Abstract
Background.
Lactate/pyruvate ratio has been introduced as a sensitive marker for ischemia in the transplanted liver. In the present study, we aimed to evaluate lactate/pyruvate ratio measured in the liver by microdialysis as a marker for ischemic complications early after liver transplantation.
Methods.
Forty-five patients undergoing liver transplantation were included in the study. A microdialysis catheter was placed in the liver graft directly following liver transplantation and the metabolites lactate and pyruvate measured for up to 6 days and the lactate/pyruvate ratio calculated. The association between increased intrahepatic lactate/pyruvate ratio and ischemic complications was studied.
Results.
One of 45 patients developed hepatic arterial thrombosis. Forty-four events with increased lactate/pyruvate ratio were identified in 24 patients. In none of the 24 patients that had a raised lactate/pyruvate ratio could we detect occurrence of any ischemic complication. In the patient that did have hepatic arterial thrombosis, the lactate/pyruvate ratio did not show a significant prolonged rise.
Conclusions.
An increase in the intrahepatic lactate/pyruvate ratio is not necessarily indicative of ischemic complications and is thus not a reliable marker for monitoring of clinically significant ischemia in the liver early after transplantation.
Early detection of graft dysfunction following liver transplantation (LT) is essential for graft survival and provides an opportunity for earlier therapeutic or surgical interventions, which should in turn lower the incidence of posttransplant morbidity and mortality. With standard monitoring techniques, ischemic complications such as hepatic arterial thrombosis and portal vein thrombosis/occlusion are not always detected and treated early enough to avoid their negative effects on long-term outcome. Hepatic arterial thrombosis is the most feared of the vascular complications post-LT, and although it occurs in only 2%–9% of liver transplanted patients, it remains one of the leading causes of early graft loss.1 The clinical and biochemical manifestation of vascular complications post-LT may be difficult to differentiate from other causes of graft dysfunction.
Microdialysis provides continuous monitoring of metabolic changes in the tissue in which it is placed. Using microdialysis, changes in the concentration of metabolic parameters can be detected in the interstitial fluid before they are detected systemically in the blood or result in clinical manifestations. Microdialysis has previously been used to study ischemic changes in brain,2 muscle,3 small intestine,4 and adipose tissue5 and has been described in detail elsewhere6 including intrahepatic microdialysis.7-12 Metabolites and inflammatory markers have been measured by intrahepatic microdialysis. Glucose, lactate, and pyruvate (markers of the oxidative metabolism in the tissue) as well as glycerol (marker of cell membrane injury) have been particularly useful as markers of ischemia-reperfusion in the liver.
Tissue glucose levels in most tissues are known to decrease during ischemia. This is attributed to a decreased blood flow and concomitant ongoing glucose utilization.13 However, in liver tissue, the hepatocytes respond to ischemia with glycogenolysis resulting in increasing tissue glucose levels.14,15 Under aerobic conditions, the metabolism of glucose results in production of pyruvate, which enters the citric acid cycle to yield energy. During ischemia, pyruvate is converted to lactate instead of entering the citric acid cycle to uphold adenosine triphosphate production. This results in an increase in lactate and a decrease in pyruvate and thus an increased lactate/pyruvate ratio (L/Pr). The L/Pr is therefore considered a sensitive marker of ischemia.16,17 Levels of lactate and pyruvate in any tissue are a product of glucose metabolism and thus should be evaluated in relation to glucose levels. In the setting of LT besides glycogenolysis, even insulin resistance in the postoperative period contributes to a systemic hyperglycemia. The interpretation of changes in L/Pr is thus complicated due to the deranged glucose metabolism after LT.
Previous experimental work from our group has shown that a porcine model of hepatic artery thrombosis causes significant changes in liver metabolism. Results show that interstitial glucose, lactate, L/Pr, and glycerol increase significantly within 60 minutes after clamping the hepatic artery as a consequence of ischemia and cell damage.9 Also, Håugaa et al18 showed that mean values of intrahepatic lactate and L/Pr were higher in patients with graft ischemia and could detect ischemic complications before standard clinical and laboratory changes became evident. In their study, they suggest cutoff values for intrahepatic L/Pr and lactate for early detection of ischemic complications following LT.18 In the present study, we aimed to evaluate the clinical usefulness of intrahepatic L/Pr measured by microdialysis as a marker for detection of ischemic complications early after LT.
