Abstract
Cytomegalovirus (CMV) gastritis is a rare opportunistic infection with diverse clinical manifestations. Our study aimed to investigate the clinical features of Chinese patients with CMV gastritis.
Six inpatients diagnosed with CMV gastritis were retrospectively enrolled, based on the finding of inclusion bodies in routine hematoxylin and eosin staining or positive anti-CMV monoclonal antibodies under immunohistochemistry in the gastric biopsy. Data, including demographics, diagnostic measurements, and medications, were collected.
Abdominal pain was the most frequently reported symptom, occurring in 4 patients. Five patients were immunocompromised with associated underlying diseases, and 3 patients had decreased leukocyte differentiation antigen 4 positive (CD4+) T lymphocyte counts. Only 3 patients had either positive cytomegalovirus (CMV)-immunoglobulin (Ig) M or increased copies of CMV-DNA peripherally. All patients had gastric lesions in the antrum of the stomach, including ulcers or erosions observed by gastroscopy. All patients received ganciclovir by intravenous injection (IV) as the first line anti-CMV therapy, and attained complete (4) or partial remission (2) during the follow-up.
CMV gastritis should be taken into consideration in patients with immunocompromised status who have abdominal pain, nausea, or vomiting. Gastroscopy and necessary biopsy are the major diagnostic methods for CMV gastritis. Early diagnosis leads to a better prognosis for these patients.
Keywords: clinical manifestation, cytomegalovirus, gastritis, infection
1. Introduction
Cytomegalovirus (CMV) is a DNA virus belonging to the Herpesviridae family. The seroprevalence of CMV, indicating previous or current CMV infection, varies from 40% to 100% depending on the population studied or detection method used.[1–3] One study from Wuhan in China revealed 76.1% of patients with inflammatory bowel disease were CMV- immunoglobulin (Ig) G positive, compared with 50.7% of healthy controls.[4] Although acute or reactivated CMV infection can occur in immunocompetent patients, immunocompromised status still presents the most important risk factor for CMV infection, especially for disseminated CMV infection.[2–5]
Gastrointestinal involvement in CMV infection is very common, with the colon being the most frequently affected site.[5] However, upper gastrointestinal tract involvement, especially CMV gastritis, has rarely been recognized or reported, and there have been only a very few case reports or case series with small sample sizes.[6] CMV gastritis can cause life-threatening complications, for example, perforation and hemorrhagic shock.[7–9] Early diagnosis and timely medication could effectively improve the prognosis of patients. Therefore, we performed this retrospective study to describe the clinical, endoscopic, and histopathologic features of CMV gastritis, and provide more information for clinical practice. To the best of our knowledge, this is the first recorded case series in Chinese patients with CMV gastritis.
2. Methods
2.1. Patients
By searching the inpatient database of Peking Union Medical College Hospital from January 2007 to December 2017, a tertiary teaching hospital in Beijing, China, patients with the diagnosis of “cytomegalovirus disease,” “cytomegalovirus pneumonitis,” “cytomegaloviral hepatitis,” “other cytomegaloviral diseases,” “cytomegalovirus disease,” or “cytomegalovirus retinitis” based on the the ninth revision of the international classification of diseases or the tenth revision of the international classification of diseases codes were screened. Finally, 6 patients with definite cytomegalovirus gastritis were retrospectively enrolled, who had either inclusion bodies in routine hematoxylin and eosin staining or stained positive with anti-CMV monoclonal antibody immunohistochemistry in gastric biopsy specimens. Disseminated CMV disease was defined as involvement of ≥1 organs besides gastrointestinal tract. All patients were followed for at least 6 months either by checking medical records or telephone connection. The study was approved by the Ethical Committee of Peking Union Medical College Hospital (S-k590).
2.2. Data collection
The following data of the participants were collected: demographics (sex and age), underlying systemic diseases, medication history of immunosuppressive agents, clinical manifestations (general and gastrointestinal symptoms), laboratory tests, radiologic features, endoscopic abnormalities, histopathologic features, treatment, and outcomes.
Laboratory tests included complete blood cell count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), serum lactate dehydrogenase (LDH), serum CMV-IgM, CMV-IgG and cytomegalovirus pp65 antigenemia (CMVpp65), CMV-DNA load, serum Ig (IgG, IgM, or IgA), and lymphocyte subsets (CD4+T cells, CD8+T cells, and activated CD8+T cells [CD38+CD8+T cells]).
Gastroscopy abnormalities were recorded, such as the type of lesions (ulcer, erosion, hyperemia, or edema), sites of ulcer involvement (the esophagus, cardia, fundus, corpus, antrum of stomach, or duodenum), solitary or multifocal ulcers, and the shape and size of ulcers. Multiple ulcers were defined as ≥2 in number, whereas a large ulcer was a solitary ulcer >2 cm in diameter.[10]
Histopathologic features of the biopsy specimen from the ulcers or erosions of the stomach or duodenum specified focused on the detection of virus inclusion bodies by routine hematoxylin and eosin staining and immunohistochemistry staining using anti-CMV monoclonal antibodies.
The medication history, including antiviral therapy (ganciclovir or foscarnet) or intravenous immunoglobulin (IVIG), was also collected. Outcome was evaluated according to the clinical symptoms (partial or complete remission) and gastroscopic appearances (partial or complete healing). Partial healing was defined as a decrease in the number and size of ulcerations and erosions, whereas complete healing was defined as clearance of a white-coated ulcer with covering epithelium or complete resolution of the lesion.
