Abstract
Claudins are the major component of tight junctions, which form a primary barrier to paracellular diffusion and maintain cell polarity in normal epithelia and endothelia. In cancer cells, claudins play additional roles besides serving as components of the tight junctions, and participate in anoikis or invasion. Among the claudin family proteins, claudin‐1 has the most promising potential, both diagnostically and prognostically, in many types of cancers, including oral, gastric, liver, and colon cancers. However, conflicting results have been reported in relation to the degree of claudin‐1 expression and the prognosis, suggesting that the expression level of claudin‐1 alone is not sufficient to analyze the relationship between claudin‐1 and cancer progression. As endocytic trafficking of claudin‐1 has been reported in several epithelial cell types in vitro, we aimed to determine whether intracellular localization of claudin‐1 is the missing aspect between claudin‐1 and cancer. We investigated the expression of claudin‐1 in 83 tongue squamous cell carcinoma (TSCC) pathological specimens. Although the expression level of claudin‐1 based on immunohistochemistry was not associated with TSCC progression, within the high claudin‐1 expression group, the incidence of intracellular localization of claudin‐1 was correlated with cervical lymph node metastasis. In an in vitro experiment, claudin‐1 was constitutively internalized in TSCC‐derived cells. Motility of TSCC‐derived cells was increased by deficiency of claudin‐1, suggesting that the decrease in cell‐surface claudin‐1 promoted the cell migration. Therefore, intracellular localization of claudin‐1 at the invasion front may represent a promising diagnostic marker of TSCC.
Keywords: claudin‐1, endocytosis, invasion front, metastasis, tongue squamous cell carcinoma
Claudin‐1 was predominantly localized at the membrane in the center of the lesion, but intracellular claudin‐1 localization was also observed at the invasion front in some tongue squamous cell carcinoma (TSCC) pathological specimens. Within the high claudin‐1 expression group, incidence of intracellular localization of claudin‐1 correlated with cervical lymph node metastasis.
Abbreviations
- CPZ
chlorpromazine
- IMP
imipramine
- OSCC
oral squamous cell carcinomas
- TSCC
tongue squamous cell carcinoma
1. INTRODUCTION
The oral cavity is the major site of head and neck cancer,1 with approximately 300 000 cases and 145 000 deaths reported annually worldwide.2 The primary sites of cancer within the oral cavity are the tongue and the floor of the mouth, or the buccal mucosa. Surgical removal is followed by postoperative declines in speech and feeding, causing a serious decline in quality of life (QOL). Histologically, most oral cancers are squamous cell carcinomas that infiltrate into mesenchymal tissues. The important factor affecting the survival prognosis is metastasis.
Claudin‐1 belongs to the claudin superfamily of proteins, which are structural and functional components of tight junctions.3 Claudin‐1 is classified as a paracellular barrier‐forming claudin. Mice deficient in claudin‐1 die of dehydration within a day of birth, indicating that claudin‐1 in skin epidermal sheets is crucial for organizing the water barrier on the entire surface of the body.4 Recent studies have suggested that claudin‐1 also plays a role unrelated to tight junctions in cancer, such as the activation of tumor necrosis factor (TNF)‐α5 or c‐Abl‐ERK signaling,5, 6 the resistance to anoikis in a Src‐dependent method7 and the enhancement of invasive activity through the activation of matrix metalloproteinases.8
Low claudin‐1 expression is associated with shorter overall survival and higher tumor grades in lung adenocarcinoma,9 prostatic adenocarcinoma,10 rectal cancer,11 and breast cancer.12, 13, 14 In these cancers, claudin‐1 is a metastasis suppressor. In contrast, in colon cancer,15 some studies have shown that claudin‐1 overexpression has a good prognostic value (fewer metastases and less aggressive),11, 16, 17 whereas other studies have shown that claudin‐1 overexpression is linked with increased invasion.15, 18, 19 Accordingly, although claudin‐1 has the potential to be a biomarker with diagnostic and prognostic values in oral, gastric, liver, and colon cancers,15 the seemingly contradictory results imply that the expression level of claudin‐1 alone is not sufficient to analyze the relationship between claudin‐1 and cancer metastasis.
The tight junction protein complex reportedly has a dynamic structure, undergoing rapid and continuous molecular remodeling in the steady state in MDCK cells.20 A functional endosomal sorting complex required for transport (ESCRT) is involved in the constitutive recycling of claudin‐1 between the plasma membrane and intracellular vesicles in MDCK cells.21 Furthermore, there is growing evidence linking endocytosis and the regulation of cell migration.22, 23 Thus, we hypothesized that a factor related to the membrane dynamics of claudin‐1 would be equivalent to the missing link between claudin‐1 and cancer metastasis.
Cancer cell morphology at the invasion front has recently attracted attention as a prognostic factor. For example, in colon cancer, the correlation between tumor budding at the invasion front of the lesion and regional lymph node metastasis has been reported.24 In oral cancer, the YK classification based on the infiltration mode has been used as one of the diagnostic criteria.25
Overexpression of the claudin‐1 (CLDN1) gene is significantly correlated with advanced disease stage in OSCC based on molecular classifications of oral cancer by cDNA microarrays.26 In line with this result, overexpression of the claudin‐1 protein is associated with invasion in OSCC.8, 27
In the present study, we investigated whether the intracellular location of claudin‐1 at the invasion front of the lesion correlates with the degree of disease progression in OSCC. When the tongue is the primary lesion, the invasion front primarily resides in soft tissue, such as muscle tissue. In contrast, when the gingiva and the floor of the mouth are the primary lesions, the invasion front infiltrates the hard tissue, and thus a decalcification process is required prior to immunohistochemical staining, which may cause variations in the results. Therefore, we concentrated on TSCC, which represents the majority of OSCC and has the advantage that the decalcification process is unnecessary. We found that, within the high claudin‐1 expression group, the incidence of intracellular claudin‐1 localization was correlated with metastasis. Furthermore, constitutive endocytosis of claudin‐1 was shown in the TSCC‐derived cell line. Endocytosis of claudin‐1 and cell motility was hindered by endocytosis inhibitors. In contrast, deficiency of claudin‐1 increased cell motility. The present study suggested that the ectopic, intracellular location of claudin‐1 at the invasion front may be an additional and promising diagnostic marker of TSCC.
2. MATERIALS AND METHODS
2.1. Clinical specimens
Primary tongue squamous cell carcinoma specimens were collected from 83 patients who had been treated at the Dental Hospital of Tokyo Medical and Dental University. All of the tissues were fixed with 10% neutral buffered formalin and embedded in paraffin according to routine laboratory protocols. All experimental procedures were conducted in accordance with the amended Declaration of Helsinki and approved by the ethics committee of Tokyo Medical and Dental University (registry no. D2015‐534). We used surgically resected, formalin‐fixed, and paraffin‐embedded human TSCC tissues for the immunohistochemical study.