PATIENTS AND METHODS
Patients
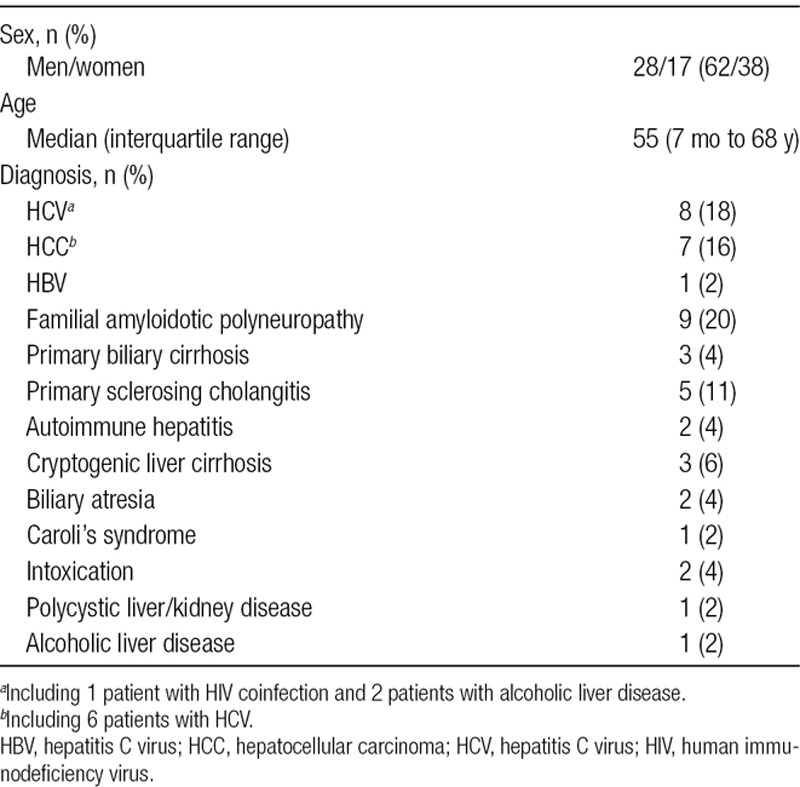
Forty-five patients undergoing LT at the Karolinska University Hospital, Stockholm, Sweden, were included in the study. Patient characteristics including the indications for LT are presented in Table 1. The median patient’s age was 55 years. Written and oral consent was obtained from all patients participating in the study. The study protocol was approved by the Ethics Committee for Clinical Studies at the Karolinska Institute in accordance with the guidelines of the Helsinki Declaration. LT was carried out using the piggyback technique as the standard and in selected cases venovenous bypass was used. Basic immunosuppression was achieved with steroids and Tacrolimus. Thrombosis prophylaxis was achieved with high molecular weight Dextran. In all patients, ultrasound to evaluate the liver circulation was performed as clinical routine, within 24 hours after surgery.
TABLE 1.
Patient characteristics
Study Protocol and Microdialysis
At the end of the operation, but before closure of the abdomen, a microdialysis catheter was inserted into segment IV of the liver graft through methods described elsewhere.12 The CMA 61 microdialysis catheter (CMA Microdialysis AB, Stockholm, Sweden), with a 60 mm shaft (0.9 mm diameter), a 30 mm membrane (0.6 mm diameter) and a molecular cutoff of 20 kilodalton was used as the intrahepatic catheter. A CMA 60 catheter reflecting systemic changes in monitored metabolites was placed subcutaneously in the right pectoral area and was used as a reference catheter. The catheters were perfused with perfusion fluid T1 (isotonic solution, Na+ 147 mmol/L, K+ 4 mmol/L, Ca2+ 2,3 mmol/L, Cl– 156 mmol/L; CMA Microdialysis AB, Stockholm, Sweden) using CMA 106 microinfusion pumps at a flow rate of 0.3 μL/min in all cases. Perfusate was analyzed in a CMA 600 Microdialysis Analyzer (CMA Microdialysis AB, Stockholm, Sweden) using enzymatic/colorimetric methods. Patients were monitored for up to 6 days after LT. Microdialysis samples were collected once every hour. Samples with collected perfusate were analyzed for glucose, lactate, and pyruvate concentrations, and the L/Pr was calculated for both the intrahepatic and subcutaneous catheters.
Clinical or laboratory suspicion of ischemic complications including raised L/Pr beyond cutoffs decided in the protocol were investigated by contrast ultrasound of the liver and if necessary a 4-phase liver CT scan. An ischemic complication was defined as vascular occlusion or graft infarction confirmed by radiology.
