Abstract
Background.
Direct-acting antivirals (DAA) allow effective and safe eradication of hepatitis C virus (HCV) in most patients. There are limited data on the long-term effects of all-oral, interferon-free DAA combination therapies in kidney transplant (KT) patients infected with HCV. Here we evaluated the long-term tolerability, efficacy, and safety of DAA combination therapies in KT patients with chronic HCV infection.
Methods.
Clinical data from KT patients treated with DAA were collected before, during, and after the treatment, including viral response, immunosuppression regimens, and kidney and liver function.
Results.
Patients (N = 226) were mostly male (65.9%) aged 56.1 ± 10.9 years, with a median time from KT to initiation of DAA therapy of 12.7 years and HCV genotype 1b (64.6%). Most patients were treated with sofosbuvir-based therapies. Rapid virological response at 1 month was achieved by 89.4% of the patients and sustained virological response by week 12 by 98.1%. Liver function improved significantly after DAA treatment. Tacrolimus dosage increased 37% from the beginning of treatment (2.5 ± 1.7 mg/d) to 1 year after the start of DAA treatment (3.4 ± 1.9 mg/d, P < 0.001). Median follow-up was 37.0 months (interquartile range, 28.4–41.9) and death-censored graft survival was 91.1%. Adverse events resulting from DAA treatment, especially anemia, were reported for 31.0% of the patients.
Conclusions.
Chronic HCV infection can be treated efficiently and safely with DAA therapy in KT patients. Most patients retained stable kidney function and improved liver function. Tacrolimus dose had to be increased in most patients, potentially as a result of better liver function.
Kidney transplant (KT) recipients who are hepatitis C virus (HCV)-positive have an increased risk of other infections, new-onset diabetes mellitus, cardiovascular diseases, liver fibrosis, graft loss, and mortality.1,2 The immunosuppressive regimen required after transplantation can promote viral replication, leading to progression of liver disease or reactivation of HCV infection and exacerbation of hepatitis. Active viral replication at transplantation is an independent risk factor for graft failure in this type of patients.3 However, kidney transplantation is still recommended, as mortality is higher if continuing on maintenance hemodialysis.4
Antiviral therapy based on interferon (IFN) or ribavirin is not recommended in patients with impaired renal function because both drugs are eliminated by the kidneys and reduced doses are required. In KT patients, the therapies based on IFN have been associated with poor efficacy, low tolerability, and increased risk of graft rejection.2,5
In recent years, the introduction of oral antiviral drugs that directly inhibit viral proteins have revolutionized the treatment of HCV-positive patients.6 The first IFN-free direct-acting antiviral (DAA) therapy implemented was sofosbuvir, an inhibitor of the viral RNA polymerase, approved by the US Food and Drug Administration in December 2013.7 Currently, there are 4 major classes of approved DAA drugs targeting 3 different nonstructural viral proteins: NS3/4A protease inhibitors (eg, simeprevir, paritaprevir, grazoprevir, glecaprevir, voxilaprevir); NS5A replication complex inhibitors (eg, ledipasvir, ombitasvir, elbasvir, daclatasvir, velpatasvir, pibrentasvir); non-nucleoside NS5B polymerase inhibitors (eg, dasabuvir); and nucleoside NS5B polymerase inhibitors (sofosbuvir). Used in combinations and with/without ribavirin, they can often lead to sustained virological responses (SVR) >90% in 12 weeks or less among patients that are treatment naïve,8 compared with curation rates of 34% in 24–48 weeks with previous treatments.9 Compared with IFN-based treatments, the safety of oral DAA therapies has been well tested on patients with renal dysfunction. Recently developed pangenotypic DAA combinations, such as glecaprevir/pibrentasvir, have emerged as major advances for patients with severe kidney impairment.10,11 However, the long-term treatment effects are still poorly known,12 as drug–drug interactions are a possibility in patients compromised by other diseases.
