Abstract
Sleep deprivation disrupted functional and structural brain areas which are associated with cognition and emotion in healthy participants. However, the effect of age on the structural changes after sleep restriction remains unclear. In the current study, gray matter volume was calculated in 43 young adults and 37 old adults before and after sleep restriction. Two-way mixed analysis of variance (between-subject factor: deprivation; within-subject factor: age) was then employed to investigate differences in gray matter volume changes between young and old adults. Gaussian random field theory was applied for multiple comparison correction. Results revealed that sleep restriction decreases gray matter volume in the right thalamus, left precuneus, and postcentral gyrus. More importantly, we found a significant deprivation × age interaction effect mainly in the right dorsal/ventral anterior insula where the gray matter volume increased in young adults after sleep restriction but showed no difference in old adults. These findings highlight the crucial role of the anterior insula in the neural mechanisms underlying sleep lose, especially among young adults. The current work provided structural evidence for describing emotional dysfunction and suggests the potential effect of age on functional and structural changes after sleep restriction.
Introduction
Sleep loss is a common and serious issue that exerts a negative effect on health. Insufficient sleep has been associated with impairment of cognition and emotion in healthy subjects [1]. The sleep–wake cycle has been observed to be disrupted in psychiatric and neurological disorders [2]. Thus, elucidating the mechanisms underlying sleep restriction is an important goal in basic and clinical neuroscience. Evidence indicates that aging has an effect on sleep. Participants with different age cohorts have been suggested to have different sensitivities to sleep lose [3].
It has been reported that better self-reported sleep is generally associated with better health outcomes, especially for mental health [4]. Sleep deprivation makes young brain resemble the old [5]. While old adults better tolerate sleep lose than young adults [3]. A study of psychomotor vigilance task found that after 40 hours sleep deprivation, the performance was declined more pronouncedly in the young than the old [6]. In addition, insufficient sleep might result in bad cognitive performance, in which the decrements were greater in the young as compared to the old [7]. A functional magnetic resonance imaging (MRI) study of verbal encoding task further reported that longer sleep time is involved in higher activation of anterior parahippocampus in old adults but low activation of the brain area in young adults, suggesting different mechanisms for maintaining cognitive performance [8]. Functional MRI studies also observed age effect on functional connectivity changes after sleep deprivation. For example, only young adults showed reduced resting-state functional connectivity of anterior insula after sleep restriction [9], and positive correlation between functional connectivity of medial temporal lobe network with sleep quality [10].
Sleep lose is associated with brain structural abnormalities. An animal study revealed that chronic sleep deprivation may negatively affect formation and maintenance of myelin [11]. A human study also suggested that acute sleep deprivation resulted in brain atrophy of distributed brain areas, which would be restored after one night sleep [12]. In healthy participants, sleep deprivation decreases thalamic gray matter volume [13], cortical thickness of medial parietal cortices [14], and prefrontal cortex [15,16]. Furthermore, the cortical atrophy was negatively correlated with sleepiness, suggesting structural plasticity after sleep deprivation [17]. Additionally, studies of sleep disorders also observed altered gray matter volumes of brain areas in the default mode network and the salience network [18,19].
Insufficient sleep was also involved in disrupted emotional processing [20]. For example, individuals with sleep deprivation had impaired dopaminergic reward functioning [21,22]. In addition, sleep-deprived participants had difficulty to discriminate between stimuli of different emotions. For instance, sleep-deprived individuals rate neutral images as more emotionally negative [23], and can not accurately identify threatening from affiliative facial signals [24]. A neuroimaging study revealed that anterior insula and anterior cingulate cortices play crucial role in impaired discrimination of negative emotions after one night of sleep deprivation [24].
Based on previous findings, the current study aims to explore the age effect on gray matter volume changes in individuals with sleep restriction. We hypothesize that (1) sleep restriction alters the gray matter volume of brain areas involved in emotional processing and (2) individuals within different age cohorts show different patterns of gray matter volume change.