Blood Glucose and Insulin Regime
Although the patients remained in the intensive care unit, blood glucose was measured every hour by arterial blood gas. During days 1 and 2, postoperatively patients were administered 5% glucose infusions at 30 mL/kg/day. On the third postoperative day, patients were given half a dose of total parenteral nutrition based on estimated caloric requirements (25 kcal/kg/day). Thereafter patients were given full dose total parenteral nutrition and were allowed to eat. Insulin was given intravenously with a target blood glucose value of 4–8 mmol/L. When patients were stabilized, they were moved to the ward where capillary blood glucose was measured 6 times daily and insulin was administered subcutaneously with a target blood glucose value of <8 mmol/L.
Statistics
In order to evaluate increased L/Pr and the correlation to ischemic complications, episodes with increased intrahepatic L/Pr were identified. Based on earlier experience with microdialysis monitoring in LT, these episodes were defined as 3 consecutive samples with increasing L/Pr where the increase was at least 30% in total. The 30% cutoff was based on our earlier studies and the assumption that such an increase would be relevant in the clinical setting.9,19 The clinical outcome was compared for patients with and without episodes with increased L/Pr.
To study the effect of systemic glucose concentrations on the L/Pr, episodes with systemic glucose increase were identified. These were defined as 3 consecutive samples with increasing glucose and a minimum increase of 30% in total, measured in the reference catheter. These episodes were studied for correlation in time before, during, and after intrahepatic L/Pr increase.
Also, the data were analyzed with respect to the optimal cutoff values for lactate and L/Pr at 3 mmol/L and 20, respectively, as observed in the study by Håugaa et al.18 Microdialysis data are presented as mean ± SE. The Statistica 13.2 Dell software program was used for data analyses and preparation of graphs.
RESULTS
Illustrative Case Reports
Patient 1
A young male recipient with cryptogenic liver cirrhosis was transplanted with a right extended lobe (split liver) from an age and blood group-matched deceased donor. Total ischemia time was 11 hours. Twenty-two hours after LT the intrahepatic L/Pr, measured by microdialysis, increased by 70% over a period of 2 hours (Figure 1A), reaching a maximum level of 17.6. Intrahepatic lactate and pyruvate measurements are presented in Figure 1B and show that the levels tend to follow each other. Analysis shows that the intrahepatic lactate remained stable around 1.2 mmol/L, while pyruvate levels decreased during the L/Pr increase mentioned above. Within 24 hours, the L/Pr returned to previous levels event though pyruvate and lactate levels continued to increase. Retrospective analysis of glucose levels in the reference catheter showed an increase 8 hours after the episode with L/Pr increase (Figure 1A). Standard liver blood tests such as prothrombin time (international normalized ratio) and transaminases reached a maximum on day 2 postoperatively. One week after LT, bilirubin increased, and an ultrasound of the liver was performed on day 10. The patient was diagnosed with thrombosis of the hepatic artery, was reoperated and a hepatic arterial thrombectomy performed. The thrombus was described by the surgeon as being “old.” Unfortunately, the patient had a complicated postoperative outcome in form of biliary strictures and was retransplanted due to cholestatic liver failure. It seems possible that the thrombosis occurred at the time of the initial increase in L/Pr. However, arterial thrombosis was expected to result in a higher and prolonged increase in L/Pr, and therefore, unfortunately, the observed changes in microdialysis did not result in any further investigation at that time.
FIGURE 1.