In KT patients with concomitant HCV infection, the benefits of DAA therapies include a reduced risk of graft rejection compared with IFN-based therapies, as well as improved liver function. HCV eradication with DAA treatments has been shown to improve significantly the quality of life of KT patients.13 Another advantage of DAA treatments is that more HCV-infected kidneys will be available for transplants, thus reducing waiting lists and time on hemodialysis for affected patients.14-16 It could also be expected that there will be a reduction in the number of severe renal impairment patients that will require a dual kidney and liver transplant.
There is a growing body of evidence regarding the efficacy and safety of DAAs in KT recipients.17-25 The review of the available data indicates that DAA therapies can cure HCV in most KT patients (>98%) with no major safety-associated concerns.17,26 They also highlighted the need for careful monitoring of immunosuppressive drug levels shortly after DAA treatment initiation, as well as the need for close collaboration between hepatologists and transplantation nephrologists. Although large cohort studies will be needed to assess the clinical and long-term benefits of DAAs in the KT patient population, the European Association for the Study of the Liver and the Spanish Association of the Liver and the Kidney encourage the use of DAA therapies for the treatment of HCV infection in chronic kidney disease.11,27
In this observational, prospective, multicenter study, we present our results of 226 cases of HCV-infected KT recipients treated with DAA. This analysis is an extension of a previous preliminary report of 119 cases.18 Our main objective here was to investigate the long-term tolerability, efficacy, and safety of a variety of IFN-free DAA combination therapies currently used in Spanish reference hospitals.
MATERIALS AND METHODS
This observational, multicentric, prospective study included KT patients from 19 reference hospitals throughout Spain from March 2013 to May 2017. All patients were ≥18 years old, HCV-positive at the time of transplant, and received DAA therapy. The study protocol was approved by the Ethics Committee of the Virgen del Rocío Hospital, Seville (Spain). All eligible patients provided written informed consent before undergoing study-related procedures. The trial was conducted in accordance with the Declaration of Helsinki.28
Patients were followed prospectively and clinical, virological, and laboratory data were collected at a basal visit before the treatment started, 1 month after treatment start (before its finalization), and 1 month, 3 months, 1 year after the treatment ended. All data collected by the investigators were placed into a single database for further analysis.
Efficacy of the therapy was defined as the SVR after 30, 90 (SVR-12), and 365 days of treatment. Safety of the therapy was assessed as a function of renal function (creatinine, estimated glomerular filtration rate [eGFR]), proteinuria, immunosuppression levels, and changes in the diabetes treatment for diabetic patients (as judged by the investigator). Risk of recurrence was also evaluated. Fibrosis stage was evaluated by transient elastography (FibroScan). Compliance with antiviral therapy and rate of adverse events were monitored through the treatment duration and follow-up by review of clinical charts by the clinicians at each of the hospitals.
Statistical analysis was performed using the SPSS 22.0 statistical software for Windows. All values were calculated from the number of valid cases (N). Quantitative variables were described as means and standard deviations or as medians with interquartile ranges (IQR). Categorical variables were presented as lists of frequencies and proportions. Comparisons of numerical variables were performed using the paired t test or the Wilcoxon test.
RESULTS
Patient Population
This study included 226 KT recipients with chronic HCV infection. The basal demographic and clinical characteristics of the patients are shown in Table 1. Most patients were male (65.9%) and the mean age was 56.1 ± 10.9 years, with a median time from KT to initiation of DAA therapy of 12.7 years (IQR, 6.3–21.9). The median time of follow-up after initiation of antiviral therapy was 37.0 months (IQR, 28.4–41.9). Nineteen patients were recipients of a combined liver and KT and 4 had simultaneous pancreatic and KTs. Seventy-four patients (32.7%) presented diabetes, which was treated with oral antidiabetics (8.4%), insulin (19.5%), or a combination of both (2.7%).
TABLE 1.