Materials and methods
Study design and participants
The dataset was obtained from the Sleepy Brain Project (https://openneuro.org/datasets/ds000201). A total of 86 participants were included and classified into the young adult group (age, 20–30 years) and the old adult group (age, 65–75 years). This study features a cross-over comparison between one night sleep restriction (3 hours sleep) and full sleep. Participants were randomized to undergo both conditions in a counterbalanced order with an interval of approximately 1 month. In the interest of ecological validity, the participants slept in their own homes in both conditions. Participants were asked to fill in sleep diaries from 3 nights before the experiment, and told to avoid coffee and alcohol on the experimental day. On the night before imaging, sleep was monitored using ambulatory polysomnography. In the sleep restriction condition, the participants were instructed to go to bed 3 hours before the time they would usually get up and then get up at their normal time. MRI imaging was performed in the evening following sleep restriction or normal sleep. Before scanning, the participants were asked to complete several questionnaires, such as the positive and negative effect schedule (PANAS), Epworth sleepiness scale, insomnia severity index, and Karolinska Sleep Questionnaire such as normal time in bed, sleep quality index and snoring symptom index. PANAS scores were again recorded once after sleep restriction of all participants. Five subjects were excluded because their T1 images before or after sleep restriction were missing. One subject was excluded due to artifacts. Finally, 80 participants remained (37 old adults, 43 young adults). Other details can be found in previous studies [25,26]. The project was preregistered at clinicaltrials.gov (https://clinicaltrials.gov/ct2/show/NCT02000076), and this study was approved by the Regional Ethics Review Board of Stockholm (2012/1870–32). All participants provided written informed consent before participating in this work, and all experiments were performed in accordance with the Declaration of Helsinki and applicable local regulations. Details of the participants are provided in Table 1.
Table 1. Participant characteristics and sleep measures.
| Young (n = 43) | Old (n = 37) | p-value | |
|---|---|---|---|
| Sex (female/male) | 21/22 | 20/17 | 0.64a |
| ESS (mean±SD) | 7.23±3.01 | 8.65±4.8 | 0.11b |
| ISI (mean±SD) | 3.49±2.07 | 2.11±1.63 | 0.002b |
| KSQ sleep quality index (mean±SD) | 5.26±0.43 | 5.19±0.48 | 0.52b |
| KSQ snoring symtom index (mean±SD) | 5.88±0.32 | 5.65±0.54 | 0.02b |
aChi-square test.
bTwo-tailed two sample t-test.
Bold p-values indicates significant difference in sleep measures between young group and old group. SD, standard deviation; ESS, Epworth Sleepiness Scale; ISI, Insomnia Severity Index; KSQ, Karolinska Sleep Questionnaire.
Scan acquisition
The MRI data of the Sleepy Brain Project were acquired using a General Electric Discovery 3T MRI scanner. T1 structural images were scanned using a sagittal BRAVO sequence, a 24 cm field of view, and a 1 mm slice thickness. Other parameters used can be referenced from previous studies [25,26].
Voxel-based morphometry analysis
All images were visually inspected for artifacts or structural abnormalities. Then, voxel-based morphometry (VBM) analysis was applied to the MRI images by using SPM12 (Wellcome Trust Centre for Neuroimaging, Institute of Neurology, UCL, London, UK; http://www.fil.ion.uncl.ac.uk/spm). Briefly, the origin of all structural images was manually set to the anterior commissure, after which the images were segmented into gray matter, white matter, and cerebrospinal fluid [27]. The segments were iteratively registered using the Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL) toolbox [28], to produce a template of a group of individuals. The gray matter images were then spatially warped into the standard Montreal Neurological Institute space using affine spatial normalization and modulated by multiplication with the Jacobian determinant of the warp field [28]. Finally, the images were smoothed with an 8 mm full-width at half maximum (FWHM) isotropic Gaussian kernel.
