expression status and immune cell infiltration densities/patterns within the tumor. A, Representative images of immunohistochemical staining for PD‐L1, CD163, CD204, CD8 or FoxP3 in PD‐L1‐low/no (PD‐L1−) or PD‐L1‐high (PD‐L1+) expression areas in PD‐L1‐positive invasive adenocarcinoma. The PD‐L1‐stained section is shown in the left panel and the rectangle PD‐L1− and PD‐L1+ areas are magnified to the right. Scale bars, 500 μm. B, Association between tumor PD‐L1 expression status and the densities of CD163‐, CD204‐, CD8‐ or FoxP3‐immunostained immune cells within the tumor (n = 27). A paired Student t test was performed. C, Representative images of PD‐L1+ carcinoma cell nests immunostained for PD‐L1, CD68, CD163, CD204, CD8 or FoxP3. Note that CD163+ or CD204+ TAM and CD8+ T cells were accumulated in PD‐L1+ carcinoma cell nests, whereas FoxP3+ T cells were mainly observed in the tumor stroma, even in PD‐L1+ areas. Dotted lines indicate PD‐L1+ cancer cell nests. Scale bar, 100 μm. D, Comparison of tumor‐infiltrating immune cell scores between PD‐L1− and PD‐L1+ areas within the tumor (n = 27). The tumor‐infiltrating immune cell score was defined as described in “Section 2”. A paired Student t test was performed
3.3. Tumor‐associated macrophage infiltration is associated with tumor programmed death‐ligand 1 positivity, independently of CD8+ T cell infiltration
CD8+ T cells are known to induce tumor PD‐L1 expression via INF‐γ production,20 but it remains unknown whether the increased TAM within tumors are associated with tumor PD‐L1 positivity. We assessed the relationships of the number of infiltrating TAM with tumor PD‐L1 positivity using univariable and multivariable logistic regression models. For these analyses, we initially included CD204+ TAM infiltration (low vs high), CD8+ T cell infiltration (low vs high), FoxP3+ T cell infiltration (low vs high) and PD‐L1 expression status (negative vs positive). Using univariable logistic regression analyses to assess possible relationships of immune cell infiltration with tumor PD‐L1 positivity, all of the increased CD204+ TAM, CD8+ T cell and FoxP3+ T cell populations were associated with tumor PD‐L1 positivity. Importantly, multivariable logistic regression analyses to assess the independent relationships of those variables revealed that increased CD204+ TAM infiltration was associated with tumor PD‐L1 positivity, independently of increased CD8+ T cell or FoxP3+ T cell infiltration (odds ratio, 3.643; 95% confidence interval, 1.300‐10.207; P = 0.014) (Table 2).
Table 2.
Associations between tumor programmed death‐ligand 1 (PD‐L1) expression status (negative vs positive) and immune cell densities
| PD‐L1(−) n (%) | PD‐L1(+) n (%) | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P‐value | Odds ratio | 95% CI | P‐value | |||
| CD204 | ||||||||
| High | 22 (27.5) | 18 (66.7) | 5.273 | 2.062‐13.480 | <.001 | 3.643 | 1.300‐10.207 | .014 |
| Low | 58 (72.5) | 9 (33.3) | ||||||
| FoxP3 | ||||||||
| High | 45 (56.3) | 23 (85.2) | 4.472 | 1.416‐14.124 | .007 | 4.068 | 1.187‐13.938 | .026 |
| Low | 35 (43.7) | 4 (14.8) | ||||||
| CD8 | ||||||||
| High | 22 (27.5) | 18 (66.7) | 5.273 | 2.062‐13.480 | <.001 | 3.367 | 1.091‐8.699 | .021 |
| Low | 58 (72.5) | 9 (33.3) | ||||||
3.4. Tumor programmed death‐ligand 1 expression was upregulated by co–culture with peripheral blood mononuclear cell‐derived M2‐differentiated macrophages
The majority of TAM show immunosuppressive M2a or M2c phenotypes.21 Using qPCR, Figure 4A shows that PBMC‐derived M2a‐differentiated or M2c‐differentiated macrophages expressed significantly higher levels of CD206 (M2a marker) or CD163 (M2c marker) mRNA, respectively, compared with unstimulated M0 macrophages. M2a macrophages also expressed remarkably higher levels of PD‐L1 than M0 macrophages. To investigate the possible extrinsic regulation of tumor PD‐L1 by TAM, we performed in vitro experiments using co–culture systems of human lung AC cell lines (A549 and H1975 cells) and macrophages prepared from PBMC (Figure 4B). Transwell indirect co–culture experiments showed that both M2a and M2c macrophages significantly increased mRNA levels of PD‐L1 in A549 and H1975 cells, when compared with the controls, using qPCR (Figure 4C). Direct co–culture experiments using Venus‐expressing A549ffLuc‐cp156 cells with M2a or M2c macrophages also showed that positive membrane staining for PD‐L1 in A549ffLuc‐cp156 cells was significantly enhanced in the presence of these macrophages (Figure 4D,E). By immunoblotting, increased PD‐L1 protein was detected in A549 cells indirectly co–cultured with PBMC‐derived M2a or M2c macrophages compared to A549 cells alone as well (Figure 4F).