Basal demographic and clinical characteristics, N = 226
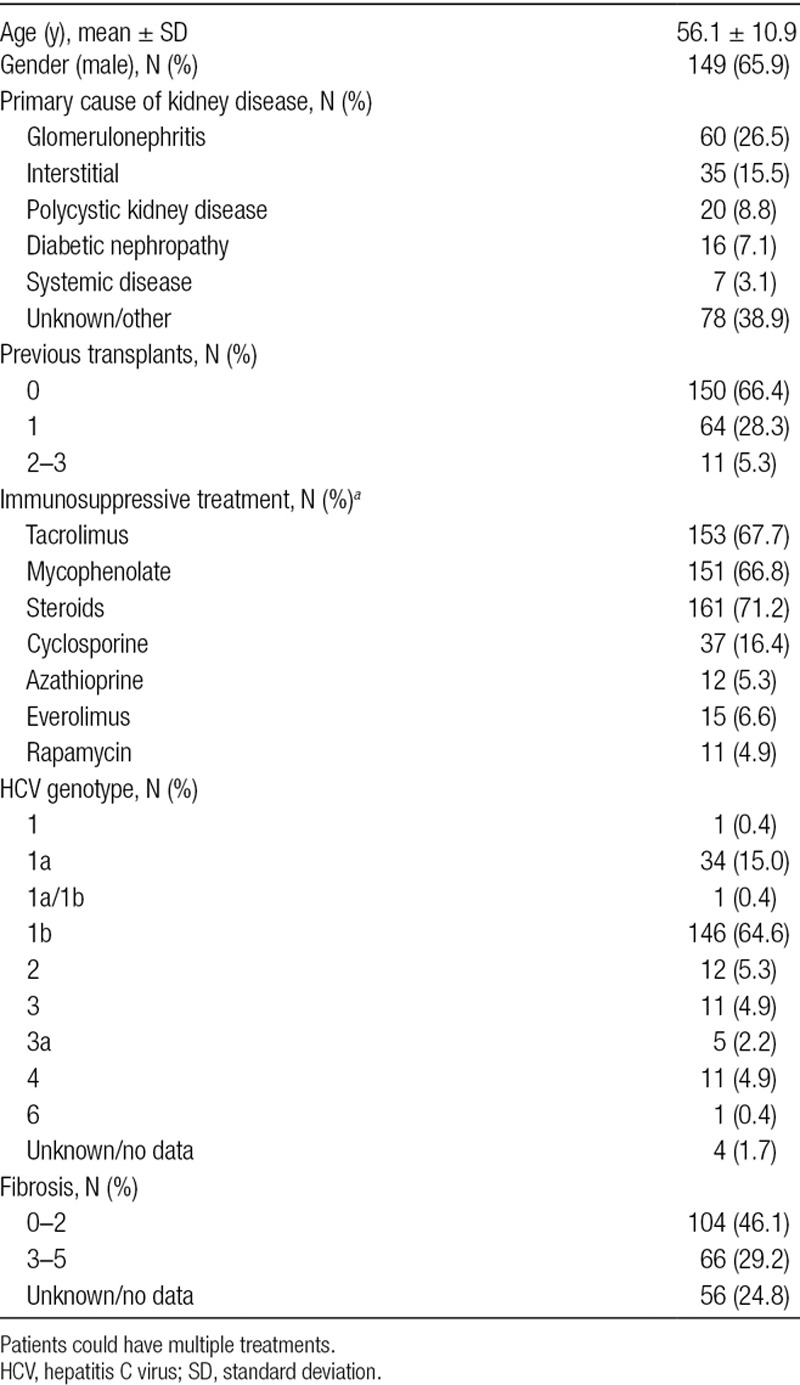
Baseline viral genotypes are shown in Table 1. Genotype 1b was the most frequent (146 patients, 64.6%). Fifty-seven patients (25.2%) had received previous HCV treatment. Further, 8 and 6 patients tested positive for hepatitis B virus and HIV, respectively. Thirty-five patients (15.5%) presented portal hypertension and 5 patients (2.2%) had hepatocellular carcinoma.
