Abstract
Background.
The most established metric for estimating graft survival from donor characteristics in liver transplantation is the liver donor risk index (LDRI). The LDRI is calculated from donor and transplant-related variables, including cold ischemic time. Because cold ischemic time is unknown at the time of organ offer, LDRI is not available for organ acceptance decisions. In contrast, the kidney donor profile index (KDPI) is derived purely from donor variables known at the time of offer and thus calculated for every deceased donor in the United States. The similarity in donor factors included in LDRI and KDPI led us to hypothesize that KDPI would reliably approximate LDRI in estimating graft survival in liver transplantation.
Methods.
The United Network of Organ Sharing registry was queried for adults who underwent deceased donor liver transplantation from 2002 to 2016. The cohort was divided into quintiles of KDPI and LDRI, and graft survival was calculated according to Kaplan Meier. Hazard ratios for LDRI and KDPI were estimated from Cox proportional hazards models, and Uno’s concordance statistic was compared.
Results.
In our analysis of 63 906 cases, KDPI closely approximated LDRI in estimating liver graft survival, with an equivalent concordance statistic of 0.56.
Conclusions.
We conclude that KDPI can serve as a reasonable alternative to LDRI in liver acceptance decisions.
In the current era, the major obstacle facing the field of liver transplantation is the severe shortage of donor organs. In response to this shortage, transplant programs are increasingly evaluating and utilizing organs from extended criteria donors, including donors of advanced age, donors with hepatic steatosis, and donors after circulatory death.1–5 As such, accurate estimation of donor risk in liver transplantation has never been more important. The most established metric for estimating graft survival from donor characteristics in liver transplantation is the liver donor risk index (LDRI), introduced by Fang et al6 in 2006. Calculated from donor characteristics (age, race, height, cause of death, and donation after circulatory death [DCD] status) and transplant factors (local/regional/national share, and cold ischemic time [CIT]), the LDRI represents a hazard ratio for graft failure in reference to a standard reference donor. Although the LDRI has provided a valuable framework for quantifying graft quality, it is not available in real time for organ acceptance decisions due to its inclusion of CIT. In particular, LDRI is not presented in United Network of Organ Sharing (UNOS) Donor Net, the online platform used for donor evaluation and organ acceptance in the United States.
In kidney transplantation, the kidney donor risk index (KDRI) was introduced by Rao et al7 to estimate donor risk. In contrast to LDRI, the KDRI does not include CIT and is based purely on donor factors known at the time of organ offer (Table 1). To make the KDRI score easier to conceptualize, it is routinely transformed to a percentile score ranging from 0% to 100%, known as the kidney donor profile index (KDPI). With this transformation, the KDPI represents the percentile of quality of a particular donor relative to all kidney donors recovered during the previous year. In contrast to LDRI, the KDPI is routinely presented in UNOS Donor Net for every deceased donor at the time of organ offer.
TABLE 1.
Donor and transplant characteristics used in the calculation of KDPI and LDRI
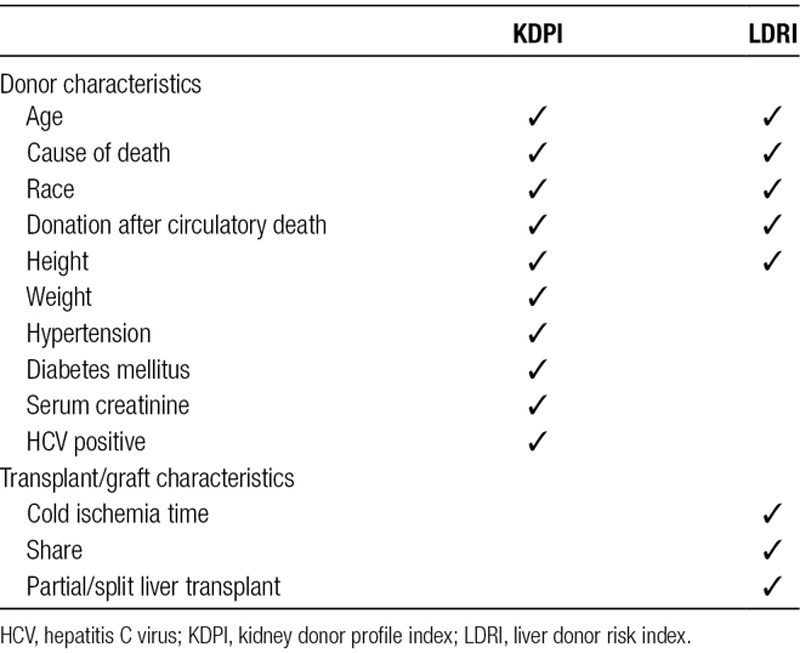
As demonstrated in Table 1, there is an overlap of several donor factors used in the calculation of LDRI and KDPI, including age, height, race, cause of death, and DCD status.8 Due to this considerable similarity, we hypothesized that KDPI would serve as a reasonable alternative to LDRI in quantifying graft quality in liver transplantation. Thus, the primary aim of this study was to compare KDPI and LDRI in estimating liver graft survival in the United States in an era of Model of End-Stage Liver Disease (MELD)-based liver allocation. A secondary aim of the study was to determine how using an assumed CIT of 8 hours would impact the LDRI calculation, which has the potential to enhance the utility of LDRI in organ acceptance decisions.