Figure 4.
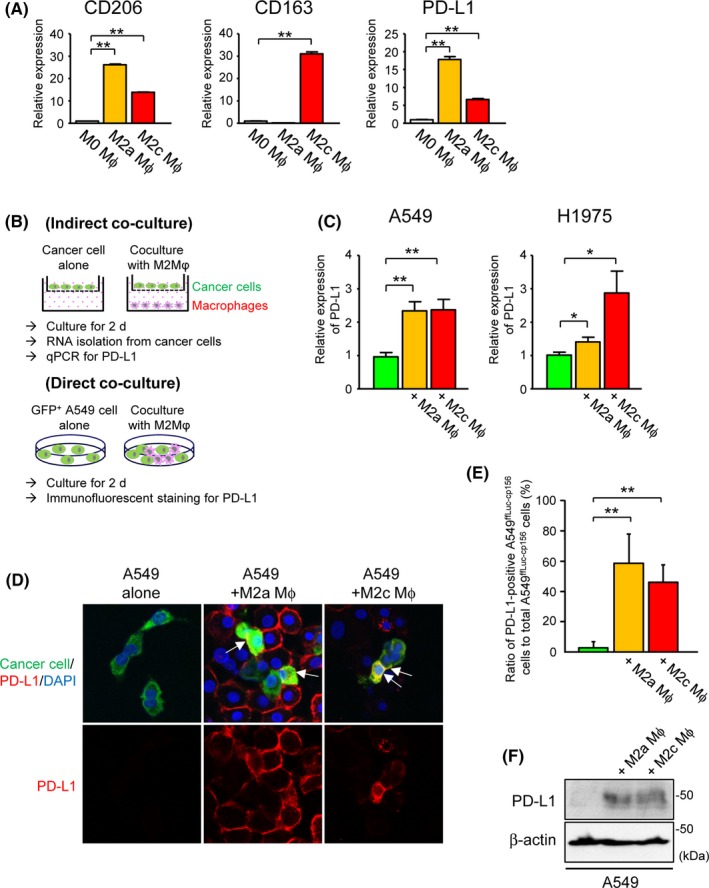
Upregulation of tumor programmed death‐ligand 1(PD‐L1) expression by co–culture with peripheral blood mononuclear cell (PBMC)‐derived M2‐differentiated macrophages. A, Relative expression of CD206, CD163 or PD‐L1 in human PBMC‐derived M0‐differentiated, M2a‐differentiated and M2c‐differentiated macrophages by real‐time quantitative PCR (qPCR) (n = 4). B, Description of co–culture schemes. For all experiments, human PBMC were differentiated into M2a or M2c macrophages prior to co–culture with human lung cancer cell lines. Following indirect or direct co–culture with these macrophages for 2 d, PD‐L1 expression in human lung cancer cell lines was evaluated by qPCR or immunofluorescent staining. C, Relative expression of PD‐L1 in A549 or H1975 cells indirectly co–cultured with human PBMC‐derived M2a‐differentiated or M2c‐differentiated macrophages by qPCR (n = 4). D, Representative images of immunofluorescent staining for PD‐L1 in Venus‐expressing A549ffLuc‐cp156 cells directly co–cultured with PBMC‐derived M2a‐differentiated or M2c‐differentiated macrophages. Arrows indicate A549ffLuc‐cp156 cells showing positive membrane staining for PD‐L1. E, Ratio of PD‐L1‐positive A549ffLuc‐cp156 cells to total A549ffLuc‐cp156 cells in the presence or absence of PBMC‐derived M2a‐ or M2c‐differentiated macrophages (n = 5). F, Representative immunoblot of PD‐L1 in A549 cells indirectly co–cultured with human PBMC‐derived M2a‐differentiated or M2c‐differentiated macrophages. β‐actin was used as a loading control. ∗ P < 0.05, ∗∗ P < 0.01
3.5. Transforming growth factor‐β1 is involved in the tumor‐associated macrophage‐mediated tumor programmed death‐ligand 1 expression
M2 macrophages are known to produce large amounts of immunosuppressive cytokines such as TGF‐β and interleukin (IL)‐10.21 Because direct and indirect co–culture experiments indicated that macrophage‐derived secreted factors were involved in tumor PD‐L1 induction, we further investigated the effect of soluble macrophage‐producing cytokines (TGF‐β1, IL‐10, TNF‐α, IL‐6, IL‐1α or IL‐1β) on PD‐L1 expression in A549 and H1975 cells. Using qPCR, PD‐L1 expression was significantly upregulated following treatment with TGF‐β1 both in A549 and H1975 cells, whereas IL‐10 modestly enhanced PD‐L1 expression only in A549 cells and TNF‐α, IL‐1α and IL‐1β upregulated PD‐L1 expression only in H1975 cells. Conversely, IL‐6 significantly decreased the expression of PD‐L1 in H1975 cells (Figure 5). Increased PD‐L1 protein was also detected in A549 and H1975 cells by treatment with TGF‐β1 by immunoblotting (Figure 6A), and flow cytometry analysis confirmed a tendency for higher PD‐L1 expression in TGF‐β1‐treated A549 and H1975 cells compared to controls (Figure 6B). Importantly, the TGF‐β concentrations in the conditioned media from A549 cells co–cultured with PBMC‐derived M2a or M2c macrophages were significantly elevated compared to those from A549 cells alone (Figure 6C). The enhanced expression of PD‐L1 in A549 cells by co–culture with M2a or M2c macrophages was reduced to basal levels following treatment with the TGF‐β inhibitor SB431542 (Figure 6D), suggesting that TGF‐β was involved in TAM‐mediated tumor PD‐L1 induction.
