Abstract
Background:
To compare the clinical outcomes of radical hysterectomy (RH) with chemoradiotherapy (CRT) in women with stage IB2-IIA cervical cancer.
Methods:
Based on articles published up to December 2017, a literature search of PubMed, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), and Chinese National Knowledge Infrastructure (CNKI) databases was conducted to identify eligible studies. Overall survival (OS), progression-free survival (PFS) with hazard ratios (HRs), and toxicities with odds ratios (ORs) were analyzed.
Results:
In total, 7 studies comprising 687 patients were identified for this meta-analysis. RH showed a significant trend toward improved survival outcomes compared with those of CRT, regardless of OS (HR = 0.49, 95% confidence interval [CI] 0.36–0.67, P < .001); or PFS (1.61, 95% CI 1.15–2.26, P = .005) for IB2-IIA cervical cancer. Subgroup analysis revealed that stage IB2 cervical cancer patients obtained better OS (HR = 0.36, 95% CI 0.23–0.56, P < .001; heterogeneity: P = .32, I2 = 13%). However, a higher incidence of grade 3/4 genitourinary abnormalities was evident with RH (OR = 2.3, 95% CI 1.42–3.87, P = .021).
Conclusion:
Our study suggested that RH had distinct advantages over CRT for carcinoma of the uterine cervix with FIGO stage IB2-IIA, especially for IB2 cervical cancer.
Keywords: chemoradiation, cervical cancer, radical hysterectomy, meta-analysis
1. Introduction
Despite the increased development of preventative methods such as screening programs and human papillomavirus vaccination, cervical cancer still accounted for an estimated 528,000 new cancer cases and 266,000 fatalities worldwide during 2012.[1] It has become a major challenge across the globe, yet there is controversy regarding the best management options in the treatment of stage IB2-IIA cervical cancer. At present, radical hysterectomy (RH) with or without tailored adjuvant therapy and CRT are the most frequent treatment modalities for affected women. Several studies have demonstrated that both treatments are equally effective for patients with early stage cervical cancer.[2–6] However, some larger scale trials have suggested patients undergoing RH achieved better survival outcomes compared to those undergoing CRT.[2,7] Additionally, there is concern that patients treated with RH plus adjuvant therapy may be at a higher risk of complications compared to CRT. Therefore, it is necessary to clarify which is the most effective treatment modality in stage IB2-IIA cervical cancer.
Consequently, we performed a meta-analysis of all eligible studies to compare the clinical treatment outcomes and toxicities of RH and CRT for stage IB2-IIA cervical cancer patients, with the hope of providing valuable evidence to inform treatment guidelines and suggestions for future trials.
2. Materials and methods
2.1. Literature search strategy
The systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines.[8] The following electronic databases were systematically searched for relevant literature: PubMed, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), and Chinese National Knowledge Infrastructure databases (CNKI). The last search was performed on December 2017, without nationality restrictions. The following terms and their combinations were applied: (chemoradiation OR concomitant radiochemotherapy OR concurrent radiochemotherapy) AND (surgery OR operations OR operative therapy) AND (uterine cervical neoplasms OR cervical cancer OR cancer of cervix). We also searched previous systematic reviews and examined the references which were included in our analysis.
2.2. Selection criteria
Studies were considered eligible for the meta-analysis if they met the following criteria: included untreated patients with histologically proven cervical cancer; involved randomized or nonrandomized controlled clinical trials that compared RH vs CRT in stage IB2-IIA cervical cancer patients, where the CRT group was designed as a control group and the experimental group was treated with RH; time to event data, including overall survival (OS) and progression-free survival (PFS), could be acquired from the paper to calculate hazard ratios (HRs) with a 95% confidence interval (CI); sufficient information should be available to evaluate odds ratios (ORs) with a 95% CI for instances of grade 3/4 adverse events; patients who received neoadjuvant chemotherapy before RH were excluded; and abstracts, conferences, reviews, letters, and case reports were excluded. The eligibility assessment was performed independently in a standardized manner by 2 reviewers. Disagreements between reviews were resolved by discussion or intervention by a 3rd reviewer.