2.2. Immunohistochemistry
For immunohistochemical staining, formalin‐fixed, paraffin‐embedded human TSCC tissue sections were used. Primary antibody was anti‐claudin‐1, rabbit polyclonal, 1:100 (ab15098; Abcam). Antigen retrieval was carried out according to the manufacturer’s protocol. EnVision + Dual Link (Dako) was used as the secondary antibody, and coloration was conducted with the diaminobenzidine substrate.
2.3. Evaluation of immunohistochemical analyses
Immunohistochemical assessment was carried out and agreed upon by two pathologists who were blinded to all patient clinical and follow‐up data. Percentages of claudin‐1‐positive neoplastic cells were semiquantitatively assessed, and proportion scores of 1, 2, 3, and 4 were given when the percentages of positive cells were 25% or less, 26%‐50%, 51%‐75% and 76%‐100%, respectively. Intensities of immunoreactivity were categorized into negative (score 0), low (score 1), moderate (score 2), and high (score 3) as shown in Figure 1. The final score of claudin‐1 immunoreactivity levels was obtained by multiplying the proportion score by the intensity score. Cases were classified into high if the scores were 4 or higher and into low if the scores were 3 or less.
Figure 1.
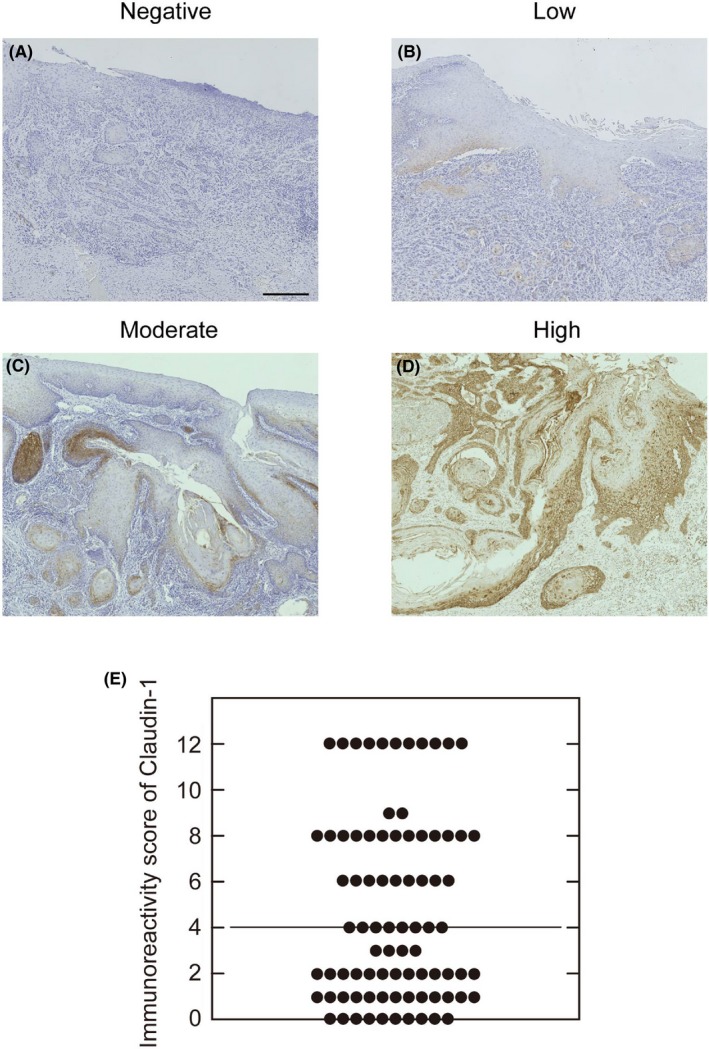
Representative photomicrographs of claudin‐1 immunohistochemistry in tongue squamous cell carcinoma (TSCC). A‐D, Immunoreactivity intensities were categorized into negative (score 0), low (score 1), moderate (score 2), and high (score 3). Scale bars, 300 μm. E, Distribution of the final scores of claudin‐1 immunoreactivity (dimensionless) in TSCC (n = 83) obtained by multiplying the proportion score by the intensity score. Horizontal line shows the median value. Cases were classified into high‐ and low‐expression groups, with final scores of 4 or more and 3 or less, respectively
For assessment of the localization of claudin‐1, five square regions were selected from the claudin‐1‐positive invasion front for each case. Cases were classified as “intracellular” when the exclusive membrane localization of claudin‐1 was not observed in three or more regions. Otherwise, they were classified as “membrane”.
2.4. Confocal laser scanning microscopy
For immunofluorescence staining, a mouse monoclonal anti‐claudin‐1 antibody (1C5‐D9)(H00009076‐M01; Novus Biologicals) and an Alexa Fluor 488‐conjugated anti‐mouse antibody were used. Immunofluorescent images were captured and analyzed with a Leica TCS SP8 confocal microscope (Leica).
2.5. Cell culture and reagents
Human tongue squamous cell carcinoma cell line (SAS) was obtained from Japanese Collection of Research Bioresources. SAS cells were maintained in DMEM: high glucose (08458; Nacalai Tesque), supplemented with 10% FBS (Sigma‐Aldrich), l‐penicillin (50 U/mL), and streptomycin (50 μg/mL), at 37°C in a 5% CO2 atmosphere. Chlorpromazine hydrochloride (C8138) was obtained from Sigma‐Aldrich and imipramine hydrochloride (I0971) was from Tokyo Chemical Industry.
2.6. Endocytosis of claudin‐1
Claudin‐1 endocytosis was evaluated based on the modified cell‐surface assay using EZ‐link sulfo‐NHS‐SS‐biotin (21 331; Pierce), as described previously.21, 28 Materials recovered by Neutravidin beads (29 200; Pierce) were subjected to 12% SDS‐PAGE under reducing conditions, and then transferred to a PVDF membrane. The membrane was incubated with 4% Block Ace (Yukijirushi) for 1 hour and then incubated with an anti‐claudin‐1, rabbit polyclonal antibody (ab15098; Abcam). An HRP‐conjugated anti‐rabbit antibody (#7074; Cell Signaling Technology) was used as the secondary antibody. Immunoblot signals of claudin‐1 were detected using the ECL Western Blotting Detection Reagent (RPN2109; GE Healthcare) and quantified by the FUSION chemiluminescence imaging system (VILBER).
2.7. Cell motility assay
SAS cells were seeded at a density of 2 × 104/well into 6‐well plates, 48 hours before the experiment. Cell motility was monitored with a BZ‐X800 time‐lapse imaging system (KEYENCE). Images were analyzed using ImageJ software (National Institutes of Health), and cell tracking was done using the Manual Tracking plugin. Total distance traveled was determined by tracking the movement of the cell gravity center, and its coordinates were used to calculate the distances.