Two-way mixed analysis of variance (between-subject factor: age, two levels; within-subject factor: deprivation, two levels) was employed to test differences between groups in terms of gray matter volume changes after sleep restriction. The individual total intracranial volume was included as covariance to remove the effect of different brain size. A Gaussian random field with p < 0.05 (voxel p < 0.01, Z > 2.3) was used for multiple comparison correction of the main effect of age, the main effect of deprivation, and the interaction effect of age × deprivation. Once a significant deprivation-related effect was observed, post-hoc analysis was employed for those brain areas showing significant effect. For the main effect of sleep restriction, we further investigated whether sleep restriction increases or decreases gray matter volume using independent two sample t-test. For the age × deprivation interaction effect, we further tested the difference in gray matter volume among young adults with and without sleep restriction, and old adults with and without sleep restriction using independent two sample t-test or paired t-test. Multiple comparisons were corrected using Bonferroni method with p<0.05/6. Notably, regions of interest (ROIs) for post-hoc analysis were selected using the “pick cluster” function in XJView (http://www.alivelearn.net/xjview8/).
Pearson correlation analysis was conducted to determine correlations between changes (post-deprivation vs. pre-deprivation) in the gray matter volume of the ROIs and changes (post-deprivation vs. pre-deprivation) in PANAS positive and negative scores in young adult and old adult separately. P values < 0.05 were considered statistically significant.
Results
Demographic results
Young and old participants had normal time in bed of 8.48±0.79 ours and 8.45±0.78 hours respectively. Analysis of ambulatory polysomnography data revealed that total sleep time in sleep restriction condition was 2.92±0.61 hours for young group, and 2.49±0.36 hours for old group. In the normal sleep condition, the total sleep time was 6.77±1.35 hours and 5.73±1.41 hours for young and old groups respectively. A mixed ANOVA was employed to test difference in PANAS scores. For positive PANAS score, we observed significant interaction effect (F(1,77) = 9.73,P = 0.003), main effect of age (F(1,77) = 18.58,P = 0.001), and main effect of deprivation (F(1,77) = 22.1,P = 0.001). Post-hoc analysis indicated that sleep restriction decreases positive PANAS score only in young adult. For negative PANAS score, we only found significant main effect of age (F(1,77) = 9,P = 0.004) with higher score in young adults. Old adults revealed a significant decrease in insomnia severity index and Karolinska Sleep Questionnaire snoring symptom index compared with young adults (Table 1).