2.3. Data extraction and quality assessment
All eligible studies were assessed and evaluated by 2 investigators working independently. During the data abstraction, a 3rd reviewer was consulted by discussion to reach a consensus when disagreement occurred. We included information regarding the name of the first author, date of publication, country, stage of cancer, number of patients in the CRT group and the RH group, details of radical hysterectomy and primary chemoradiation, follow-up time, and quality of the trials. The endpoints for evaluation were OS and PFS. OS was defined as the time from diagnosis until death, or the latest day the patient was known to be alive. The duration of time to distant metastasis or recurrence was counted from the initiation of treatment to treatment failure. Grade 3/4 complications were also assessed in 2 groups. Additionally, the methodological quality of included retrospective studies was evaluated using the 9-star Newcastle–Ottawa scale.[9] The quality of each retrospective study was scored from 0 to 9, and studies with scores of 6 or above were considered high quality.
2.4. Statistical analysis
The OS and PFS were assessed by HRs and a 95% CI, and the results were pooled using STATA 14 (STATA Corporation, College Station, TX). For each study, the HRs for OS and RFS were extracted directly from the original report. If not available, we obtained the data by reading off Kaplan–Meier survival curves to estimate the HRs of survival or by the indirect method which was suggested by Parmar and colleagues.[10] Additionally, the results of grade 3/4 complications were calculated as ORs and presented with a 95% CI. The Cochrane Q test and I2 statistic were used to assess statistical heterogeneity among trials. If a P-value was <.1 or the I2 >50%, results were reported using a random-effects model. If not, a fixed-effects model was used.
Sensitivity analysis was applied by excluding the trials that potentially biased the results. The Egger test was conducted to assess potential publication bias in the meta-analysis, and a P-value of >.1 was considered to have no potential publication bias.[11,12]
3. Results
3.1. Study selection and characteristics
The baseline characteristics of eligible studies are summarized in Table 1. A total of 1026 records were identified from the databases and references. After excluding 208 duplicate publications, 781 nonrelevant studies were discarded by screening their titles and/or abstracts. Of the 37 full-text articles assessed for eligibility, 4 were abandoned for no matched comparison, 12 for not involving RH modality, and 13 for including patients with III or IV stages of cervical cancer. The flow diagram is presented in Figure 1. Consequently, a total of 7 studies were identified for inclusion in the meta-analysis.[13–19] Of 683 total patients included in our meta-analysis, 411 received RH and 272 received CRT. Most of studies were performed in Korea and all trials were retrospective studies. Four studies[13,17–19] only recruited stage IB2 cervical cancer patients and 3 studies[14–16] included a small fraction of stage IIA patients. The general quality of the 7 studies was evaluated, and 6 were classified as high quality.
Table 1.
Characteristics of the included studies.
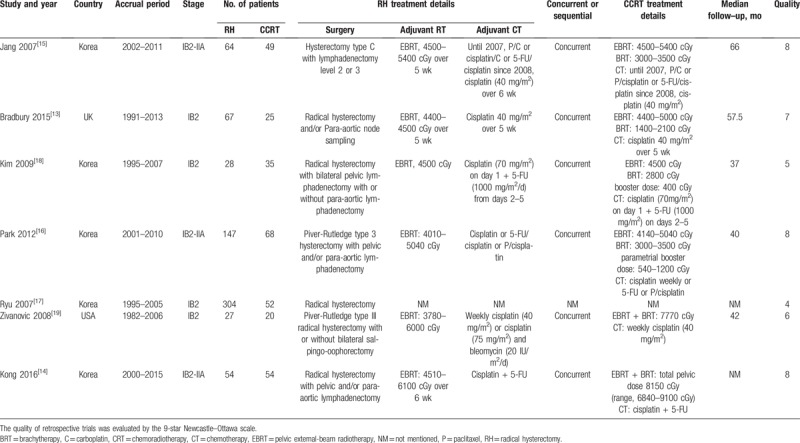
Figure 1.
A flowchart showing the selection procedure of relevant studies in this meta-analysis.