2.8. Establishment of SAS cells deficient for the claudin‐1 gene
Claudin‐1‐knockout SAS cells were established using the clustered regularly interspaced short palindromic repeats/CRISPR associated protein 9 (CRISPR/CAS9)‐mediated genome editing system.29 Guide RNA (gRNA) target sequences for claudin‐1: 5′‐CCTATGCCGGCGACAACATCGTG‐3′ (gRNA #1) and 5′‐CCTGCGTGTCGCAGAGCACCGGG‐3′ (gRNA #2) (PAM sequence underlined) were designed with CRISPRdirect (https://crispr.dbcls.jp). Expression cassette of each gRNA was incorporated into the pX330 plasmid, carrying the human Cas9 expression cassette. Efficiency of both gRNAs to induce double strand breaks was validated by reconstituted EGFP fluorescence, as described previously.30 After transfection of SAS cells with the pX330 plasmid, knockout clones were isolated by carrying out limiting dilution. Effective knockout of claudin‐1 was confirmed at the protein level by immunoblotting with an anti‐human claudin‐1 antibody (ab15098).
2.9. Statistical analysis
In clinical specimens, Fisher’s exact test or Pearson’s chi‐squared test were carried out with the statistical software ‘EZR’ (Easy R).31 For the in vitro experiments, significant differences between means were determined using Student’s t test. P <.05 was considered statistically significant.
3. RESULTS
3.1. Claudin‐1 expression level is not associated with TSCC progression
Paraffin‐embedded sections of TSCC specimens from 83 individual patients were examined for claudin‐1 immunoreactivity. Patient details are provided in Table 1. Various degrees of claudin‐1 immunoreactivities were observed within the basal layers of the epithelium and metastatic lesions as shown in Figure 1. Claudin‐1 expression was not observed in the surrounding mesenchymal tissue. All of the cases were classified into either the high or low expression group, based on both the intensity and proportion scores. Results are shown in Table 2. There were no significant differences in any of the evaluation items between the two groups.
Table 1.
Clinicopathological features of 83 tongue squamous cell carcinoma patients
| No. of patients | (%) | |
|---|---|---|
| Gender | ||
| Male | 50 | |
| Female | 33 | |
| Age (y) | ||
| Median | 65.36 | |
| SD | 15.41 | |
| T stage | ||
| Carcinoma in situ (cis) | 5 | 6.0 |
| T1 | 43 | 51.8 |
| T2 | 25 | 30.1 |
| T3 | 9 | 10.8 |
| T4a | 1 | 1.2 |
| T4b | 0 | 0.0 |
| TNM stage | ||
| I | 43 | 51.8 |
| II | 18 | 21.7 |
| III | 5 | 6.0 |
| IVa | 3 | 3.6 |
| IVb | 9 | 10.8 |
| IVc | 0 | 0.0 |
| YK classification | ||
| YK ‐1 | 2 | 2.4 |
| YK‐2 | 10 | 12.0 |
| YK‐3 | 35 | 42.2 |
| YK‐4c | 25 | 30.1 |
| YK‐4d | 6 | 7.2 |
| Differentiation | ||
| Well | 42 | 50.6 |
| Moderate | 29 | 34.9 |
| Poor | 7 | 8.4 |
| Local recurrence | 7 | 8.4 |
| Cervical lymph node metastasis | 27 | 32.5 |
| Distant organ metastasis | 3 | 3.6 |
Table 2.
Relationship between claudin‐1 expression and clinicopathological features of 83 tongue squamous cell carcinoma patients
| No. of patients | |||
|---|---|---|---|
| Claudin‐1 expression | |||
| High | Low | ||
| Gender | |||
| Male | 24 | 26 | |
| Female | 19 | 14 | P = .52 |
| Age (y) | |||
| ≦69 | 20 | 15 | |
| >69 | 23 | 25 | P = .54 |
| T stage | |||
| T1 | 24 | 19 | |
| T2‐4 | 18 | 17 | P = .87 |
| TNM stage | |||
| I | 24 | 19 | |
| I‐IV | 18 | 17 | P = .87 |
| Differentiation | |||
| Well | 24 | 18 | |
| Moderate | 14 | 15 | |
| Poor | 4 | 3 | P = .79 |
| YK classification | |||
| YK ‐1 | 0 | 2 | |
| YK‐2 | 3 | 7 | |
| YK‐3 | 26 | 9 | |
| YK‐4c | 10 | 15 | |
| YK‐4d | 3 | 3 | P = .008 |
| Local recurrence | |||
| Positive | 5 | 2 | |
| Negative | 38 | 38 | P = .43 |
| Cervical lymph node metastasis | |||
| Positive | 17 | 10 | |
| Negative | 26 | 30 | P = .23 |
| Distant organ metastasis | |||
| Positive | 2 | 1 | |
| Negative | 41 | 39 | P = .00 |
3.2. Intracellular localization of claudin‐1 at the invasion front is associated with cervical lymph node metastasis
In some specimens, we noticed a difference in claudin‐1 immunostaining between the center and the invasion front of the lesion, which may be due to an altered localization of claudin‐1 (Figure 2A). Therefore, claudin‐1 immunoreactivity was observed by immunofluorescence using confocal microscopy. Claudin‐1 was predominantly localized at the membrane in the center of the lesion (Figure 2B,C), but intracellular claudin‐1 localization was also observed at the invasion front (Figure 2D,E), as detected by 3‐D images (Videos S1 and S2, respectively). Claudin‐1 is likely localized to intracellular vesicles. Although nuclear localization of claudin‐1 was reported in the case of colon cancer,18 the nucleus was not the main site of localization in the present study. Although intracellular localization of claudin‐1 was observed in some specimens, exclusive membrane localization was maintained at the invasion front in other specimens (Figure 2F‐H and Video S3).
Figure 2.
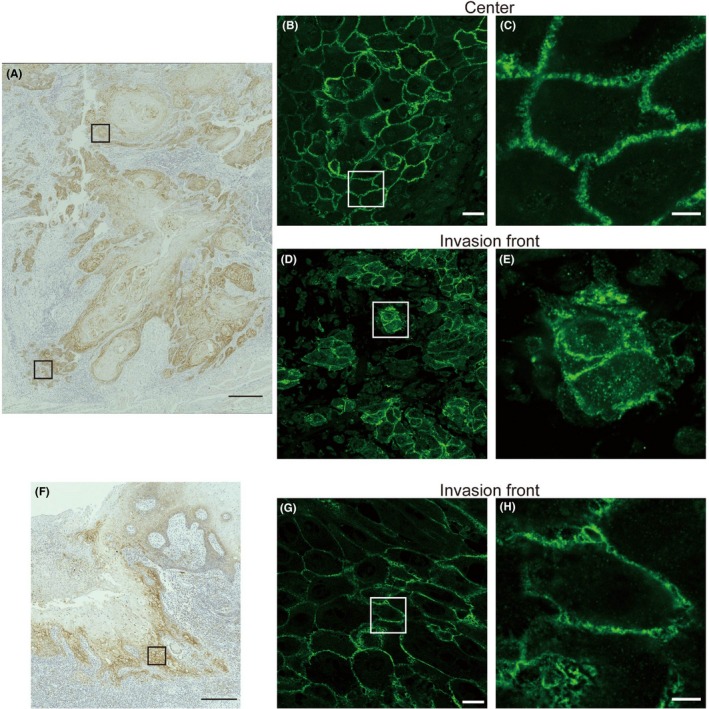
Typical cases of tongue squamous cell carcinoma (TSCC) showing either intracellular (A‐E) or membrane‐confined localization (F‐H) of claudin‐1 at the invasion front. Immunohistochemical (A, F) and immunofluorescence (B‐E, G, H) staining of claudin‐1 in two cases of TSCC. Upper and lower boxed areas in (A) show the center and the invasion front of the lesion, which are magnified in (B) and (D), respectively. The boxed area in (F) shows the invasion front of the lesion in another TSCC, which is magnified in (G). The boxed areas in (B, D, G) are further magnified in (C, E, H), respectively. See also Videos [Link], [Link], [Link], which correspond to (C, E, H), respectively. Scale bars: 300 μm (A, F), 25 μm (B, D, G), and 5 μm (C, E, H)
Thus, we developed a scoring system by which the localization of claudin‐1 at the invasion front was evaluated as either “membrane” (Figure 3) or “intracellular” (Figure 4). Among the cases categorized as having high claudin‐1 expression, the frequency of cervical lymph node metastasis was significantly higher in the “intracellular claudin‐1” group than in the “membrane claudin‐1” group, as shown in Table 3.