MATERIALS AND METHODS
Data Source
The Duke University Institutional Review Board granted exempt status for this retrospective analysis of the UNOS/OPTN Standard Transplant Analysis Research file. KDPI scores were obtained directly from the database, and LDRI scores were calculated as described by Fang et al6 when all data elements were available for analysis.
Study Design
All adult recipients (≥18 y of age) who underwent initial deceased donor liver transplantation between February 27, 2002, and March 31, 2016, were included in the analysis. The year 2002 was selected as the starting point for our analysis due to the introduction of MELD-based liver allocation in the United States. Patients undergoing multiorgan transplants, retransplants, and machine preservation of donor livers and those with missing data were excluded from the study.
Outcome
Graft survival was calculated as the time from initial transplant to graft failure, retransplant, or all-cause death. If a recipient was alive or lost to follow-up at time of last contact, then survival time was censored at time of last contact.
Statistical Analysis
Donor characteristics included in the calculation of LDRI were age, race, height, cause of death, DCD status, graft type (split versus whole), organ origin (local versus regional versus national share), and CIT. Because CIT is unknown at the time of organ offer, LDRI was calculated in 2 ways: (1) using the actual CIT and (2) using an assumed CIT of 8 hours. This comparison was made to determine whether using an assumed value of 8 hours for CIT would change the LDRI value substantially. If the 2 versions of LDRI closely agree, there would be rationale to calculate and present LDRI with an assumed CIT of 8 hours in UNOS DonorNet at the time of organ offer. KDPI was recorded for each donor. Data elements are summarized using mean and SD or frequency and percentage. LDRI takes on values ≥0 and KDPI ranges from 0 to 1.
First, a scatterplot depicting KDPI and LDRI for each subject was generated to allow visualization of the relationship between the 2 scores, and linear correlation was estimated using Pearson’s correlation. To assess graft survival as a function of KDPI and LDRI, the study cohort was divided into quintiles for each index and Kaplan-Meier estimates were calculated. Uno’s concordance statistic,9 as implemented by PROC PHREG, was computed to estimate the predictive ability of these indices in estimating donor risk. Concordance statistics range between 0 and 1 with values closer to 0.5 indicating poor concordance and values closer to 1 indicating strong concordance. Receiver operating characteristic (ROC) curves and area under the curve were approximated using a logistic regression model. P < 0.05 was regarded as significant. All analyses were performed in R version 3.5.0 (Vienna, Austria) and SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Study Population
The final study population consisted of 63 906 patients who met inclusion criteria. We excluded 31 patients with no survival time, and 3737 cases with missing data needed to calculate either KDPI or LDRI. Baseline donor and transplant characteristics of the study are displayed in Table 2. The mean LDRI for the cohort was 1.4 (SD = 0.4), while the mean KDPI was 0.5 (SD = 0.3). A scatterplot demonstrating KDPI and LDRI for each subject in the study is shown in Figure 1. Pearson’s correlation coefficient is 0.74, indicating a strong correlation between KDPI and LDRI.
TABLE 2.