Antiviral Treatments and Virological Response
A total of 11 different antiviral regimens were prescribed in our cohort study (Table 2). More than half of the patients were treated with the combination of sofosbuvir and ledipasvir (118 patients, 52.2%). The combination of sofosbuvir and ribavirin, with or without other DAA, was used in 38 patients (16.8%). The median treatment duration was 12.3 weeks (IQR, 11.9–15.7 wk). Of the 207 patients who completed the treatment, 185 (89.4%) patients achieved rapid virological response after 1 month of treatment, with undetectable viral load. After completion of the 12-week DAA treatment, 203 patients (98.1%) achieved SVR-12. One year after the start of treatment, there were no cases recorded of infection relapse or retreatment with antivirals.
TABLE 2.
DAA treatments and adverse events, N (%)
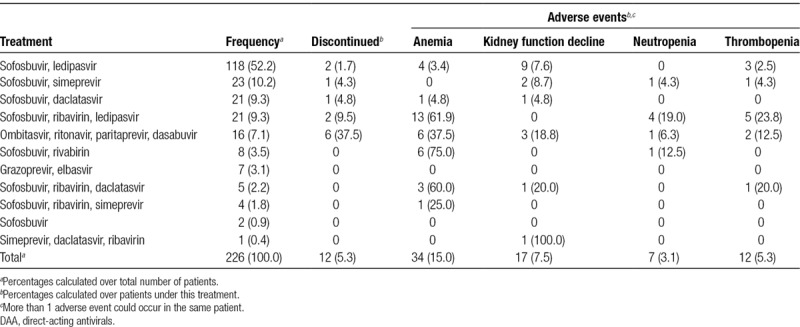
There were 12 patients (5.3%) who discontinued treatment; half of them had been treated with the combination of ombitasvir, ritonavir, paritaprevir, and dasabuvir. Discontinuation was due to pancytopenia in 2 patients, neural toxicity in 3 patients, hepatic toxicity in 2 patients, and gastrointestinal toxicity in 1 patient; in 1 patient, the reason was not registered and there were 3 deaths: 2 due to sepsis and 1 due to acute myocardial infarction. In these cases, the median treatment duration was 3.3 weeks (IQR, 1.9–12.2).
Graft Function and Immunosuppression
Clinical renal and hepatic parameters before and 1 year after the start of DAA treatment are shown in Table 3. The indicators of kidney function worsened, as eGFR decreased significantly (P = 0.003, paired t test). Renal function was not significantly different between DAA treatment regimens. All indicators of liver function presented very significant improvements (P < 0.001).
TABLE 3.
Clinical parameters of kidney and liver function
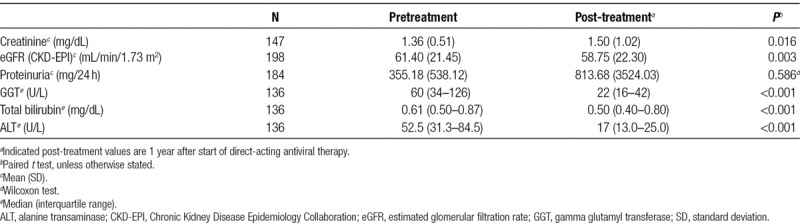
All patients were on 1 or more immunosuppressive agents, of which tacrolimus was the most frequent (67.7%, Table 1). Tacrolimus trough levels were reduced 1 year after the start of DAA therapy (7.2 ± 1.9 ng/mL at start versus 6.6 ± 2.3 ng/mL, P = 0.041). The total daily dose of tacrolimus significantly increased 1 year after the start of the treatment (2.5 ± 1.7 versus 3.4 ± 1.9 mg/d; P < 0.001).