Figure 3.
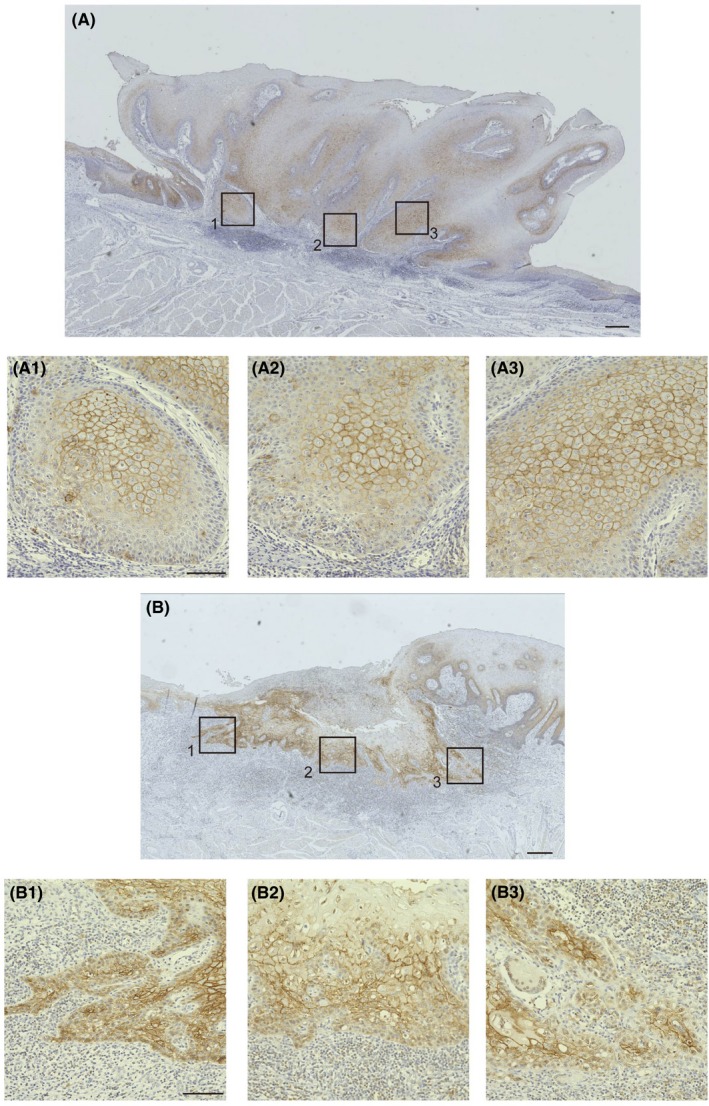
Assessment of tongue squamous cell carcinoma according to membrane localization of claudin‐1 at the invasion front. For assessment of claudin‐1 localization, five square regions were selected from the claudin‐1 positive invasion front for each case. The case was classified as “membrane” when exclusive membrane localization of claudin‐1 was observed based on immunohistochemical staining in more than three regions. This figure shows examples of membrane localization of claudin‐1. Three boxed areas out of five in (A, B) are magnified (A‐1/A‐2/A‐3, B‐1/B‐2/B‐3), respectively, and show the membrane localization of claudin‐1. Scale bars, 300 μm (A, B) and 100 μm (A‐1/A‐2/A‐3, B‐1/B‐2/B‐3). The case shown in Figure 3A is the same as that in Figure 2F‐H
Figure 4.
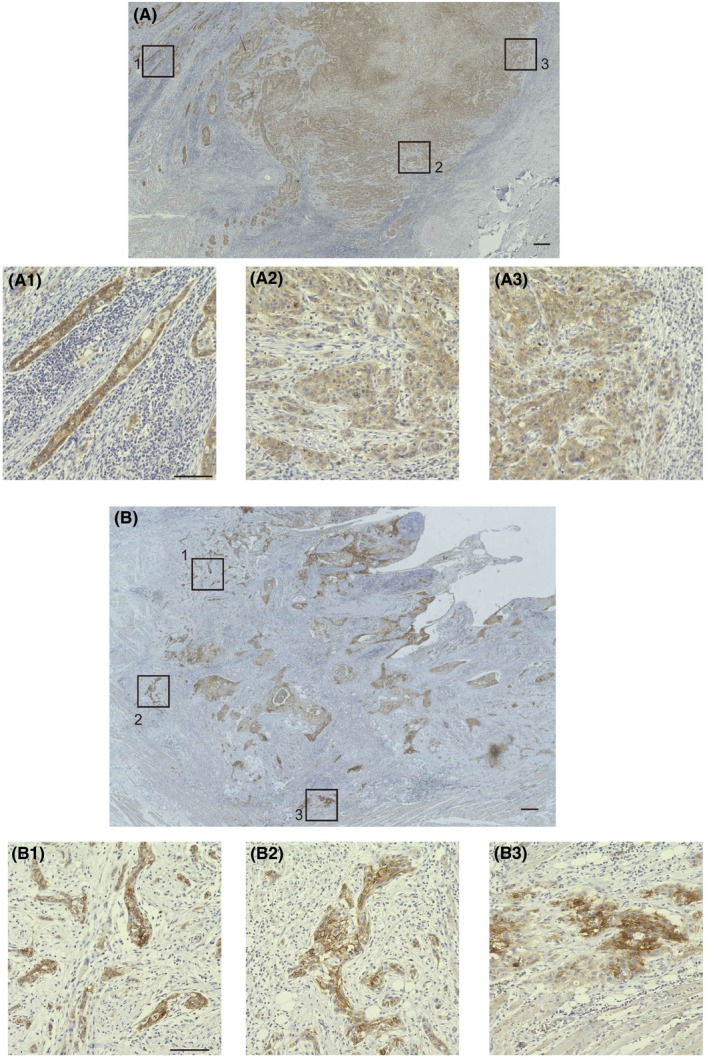
Assessment of tongue squamous cell carcinoma according to intracellular localization of claudin‐1 at the invasion front. This figure shows examples of the intracellular localization of claudin‐1. Three boxed areas out of five in (A, B) are magnified in (A‐1/A‐2/A‐3, B‐1/B‐2/B‐3), respectively, and show the intracellular localization of claudin‐1. Scale bars, 300 μm (A, B) and 100 μm (A‐1/A‐2/A‐3, B‐1/B‐2/B‐3)
Table 3.