Figure 5.

Effect of macrophage‐producing cytokines on programmed death‐ligand 1 (PD‐L1) expression in human lung adenocarcinoma cell lines. Relative expression of PD‐L1 in A549 or H1975 cells by treatment with transforming growth factor‐β1 or interleukin (IL)‐10, tumor necrosis factor‐α, IL‐6, IL‐1α or IL‐1β for 12 or 24 h by real‐time quantitative PCR (n = 4). ∗ P < 0.05, ∗∗ P < 0.01
Figure 6.

Transforming growth factor (TGF)‐β1 is involved in tumor‐associated macrophage (TAM)‐mediated programmed death‐ligand 1 (PD‐L1) expression in human lung cancer cell lines. A, Representative immunoblot of PD‐L1 in A549 or H1975 cells by treatment with TGF‐β1 or vehicle alone for 72 h. β‐actin was used as a loading control. B, Representative flow cytometry data showing cell surface PD‐L1 expression in A549 or H1975 cells by treatment with TGF‐β1 or vehicle alone for 48 h. C, The concentration of TGF‐β in the conditioned media from A549 cells alone or indirectly co–cultured with human peripheral blood mononuclear cell (PBMC)‐derived M2a or M2c macrophages for 3 d, determined by ELISA (n = 4). D, Relative expression of PD‐L1 in A549 cells co–cultured with human PBMC‐derived M2a‐differentiated or M2c‐differentiated macrophages in the presence of a TGF‐β inhibitor, SB431542 or vehicle alone by real‐time quantitative (n = 3). E, Schematic presentation of the possible role of TAM in the tumor microenvironment. In this study, we showed that infiltration of TAM is involved in tumor PD‐L1 induction through production of cytokines such as TGF‐β in early lung adenocarcinoma. Upregulated PD‐L1 in macrophages during M2 differentiation may also directly contribute to immune suppression. In addition, TGF‐β is known to promote FoxP3+ regulatory T cell development and inhibit CD8+ T cell functions, suggesting that TAM infiltration within the tumor is a key factor for creation of the immunosuppressive tumor microenvironment. ∗ P < 0.05, ∗∗ P < 0.01
4. DISCUSSION
Immunotherapies targeting the PD‐1/PD‐L1 pathway have been a recent breakthrough in the treatment of human malignant diseases, including NSCLC. Although PD‐L1 expression has been shown to be associated with the clinical response to anti–PD‐L1 antibodies, its concise regulatory mechanisms remain elusive. In this study, we showed that tumor PD‐L1 positivity was associated with stromal invasion accompanied by increased immune cell infiltration in early lung AC. Among these immune cells, TAM infiltration was an additional factor related to tumor PD‐L1 expression, independent of CD8+ T cell infiltration. Our in vitro experiments showed that M2‐differentiated macrophages facilitated tumor PD‐L1 expression through TGF‐β. Together, these results suggested that TAM were extrinsic regulators of tumor PD‐L1 expression and could serve as potential therapeutic targets.