3.2. Survival outcomes
The meta-analysis of OS was based on 7 trials with 683 patients. No obvious heterogeneity was observed among these trials (P = .137, I2 = 38%). Analysis using a fixed-effects model showed that the RH group had improved OS compared with that of the CRT group (HR = 0.49, 95% CI 0.36–0.67, P < .001; Fig. 2A). Six trials with 629 patients reported PFS (Fig. 3). The merge HR was 1.61 (95% CI 1.15–2.26, P = .005; heterogeneity: P = .89, I2 = 0%), indicating that the RH group did demonstrate improved PFS in comparison to the CRT group. Four studies that only included IB2 stage cervical cancer patients reported OS. Figure 2B shows that stage IB2 cervical cancer patients can obtain a better OS (HR = 0.36, 95% CI 0.23–0.56, P < .001; heterogeneity: P = .32, I2 = 13%).
Figure 2.
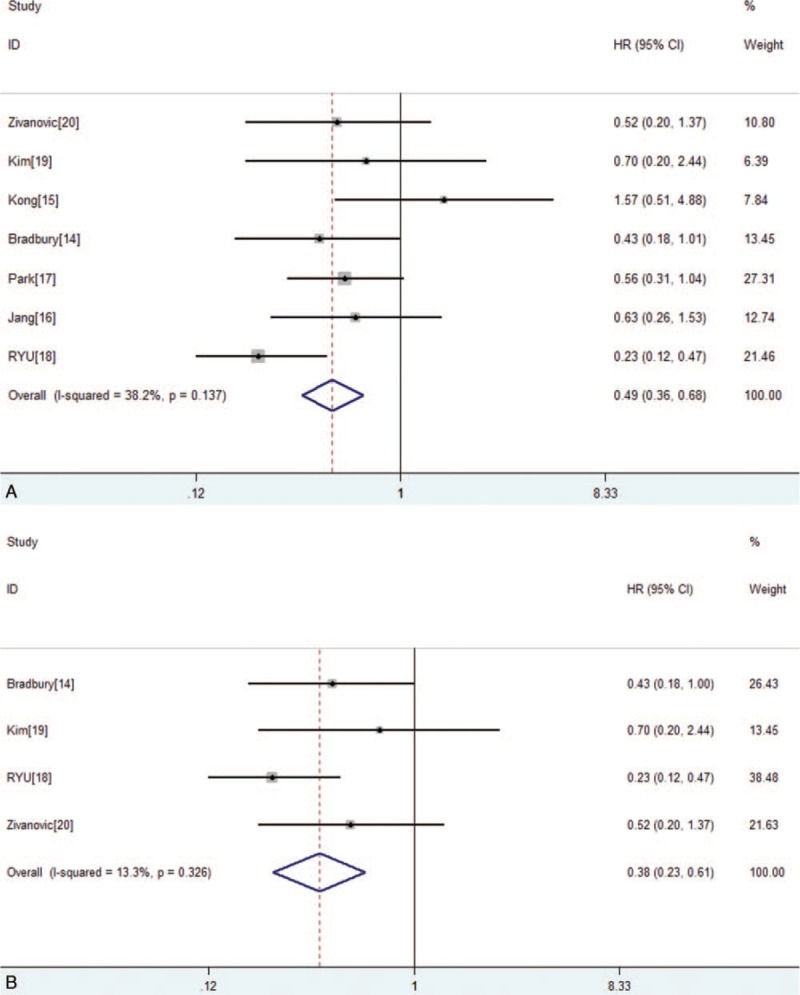
(A) Forest plot of the hazard ratios of overall survival (OS) between the radical hysterectomy (RH) group and chemoradiotherapy (CRT) group in all papers. (B) Forest plot of the hazard ratios of OS between the RH group and CRT group in stage IB2 patients. CI = confidence interval, HR = hazard ratio.
Figure 3.