During the median 37 months of follow-up, the death-censored graft survival was 96.9% after 1 year, 96.4% after 2 years, and 91.1% after 37 months. The causes of graft loss were interstitial fibrosis plus tubular atrophy in 10 patients, chronic humoral rejection in 3 patients, and cryoglobulinemia in 3 patients. Patient survival (DAA-treated) was 96.4% after 1 year, 95.8% after 2 years, and 89.1% after 37 months. Causes of death were cancer (lung and cholangiocarcinoma) in 6 patients, cardiovascular events in 6 patients, sepsis in 5 patients, liver failure due to cirrhosis in 2 patients, and other causes in 4 patients.
Adverse Events
A total of 70 patients (31.0%) reported adverse events while on DAA treatment (Table 2). The most common adverse event was anemia, which was serious in 25 of the cases and highly prevalent in patients treated with ribavirin (>60% of patients). A decline of kidney function was observed in 17 patients (7.5%). Neutropenia and thrombopenia were detected in 3.1% and 5.3% of patients, respectively. In addition to the adverse events shown in Table 2, there were also reported cases of asthenia (4 patients), nausea and vomiting (3 patients), headache (3 patients), skin rash (2 patients), tacrolimus toxicity (2 patients), and hepatotoxicity (2 patients). Toxicity due to tacrolimus was observed in 2 of the 16 patients treated with ombitasvir, ritonavir, paritaprevir, and dasabuvir, but we found no other cases of toxicity in any of the other DAA treatments.
DISCUSSION
In this study, we describe the results of the medium to long-term treatment of HCV-infected KT recipients with the recently developed DAA therapies. The virological response was very high (98.1% achieved SVR-12) and, after a median follow-up of 26.6 months, patient and graft survival were >95%. SVR was similar in double liver/kidney or pancreas/KT patients, suggesting that immunosuppression did not affect the effectiveness of the therapy. Additionally, we observed no post-treatment relapses. One year after the antiviral treatment, hepatic function experienced significant improvements in most patients.
The SVR-12 observed in our study is comparable to that found in other series, which range from 91% to 100% in this patient population.20-22,25,29–31 In our study, some patients developed mild allograft dysfunction and some patients required immunosuppression dose adjustment. One year after initiation of DAA treatment, our study showed that tacrolimus dose had to be significantly increased to maintain levels within target ranges. Immunosuppressive dose adjustment in KT or liver transplant recipients receiving DAA has been observed previously.32-34 It has been suggested that drug–drug interactions could develop during DAA therapy, as the NS3/4A protease inhibitors are degraded in the liver by cytochrome P450, which also metabolizes calcineurin inhibitors. However, in our study, only 50 patients (22%) received NS3/4A protease inhibitors simeprevir, paritaprevir, or grazoprevir. Another possibility is that enhanced liver function is the result of improved metabolism of the calcineurin inhibitors as a consequence of the DAA treatment. Proinflammatory cytokines may inhibit cytochrome P450 enzymes during HCV infection, which are then restored to normal function after virus clearance.32 This apparent DAA-induced reversibility of liver function could be more pronounced in KT patients with lower degrees of fibrosis compared with those with severe fibrosis or cirrhosis.33 In the non-KT population, the biochemical parameters of liver function improve shortly after DAA therapy,35 with regression of fibrosis, normalization of portal hypertension and liver stiffness, and increase in skeletal muscle mass.36-38
Independent of intervention, some functional deterioration is expected in KT patients followed in time, as reflected in their clinical parameters. In our study, we could not determine if there had been a prior decline in renal function, or even an improvement, as no data were available before DAA treatment. The observed variation in renal function was not clinically significant and the survival of the transplanted kidney censored for death, 91.1% after a median follow-up of 37.0 months, was high. Likely the elimination of the virus suppresses or limits its negative impact, improving the survival of the patient and the transplanted organ until it equals or nears that of the negative HCV receptors. Our study and others were not designed to assess the functional evolution of the kidney in the same patient before and after treatment (comparing the slope of loss of eGFR as a predictor of its long-term survival). Possibly this issue cannot be resolved directly and conclusively, although large registries could be used to develop retrospective studies by following large cohorts of patients.