VBM results
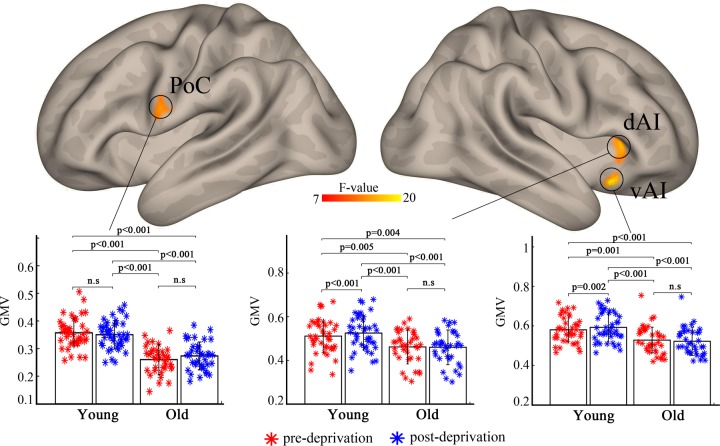
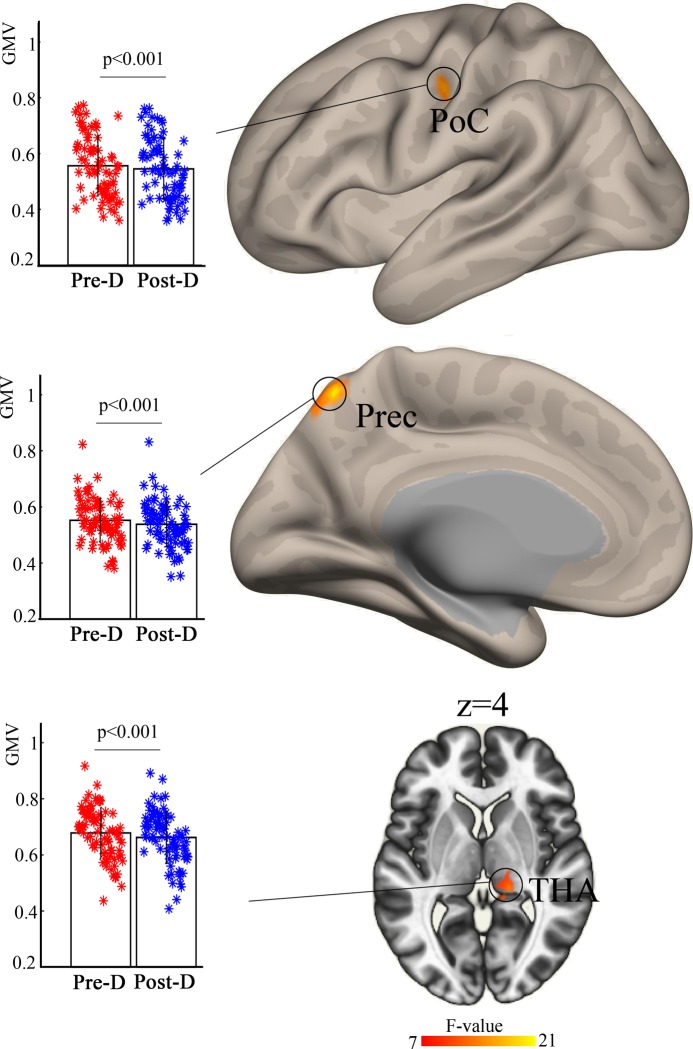
Analysis of variance showed a significant age × deprivation interaction effect on the left postcentral gyrus (F(1,77) = 11.1, partial η2 = 0.127), right dorsal anterior insula (dAI) (F(1,77) = 11.8, partial η2 = 0.113), and ventral anterior insula (vAI) (F(1,77) = 19.8, partial η2 = 0.159). Post-hoc analysis revealed increased gray matter volume in the right dAI and vAI after sleep restriction in young individuals but not in old individuals (Fig 1). A significant main effect of deprivation was observed in the left postcentral gyrus (F(1,77) = 16.1, partial η2 = 0.171), left precuneus (F(1,77) = 10.6, partial η2 = 0.173), and right thalamus (F(1,77) = 20.8, partial η2 = 0.159) (Fig 2). Post-hoc analysis showed that these three brain areas exhibit decreased gray matter volume after sleep restriction.
Fig 1. Significant age × deprivation interaction effect on gray matter volume in the left postcentral gyrus and right dorsal/ventral anterior insula.
Post-hoc analysis revealed a larger volume of gray matter after sleep restriction in young adults but no difference in older adults. PoC, postcentral gyrus; dAI, dorsal anterior insula; vAI, ventral anterior insula.
Fig 2. Significant main effect of deprivation on the left postcentral and precuneus and right thalamus.
Post-hoc analysis indicated reduced gray matter volume in these brain areas. post-D, post-deprivation; pre-D, pre-deprivation; PoC, postcentral gyrus; Prec, precuneus; THA, thalamus.
Finally, we observed a significant main effect of age, that is, most brain areas of old adult exhibit decreased gray matter volume in comparison with those of young adults (S1 Fig). We did not find significant correlation (p>0.05) between gray matter volume change of those ROIs and positive or negative PANAS scores both in young and old groups.
Discussion
In the present study, we observed a significant interaction effect between deprivation and age on the right anterior insula and a main effect of deprivation on the left postcentral gyrus and precuneus and right thalamus. These results provide structural evidence for the functional dysfunction of brain networks and suggest an age effect on morphological changes after sleep restriction.