Relationship between localization of claudin‐1 expression and clinicopathological features of claudin‐1‐high patients
| No. of patients | |||
|---|---|---|---|
| Intracellular | Membrane | ||
| Differentiation | |||
| Well | 9 | 15 | |
| Moderate | 8 | 6 | |
| Poor | 4 | 0 | P = .06 |
| YK classification | |||
| YK‐1 | 0 | 0 | |
| YK‐2 | 0 | 3 | |
| YK‐3 | 12 | 14 | |
| YK‐4c | 6 | 4 | |
| YK‐4d | 3 | 0 | P = .10 |
| Local recurrence | |||
| Positive | 4 | 1 | |
| Negative | 17 | 20 | P = .34 |
| Cervical lymph node metastasis | |||
| Positive | 15 | 2 | |
| Negative | 6 | 19 | P = .0000929 |
| Distant organ metastasis | |||
| Positive | 2 | 0 | |
| Negative | 19 | 21 | P = .48 |
3.3. Claudin‐1 is constitutively endocytosed in TSCC‐derived cells
SAS cells are derived from TSCC. Using these cells, we investigated the ability of claudin‐1 to be endocytosed. For this purpose, cell‐surface claudin‐1 was biotinylated by a cleavable, cell‐impermeable crosslinker (Figure 5A). After incubation, biotinylated residues remaining on the cell surface were either left or stripped off. The biotinylated claudin‐1 was then recovered with Neutravidin beads and evaluated by immunoblotting. The biotinylated claudin‐1 that remained after stripping was considered to be endocytosed from the cell surface. As shown in Figure 5A,B, a substantial part of the biotinylated claudin‐1 was resistant to stripping, which is a biochemical demonstration of the endocytosis of claudin‐1 in SAS cells. In addition, endocytosis of claudin‐1 in SAS cells was investigated in the presence of an inhibitor of clathrin‐dependent endocytosis, CPZ,32 and an inhibitor of macropinocytosis, IMP.33 Addition of IMP decreased the extent of stripping‐resistant claudin‐1, suggesting that IMP inhibited claudin‐1 endocytosis. Although the addition of CPZ was also found to decrease the extent of stripping‐resistant claudin‐1, the effect was not statistically significant. However, immunofluorescence staining of claudin‐1 in SAS cells revealed that the abundant intracellular staining of claudin‐1 was suppressed in the presence of CPZ or IMP (Figure 5C). These results suggested that claudin‐1 is constitutively endocytosed in TSCC cells. We considered that the loss of membrane location of claudin‐1 is mainly caused by macropinocytosis with some contribution of clathrin‐dependent endocytosis.
Figure 5.
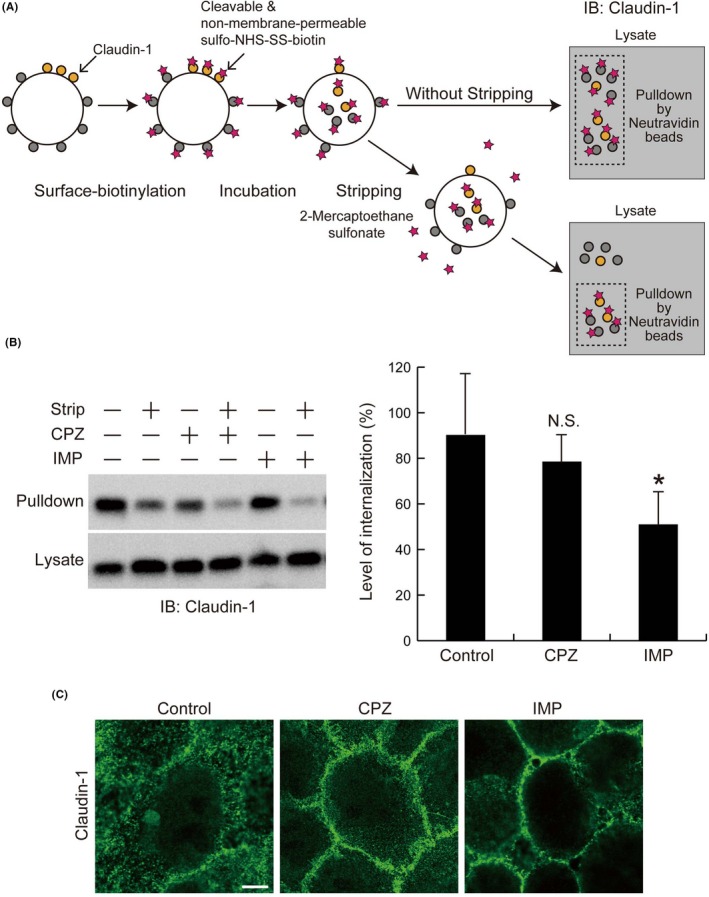
Claudin‐1 is constitutively endocytosed in SAS cells. A, The protocol for evaluating the endocytosis of cell‐surface proteins was described previously.21, 28 Cell‐surface proteins were labeled by sulfo‐NHS‐SS‐biotin. After incubation to allow the endocytosis of cell‐surface molecules, the remaining biotin conjugated to the cell‐surface molecules was either retained (without stripping) or cleaved by reduction (stripping). The cells were then lysed and the biotinylated materials were recovered by Neutravidin beads (Pierce) (pulldown). B, SAS cells were seeded in 35‐mm dishes (2 × 105 cells/dish). The day after cells became confluent, the cells were subjected to surface‐biotinylation assay for evaluation of endocytosis. During the incubation, the cells were treated without or with 10 μM chlorpromazine (CPZ) or 1 μM imipramine (IMP). After the amounts of Neutravidin‐captured (pulldown) claudin‐1 were normalized to their respective values in the lysates, level of internalization was expressed as the ratio between the values with and without stripping, as shown in the graph. Data are expressed as means + SD (n = 4). Student’s t test with two biological independent replicates was used to determine statistical significance; *P < .05; N.S., not significant compared with control. C, SAS cells were seeded on 15‐mm round glass coverslips in 12‐well plates (3 × 104 cells/well). After 72‐96 h, cells were treated without or with 10 μM CPZ or 1 μM IMP for 2 h. The cells were fixed with 4% PFA for 10 min, permeabilized with 0.1% Triton X‐100 for 5 min, and then labeled with an anti‐claudin‐1 antibody. Scale bars, 5 μm
3.4. Inhibition of endocytosis suppresses the motility of SAS cells
The molecular machineries required for clathrin‐dependent endocytosis are reportedly involved in the regulation of migration.22, 23, 34 As constitutive endocytosis of claudin‐1 was shown in SAS cells (Figure 5), we examined the effects of endocytosis inhibitors (CPZ and IMP) on the migration of SAS cells. For this purpose, we used the cell tracking method of recent studies.35, 36, 37 As shown in Figure 6 (Videos [Link], [Link], [Link]), total migrated distance was decreased by the addition of CPZ and IMP, suggesting that inhibition of endocytosis suppresses cell migration.