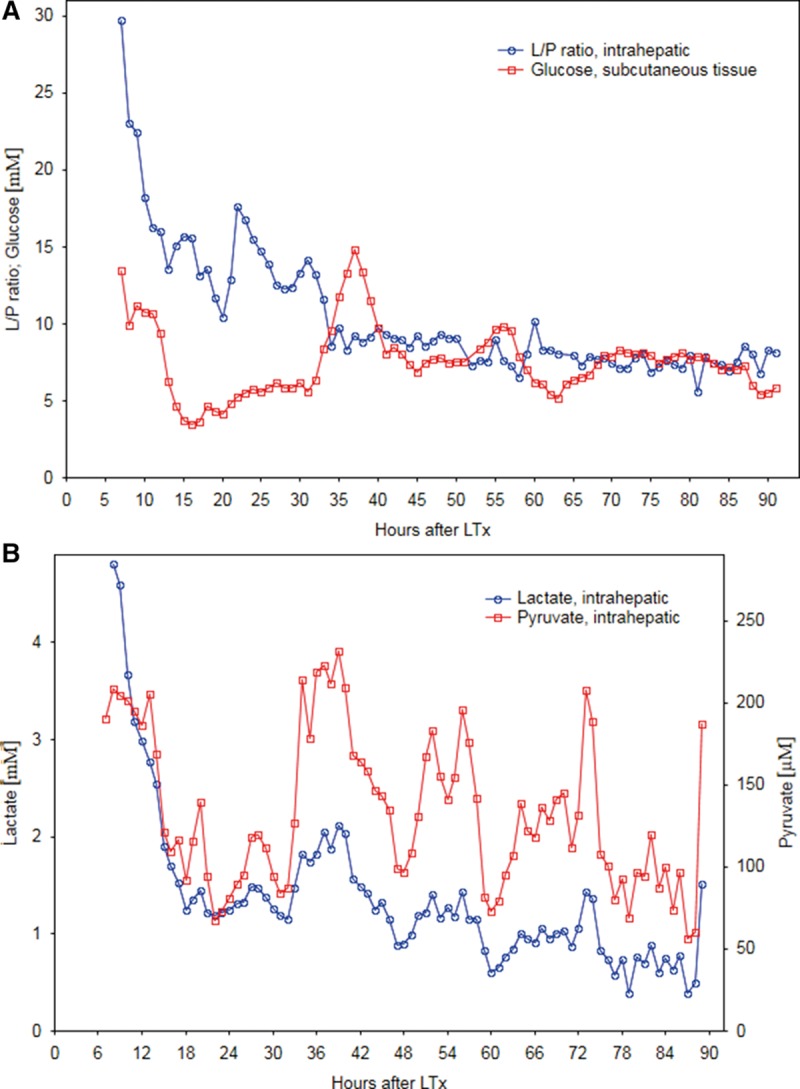
A, Intrahepatic lactate/pyruvate ratio (L/Pr) and subcutaneous glucose; (B) intrahepatic lactate and pyruvate, measured by microdialysis, over time after liver transplantation (LT) in case report 1.
Patient 2
A middle-aged male with HCV cirrhosis underwent LT using the liver from an age and blood group-matched deceased female donor. Total ischemia time was 13 hours and the initial recovery was uneventful. Sixty-five hours after LT, an increase of 50% in the L/Pr was seen over a period of 3 hours (Figure 2A), reaching a maximum level of 10.8. The patient was suspected of having hepatic arterial or portal vein thrombosis and underwent a CT angiography (due to nonconclusive ultrasonography), which showed normal arterial and portal circulation in the liver. The outcome of the LT itself was uneventful. Analysis of the intrahepatic lactate and pyruvate measurements shows a similar pattern where both metabolite levels follow each other and during the L/Pr rise pyruvate levels decreased while lactate levels remained essentially stable (Figure 2B). Further review of the patient’s microdialysis results showed that he had experienced an increase in systemic glucose 3 hours after the time of elevated L/Pr levels as a result of a stop in intravenous insulin infusion. As measured in the reference catheter (adipose tissue), glucose increased by 7.3 units (560%) over a period of 6 hours with a baseline level of 1.3 mmol/L in the subcutaneous tissue (Figure 2A).
FIGURE 2.