We firstly observed a significant deprivation × age interaction effect on the anterior insula. Post-hoc analysis revealed that the gray matter volume of the anterior insula increases in the young cohort after sleep restriction but showed no change in the old cohort, suggesting a pathological mechanism underlying the increase in gray matter volume. The anterior insula, a key brain area of the salience network, is involved in attention and interoceptive and affective processes [29]. Neuroimaging evidence suggests that sleep deprivation can over-generalize the responsivity of the affective salience networks [30], which means participants with sleep deprivation cannot discriminate between stimuli of different emotional strengths. For example, deprivation individuals usually rate neutral images as more emotionally negative than their non-deprivation counterparts [23,24]. In addition, sleep deprivation alters the brain anticipation of cued emotional experience via the elevated activation of the anterior insula and anterior cingulate cortex [31]. A resting study revealed altered spontaneous neural activity of the insula of participants after sleep deprivation [32]. The enhanced gray matter volume of the anterior insula observed in the present study may suggest disrupted emotion discrimination and expression after sleep restriction. However, the gray matter volume of this area in old adults was preserved after sleep restriction. A possible interpretation is that old adults are more tolerant of sleep deprivation than young adults, which means the former are less affected by sleep restriction [6,33]. The decreased insomnia severity index in old adults compared with that in young adults observed in the current study provides direct evidence of this supposition. Another interpretation is that old adults may have less cortical plasticity than young adults. For example, a study of training task reported that motor training resulted in greater improvements in corticomotor excitability in young subjects compared with old subjects, suggesting reduced corticomotor plasticity with aging [34].
The significant main effect of deprivation was observed in the left precuneus, right thalamus, and left postcentral gyrus. These areas showed decreased gray matter volume after sleep restriction. Sleep deprivation has been suggested to impair short-term memory, which is associated with the reduced deactivation of the precuneus in participants with sleep deprivation [35]. Visual memory and novelty processing tasks demonstrate that sleep deprivation decreases the activation of the precuneus and ventral stream regions when processing object location [36,37]. Moreover, this decrease is correlated with poor recognition performance. The precuneus belongs to the default mode network. A resting study revealed that the spontaneous activity of the default mode network decreases after long-term total sleep deprivation [38]. Functional connectivity analyses further report increased functional connectivity between the precuneus and cerebellum [39] and amygdala [40]. The cortical thickness of the precuneus has been reported to decrease after 24 hours of sleep deprivation [14]. The decrease in gray matter volume observed in our study is consistent with previous results and suggests the dysfunction of the default mode network after sleep restriction.
The thalamus is believed to be a pivotal gating hub that integrates the flow of information from the periphery to the cortex [41]. Neuroimaging studies have demonstrated that sleep deprivation alters the thalamic activity during sustained attention. However, the profile of activity change in the thalamus is inconsistent. Some studies report increased activity under sleep loss conditions [42,43], while others observe diminished thalamic activity [44,45]. These inconsistencies may be due to differences in performance and cortical arousal provided by thalamic activity. The thalamus shows specific functional and structural connectivity with different cortices [46]. A resting study demonstrated that sleep deprivation decreases the thalamic functional connectivity with the cortex, mainly in the frontal and temporal cortex [47]. A morphological study reported reduced thalamic gray matter volume after long-term sleep deprivation [13], which is consistent with the results of the current study. The decreased gray matter volume of the postcentral gyrus after sleep restriction found in the present study is in accordance with that of a previous resting state study reporting altered activity and functional connectivity in this area [32,48].
Several limitations must be addressed. First, another independent datasets were needed to validate the findings observed in the current study. Second, the negative results (i.e., no difference in gray matter volume) found in old adults may be due to short-term sleep deprivation. Future studies must investigate the age effect on gray matter volume changes by applying long-term sleep deprivation. Third, this study only employed structural imaging data for analysis; thus, a relationship between brain function and structure under sleep restriction could not be found.
In conclusion, the current study investigated the effect of age on changes in gray matter volume after sleep restriction. We found a significant age × deprivation interaction effect on the anterior insula that suggests the dysfunction of this brain area in emotion discrimination and expression after sleep restriction. Moreover, sleep restriction decreased the gray matter volume of the default mode network and thalamus. This study provides a structural basis for the functional disturbance of the default mode network and salience network in sleep restriction individuals and highlights the role of age in sleep deprivation studies.
Supporting information
(TIF)
Acknowledgments
We thank the Stockholm Sleepy Brain study for providing the online datasets.