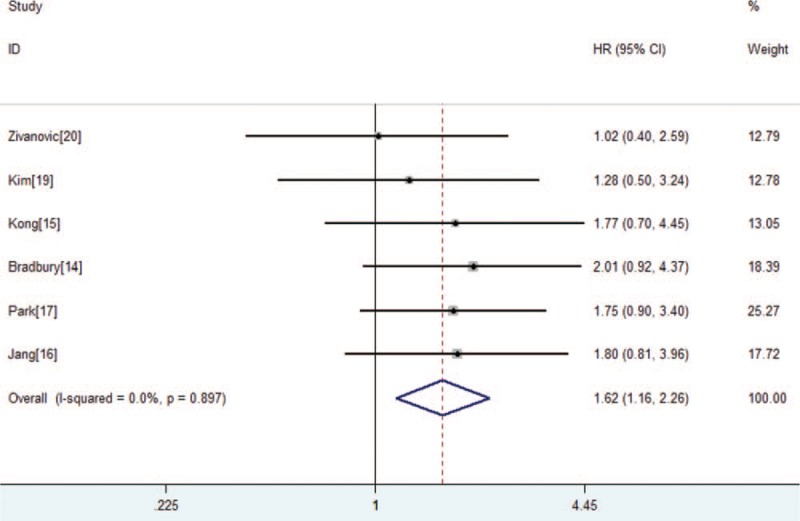
Forest plot of the hazard ratios of progression-free survival between the radical hysterectomy group and chemoradiotherapy group in all papers. CI = confidence interval, HR = hazard ratio.
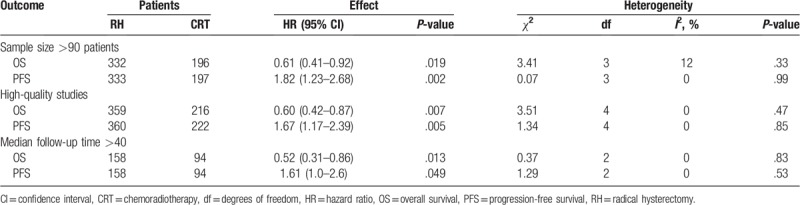
A sensitivity analysis was performed to identify whether the survival results were sharply influenced by certain trials. As showed in Table 2, the survival outcomes remained stable after separately excluding three trials that recruited <90 patients,[17–19] 2 low-quality studies,[17,18] 1 trial without the median follow-up time and enough information about the treatment of RH or CRT group,[17] and 1 trial that enrolled a small number of cervical cancer patients and had a median follow-up time of <40 months.[18] As we all know, the weight assigned to each study was influenced by the number of patients. One trial[16] received the largest weight in this analysis. After we removed this largest study and analyzed a combined HR estimate from the remaining papers, the combined HRs for OS and PFS were 0.47 (95% CI 0.32–0.68, P < .001) and 1.57 (95% CI 1.07–2.32, P = .021), suggesting a similar result as the main meta-analysis. Generally speaking, the survival results of RH vs CRT were of high stability. We performed Egger tests and no obvious publication bias was observed in OS or PFS (Egger test, P = .197, .196).
Table 2.
Sensitivity analysis for the comparison of RH group with CRT group.
3.2.1. Grade 3/4 complication
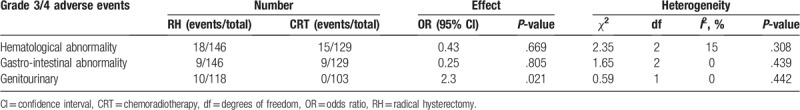
The grade 3/4 adverse events that were available for pooled analysis are presented in Table 3. No significant difference was observed between the 2 arms in terms of the incidence of hematologic abnormality (OR = 0.43, 95% CI 0.56–2.45, P = .669) or gastrointestinal abnormality (OR = 0.25, 95% CI 0.33–2.33, P = .805). However, compared with CRT, RH notably increased the risk of genitourinary abnormality (OR = 2.3, 95% CI 1.42–3.87, P = .021).
Table 3.
Grade 3/4 adverse events of RH vs CRT for stage IB2-IIA cervical cancer.