Donor and transplant characteristics of the study population
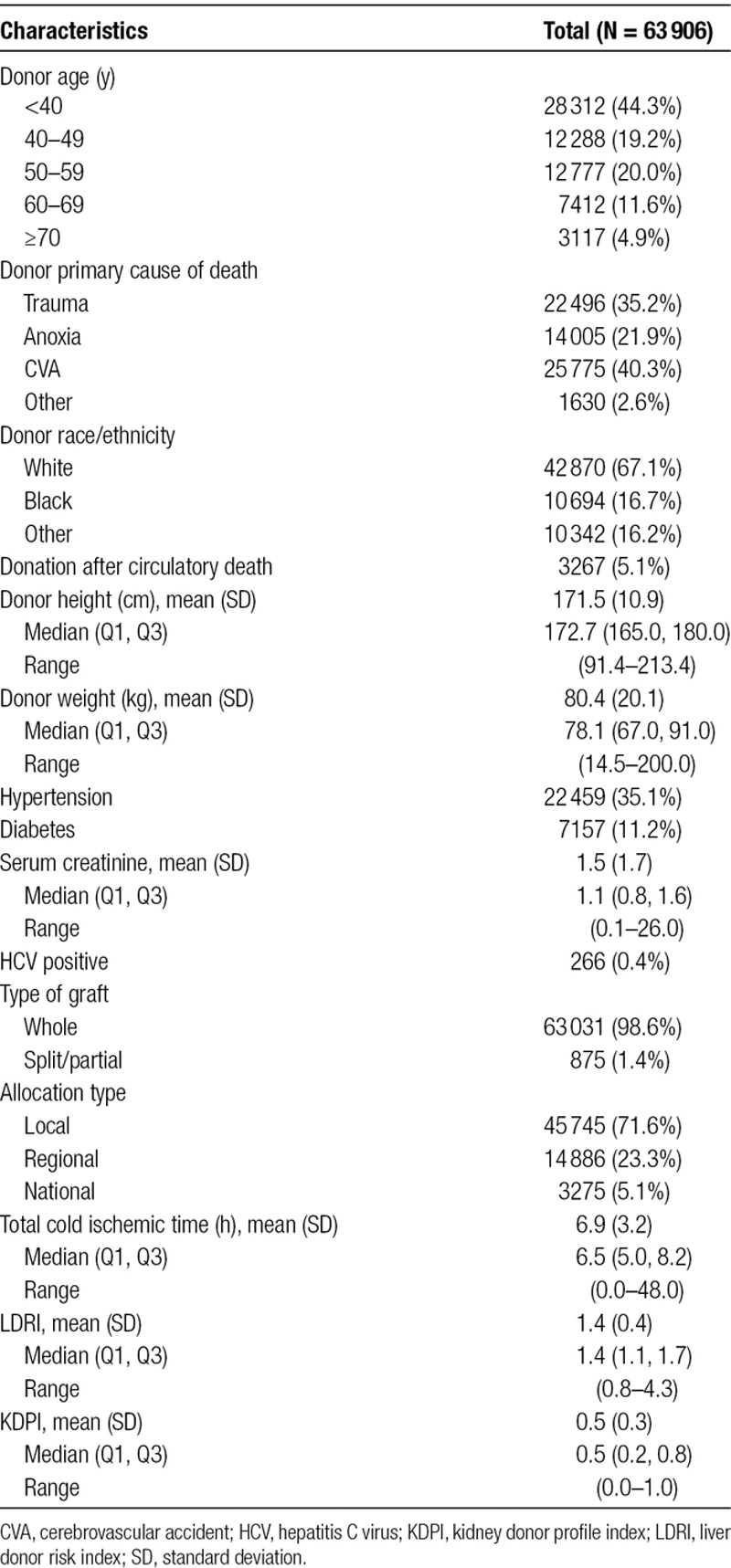
FIGURE 1.