Data Availability
The minimal data set underlying this study is available from the Sleepy Brain Project: https://openneuro.org/datasets/ds000201
Funding Statement
This work was supported by the Fundamental Research Funds for the Central Universities (No. SWU2120132007)
References
- 1.Lim J, Dinges DF (2010) A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull 136: 375–389. 10.1037/a0018883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Videnovic A, Lazar AS, Barker RA, Overeem S (2014) 'The clocks that time us'—circadian rhythms in neurodegenerative disorders. Nat Rev Neurol 10: 683–693. 10.1038/nrneurol.2014.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffy JF, Willson HJ, Wang W, Czeisler CA (2009) Healthy older adults better tolerate sleep deprivation than young adults. J Am Geriatr Soc 57: 1245–1251. 10.1111/j.1532-5415.2009.02303.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gadie A, Shafto M, Leng Y, Kievit RA (2017) How are age-related differences in sleep quality associated with health outcomes? An epidemiological investigation in a UK cohort of 2406 adults. BMJ Open 7: e014920 10.1136/bmjopen-2016-014920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou X, Wu T, Yu J, Lei X (2017) Sleep Deprivation Makes the Young Brain Resemble the Elderly Brain: A Large-Scale Brain Networks Study. Brain Connect 7: 58–68. 10.1089/brain.2016.0452 [DOI] [PubMed] [Google Scholar]
- 6.Blatter K, Graw P, Munch M, Knoblauch V, Wirz-Justice A, et al. (2006) Gender and age differences in psychomotor vigilance performance under differential sleep pressure conditions. Behav Brain Res 168: 312–317. 10.1016/j.bbr.2005.11.018 [DOI] [PubMed] [Google Scholar]
- 7.Richards A, Inslicht SS, Metzler TJ, Mohlenhoff BS, Rao MN, et al. (2017) Sleep and Cognitive Performance From Teens To Old Age: More Is Not Better. Sleep 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jonelis MB, Drummond SP, Salamat JS, McKenna BS, Ancoli-Israel S, et al. (2012) Age-Related Influences of Prior Sleep on Brain Activation during Verbal Encoding. Front Neurol 3: 49 10.3389/fneur.2012.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long Z, Cheng F (2019) Age effect on functional connectivity changes of right anterior insula after partial sleep deprivation. Neuroreport 30: 1246–1250. 10.1097/WNR.0000000000001347 [DOI] [PubMed] [Google Scholar]
- 10.Liu YR, Fan DQ, Gui WJ, Long ZL, Lei X, et al. (2018) Sleep-related brain atrophy and disrupted functional connectivity in older adults. Behav Brain Res 347: 292–299. 10.1016/j.bbr.2018.03.032 [DOI] [PubMed] [Google Scholar]
- 11.Bellesi M, Haswell JD, de Vivo L, Marshall W, Roseboom PH, et al. (2018) Myelin modifications after chronic sleep loss in adolescent mice. Sleep 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai XJ, Jiang J, Zhang Z, Nie X, Liu BX, et al. (2018) Plasticity and Susceptibility of Brain Morphometry Alterations to Insufficient Sleep. Front Psychiatry 9: 266 10.3389/fpsyt.2018.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C, Kong XZ, Liu X, Zhou R, Wu B (2014) Long-term total sleep deprivation reduces thalamic gray matter volume in healthy men. Neuroreport 25: 320–323. 10.1097/WNR.0000000000000091 [DOI] [PubMed] [Google Scholar]
- 14.Elvsashagen T, Zak N, Norbom LB, Pedersen PO, Quraishi SH, et al. (2017) Evidence for cortical structural plasticity in humans after a day of waking and sleep deprivation. Neuroimage 156: 214–223. 10.1016/j.neuroimage.2017.05.027 [DOI] [PubMed] [Google Scholar]