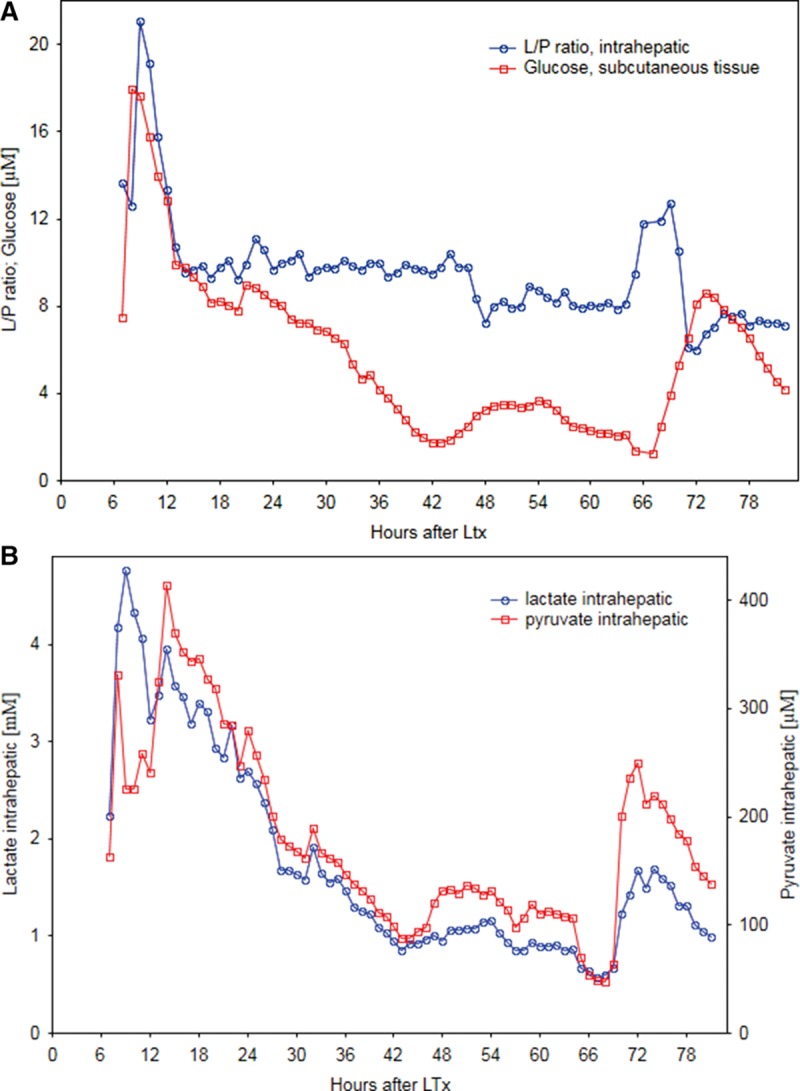
A, Intrahepatic lactate/pyruvate ratio (L/Pr) and subcutaneous glucose; (B) intrahepatic lactate and pyruvate, measured by microdialysis, over time after liver transplantation (LT) in case report 2.
Overall Results
No major events related to the microdialysis procedure were observed during transplantation or in the postoperative monitoring period. Liver graft cold ischemia time ranged from 322–960 minutes with a median of 718 minutes.
The chronologic changes in glucose, lactate, pyruvate, and the L/Pr in the entire cohort are shown in Figures 3A and B and followed the usual trend seen post-LT. There was no correlation in time between episodes with increased intrahepatic L/Pr and episodes of increase in systemic glucose as measured in the reference catheter. No correlation was found between intrahepatic glucose and lactate.
FIGURE 3.
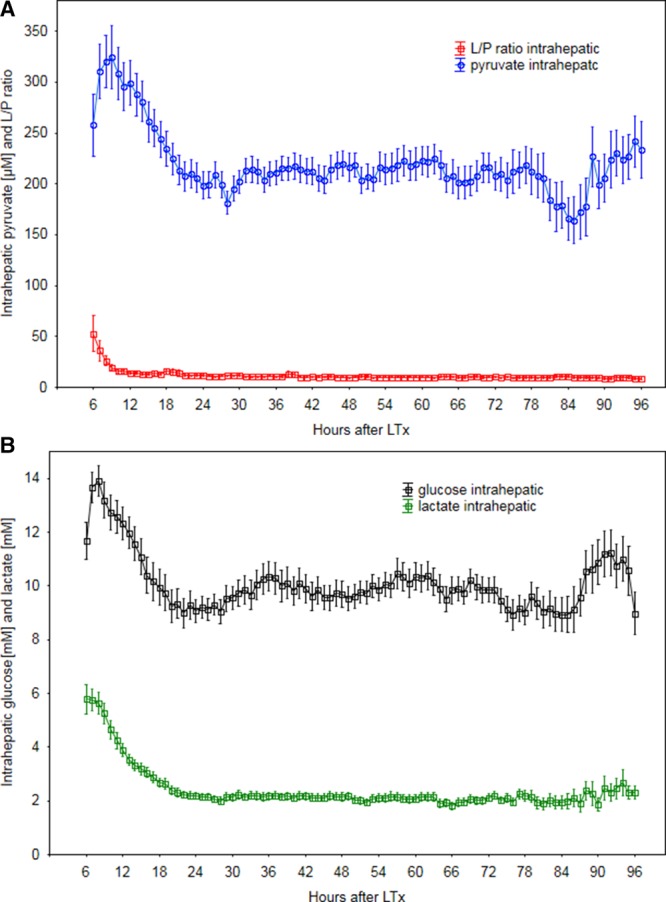
A, Chronologic changes of intrahepatic pyruvate and lactate/pyruvate ratio (L/Pr) following liver transplantation (LT) in the study cohort (45 patients). B, Chronologic changes of intrahepatic lactate and glucose following LT in the study cohort (45 patients).