4. Discussion
At present, the 1st strategy for FIGO stage IB2 and IIA cervical cancer has been CRT, which is classified as category 1 level of evidence according to the NCCN guidelines.[20,21] However, surgery has also been proven a highly effective method for early stage disease.[22–25] In our systematic review and meta-analysis, we compared the survival outcomes of primary radical hysterectomy and chemoradiation. The results reported that the RH group obtained better survival conditions for stage IB2-IIA cervical cancer, especially for IB2 patients.
Several large-scaled studies have revealed that RH facilitated improved rates of survival compared to CRT in early stage patients with bulky disease. One study demonstrated a 49% improvement in survival with an RH group, consistent with our meta-analysis.[2,3,17] There are several possible explanations for the significant difference in survival. First, a bulky and especially barrel-shaped disease has a poor radiation dose distribution, which may lead to increased local failure and decreased survival outcomes.[26,27] However, surgery can remove the part of the disease which is too far from the radium system to receive an effective dose. Therefore, women with this type of tumor may benefit from radical hysterectomy.[20,28] Next, surgery can confidently permit the status of lymph node and parametrial invasion, the most dependent factor associated with survival outcomes. A retrospective study[16] reported that lymph node failure rate was higher in the CRT group compared to the RH group, although it was not statistically significant (14.7% vs 8.2%). Delpech et al[29] observed a high rate of positive para-aortic nodes (18%) in patients with stage IB2/II cervical cancer. All these data were in good agreement. In addition, several studies[30,31] reported that performing a pelvic lymphadenectomy along with removal of the primary disease could reduce the rate of lymph node recurrence in patients with a bulky, early stage disease. This may be the reason for better PFS in the RH group. Our subgroup analysis found that stage IB2 cervical cancer patients can achieve better OS from radical surgery with tailored therapy. In our study, at least half of stage IB2 patients received adjuvant to promote local control. The greatest concern for bulky stage IB2 cervical cancer is poor local control rate; therefore, radical surgery with adjuvant therapy is strongly recommended compared to chemoradiation.
There is concern that patients treated with radical hysterectomy, followed by adjuvant therapy, may be at higher risk of complication incidences. However, the pooled analysis showed no significant difference between the 2 arms regarding the incidence of grade 3/4 toxicity reactions, except for genitourinary abnormalities (OR = 2.3, 95% CI 1.42–3.87, P = .021). A retrospective study conducted by Park et al[16] found that the incidence of grade 3/4 early complications is similar between RH with radiation therapy and the CRT group. More interestingly, they also suggest that the rate of grade 3/4 late complications is lower in the RH group. In addition, several recent trials[32,33] have reported similar results in terms of grade 3/4 complications. However, considering the genitourinary complications, the management of RH should be considered with caution.
This systematic review and meta-analysis had several limitations. First, the included trials were retrospective, which made biases inevitable. Second, our meta-analysis evaluated the differences in survival outcomes between RH and CRT. We must admit that the RH group had a lower risk for recurrence, which may have influenced our final conclusions. Third, the included studies were performed in Korea, which may be attributed to the epidemiologic characteristics of cervical cancer. Undeniably, the generalization of our conclusions must be carefully considered. Despite these drawbacks, this meta-analysis may provide some significant guidance and reference in identifying the optimal treatment strategies for stage IB2-IIA cervical cancer patients.
5. Conclusion
This was the 1st meta-analysis to provide conservative estimates of the clinical treatment effectiveness of radical hysterectomy compared with chemoradiation, in bulky early stage cervical cancer patients. Our study revealed that the RH group had significant superiority over the CRT group among the IB2-IIA patients, especially for IB2 cervical cancer. However, considering the evidence of genitourinary complications, the management of RH should proceed with caution. Prospective, randomized controlled clinical trials with large sample sizes are still required.
Author contributions
Conceptualization: Han-lin Gong, Lei Liu.
Formal analysis: Ruo-nan Yan.
Methodology: Yuan-yuan Zeng.
Software: Tao He, Zhong-zheng Xiang, Bai-lu Zhang.
Supervision: Fang Liu.
Writing – original draft: Ruo-nan Yan, Zhen Zeng.