In the present study, tumor PD‐L1 expression was detected in 27 of 146 patients (18.5%). Tumor PD‐L1 positivity in NSCLC varied among studies, and these differences were possibly due to the definition of positivity, sample size or heterogeneity of the study populations, as well as the use of different PD‐L1 antibodies. Indeed, although there are currently four approved therapeutic agents on the market targeting the PD1/PD‐L1 pathway in NSCLC patients, their cut‐offs of PD‐L1 tumor proportion score by immunohistochemistry are various: ie 1% for 28‐8 (nivolumab), 1% for SP263 (durvalumab), 50% for SP142 (atezolizumab) and 1% (second‐line criteria) or 50% (first‐line criteria) for 22C3 (pembrolizumab).22 The antibody used here (E1L3N) is not related to drugs, but its specificity and sensitivity were validated in a previous report by Inamura et al13 and we defined a score of 5% or more as “PD‐L1‐positive,” based on previous studies.13, 23 Inamura et al showed that tumor PD‐L1 positivity was associated with less tumor differentiation and the EGFR wild‐type status,13 similar to our findings, and we further identified “stromal invasion” as an additional factor related to tumor PD‐L1 positivity in early lung AC. Stromal invasion is known to affect stromal remodeling and immune responses,19 and our study showed that tumor PD‐L1 expression was closely associated with increased numbers of tumor‐infiltrating TAM. TAM are known to show an immunosuppressive phenotype by attracting other immunosuppressive cells such as regulatory T cells, myeloid‐derived suppressor cells and type 2 helper T cells.21, 24, 25 Recently, TAM infiltration has been reported to be associated with the upregulation of PD‐L1 expression in gastric26 and pancreatic27 cancer, but its molecular mechanism is limited. The present study is the first report describing the association between TAM infiltration and tumor PD‐L1 expression in early lung AC, and to further show that TAM induced PD‐L1 expression on tumor cells via TGF‐β using an in vitro co–culture system.
M2 macrophages do not constitute a uniform population and are further subdivided into M2a, M2b, M2c and M2d categories.21 TAM are mainly composed of M2a and M2c macrophages, and the common denominator of these subpopulations is the production of large amounts of immunosuppressive cytokines, such as IL‐10 and TGF‐β.28 Our in vitro studies showed that tumor PD‐L1 expression was induced by both direct and indirect co–culture with M2a or M2c macrophages, suggesting that tumor PD‐L1 was induced by soluble factors commonly secreted by M2a and M2c macrophages. Although IL‐10 has been reported to upregulate PD‐L1 on TAM,29 the modest effect of IL‐10 on tumor PD‐L1 expression was observed only in A549 cells. This may be due to different mechanisms of PD‐L1 induction by macrophages in cancer cells. It has also been reported that tumor PD‐L1 expression is enhanced by TNF‐α from TAM in pancreatic cancer27 and NF‐κB signal elicited by macrophage inflammatory responses, including production of TNF‐α and IL‐1β, can trigger PD‐L1 expression in hepatocellular carcinoma cells.30 In this study, we identified TGF‐β as an additional key inducer of PD‐L1 in both A549 and H1975 cells. Inhibition studies using a TGF‐β inhibitor showed that TGF‐β was dispensable for upregulation of PD‐L1 in tumor cells by co–culture with M2 macrophages. Notably, recent studies have reported that TGF‐β restrains the anti–tumor immune response by blocking T cells from infiltrating the tumor.31, 32 TGF‐β has also been reported to promote regulatory T cell development,33 which may explain the enhanced FoxP3+ T cell infiltration in TAM‐rich PD‐L1+ tumor areas in our study. TAM‐derived TGF‐β is, therefore, thought to be a key factor in the creation of the immunosuppressive tumor microenvironment (Figure 6E).