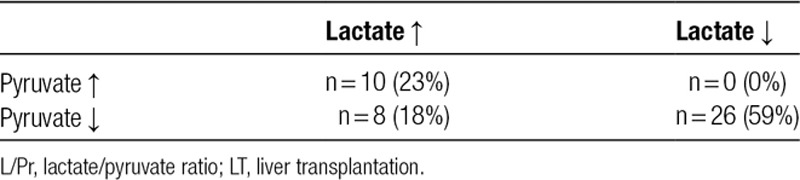
Results are presented in Tables 2 and 3. Of a total of 45 patients included in the study, only one had an ischemic complication in the form of a hepatic arterial thrombosis (patient 1). A total of 44 events of increased L/Pr (as defined by the study protocol) were identified in 24 patients in the study. None of these patients had any clinical, biochemical, or radiological signs of ischemic complications and had an uneventful recovery. Detailed analysis of the episodes of increase in L/Pr showed that in 26 cases (of total 44), the increase was seen during decreasing lactate and pyruvate levels. In 10 cases, the increase in L/Pr was seen during increase in lactate and pyruvate levels. In 8 cases, the increase in L/Pr was due to an increase in lactate and decrease in pyruvate levels (Table 3).
TABLE 2.
Metabolic events and ischemic complications
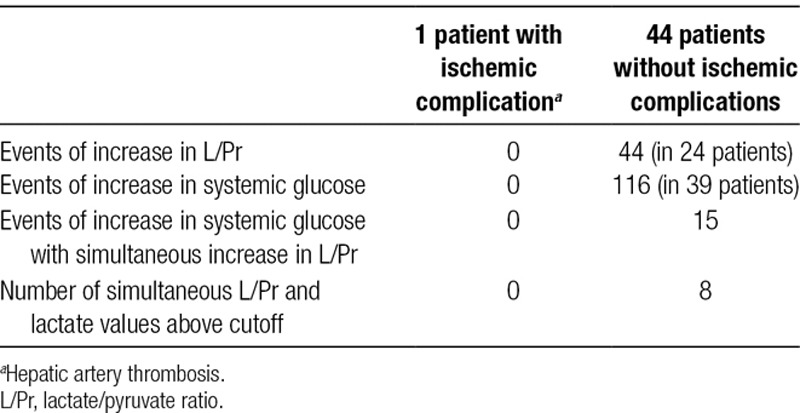
TABLE 3.
Number of cases of increased intrahepatic L/Pr explained by simultaneous increase, simultaneous decrease or increase and decrease of lactate and pyruvate respectively early after LT
Eight patients (17%) had at least one L/Pr value above the by Håugaa et al18 suggested cutoff of 20 and a simultaneous lactate value above the suggested cutoff of 3 mmol/L without showing any clinical or radiological signs of ischemic complications. The patient with artery thrombosis did not reach these suggested L/Pr and lactate cutoff values.
DISCUSSION
Ischemic complications after LT are an important cause of graft dysfunction and their diagnosis is often delayed. Hepatic artery thrombosis after LT, al though relatively rare, is a life-threatening vascular complication and detection methods should ideally have a high sensitivity. In our study, only 1 of 45 patients (~2%) in the study population suffered from hepatic arterial thrombosis. Thus, making statistically valid conclusions regarding patterns of metabolic change during ischemic complications after LT is difficult. However, this patient did not have an intrahepatic L/Pr or lactate increase great enough to indicate ischemia as defined by our study protocol or by the cutoff values suggested by Håugaa et al.18
The changes observed in patient 1 with hepatic arterial thrombosis indicate that increased L/Pr in the liver early after LT could be related to ischemic events such as arterial thrombosis. However, similar changes in patient 2 were seen without verified hepatic artery thrombosis. In both patients, the rise in L/Pr was the result of decreasing pyruvate levels and stable lactate levels. In both patients, the episode of raised L/Pr was followed by increasing pyruvate and lactate levels and consequently decreasing L/Pr suggestive of hypermetabolism. Intrahepatic glucose metabolism is complex and differs from other tissues. Specificity of liver enzymes involved in glucose metabolism account for the liver being more sensitive to changes in glucose levels as a result of ischemia during LT. Due to properties of enzymes involved in glycolysis in the liver, the initial metabolism of glucose is carried out at a 15 times higher rate than in for, for example, the kidney and with no feedback inhibition.15 This results in a low intracellular concentration of glucose allowing for continuous influx of glucose into the cell and “overload” hepatocytes with glucose. Furthermore, the isoenzyme form of lactate dehydrogenase found in liver is active under conditions of low pH and high lactate concentrations, unlike the isoenzyme form of lactate dehydrogenase found in the kidney.15 The metabolic patters of lactate and L/Pr seen by microdialysis early after LT may be the effect of the primary ischemia-reperfusion injury on enzyme activity regulating glucose metabolism in the liver after LT and may not necessarily be indicative of ongoing ischemia.20