Figure 6.
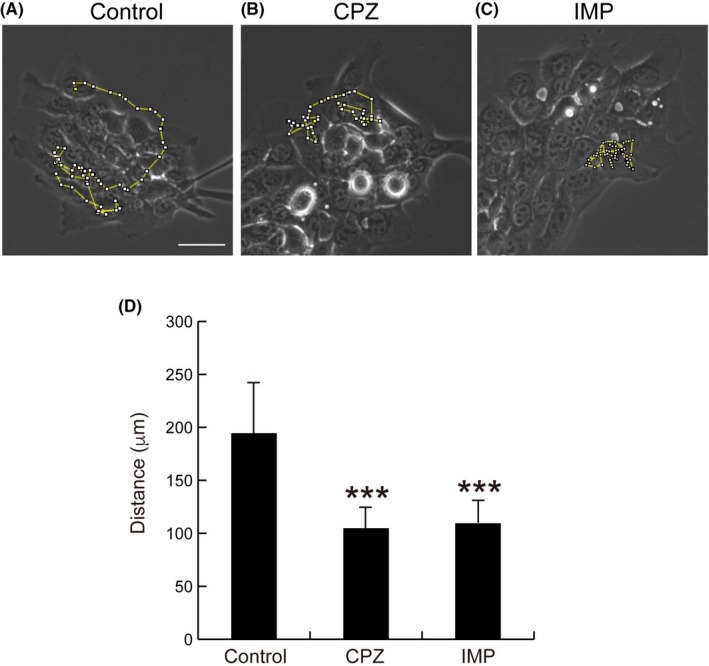
Endocytosis inhibitors chlorpromazine (CPZ) and imipramine (IMP) reduce the motility of SAS cells based on cell motility analysis. SAS cells were seeded in 6‐well plates (2 × 104 cells/well). A‐C, After 48 h, cells were incubated without or with 10 μM CPZ or 1 μM IMP, and cell motility was recorded. Representative random moving traces of 61 cells during a 10‐min recoding period over 10 h are presented. Scale bar, 50 μm. D, Summarized data of the migrated distances within 10 h. Data are expressed as means + SD (n = 10). Student’s t test with two biological independent replicates was used to determine statistical significance; ***P < .001, compared with control
3.5. CRISPR/CAS9‐mediated knockout of claudin‐1 in SAS cells accelerates cell motility
Finally, we investigated whether claudin‐1 is involved in cell motility in SAS cells. For this purpose, claudin‐1‐knockout SAS cells were established by CRISPR/CAS9‐mediated genome editing (Figure 7A). As shown in Figure 7B‐E (Videos [Link], [Link], [Link], respectively), total migrated distance was increased in two independent clones of claudin‐1‐knockout SAS cells. These results suggested that claudin‐1 has a great impact on the migration of SAS cells.
Figure 7.
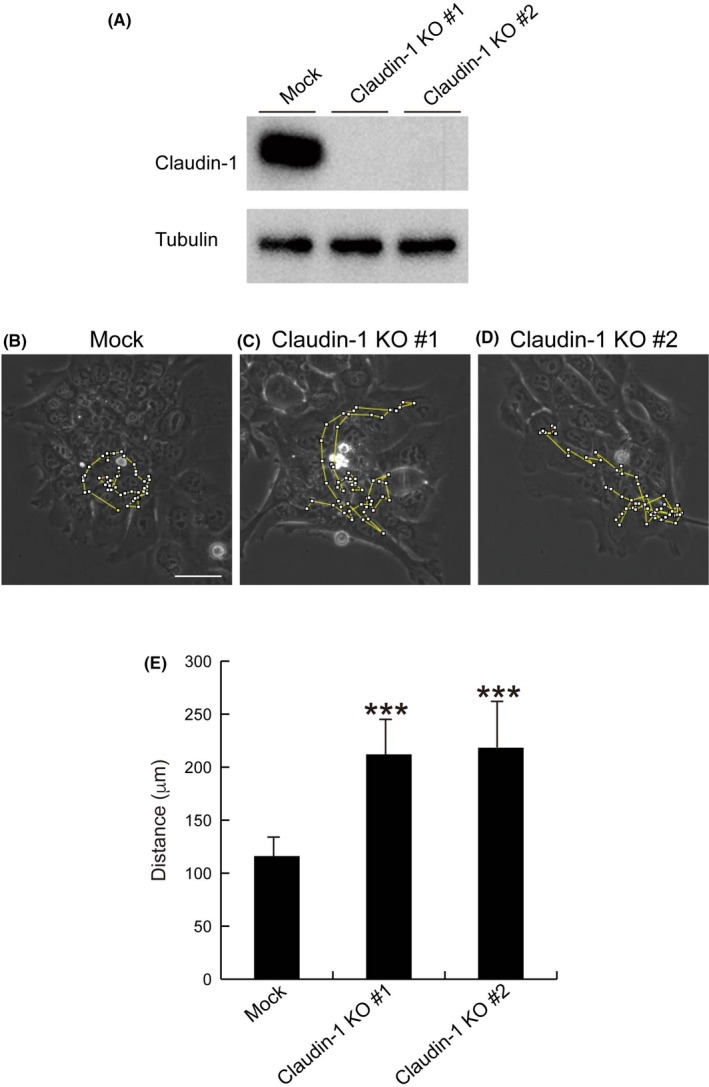
Deficiency of claudin‐1 promotes motility of SAS cells based on cell motility analysis. A, Claudin‐1 expression in mock or claudin‐1‐knockout SAS cells (generated by gRNA #1 and gRNA #2) was determined by immunoblotting. B‐D, Mock or claudin‐1‐knockout SAS cells (generated by gRNA #1 and gRNA #2) were seeded in 6‐well plates (2 × 104 cells/well). After 48 h, cell motility was recorded. Representative random moving traces of 61 cells during a 10‐min recoding period over 10 h are presented. Scale bar, 50 μm. (E) Summarized data of the migrated distances within 10 h. Data are expressed as means + SD (n = 10). Student’s t test with two biological independent replicates was used to determine statistical significance; ***P < .001, compared with control
Both clathrin‐dependent endocytosis and micropinocytosis38 are involved in the regulation of cell migration. Thus, the possibility remained that the effects of CPZ and IMP (Figure 6) are independent of their actions on the endocytosis of claudin‐1. However, considering that deficiency of claudin‐1 promoted cell motility (Figure 7), it can be speculated that the disappearance of claudin‐1 from the membrane by endocytosis promotes cell migration.