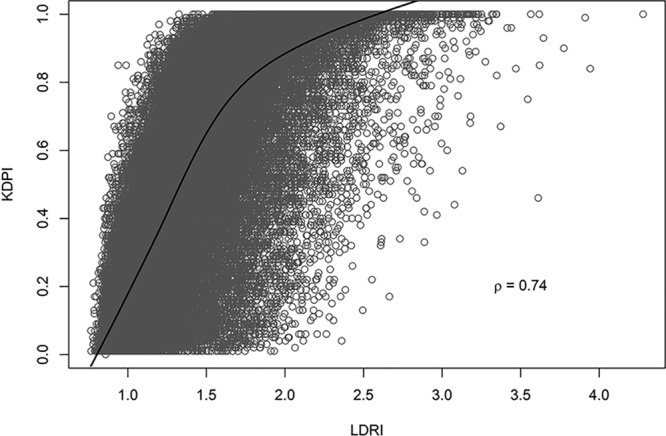
Scatterplot demonstrating KDPI and LDRI for each study subject. A strong correlation is observed between the 2 scores, with a Pearson correlation coefficient of 0.74. KDPI, kidney donor profile index; LDRI, liver donor risk index.
Comparison of Long-term Graft Survival by Quintiles of KDPI and LDRI
Graft survival for the entire cohort at 1, 5, and 10 was 87.2%, 76.2%, and 71.2%, respectively. Kaplan-Meier curves demonstrated that graft survival was strongly associated with quintile of donor quality for both KDPI and LDRI (Figure 2). One-, 5-, and 10-year graft survival was similar for KDPI and LDRI by quintile. For example, 1-year graft survival by quintile for KDPI versus LDRI was 90% versus 91% (quintile 1), 89% versus 89% (quintile 2), 87% versus 87% (quintile 3), 85% versus 85% (quintile 4), and 82% versus 82% (quintile 5).
FIGURE 2.
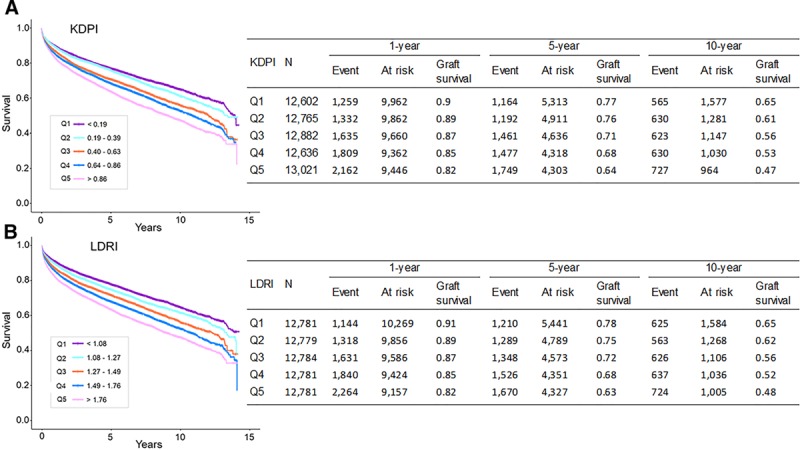
Graft survival as a function of KDPI and LDRI. Kaplan-Meier curves of graft survival by quintiles of (A) KDPI and (B) LDRI. Graft survival at 1, 5, and 10 years for quintiles of KDPI closely approximates graft survival for quintiles of LDRI. KDPI, kidney donor profile index; LDRI, liver donor risk index.
Hazard Ratios for Graft Failure by KDPI and LDRI
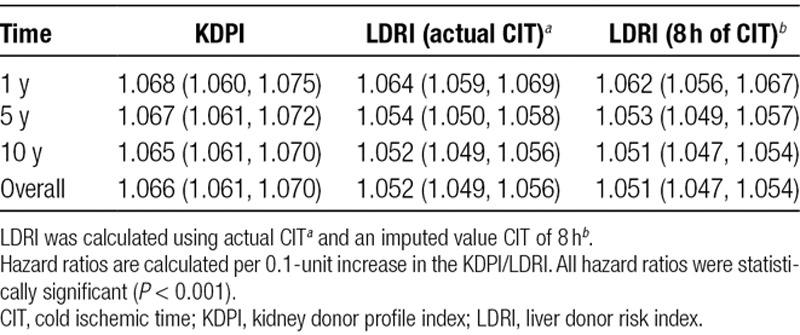
Hazard ratios for graft failure at 1, 5, and 10 years per 0.1 unit increase in KDPI and LDRI are shown in Table 3. For KDPI and both versions of LDRI (using actual CIT and imputing 8 h of CIT), each 0.1 unit increase in score was significantly associated with an increased risk of graft failure at 1, 5, and 10 years. There did not appear to be a substantial change in LDRI hazard ratios when imputing 8 hours of CIT in the formula instead of the actual CIT.