Writing – review & editing: Fang Liu, Han-lin Gong, Lei Liu.
Footnotes
Abbreviations: BRT = brachytherapy, CRT = chemoradiotherapy, EBRT = pelvic external-beam radiotherapy, HR = hazard ratio, OR = odds ratio, RH = radical hysterectomy.
How to cite this article: Yan Rn, Zeng Z, Liu F, Zeng Yy, He T, Xiang Zz, Zhang Bl, Gong Hl, Liu L. Primary radical hysterectomy vs chemoradiation for IB2-IIA cervical cancer: a systematic review and meta-analysis. Medicine. 2020;99:5(e18738).
RnY and ZZ contributed equally to the work.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Cervical cancer. Estimated incidence, mortality and prevalence worldwide in 2012. Accessed March 18, 2015. [Google Scholar]
- [2].Rungruang B, Courtney-Brooks M, Beriwal S, et al. Surgery versus radiation therapy for stage IB2 cervical carcinoma: a populationbased analysis. Int J Gynecol Cancer 2012;22:484–9. [DOI] [PubMed] [Google Scholar]
- [3].Jewell EL, Kulasingam S, Myers ER, et al. Primary surgery versus chemoradiation in the treatment of IB2 cervical carcinoma: a cost effectiveness analysis. Gynecol Oncol 2007;107:532–40. [DOI] [PubMed] [Google Scholar]
- [4].Havrilesky LJ, Leath CA, Huh W, et al. Radical hysterectomy and pelvic lymphadenectomy for stage IB2 cervical cancer. Gynecol Oncol 2004;93:429–34. [DOI] [PubMed] [Google Scholar]
- [5].Landoni F, Maneo A, Colombo A, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 1997;350:535–40. [DOI] [PubMed] [Google Scholar]
- [6].Keys HM, Bundy BN, Stehman FB, et al. Radiation therapy with and without extrafascial hysterectomy for bulky stage IB cervical carcinoma: a randomized trial of the Gynecologic Oncology Group. [DOI] [PubMed] [Google Scholar]
- [7].Bansal N, Herzog TJ, Shaw RE, et al. Primary therapy for early-stage cervical cancer: radical hysterectomy vs radiation. Am J Obstet Gynecol 2009;201:485.e1–9. [DOI] [PubMed] [Google Scholar]
- [8].L A, A DG, T J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700 Epidemiology Biostatistics & Public Health. 2009; 6(4):e1-e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Erin K, Greenleaf MD, Afif N, et al. Timing of adjuvant chemotherapy and impact on survival for resected gastric cancer. Ann Surg Oncol 2016;23:4203–13. [DOI] [PubMed] [Google Scholar]
- [10].Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815–34. [DOI] [PubMed] [Google Scholar]
- [11].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [12].Schnee S, Enoch M, Noriega-Crespo A, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bradbury M, Founta C, Taylor W, et al. Pathological risk factors and outcomes in women with stage IB2 cervical cancer treated with primary radical surgery versus chemoradiotherapy. Int J Gynecol Cancer 2015;25:1476–83. [DOI] [PubMed] [Google Scholar]
- [14].Kong TW, Lee JD, Son JH, et al. Treatment outcomes in patients with FIGO stage IB–IIA cervical cancer and a focally disrupted cervical stromal ring on magnetic resonance imaging: a propensity score matching study. YGYNO-976395; No. of pages: 6; 4C [DOI] [PubMed] [Google Scholar]
- [15].Jang TK, Shin SJ, Chung H, et al. A retrospective comparison of outcome in IB2 and IIA cervical cancer patients treated with primary concurrent chemoradiation versus radical hysterectomy with or without tailored adjuvant therapy. Obstet Gynecol Sci 2017;60:549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Park JY, Kim DY, Kim JH, et al. Comparison of outcomes between radical hysterectomy followed by tailored adjuvant therapy versus primary chemoradiation therapy in IB2 and IIA2 cervical cancer. J Gynecol Oncol 2012;23:226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ryu HS, Kang SB, Kim KT, et al. Efficacy of different types of treatment in FIGO stage IB2 cervical cancer in Korea: results of a multicenter retrospective Korean study (KGOG-1005). Int J Gynecol Cancer 2007;17:132–6. [DOI] [PubMed] [Google Scholar]