4. DISCUSSION
Numerous epithelial‐derived cancers show altered expression patterns of claudins.39 In the present study, the expression level of claudin‐1, as assessed by immunohistochemistry, was not associated with disease progression in 83 TSCC. However, within the high claudin‐1 expression group, the loss of the exclusive membrane localization of claudin‐1 (intracellular localization of claudin‐1) at the invasion front was associated with cervical lymph node metastasis. This result suggested that the intracellular localization of claudin‐1 at the invasion front could be a potential marker of metastasis in TSCC.
Previously, claudin‐1 overexpression was reported to be associated with invasive pathological characteristics in OSCC in two independent studies which analyzed various regions in the oral cavity.27, 40 The areas in one study (total 99 patients) included the tongue (44%), floor of mouth (24%), floor of mouth and tongue (13%), alveoli (13%), buccal mucosa (5%), and retromolar trigone (1%).27 Those of the other study (total 45 patients) included the gingiva (38%), tongue (16%), floor of mouth (18%), buccal mucosa (13%), hard palate (7%), and alveolar mucosa (9%).40 In the present study, we focused on TSCC, which may explain the different conclusions obtained between the previous and present studies. As for our study, considering that the opposite conclusions of poor18, 19 and good11, 16, 17 prognoses have been reported for high expression of claudin‐1 in colon cancer,15 it is possible that opposite types of TSCC coexist.
As the immunoreactivity of claudin‐1 was restricted to the lesion in TSCC, claudin‐1 in the cancer lesion may have a direct effect on invasion, rather than on the surrounding mesenchymal tissue. Expression of claudin‐1 in cancer is regulated in various ways: claudin‐1 is upregulated by β‐catenin/Tcf signaling in human colorectal cancers,19 and epigenetically silenced in estrogen receptor‐positive breast cancer.41 Thus, the expression level of claudin‐1 in cancer cells could be context‐dependent. During epithelial‐mesenchymal transition (EMT), transcription factors Slug and Snail act as repressors of claudin‐1, leading to reduction of the expression of claudin‐1.42 In contrast, claudin‐1 reportedly promotes EMT through activation of the c‐Abl/ERK signaling pathway6 or facilitates invasion by disrupting interaction with the extracellular matrix through MMP.8 In this context, high claudin‐1 expression is predicted to promote invasion. In the present study, there was no relationship between the expression levels of claudin‐1 and metastasis in TSCC patients (Table 2), suggesting that pathological TSCC belonging to “claudin‐1‐low” type and “claudin‐1‐high” type coexisted.
When we focused on the “claudin‐1‐high” type, the frequency of cervical lymph node metastasis was significantly higher in the “intracellular claudin‐1” group than in the “membrane claudin‐1” group (Table 3). The role of claudin‐1 as a component of the tight junction may inhibit the invasion.43 In SAS cells, endocytosis of claudin‐1, as well as cell motility, was hindered by the endocytosis inhibitors (Figures 5 and 6). However, the deficiency of claudin‐1 in SAS cells increased cell motility (Figure 7). Combining these results, it is possible that the endocytosis‐mediated decrease in claudin‐1 on the membrane promotes cell motility. Thus, we propose that the endocytosis of claudin‐1 at the invasion front could relieve the effect of the persistence of tight junctions to inhibit cell motility in the “claudin‐1‐high” type.
We also noticed that membrane localization of claudin‐1 at the invasion front tended to correlate with higher differentiation (Table 3). Cell polarity is an important aspect of cell differentiation. As claudin‐1 is reportedly important in maintaining cell polarity,21 we speculate that membrane localization of claudin‐1 at the invasion front may contribute to maintaining the cell polarity and suppress dedifferentiation.
As tight junction proteins are overexpressed in several types of carcinomas, different approaches using monoclonal antibodies, enterotoxins, and therapeutic gene delivery have been tested as candidates for anticancer drug therapy.43 The present study suggests that a specific inhibitor of the endocytosis of claudin‐1 will be therapeutically valuable to suppress some cases of cancer metastasis, including TSCC.
CONFLICTS OF INTEREST
Authors declare no conflicts of interest for this article.
Supporting information
ACKNOWLEDGMENTS
The authors would like to thank Dr Satoshi Yamaguchi, Ms Miwako Hamagaki, Mr Kazuki Takahashi, Dr Yuta Ikami, and Ms Megumi Naito at Tokyo Medical and Dental University (TMDU) for valuable discussions and great support in carried out the present study. This research was supported by the Project for Promoting Leading‐edge Research in Oral Science at Tokyo Medical and Dental University (TMDU) (to KK, KS, HH, SY, TY, TW, HY) and Grants‐in‐Aid for Scientific Research 17K12005 (to KK, KS, TI, HH, TY, TW, MH).
Yamamoto D, Kayamori K, Sakamoto K, et al. Intracellular claudin‐1 at the invasive front of tongue squamous cell carcinoma is associated with lymph node metastasis. Cancer Sci. 2020;111:700–712. 10.1111/cas.14249
REFERENCES
- 1. Casiglia J, Woo SB. A comprehensive review of oral cancer. Gen Dent. 2001;49:72‐82. [PubMed] [Google Scholar]
- 2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359‐E386. [DOI] [PubMed] [Google Scholar]
- 3. Tamura A, Tsukita S. Paracellular barrier and channel functions of TJ claudins in organizing biological systems: advances in the field of barriology revealed in knockout mice. Semin Cell Dev Biol. 2014;36:177‐185. [DOI] [PubMed] [Google Scholar]
- 4. Furuse M, Hata M, Furuse K, et al. Claudin‐based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin‐1‐deficient mice. J Cell Biol. 2002;156:1099‐1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhat AA, Ahmad R, Uppada SB, Singh AB, Dhawan P. Claudin‐1 promotes TNF‐alpha‐induced epithelial‐mesenchymal transition and migration in colorectal adenocarcinoma cells. Exp Cell Res. 2016;349:119‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suh Y, Yoon C‐H, Kim R‐K, et al. Claudin‐1 induces epithelial‐mesenchymal transition through activation of the c‐Abl‐ERK signaling pathway in human liver cells. Oncogene. 2013;32:4873‐4882. [DOI] [PubMed] [Google Scholar]
- 7. Singh AB, Sharma A, Dhawan P. Claudin‐1 expression confers resistance to anoikis in colon cancer cells in a Src‐dependent manner. Carcinogenesis. 2012;33:2538‐2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oku N, Sasabe E, Ueta E, Yamamoto T, Osaki T. Tight junction protein claudin‐1 enhances the invasive activity of oral squamous cell carcinoma cells by promoting cleavage of laminin‐5 gamma2 chain via matrix metalloproteinase (MMP)‐2 and membrane‐type MMP‐1. Can Res. 2006;66:5251‐5257. [DOI] [PubMed] [Google Scholar]