DAA therapy was well tolerated, as in previously reported series,17-19,21-25 with only 5.3% of the patients discontinuing treatment. Most common adverse event was anemia in patients treated with ribavirin, a well-known effect of this antiviral.39 The highest proportion of discontinuation due to DAA adverse effects was seen for patients taking the combination of ombitasvir, ritonavir, paritaprevir, and dasabuvir (6 patients, 37.5% of all discontinuations). We did not register the emergence of serious infections that required hospitalization.
Currently, the European Association for the Study of the Liver recommends a fixed-dose combination of sofosbuvir and ledipasvir (genotypes 1, 4, 5, and 6) without the need for immunosuppressant drug dose adjustments in case of KT patients with acceptable kidney function.11 However, the fixed-dose combination of glecaprevir and pibrentasvir for 12 weeks, with immunosuppressant drug adjustments as needed, is recommended for patients with severe kidney impairment (eGFR <30 mL/min/1.73 m2).11 Although current therapeutic protocols for KT patients seem highly effective and safe, it is likely that novel combination DAA therapies will be developed in the future for those patients with comorbidities or refractory to treatment.
To date, this multicentric observational study of 226 patients reflecting current clinical practices is the largest of its kind in Europe and with the longest follow-up (37 mo). However, the study was limited by patient heterogeneity (eg, genotypes, degree of fibrosis) and diversity in DAA regimes, which prevented comparative and statistically significant analysis of effectiveness or safety.
CONCLUSIONS
Our study confirms that HCV infection can be successfully and safely treated after kidney transplantation with a 12-week course of treatment with DAAs. After a median follow-up of 37 months, most patients improved liver function and retained clinically stable kidney activity. Patients required a significant increase in tacrolimus dose to maintain trough levels without changes in other immunosuppressive drugs.
ACKNOWLEDGMENTS
The authors thank Francisco López de Saro, PhD, for medical writing support with the preparation of this article.
Footnotes
Published online 18 November, 2019.
All authors participated in research design and contributed with data from their respective hospitals. J.C.R. collected data and performed the initial data analysis. All authors provided feedback on the data results. J.C.R. wrote draft of the manuscript and all authors contributed to and approved the final version.
The authors declare no conflicts of interest.
This work was partially supported by a grant from the Instituto de Salud Carlos III co-funded by the Fondo Europeo de Desarrollo Regional-FEDER, RETICS (REDinREN RD16/0009). Astellas Pharma S.A. provided financial support for medical writing and editing of the manuscript.
REFERENCES
- 1.Heo NY, Mannalithara A, Kim D, et al. Long-term patient and graft survival of kidney transplant recipients with hepatitis C virus infection in the United States. Transplantation 2018102454–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fabrizi F, Martin P, Dixit V, et al. Meta-analysis of observational studies: hepatitis C and survival after renal transplant. J Viral Hepat 201421314–324 [DOI] [PubMed] [Google Scholar]
- 3.Gentil Govantes MA, Esforzado N, Cruzado JM, et al. Harmful effects of viral replication in seropositive hepatitis C virus renal transplant recipients. Transplantation 2012941131–1137 [DOI] [PubMed] [Google Scholar]
- 4.Ingsathit A, Kamanamool N, Thakkinstian A, et al. Survival advantage of kidney transplantation over dialysis in patients with hepatitis C: a systematic review and meta-analysis. Transplantation 201395943–948 [DOI] [PubMed] [Google Scholar]