In our study, we identified 44 events with protocol-defined L/Pr increase. These 44 events occurred in 24 patients. Detailed analyses show that 59% of the cases with increased L/Pr are seen during decrease in levels of lactate and pyruvate, with pyruvate decreasing at a faster rate than lactate. The decrease in metabolites suggests a recovery of metabolism rather than ischemia. There are several possible explanations for why pyruvate can be metabolized at a faster rate than lactate. Pyruvate acts as a positive regulator of its own metabolism by regulating pyruvate dehydrogenase.21 Also, there are more pathways of metabolism for pyruvate than for lactate.21 This enables pyruvate levels to decrease at a higher rate than lactate levels which would lead to an increase in the L/Pr. Directly after LT, there is an increase in the levels of intrahepatic pyruvate which normalizes within hours.19 A faster decrease in pyruvate levels compared with that of lactate may in fact be a sign of restoration of aerobic metabolism in the transplanted liver.
Twenty-three percent of events with increased L/Pr as defined by the study protocol were seen during increase in both lactate and pyruvate. The fact that pyruvate increases strongly suggests that there is no ongoing ischemia. This indicates accumulation of lactate at a higher rate than pyruvate and could be explained by differences in metabolic pathways favoring a faster metabolism of pyruvate. Increasing pyruvate and lactate levels may be thus a result of hypermetabolism.
Only 18% of the events with increased L/Pr were due to an increase in lactate and a decrease in pyruvate (suggesting ischemia), but no clinical or radiologically detected ischemic complications were diagnosed in these patients. Seventeen percent of the patients in our series had episodes with increased L/Pr and lactate above the cutoff values suggested by Håugaa et al18 but did not have any detectable ischemic complications. This suggests that intrahepatic L/Pr may increase for reasons other than graft ischemia. It is also possible that in these patients, there were some ischemic events, which were reflected in the increased L/Pr but were of no clinical significance since radiological evaluation did not detect any complication and the further clinical course was uneventful. The patient diagnosed with hepatic artery thrombosis (mentioned as patient 1) was not among the 25 patients with events of increased L/Pr as defined by the study protocol. Using the suggested cutoff values results in too many false positives and they are therefore suboptimal for detection of ischemia in the clinical setting.
In conclusion, our results show that an increase in the intrahepatic L/Pr is not a reliable or specific marker of clinically significant ischemic complications early after LT. Hepatic L/Pr is a product of the complex interplay of changes in hepatic metabolism, systemic glucose levels, primary ischemia-reperfusion injury, and recovery of glucose anaerobic metabolism in the posttransplant phase. The interpretation of L/Pr is complex and dependant on a number of factors in the transplanted liver and by itself is thus a poor marker of clinically relevant ischemia early after LT. To detect and treat vascular complications early after LT specific markers of ischemia, such as glutathione or products of nitric oxide metabolism, maybe more reliable.8 Microdialysis could perhaps be helpful in detecting other complications after LT such as rejection, if specific and sensitive markers are identified.
ACKNOWLEDGMENTS
The authors thank Johan Waern for expert biomedical assistance and Per Nasman for statistical assistance.
Footnotes
Published online 15 November, 2019.
A.v.P. involved in collection and analyzing of data and writing article. M.A.D.S. involved in analyzing of data and writing article. O.R. involved in writing article. G.N. involved in study design, collection and analyzing of data, and writing article
This work was supported by CMA Microdialysis AB, Stockholm, Sweden. Pregraduate School of Clinical Science, Karolinska Institute.
The authors declare no conflicts of interest.