Consistent with previous studies, we have shown that macrophages significantly express PD‐L1 during TAM‐like M2 differentiation (Figure 4A). Recent studies using various mouse tumor models have reported that both tumor‐derived and host‐derived PD‐L1 play critical roles in immune suppression, and their relative contributions appear to be context‐dependent.34, 35 Gordon et al reported that PD‐1/PD‐L1 inhibition increased macrophage phagocytosis and tumor immunity, reduced tumor growth and prolonged macrophage survival,36 suggesting that TAM‐derived PD‐L1 is actively involved in antigen‐specific tolerance induction in tumor‐bearing hosts. In addition, multiple clinical trials have reported that some patients with tumor PD‐L1‐negative tumors also respond to PD‐1/PD‐L1 blockade therapy,37, 38 which may indicate the potential contribution of stromal PD‐L1. Therefore, comprehensively evaluating global PD‐L1 expression, rather than monitoring PD‐L1 expression on tumor cells alone, may be a more accurate way to predict responses during PD‐1/PD‐L1 blockade therapy in cancer patients. Understanding regulatory mechanisms of PD‐L1 in immune cells, which can be induced by IL‐1029 and under hypoxic conditions,39, 40, 41 is also important in this field.
There are several limitations in this study. First, in many tumors, the microsatellite instability subtype is linked to PD‐L1 positivity, and is considered a key factor indicating suitability for checkpoint blockade therapy.42, 43 PD‐L1 overexpression in tumor cells is also related to oncogenic mutations in phosphatase and tensin homologues44 and nucleophosmin‐ALK.45 In this study, these genetic/epigenetic alterations in tumor tissues were not evaluated, except for EGFR mutations. Second, based on our data, we speculate that tumor‐infiltrating TAM are extrinsic regulators of tumor PD‐L1 expression and contribute to heterogeneity of tumor PD‐L1 expression. However, it is still difficult to determine to what extent intrinsic and extrinsic factors contribute to heterogenous and/or homogeneous PD‐L1 staining patterns by immunohistochemistry. Further studies are necessary to address possible associations among immunohistochemical tumor PD‐L1 positivity, intrinsic factors and immune cell infiltration patterns. Third, as for the assessment of the immune cell predictors of tumor PD‐L1 positivity by multivariable logistic regression analyses, we cannot exclude the possibility that the same types of chemokines regulate recruitment of various types of immune cells simultaneously. We did not measure the concentration of chemokines in tumor tissues in this study and potential confounders such as chemokines need to be considered in future studies.
Although PD‐1/PD‐L1 blockade is revolutionizing the clinical management of various tumors, not all cancer patients respond to this therapy. To predict its efficacies and/or adverse events and to optimize and personalize anti–PD‐1/PD‐L1 therapy, alone or in combination, it is essential to understand the complexity of the tumor immune microenvironment regulating the biology of the PD‐1/PD‐L1 pathway. The results of the present study showing that tumor‐infiltrating TAM are extrinsic regulators of tumor PD‐L1 expression provide important mechanistic insight into tumor immune evasion, which may contribute to the future development of ongoing combination therapies of PD‐1/PD‐L1 blockade.
DISCLOSURE
The authors have no conflict of interest to declare.
Supporting information
ACKNOWLEDGMENTS
We thank Ms H Inomata (Department of Pathology, Keio University School of Medicine) for the technical assistance. This work was supported by JSPS KAKENHI Grant Numbers 16K08719 (to MS) and 17K16617 (to TS), Keio Gijuku Academic Development Funds, the Public Trust Fund for Clinical Cancer Research, a research grant of the Princess Takamatsu Cancer Research Fund and grants from the Takeda Science Foundation (to MS).