- 5.Fabrizi F, Lunghi G, Dixit V, et al. Meta-analysis: anti-viral therapy of hepatitis C virus-related liver disease in renal transplant patients. Aliment Pharmacol Ther 2006241413–1422 [DOI] [PubMed] [Google Scholar]
- 6.Fabrizi F, Messa P. Treatment choices for hepatitis C in patients with kidney disease. Clin J Am Soc Nephrol 201813793–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noell BC, Besur SV, deLemos AS. Changing the face of hepatitis C management - the design and development of sofosbuvir. Drug Des Devel Ther 201592367–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pawlotsky JM. New hepatitis C virus (HCV) drugs and the hope for a cure: concepts in anti-HCV drug development. Semin Liver Dis 20143422–29 [DOI] [PubMed] [Google Scholar]
- 9.Fabrizi F, Penatti A, Messa P, et al. Treatment of hepatitis C after kidney transplant: a pooled analysis of observational studies. J Med Virol 201486933–940 [DOI] [PubMed] [Google Scholar]
- 10.Zeuzem S, Foster GR, Wang S, et al. Glecaprevir-pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N Engl J Med 2018378354–369 [DOI] [PubMed] [Google Scholar]
- 11.EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol 201869461–511 [DOI] [PubMed] [Google Scholar]
- 12.Jakobsen JC, Nielsen EE, Feinberg J, et al. Direct-acting antivirals for chronic hepatitis C. Cochrane Database Syst Rev. 2017;9:CD012143. doi: 10.1002/14651858.CD012143.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabbatini M, Capuano I, Camera S, et al. Eradication of HCV in renal transplant recipients and its effects on quality of life. Biomed Res Int. 2018;2018:8953581. doi: 10.1155/2018/8953581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franco A, Moreso F, Merino E, et al. Renal transplantation from seropositive hepatitis C virus donors to seronegative recipients in Spain: a prospective study. Transpl Int 201932710–716 [DOI] [PubMed] [Google Scholar]
- 15.Durand CM, Bowring MG, Brown DM, et al. Direct-acting antiviral prophylaxis in kidney transplantation from hepatitis C virus-infected donors to noninfected recipients: an open-label nonrandomized trial. Ann Intern Med 2018168533–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg DS, Abt PL, Blumberg EA, et al. Trial of transplantation of HCV-infected kidneys into uninfected recipients. N Engl J Med 20173762394–2395 [DOI] [PubMed] [Google Scholar]
- 17.Chen K, Lu P, Song R, et al. Direct-acting antiviral agent efficacy and safety in renal transplant recipients with chronic hepatitis C virus infection: a PRISMA-compliant study. Medicine (Baltimore) 2017;96:e7568. doi: 10.1097/MD.0000000000007568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gentil MA, González-Corvillo C, Perelló M, et al. Hepatitis C treatment with direct-acting antivirals in kidney transplant: preliminary results from a multicenter study. Transplant Proc 2016482944–2946 [DOI] [PubMed] [Google Scholar]
- 19.Goel A, Bhadauria DS, Kaul A, et al. Experience with direct acting anti-viral agents for treating hepatitis C virus infection in renal transplant recipients. Indian J Gastroenterol 201736137–140 [DOI] [PubMed] [Google Scholar]
- 20.Fernández I, Muñoz-Gómez R, Pascasio JM, et al. Efficacy and tolerability of interferon-free antiviral therapy in kidney transplant recipients with chronic hepatitis C. J Hepatol 201766718–723 [DOI] [PubMed] [Google Scholar]
- 21.Kamar N, Marion O, Rostaing L, et al. Efficacy and safety of sofosbuvir-based antiviral therapy to treat hepatitis C virus infection after kidney transplantation. Am J Transplant 2016161474–1479 [DOI] [PubMed] [Google Scholar]
- 22.Lin MV, Sise ME, Pavlakis M, et al. Efficacy and safety of direct acting antivirals in kidney transplant recipients with chronic hepatitis C virus infection. PLoS One. 2016;11:e0158431. doi: 10.1371/journal.pone.0158431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reddy S, Sharma RK, Mehrotra S, et al. Efficacy and safety of sofosbuvir-based antiviral therapy to treat hepatitis C virus infection after kidney transplantation. Clin Kidney J 201811429–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawinski D, Kaur N, Ajeti A, et al. Successful treatment of hepatitis C in renal transplant recipients with direct-acting antiviral agents. Am J Transplant 2016161588–1595 [DOI] [PubMed] [Google Scholar]