TABLE 3.
Hazard ratios and 95% confidence intervals for KDPI and LDRI for 1-, 5-, and 10-year graft failure
Concordance Statistics and ROC Curves
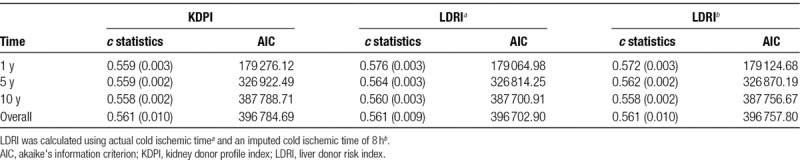
The ability of each score to discriminate between graft failure and survival was then analyzed by calculating the respective concordance statistics (c statistics), shown in Table 4. Due to the inclusion of only donor/transplant factors (and exclusion of recipient characteristics), both KDPI and LDRI demonstrate similarly modest c statistics (0.56–0.58) for graft survival at 1, 5, and 10 years. Again, using an imputed 8 hours of CIT for LDRI did not appear to change the c statistic substantially in comparison to actual LDRI.
TABLE 4.
Concordance statistics (SE) and AIC for KDPI and LDRI for 1-, 5-, and 10-year graft survival
Uno’s statistic, which was used to calculate concordance while accounting for censoring, does not allow for direct generation of an ROC curve. For this reason, ROC curves were based on logistic regression for graft survival (Figure 3). The area under the curve was 0.56 for KDPI versus 0.57 for LDRI, indicating similar performance of both scores. It is noteworthy that these results were similar to the c statistics for graft survival presented in Table 4, which did account for censoring.
FIGURE 3.
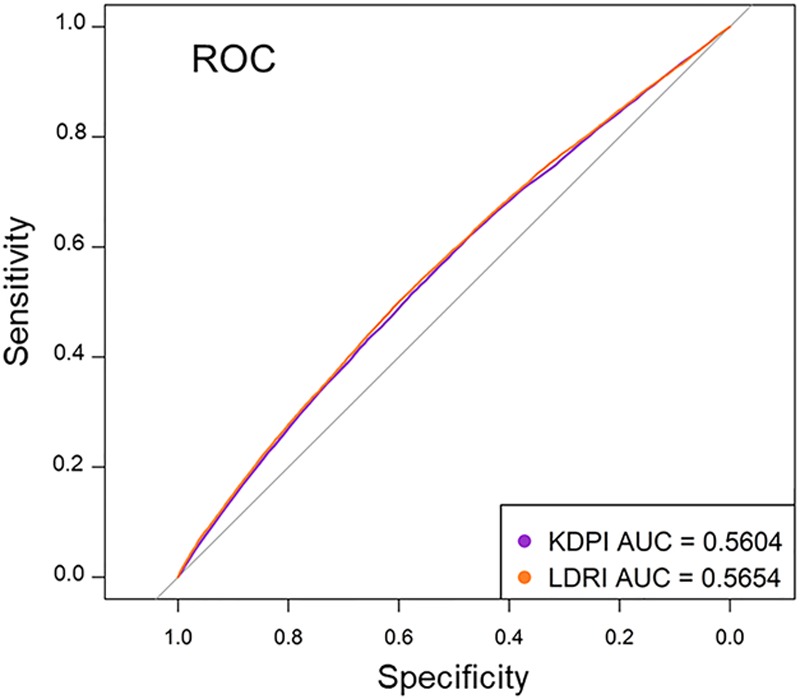
ROC curves for KDPI and LDRI using logistic regression for graft survival. ROC curves for KDPI and LDRI overlap across the range of the respective index values, with an AUC of 0.56 for KDPI vs 0.57 for LDRI. AUC, area under the curve; KDPI, kidney donor profile index; LDRI, liver donor risk index; ROC, receiver operating characteristic.
DISCUSSION
The most established metric for estimating graft survival from donor characteristics in liver transplantation is the LDRI, introduced by Feng et al.6 It has been validated in Europe10 and has led to the development of both Eurotransplant-Donor Risk Index (ET-DRI) and United Kingdom (Donor Liver Index) counterparts.11,12 At present, however, the utilization of LDRI in organ acceptance decisions is limited by its dependence on CIT, which is unknown at the time of organ offer. As such, LDRI is not presented in UNOS DonorNet, the online system for organ evaluation and allocation in the United States. In contrast, the KDPI is calculated from donor factors known at the time of organ offer and thus presented for every deceased donor on UNOS DonorNet.