Shima T, Shimoda M, Shigenobu T, et al. Infiltration of tumor‐associated macrophages is involved in tumor programmed death‐ligand 1 expression in early lung adenocarcinoma. Cancer Sci. 2020;111:727–738. 10.1111/cas.14272
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Anagnostou VK, Brahmer JR. Cancer immunotherapy: a future paradigm shift in the treatment of non–small cell lung cancer. Clin Cancer Res. 2015;21:976‐984. [DOI] [PubMed] [Google Scholar]
- 3. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974‐1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi L, Chen S, Yang L, Li Y. The role of PD‐1 and PD‐L1 in T‐cell immune suppression in patients with hematological malignancies. J Hematol Oncol. 2013;6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ohaegbulam KC, Assal A, Lazar‐Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD‐1 and PD‐L1 pathway. Trends Mol Med. 2015;21:24‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yarchoan M, Johnson BA 3rd, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer. 2017;17:209‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39‐51. [DOI] [PubMed] [Google Scholar]
- 9. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243‐1260. [DOI] [PubMed] [Google Scholar]
- 10. Borghaei H, Paz‐Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small‐cell lung cancer. N Engl J Med. 2015;373:1627‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang CY, Lin MW, Chang YL, Wu CT, Yang PC. Programmed cell death‐ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer. 2014;50:1361‐1369. [DOI] [PubMed] [Google Scholar]
- 12. Yang CY, Lin MW, Chang YL, Wu CT, Yang PC. Programmed cell death‐ligand 1 expression is associated with a favourable immune microenvironment and better overall survival in stage I pulmonary squamous cell carcinoma. Eur J Cancer. 2016;57:91‐103. [DOI] [PubMed] [Google Scholar]
- 13. Inamura K, Yokouchi Y, Sakakibara R, et al. Relationship of tumor PD‐L1 expression with EGFR wild‐type status and poor prognosis in lung adenocarcinoma. Jpn J Clin Oncol. 2016;46:935‐941. [DOI] [PubMed] [Google Scholar]
- 14. Ogino S, Nosho K, Irahara N, et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res. 2009;15:6412‐6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nishimura T, Ota M, Mori M, et al. Risk of tuberculosis infection among health care workers and nursing students in Japan. J Infect Chemother. 2018;24:921‐924. [DOI] [PubMed] [Google Scholar]
- 16. Ohlsson SM, Linge CP, Gullstrand B, et al. Serum from patients with systemic vasculitis induces alternatively activated macrophage M2c polarization. Clin Immunol. 2014;152:10‐19. [DOI] [PubMed] [Google Scholar]
- 17. Ota M, Mochizuki S, Shimoda M, et al. ADAM23 is downregulated in side population and suppresses lung metastasis of lung carcinoma cells. Cancer Sci. 2016;107:433‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shimoda M, Hashimoto G, Mochizuki S, et al. Binding of ADAM28 to P‐selectin glycoprotein ligand‐1 enhances P‐selectin‐mediated leukocyte adhesion to endothelial cells. J Biol Chem. 2007;282:25864‐25874. [DOI] [PubMed] [Google Scholar]
- 19. Clark AG, Vignjevic DM. Modes of cancer cell invasion and the role of the microenvironment. Curr Opin Cell Biol. 2015;36:13‐22. [DOI] [PubMed] [Google Scholar]
- 20. Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T‐cell infiltration and PD‐L1. Cancer Res. 2015;75(11):2139‐2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chanmee T, Ontong P, Konno K, Itano N. Tumor‐associated macrophages as major players in the tumor microenvironment. Cancers. 2014;6:1670‐1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O'Malley DP, Yang Y, Boisot S, et al. Immunohistochemical detection of PD‐L1 among diverse human neoplasms in a reference laboratory: observations based upon 62,896 cases. Modern Pathol. 2019;32:929‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ilie M, Hofman V, Dietel M, Soria JC, Hofman P. Assessment of the PD‐L1 status by immunohistochemistry: challenges and perspectives for therapeutic strategies in lung cancer patients. Virchows Arch. 2016;468:511‐525. [DOI] [PubMed] [Google Scholar]