- 25.Saxena V, Khungar V, Verna EC, et al. Safety and efficacy of current direct-acting antiviral regimens in kidney and liver transplant recipients with hepatitis C: results from the HCV-TARGET study. Hepatology 2017661090–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chute DF, Chung RT, Sise ME. Direct-acting antiviral therapy for hepatitis C virus infection in the kidney transplant recipient. Kidney Int 201893560–567 [DOI] [PubMed] [Google Scholar]
- 27.Aoufi-Rabih S, García-Agudo R, Londoño MC, et al. ; on behalf of the Spanish Association of the Liver and the Kidney (AEHR) Recommendations for the treatment of hepatitis C virus infection in chronic kidney disease: a position statement by the Spanish association of the liver and the kidney. J Nephrol 2018311–13 [DOI] [PubMed] [Google Scholar]
- 28.World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 20133102191–2194 [DOI] [PubMed] [Google Scholar]
- 29.Morales AL, Liriano-Ward L, Tierney A, et al. Ledipasvir/sofosbuvir is effective and well tolerated in postkidney transplant patients with chronic hepatitis C virus. Clin Transplant. 2017;31:e12941. doi: 10.1111/ctr.12941. [DOI] [PubMed] [Google Scholar]
- 30.Lubetzky M, Chun S, Joelson A, et al. Safety and efficacy of treatment of hepatitis C in kidney transplant recipients with directly acting antiviral agents. Transplantation 20171011704–1710 [DOI] [PubMed] [Google Scholar]
- 31.Colombo M, Aghemo A, Liu H, et al. Treatment with ledipasvir-sofosbuvir for 12 or 24 weeks in kidney transplant recipients with chronic hepatitis C virus genotype 1 or 4 infection: randomized trial. Ann Intern Med 2017166109–117 [DOI] [PubMed] [Google Scholar]
- 32.Smolders EJ, Pape S, de Kanter CT, et al. Decreased tacrolimus plasma concentrations during HCV therapy: a drug-drug interaction or is there an alternative explanation? Int J Antimicrob Agents 201749379–382 [DOI] [PubMed] [Google Scholar]
- 33.Fernández-Ruiz M, Polanco N, García-Santiago A, et al. Impact of anti-HCV direct antiviral agents on graft function and immunosuppressive drug levels in kidney transplant recipients: a call to attention in the mid-term follow-up in a single-center cohort study. Transpl Int 201831887–899 [DOI] [PubMed] [Google Scholar]
- 34.Kwo PY, Mantry PS, Coakley E, et al. An interferon-free antiviral regimen for HCV after liver transplantation. N Engl J Med 20143712375–2382 [DOI] [PubMed] [Google Scholar]
- 35.van der Meer AJ, Berenguer M. Reversion of disease manifestations after HCV eradication. J Hepatol 2016651 SupplS95–S108 [DOI] [PubMed] [Google Scholar]
- 36.Knop V, Hoppe D, Welzel T, et al. Regression of fibrosis and portal hypertension in HCV-associated cirrhosis and sustained virologic response after interferon-free antiviral therapy. J Viral Hepat 201623994–1002 [DOI] [PubMed] [Google Scholar]
- 37.Pietsch V, Deterding K, Attia D, et al. Long-term changes in liver elasticity in hepatitis C virus-infected patients with sustained virologic response after treatment with direct-acting antivirals. United European Gastroenterol J 201861188–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugimoto R, Iwasa M, Hara N, et al. Changes in liver function and body composition by direct-acting antiviral therapy for hepatitis C virus infection. Hepatol Res 201848337–344 [DOI] [PubMed] [Google Scholar]
- 39.Russmann S, Grattagliano I, Portincasa P, et al. Ribavirin-induced anemia: mechanisms, risk factors and related targets for future research. Curr Med Chem 2006133351–3357 [DOI] [PubMed] [Google Scholar]