There are 2 primary findings in our analysis. First, we demonstrate that KDPI closely approximates LDRI in estimating graft survival in liver transplantation. Quintiles of KDPI and LDRI exhibit similar graft survival at 1, 5, and 10 years. Moreover, the models have identical overall c statistics. Second, we demonstrate that imputing an 8-hour CIT into the LDRI formula does not significantly change its predictive value. Adopting this methodology would allow the calculation of LDRI for all potential liver donors in UNOS DonorNet and may represent a practical solution to enhance the utility of LDRI in organ acceptance decision making.
While KDPI appears to approximate LDRI very closely, it must be noted that neither metric appears to be particularly robust in prediction of liver graft survival, with c statistics in the 0.56–0.58 range. This observation is not surprising given that both KDPI and LDRI are based purely on donor factors and do not include any of the important recipient variables known to impact graft survival, including recipient age, MELD score, and patient acuity.13–18 Other risk scoring systems have been introduced in liver transplantation that incorporate both donor and recipient factors, including D-MELD,19 Survival outcomes following liver transplantation score,15,17 and Balance of Risk score.16 The c statistics for these scoring systems are higher at 0.6, 0.7, and 0.7, respectively, reflecting the important contribution of recipient factors in determining post-transplant outcomes.16 Even so, c statistics in the 0.6–0.7 range are rather modest and highlight the difficulty of accurately predicting liver transplant outcomes based purely on clinical parameters. In general, there has not been widespread adoption of such scoring systems in day-to-day clinical practice. Barriers to greater utilization of the available scoring systems include the requirement for recipient chart review and subsequent calculation and interpretation of a numeric score, which present challenges when making organ acceptance decisions in the middle of the night.
In clinical practice, organ acceptance decisions are made based on the judgment of transplant clinicians and include careful consideration of recipient status in parallel with donor and graft characteristics. Given the close overlap with LDRI, we believe KDPI can act as an immediately available surrogate to facilitate donor assessment. In light of the rising incidence of donor hepatic steatosis and its detrimental impact on graft survival, the inclusion of known risk factors (Diabetes Mellitus and Hypertension) in the KDPI may become more impactful in the future. That said, KDPI should certainly not be the only factor considered in organ acceptance. Inappropriate reliance on a particular clinical scoring system may have unintended consequences with regard to increased organ discard, as has been demonstrated for high KDPI kidneys.20
There are some notable limitations of this study that should be mentioned, related to data source and study design. Due to missing data (either survival data or parameters needed to calculate KDPI or LDRI), we excluded a small percentage (5.5%) of all eligible patients. Even though the potential for bias in the final study population is minimal, it cannot be excluded entirely. In addition, the KDPI is calculated in reference to the pool of donors recovered in the previous calendar year. As such, there may be some amount of variation year to year with changes in the demographics of the donor population.
In conclusion, estimating donor quality in liver transplantation remains a critical issue, particularly as the organ shortage forces transplant programs to increase consideration of extended criteria donors. KDPI appears to be an acceptable alternative to LDRI that is readily available at the time of organ offer and maybe a practical tool to use in organ acceptance decisions.
Footnotes
Published online 25 November, 2019.
U.S. contributed to research design, writing, performance of research, and data analysis. T.T. contributed to research design, data analysis, and critical review. E.R.S. contributed to data analysis and critical review. K.F. contributed to data analysis and critical review. Q.G. contributed to data analysis and critical review. J.Y. contributed to data analysis and critical review. B.E. contributed to data analysis and critical review. R.P.D. contributed to data analysis and critical review. P.M.S. contributed to data analysis and critical review. S.B.P. contributed to research design, data analysis, and critical review. A.S.B. contributed to research design, writing, data analysis, and critical review.
The authors declare no funding or conflicts of interest.