- 24. Komohara Y, Fujiwara Y, Ohnishi K, Takeya M. Tumor‐associated macrophages: potential therapeutic targets for anti–cancer therapy. Adv Drug Deliv Rev. 2016;99:180‐185. [DOI] [PubMed] [Google Scholar]
- 25. Sica A, Schioppa T, Mantovani A, Allavena P. Tumour‐associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti–cancer therapy. Eur J Cancer. 2006;42:717‐727. [DOI] [PubMed] [Google Scholar]
- 26. Harada K, Dong X, Estrella JS, et al. Tumor‐associated macrophage infiltration is highly associated with PD‐L1 expression in gastric adenocarcinoma. Gastric Cancer. 2018;21:31‐40. [DOI] [PubMed] [Google Scholar]
- 27. Tsukamoto M, Imai K, Ishimoto T, et al. PD‐L1 expression enhancement by infiltrating macrophage‐derived tumor necrosis factor‐alpha leads to poor pancreatic cancer prognosis. Cancer Sci. 2019;110:310‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Noy R, Pollard JW. Tumor‐associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jiang C, Yuan F, Wang J, Wu L. Oral squamous cell carcinoma suppressed antitumor immunity through induction of PD‐L1 expression on tumor‐associated macrophages. Immunobiology. 2017;222:651‐657. [DOI] [PubMed] [Google Scholar]
- 30. Wei Y, Zhao Q, Gao Z, et al. The local immune landscape determines tumor PD‐L1 heterogeneity and sensitivity to therapy. J Clin Invest. 2019;129:3347‐3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tauriello DVF, Palomo‐Ponce S, Stork D, et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538‐543. [DOI] [PubMed] [Google Scholar]
- 32. Mariathasan S, Turley SJ, Nickles D, et al. TGFβ attenuates tumour response to PD‐L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dardalhon V, Awasthi A, Kwon H, et al. IL‐4 inhibits TGF‐beta‐induced Foxp3+ T cells and together with TGF‐beta, generates IL‐9+ IL‐10+ Foxp3(‐) effector T cells. Nat Immunol. 2008;9:1347‐1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tang H, Liang Y, Anders RA, et al. PD‐L1 on host cells is essential for PD‐L1 blockade‐mediated tumor regression. J Clin Invest. 2018;128:580‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Noguchi T, Ward JP, Gubin MM, et al. Temporally distinct PD‐L1 expression by tumor and host cells contributes to immune escape. Cancer Immunol Res. 2017;5:106‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gordon SR, Maute RL, Dulken BW, et al. PD‐1 expression by tumour‐associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti–PD‐L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558‐562. [DOI] [PubMed] [Google Scholar]
- 38. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320‐330. [DOI] [PubMed] [Google Scholar]
- 39. Cubillos‐Zapata C, Avendano‐Ortiz J, Hernandez‐Jimenez E, et al. Hypoxia‐induced PD‐L1/PD‐1 crosstalk impairs T‐cell function in sleep apnoea. Eur Respir J. 2017;50(4): pii: 1700833. [DOI] [PubMed] [Google Scholar]
- 40. Noman MZ, Desantis G, Janji B, et al. PD‐L1 is a novel direct target of HIF‐1alpha, and its blockade under hypoxia enhanced MDSC‐mediated T cell activation. J Exp Med. 2014;211:781‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia‐mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665‐674. [DOI] [PubMed] [Google Scholar]
- 42. Xiao Y, Freeman GJ. The microsatellite instable subset of colorectal cancer is a particularly good candidate for checkpoint blockade immunotherapy. Cancer Discov. 2015;5:16‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dudley JC, Lin MT, Le DT, Eshleman JR. Microsatellite instability as a biomarker for PD‐1 blockade. Clin Cancer Res. 2016;22:813‐820. [DOI] [PubMed] [Google Scholar]
- 44. Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7–H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84‐88. [DOI] [PubMed] [Google Scholar]
- 45. Marzec M, Zhang Q, Goradia A, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD‐L1, B7‐H1). Proc Natl Acad Sci USA. 2008;105:20852‐20857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