- 9. Chao Y‐C, Pan S‐H, Yang S‐C, et al. Claudin‐1 is a metastasis suppressor and correlates with clinical outcome in lung adenocarcinoma. Am J Respir Crit Care Med. 2009;179:123‐133. [DOI] [PubMed] [Google Scholar]
- 10. Sheehan GM, Kallakury BV, Sheehan CE, Fisher HA, Kaufman RP Jr, Ross JS. Loss of claudins‐1 and ‐7 and expression of claudins‐3 and ‐4 correlate with prognostic variables in prostatic adenocarcinomas. Hum Pathol. 2007;38:564‐569. [DOI] [PubMed] [Google Scholar]
- 11. Yoshida T, Kinugasa T, Akagi Y, et al. Decreased expression of claudin‐1 in rectal cancer: a factor for recurrence and poor prognosis. Anticancer Res. 2011;31:2517‐2525. [PubMed] [Google Scholar]
- 12. Morohashi S, Kusumi T, Sato F, et al. Decreased expression of claudin‐1 correlates with recurrence status in breast cancer. Int J Mol Med. 2007;20:139‐143. [PubMed] [Google Scholar]
- 13. Ma F, Ding X, Fan Y, et al. A CLDN1‐negative phenotype predicts poor prognosis in triple‐negative breast cancer. PLoS ONE. 2014;9:e112765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Szasz AM, Tokes AM, Micsinai M, et al. Prognostic significance of claudin expression changes in breast cancer with regional lymph node metastasis. Clin Exp Metas. 2011;28:55‐63. [DOI] [PubMed] [Google Scholar]
- 15. Ouban A. Claudin‐1 role in colon cancer: An update and a review. Histol Histopathol. 2018;33:1013‐1019. [DOI] [PubMed] [Google Scholar]
- 16. Shibutani M, Noda E, Maeda K, Nagahara H, Ohtani H, Hirakawa K. Low expression of claudin‐1 and presence of poorly‐differentiated tumor clusters correlate with poor prognosis in colorectal cancer. Anticancer Res. 2013;33:3301‐3306. [PubMed] [Google Scholar]
- 17. Nakagawa S, Miyoshi N, Ishii H, et al. Expression of CLDN1 in colorectal cancer: a novel marker for prognosis. Int J Oncol. 2011;39:791‐796. [DOI] [PubMed] [Google Scholar]
- 18. Dhawan P, Singh AB, Deane NG, et al. Claudin‐1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Investig. 2005;115:1765‐1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miwa N, Furuse M, Tsukita S, Niikawa N, Nakamura Y, Furukawa Y. Involvement of claudin‐1 in the beta‐catenin/Tcf signaling pathway and its frequent upregulation in human colorectal cancers. Oncol Res. 2001;12:469‐476. [DOI] [PubMed] [Google Scholar]
- 20. Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol. 2008;181:683‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dukes JD, Fish L, Richardson JD, et al. Functional ESCRT machinery is required for constitutive recycling of claudin‐1 and maintenance of polarity in vertebrate epithelial cells. Mol Biol Cell. 2011;22:3192‐3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lakoduk AM, Roudot P, Mettlen M, Grossman HM, Schmid SL, Chen PH. Mutant p53 amplifies a dynamin‐1/APPL1 endosome feedback loop that regulates recycling and migration. J Cell Biol. 2019;218:1928‐1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsygankova OM, Keen JH. A unique role for clathrin light chain A in cell spreading and migration. J Cell Sci. 2019;132:jcs224030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koelzer VH, Zlobec I, Lugli A. Tumor budding in colorectal cancer–ready for diagnostic practice? Hum Pathol. 2016;47:4‐19. [DOI] [PubMed] [Google Scholar]
- 25. Yamamoto E, Miyakawa A, Kohama G. Mode of invasion and lymph node metastasis in squamous cell carcinoma of the oral cavity. Head Neck Surg. 1984;6:938‐947. [DOI] [PubMed] [Google Scholar]
- 26. Warner GC, Reis PP, Jurisica I, et al. Molecular classification of oral cancer by cDNA microarrays identifies overexpressed genes correlated with nodal metastasis. Int J Cancer. 2004;110:857‐868. [DOI] [PubMed] [Google Scholar]
- 27. dos Reis PP, Bharadwaj RR, Machado J, et al. Claudin 1 overexpression increases invasion and is associated with aggressive histological features in oral squamous cell carcinoma. Cancer. 2008;113:3169‐3180. [DOI] [PubMed] [Google Scholar]
- 28. Nishimura N, Sasaki T. Cell‐surface biotinylation to study endocytosis and recycling of occludin. Methods Mol Biol. 2008;440:89‐96. [DOI] [PubMed] [Google Scholar]
- 29. Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mashiko D, Fujihara Y, Satouh Y, Miyata H, Isotani A, Ikawa M. Generation of mutant mice by pronuclear injection of circular plasmid expressing Cas9 and single guided RNA. Sci Rep. 2013;3:3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kanda Y. Investigation of the freely available easy‐to‐use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang LH, Rothberg KG, Anderson RG. Mis‐assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123:1107‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin H‐P, Singla B, Ghoshal P, et al. Identification of novel macropinocytosis inhibitors using a rational screen of Food and Drug Administration‐approved drugs. Br J Pharmacol. 2018;175:3640‐3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ezratty EJ, Bertaux C, Marcantonio EE, Gundersen GG. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol. 2009;187:733‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu Y‐L, Li H‐Y, Zhao X‐P, et al. Mesenchymal stem cell‐derived CCN2 promotes the proliferation, migration and invasion of human tongue squamous cell carcinoma cells. Cancer Sci. 2017;108:897‐909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gu M‐M, Gao D, Yao P‐A, et al. p53‐inducible gene 3 promotes cell migration and invasion by activating the FAK/Src pathway in lung adenocarcinoma. Cancer Sci. 2018;109:3783‐3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miyata T, Yamashita YI, Yoshizumi T, et al. CXCL12 expression in intrahepatic cholangiocarcinoma is associated with metastasis and poor prognosis. Cancer Sci. 2019;110:3197‐3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Veltman DM. Drink or drive: competition between macropinocytosis and cell migration. Biochem Soc Trans. 2015;43:129‐132. [DOI] [PubMed] [Google Scholar]
- 39. Tabaries S, Siegel PM. The role of claudins in cancer metastasis. Oncogene. 2017;36:1176‐1190. [DOI] [PubMed] [Google Scholar]
- 40. Sappayatosok K, Phattarataratip E. Overexpression of claudin‐1 is associated with advanced clinical stage and invasive pathologic characteristics of oral squamous cell carcinoma. Head Neck Pathol. 2015;9:173‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Di Cello F, Cope L, Li H, et al. Methylation of the claudin 1 promoter is associated with loss of expression in estrogen receptor positive breast cancer. PLoS ONE. 2013;8:e68630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martinez‐Estrada OM, Culleres A, Soriano FX, et al. The transcription factors Slug and Snail act as repressors of Claudin‐1 expression in epithelial cells. Biochem J. 2006;394:449‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leech AO, Cruz RG, Hill AD, Hopkins AM. Paradigms lost‐an emerging role for over‐expression of tight junction adhesion proteins in cancer pathogenesis. Ann Transl Med. 2015;3:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