REFERENCES
- 1.Shay R, Taber D, Pilch N, et al. Early aspirin therapy may reduce hepatic artery thrombosis in liver transplantation. Transplant Proc 201345330–334 [DOI] [PubMed] [Google Scholar]
- 2.Hutchinson PJ, O’Connell MT, Al-Rawi PG, et al. Clinical cerebral microdialysis: a methodological study. J Neurosurg 20009337–43 [DOI] [PubMed] [Google Scholar]
- 3.Gutierrez A, Anderstam B, Alvestrand A. Amino acid concentration in the interstitium of human skeletal muscle: a microdialysis study. Eur J Clin Invest 199929947–952 [DOI] [PubMed] [Google Scholar]
- 4.Ungerstedt J, Nowak G, Ericzon BG, et al. Intraperitoneal microdialysis (IPM): a new technique for monitoring intestinal ischemia studied in a porcine model. Shock 20032091–96 [DOI] [PubMed] [Google Scholar]
- 5.Bolinder J, Ungerstedt U, Arner P. Microdialysis measurement of the absolute glucose concentration in subcutaneous adipose tissue allowing glucose monitoring in diabetic patients. Diabetologia 1992351177–1180 [DOI] [PubMed] [Google Scholar]
- 6.Müller M. Science, medicine, and the future: microdialysis. BMJ 2002324588–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nowak G, Ungerstedt J, Wernerson A, et al. Hepatic cell membrane damage during cold preservation sensitizes liver grafts to rewarming injury. J Hepatobiliary Pancreat Surg 200310200–205 [DOI] [PubMed] [Google Scholar]
- 8.Rooyackers O, Thorell A, Nygren J, et al. Microdialysis methods for measuring human metabolism. Curr Opin Clin Nutr Metab Care 20047515–521 [DOI] [PubMed] [Google Scholar]
- 9.Ungerstedt J, Nowak G, Ungerstedt U, et al. Microdialysis monitoring of porcine liver metabolism during warm ischemia with arterial and portal clamping. Liver Transpl 200915280–286 [DOI] [PubMed] [Google Scholar]
- 10.Waelgaard L, Thorgersen EB, Line PD, et al. Microdialysis monitoring of liver grafts by metabolic parameters, cytokine production, and complement activation. Transplantation 2008861096–1103 [DOI] [PubMed] [Google Scholar]
- 11.Isaksson B, D’Souza MA, Jersenius U, et al. Continuous assessment of intrahepatic metabolism by microdialysis during and after portal triad clamping. J Surg Res 2011169214–219 [DOI] [PubMed] [Google Scholar]
- 12.Nowak G, Ungerstedt J, Wernerman J, et al. Clinical experience in continuous graft monitoring with microdialysis early after liver transplantation. Br J Surg 2002891169–1175 [DOI] [PubMed] [Google Scholar]
- 13.Hovda DA, Lee SM, Smith ML, et al. The neurochemical and metabolic cascade following brain injury: moving from animal models to man. J Neurotrauma 199512903–906 [DOI] [PubMed] [Google Scholar]
- 14.Gillispie A, Rooyackers O, Wernerman J, et al. Effect of extended cold ischemia time on glucose metabolism in liver grafts: experimental study in pigs. J Hepatobiliary Pancreat Surg 200714183–188 [DOI] [PubMed] [Google Scholar]
- 15.Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation 198845673–676 [DOI] [PubMed] [Google Scholar]
- 16.Hillered L, Persson L. Neurochemical monitoring of the acutely injured human brain. Scand J Clin Lab Invest Suppl 19992299–18 [DOI] [PubMed] [Google Scholar]
- 17.Nilsson OG, Brandt L, Ungerstedt U, et al. Bedside detection of brain ischemia using intracerebral microdialysis: subarachnoid hemorrhage and delayed ischemic deterioration. Neurosurgery 1999451176–1184discussion 1184–1185 [DOI] [PubMed] [Google Scholar]
- 18.Håugaa H, Thorgersen EB, Pharo A, et al. Early bedside detection of ischemia and rejection in liver transplants by microdialysis. Liver Transpl 201218839–849 [DOI] [PubMed] [Google Scholar]
- 19.Nowak G, Ungerstedt J, Wernerman J, et al. Metabolic changes in the liver graft monitored continuously with microdialysis during liver transplantation in a pig model. Liver Transpl 20028424–432 [DOI] [PubMed] [Google Scholar]
- 20.Inomoto T, Tanaka A, Awane M, et al. Changes in glucose transporter 2 and carbohydrate-metabolizing enzymes in the liver during cold preservation and warm ischemia. Transplantation 199661869–874 [DOI] [PubMed] [Google Scholar]
- 21.Voet D, Voet JG. Biochemistry. 2nd ed. New York, NY: John Wiley & Sons Inc.; 1995. [Google Scholar]