REFERENCES
- 1.Gao Q, Mulvihill MS, Scheuermann U, et al. Improvement in liver transplant outcomes from older donors: a US national analysis. Ann Surg 2019270333–339 [DOI] [PubMed] [Google Scholar]
- 2.Scalea JR, Redfield RR, Foley DP. Liver transplant outcomes using ideal donation after circulatory death livers are superior to using older donation after brain death donor livers. Liver Transpl 2016221197–1204 [DOI] [PubMed] [Google Scholar]
- 3.Bohorquez H, Seal JB, Cohen AJ, et al. Safety and outcomes in 100 consecutive donation after circulatory death liver transplants using a protocol that includes thrombolytic therapy. Am J Transplant 2017172155–2164 [DOI] [PubMed] [Google Scholar]
- 4.Halazun KJ, Quillin RC, Rosenblatt R, et al. Expanding the margins: high volume utilization of marginal liver grafts among >2000 liver transplants at a single institution. Ann Surg 2017266441–449 [DOI] [PubMed] [Google Scholar]
- 5.Halazun KJ, Rana AA, Fortune B, et al. No country for old livers? Examining and optimizing the utilization of elderly liver grafts. Am J Transplant 201818669–678 [DOI] [PubMed] [Google Scholar]
- 6.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant 20066783–790 [DOI] [PubMed] [Google Scholar]
- 7.Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation 200988231–236 [DOI] [PubMed] [Google Scholar]
- 8.Akkina SK, Asrani SK, Peng Y, et al. Development of organ-specific donor risk indices. Liver Transpl 201218395–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uno H, Cai T, Pencina MJ, et al. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med 2011301105–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blok JJ, Braat AE, Adam R, et al. ; European Liver Intestine Transplant Association Eurotransplant Liver Intestine Advisory Committee; Eurotransplant Liver Intestine Advisory Committee Validation of the donor risk index in orthotopic liver transplantation within the Eurotransplant region. Liver Transpl 201218112–119 [DOI] [PubMed] [Google Scholar]
- 11.Collett D, Friend PJ, Watson CJ. Factors associated with short- and long-term liver graft survival in the United Kingdom: development of a UK donor liver index. Transplantation 2017101786–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braat AE, Blok JJ, Putter H, et al. ; European Liver and Intestine Transplant Association (ELITA) and Eurotransplant Liver Intestine Advisory Committee (ELIAC) The Eurotransplant donor risk index in liver transplantation: ET-DRI. Am J Transplant 2012122789–2796 [DOI] [PubMed] [Google Scholar]
- 13.Ghobrial RM, Gornbein J, Steadman R, et al. Pretransplant model to predict posttransplant survival in liver transplant patients. Ann Surg 2002236315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ioannou GN. Development and validation of a model predicting graft survival after liver transplantation. Liver Transpl 2006121594–1606 [DOI] [PubMed] [Google Scholar]
- 15.Rana A, Hardy MA, Halazun KJ, et al. Survival outcomes following liver transplantation (SOFT) score: a novel method to predict patient survival following liver transplantation. Am J Transplant 200882537–2546 [DOI] [PubMed] [Google Scholar]
- 16.Dutkowski P, Oberkofler CE, Slankamenac K, et al. Are there better guidelines for allocation in liver transplantation? A novel score targeting justice and utility in the model for end-stage liver disease era. Ann Surg 2011254745–753 [DOI] [PubMed] [Google Scholar]
- 17.Rana A, Jie T, Porubsky M, et al. The survival outcomes following liver transplantation (SOFT) score: validation with contemporaneous data and stratification of high-risk cohorts. Clin Transplant 201327627–632 [DOI] [PubMed] [Google Scholar]
- 18.Blok JJ, Putter H, Rogiers X, et al. ; Eurotransplant Liver Intestine Advisory Committee Combined effect of donor and recipient risk on outcome after liver transplantation: research of the Eurotransplant database. Liver Transpl 2015211486–1493 [DOI] [PubMed] [Google Scholar]
- 19.Halldorson JB, Bakthavatsalam R, Fix O, et al. D-MELD, a simple predictor of post liver transplant mortality for optimization of donor/recipient matching. Am J Transplant 20099318–326 [DOI] [PubMed] [Google Scholar]
- 20.Bae S, Massie AB, Luo X, et al. Changes in discard rate after the introduction of the Kidney Donor Profile Index (KDPI). Am J Transplant 2016162202–2207 [DOI] [PMC free article] [PubMed] [Google Scholar]