- 15.Rahayel S, Postuma RB, Montplaisir J, Genier Marchand D, Escudier F, et al. (2018) Cortical and subcortical gray matter bases of cognitive deficits in REM sleep behavior disorder. Neurology 90: e1759–e1770. 10.1212/WNL.0000000000005523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macey PM, Kheirandish-Gozal L, Prasad JP, Ma RA, Kumar R, et al. (2018) Altered Regional Brain Cortical Thickness in Pediatric Obstructive Sleep Apnea. Front Neurol 9: 4 10.3389/fneur.2018.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Killgore WD, Schwab ZJ, Kipman M, DelDonno SR, Weber M (2012) Voxel-based morphometric gray matter correlates of daytime sleepiness. Neurosci Lett 518: 10–13. 10.1016/j.neulet.2012.04.029 [DOI] [PubMed] [Google Scholar]
- 18.Joo EY, Tae WS, Lee MJ, Kang JW, Park HS, et al. (2010) Reduced brain gray matter concentration in patients with obstructive sleep apnea syndrome. Sleep 33: 235–241. 10.1093/sleep/33.2.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altena E, Vrenken H, Van Der Werf YD, van den Heuvel OA, Van Someren EJ (2010) Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol Psychiatry 67: 182–185. 10.1016/j.biopsych.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 20.Krause AJ, Simon EB, Mander BA, Greer SM, Saletin JM, et al. (2017) The sleep-deprived human brain. Nat Rev Neurosci 18: 404–418. 10.1038/nrn.2017.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullin BC, Phillips ML, Siegle GJ, Buysse DJ, Forbes EE, et al. (2013) Sleep deprivation amplifies striatal activation to monetary reward. Psychol Med 43: 2215–2225. 10.1017/S0033291712002875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkatraman V, Chuah YM, Huettel SA, Chee MW (2007) Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep 30: 603–609. 10.1093/sleep/30.5.603 [DOI] [PubMed] [Google Scholar]
- 23.Tempesta D, Couyoumdjian A, Curcio G, Moroni F, Marzano C, et al. (2010) Lack of sleep affects the evaluation of emotional stimuli. Brain Res Bull 82: 104–108. 10.1016/j.brainresbull.2010.01.014 [DOI] [PubMed] [Google Scholar]
- 24.Goldstein-Piekarski AN, Greer SM, Saletin JM, Walker MP (2015) Sleep Deprivation Impairs the Human Central and Peripheral Nervous System Discrimination of Social Threat. J Neurosci 35: 10135–10145. 10.1523/JNEUROSCI.5254-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamm S, Nilsonne G, Schwarz J, Lamm C, Kecklund G, et al. (2017) The effect of sleep restriction on empathy for pain: An fMRI study in younger and older adults. Sci Rep 7: 12236 10.1038/s41598-017-12098-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nilsonne G, Tamm S, Schwarz J, Almeida R, Fischer H, et al. (2017) Intrinsic brain connectivity after partial sleep deprivation in young and older adults: results from the Stockholm Sleepy Brain study. Sci Rep 7: 9422 10.1038/s41598-017-09744-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashburner J, Friston KJ (2000) Voxel-based morphometry—the methods. Neuroimage 11: 805–821. 10.1006/nimg.2000.0582 [DOI] [PubMed] [Google Scholar]
- 28.Ashburner J (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38: 95–113. 10.1016/j.neuroimage.2007.07.007 [DOI] [PubMed] [Google Scholar]
- 29.Sridharan D, Levitin DJ, Menon V (2008) A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A 105: 12569–12574. 10.1073/pnas.0800005105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein AN, Walker MP (2014) The role of sleep in emotional brain function. Annu Rev Clin Psychol 10: 679–708. 10.1146/annurev-clinpsy-032813-153716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein AN, Greer SM, Saletin JM, Harvey AG, Nitschke JB, et al. (2013) Tired and apprehensive: anxiety amplifies the impact of sleep loss on aversive brain anticipation. J Neurosci 33: 10607–10615. 10.1523/JNEUROSCI.5578-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L, Qi X, Zheng J (2018) Altered Regional Cortical Brain Activity in Healthy Subjects After Sleep Deprivation: A Functional Magnetic Resonance Imaging Study. Front Neurol 9: 588 10.3389/fneur.2018.00588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Philip P, Taillard J, Sagaspe P, Valtat C, Sanchez-Ortuno M, et al. (2004) Age, performance and sleep deprivation. J Sleep Res 13: 105–110. 10.1111/j.1365-2869.2004.00399.x [DOI] [PubMed] [Google Scholar]