- [18].Kim WY, Chang SJ, Changa KH, et al. Treatment patterns and outcomes in bulky stage IB2 cervical cancer patients: a single institution's experience over 14 years. Gynecol Obstet Invest 2011;71:19–23. [DOI] [PubMed] [Google Scholar]
- [19].Zivanovic O, Alektiar KM, Sonoda Y, et al. Treatment patterns of FIGO Stage IB2 cervical cancer: a single-institution experience of radical hysterectomy with individualized postoperative therapy and definitive radiation therapy. Gynecol Oncol 2008;111:265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med 1999;340:1154–61. [DOI] [PubMed] [Google Scholar]
- [21].Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med 1999;340:1137–43. [DOI] [PubMed] [Google Scholar]
- [22].Walji N, Chue AL, Yap C, et al. Is there a role for adjuvant hysterectomy after suboptimal concurrent chemoradiation in cervical carcinoma? Clin Oncol (R Coll Radiol) 2010;22:140–6. [DOI] [PubMed] [Google Scholar]
- [23].Henry M, Keys MD, Brian N, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med 1999;340:1154–61. [DOI] [PubMed] [Google Scholar]
- [24].Gallion HH, van Nagell JR, Jr, Donaldson ES, et al. Combined radiation therapy and extra-fascial hysterectomy in the treatment of stage IB barrel-shaped cervical cancer. Cancer 1985;56:262–5. [DOI] [PubMed] [Google Scholar]
- [25].Perez CA, Breaux S, Askin F, et al. Irradiation alone or in combination with surgery in stage IB and IIA carcinoma of the uterine cervix: a nonrandomized comparison. Cancer 1979;43:1062–72. [DOI] [PubMed] [Google Scholar]
- [26].Nelson AJ, III, Fletcher GH, Wharton JT. Indications for adjunctive conservative extrafascial hysterectomy in selected cases of carcinoma of the uterine cervix. Am J Roentgenol Radium Ther Nucl Med 1975;123:91–9. [DOI] [PubMed] [Google Scholar]
- [27].Beadle BM, Jhingran A, Yom SS, et al. Patterns of regional recurrence after definitive radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys 2010;76:1396–403. [DOI] [PubMed] [Google Scholar]
- [28].Eifel PJ, Morris M, Wharton JT, et al. The influence of tumor size and morphology on the outcome of patients with FIGO stage IB squamous cell carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys 1994;29:9–16. [DOI] [PubMed] [Google Scholar]
- [29].Delpech Y, Haie-Meder C, Rey A, et al. Para-aortic involvement and interest of paraaortic lymphadenectomy after chemoradiation therapy in patients with stage IB2 and II cervical carcinoma radiologically confined to the pelvic cavity. Ann Surg Oncol 2007;14:3223–31. [DOI] [PubMed] [Google Scholar]
- [30].Ferrandina G, Distefano M, Ludovisi M, et al. Lymph node involvement in locally advanced cervical cancer patients administered preoperative chemoradiation versus chemotherapy. Ann Surg Oncol 2007;14:1129–35. [DOI] [PubMed] [Google Scholar]
- [31].Houvenaeghel G, Lelievre L, Rigouard AL, et al. Residual pelvic lymph node involvement after concomitant chemoradiation for locally advanced cervical cancer. Gynecol Oncol 2006;102:74–9. [DOI] [PubMed] [Google Scholar]
- [32].Gruen A, Musik T, Köhler C, et al. Adjuvant chemoradiation after laparoscopically assisted vaginal radical hysterectomy (LARVH) in patients with cervical cancer: oncologic outcome and morbidity. Strahlenther Onkol 2011;187:344–9. [DOI] [PubMed] [Google Scholar]
- [33].Sedlis A, Bundy BN, Rotman MZ, et al. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group Study. Gynecol Oncol 1999;73:177–83. [DOI] [PubMed] [Google Scholar]