- 34.Rogasch NC, Dartnall TJ, Cirillo J, Nordstrom MA, Semmler JG (2009) Corticomotor plasticity and learning of a ballistic thumb training task are diminished in older adults. J Appl Physiol (1985) 107: 1874–1883. 10.1152/japplphysiol.00443.2009 [DOI] [PubMed] [Google Scholar]
- 35.Chee MW, Chuah YM (2007) Functional neuroimaging and behavioral correlates of capacity decline in visual short-term memory after sleep deprivation. Proc Natl Acad Sci U S A 104: 9487–9492. 10.1073/pnas.0610712104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beauchamp MS, Petit L, Ellmore TM, Ingeholm J, Haxby JV (2001) A parametric fMRI study of overt and covert shifts of visuospatial attention. Neuroimage 14: 310–321. 10.1006/nimg.2001.0788 [DOI] [PubMed] [Google Scholar]
- 37.Burgess N, Maguire EA, Spiers HJ, O'Keefe J (2001) A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage 14: 439–453. 10.1006/nimg.2001.0806 [DOI] [PubMed] [Google Scholar]
- 38.Dai XJ, Liu CL, Zhou RL, Gong HH, Wu B, et al. (2015) Long-term total sleep deprivation decreases the default spontaneous activity and connectivity pattern in healthy male subjects: a resting-state fMRI study. Neuropsychiatr Dis Treat 11: 761–772. 10.2147/NDT.S78335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X, Yan Z, Wang T, Yang X, Feng F, et al. (2015) Connectivity pattern differences bilaterally in the cerebellum posterior lobe in healthy subjects after normal sleep and sleep deprivation: a resting-state functional MRI study. Neuropsychiatr Dis Treat 11: 1279–1289. 10.2147/NDT.S84204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao Y, Lei Y, Wang L, Zhai T, Jin X, et al. (2014) Altered resting-state amygdala functional connectivity after 36 hours of total sleep deprivation. PLoS One 9: e112222 10.1371/journal.pone.0112222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherman SM (2007) The thalamus is more than just a relay. Curr Opin Neurobiol 17: 417–422. 10.1016/j.conb.2007.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chee MW, Tan JC, Parimal S, Zagorodnov V (2010) Sleep deprivation and its effects on object-selective attention. Neuroimage 49: 1903–1910. 10.1016/j.neuroimage.2009.08.067 [DOI] [PubMed] [Google Scholar]
- 43.Tomasi D, Wang RL, Telang F, Boronikolas V, Jayne MC, et al. (2009) Impairment of attentional networks after 1 night of sleep deprivation. Cereb Cortex 19: 233–240. 10.1093/cercor/bhn073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chee MW, Tan JC (2010) Lapsing when sleep deprived: neural activation characteristics of resistant and vulnerable individuals. Neuroimage 51: 835–843. 10.1016/j.neuroimage.2010.02.031 [DOI] [PubMed] [Google Scholar]
- 45.Chee MW, Tan JC, Zheng H, Parimal S, Weissman DH, et al. (2008) Lapsing during sleep deprivation is associated with distributed changes in brain activation. J Neurosci 28: 5519–5528. 10.1523/JNEUROSCI.0733-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME (2010) Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex 20: 1187–1194. 10.1093/cercor/bhp182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shao Y, Wang L, Ye E, Jin X, Ni W, et al. (2013) Decreased thalamocortical functional connectivity after 36 hours of total sleep deprivation: evidence from resting state FMRI. PLoS One 8: e78830 10.1371/journal.pone.0078830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu Y, Feng Z, Xu J, Fu C, Sun J, et al. (2016) Increased interhemispheric resting-state functional connectivity after sleep deprivation: a resting-state fMRI study. Brain Imaging Behav 10: 911–919. 10.1007/s11682-015-9490-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Data Availability Statement
The minimal data set underlying this study is available from the Sleepy Brain Project: https://openneuro.org/datasets/ds000201