2.3. Statistical analysis
IBM SPSS Statistics 22.0 (IBM Corp., Illinois, USA) was used for data analysis. Continuous variables with normal distribution and non-normal distribution are presented as mean ± standard deviation (SD) and median (range), respectively.
3. Results
3.1. Demographics, clinical, and laboratory features of patients with CMV gastritis
Table 1 shows the demographic, clinical, and routine laboratory test data of 6 patients with CMV gastritis.
Table 1.
Demographic, clinical, and laboratory features of 6 patients with cytomegalovirus gastritis.

The average age at diagnosis was 54 ± 17 years. The ratio of male to female patients was 2:1. Abdominal pain was the most frequently reported symptom, occurring in 4 patients. Other symptoms included fatigue (4), nausea (3), vomiting (3), diarrhea (2), fever (2), and weight loss (2). Patient 4 only experienced fatigue and consented to gastroscopy due to a high level of cancer antigen 72–4 (CA72–4). Three patients had disseminated CMV infection, including pneumonia, retinitis, or hypocytosis.
Five patients were immunocompromised with underlying associated diseases as follows: dermatomyositis (2), combined variable immune deficiency (CVID, 1), type I membranoproliferative glomerulonephritis (MPGN) (1), and primary gastric diffuse large B-cell lymphoma (1). Upon the onset of CMV gastritis, 3 patients were still receiving corticosteroid or immunosuppressants.
Four patients had moderate to severe anemia and 3 patients had lymphocytopenia. Four patients had elevated levels of ESR and LDH and 3 patients had increased CRP. The lymphocyte subsets of 5 patients were analyzed. Three patients had decreased CD4+ T lymphocyte counts, which were <200/μL in patients 1 and 2. Three out of 5 patients had an increased percentage of CD38+CD8+ T lymphocytes among the CD8+ T lymphocytes. Three patients had decreased B lymphocyte counts. For the serum level of immunoglobulin, only patient 2 with CVID had very low levels of IgG, IgM, and IgA. All patients were negative for HIV, hepatitis B, and hepatitis C on screening.
3.2. Diagnostic measurements and medications of patients with CMV gastritis
The diagnostic measurements (serology CMV test and histopathologic findings), medications, and outcomes of patients are presented in Table 2.
Table 2.
Diagnostic measurements and medications of the 6 cases of cytomegalovirus gastritis.

For the serology tests of CMV, only 1 patient out of the 5 tested had positive CMV-IgM, and none had positive CMVpp65 antigen. Two patients had increased copy numbers of peripheral CMV-DNA. In all, 3 of the 6 patients had either positive CMV-IgM or increased copies of CMV-DNA.
Nonspecific abdominal adenopathy was the most common finding in enhanced computerized tomography (CT) scans of the abdomen. Gastric wall thickening was found in 2 patients. Patient 6 had a striking thickness of the gastric antrum wall of >2.0 cm, with hypermetabolic uptake and an standardized uptake value max of 30.45 under positron emission tomography/computed tomography (PET/CT), as shown in Fig. 1. Histopathological examination of >5 repeated biopsies taken by different methods, including endoscopic mucosal resection (EMR) and deep bites, did not find any dysplasia or malignant cells.
Figure 1.
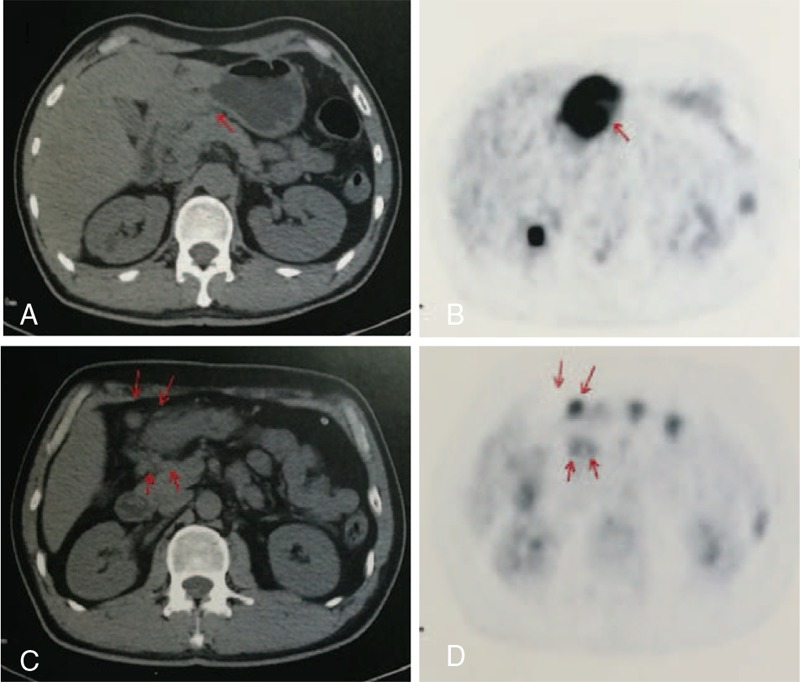
Positron Emission Tomography/Computed Tomography (PET/CT) of Patient 6. PET/CT revealed a thickened antrum wall (A, arrow) by CT scan and hypermetabolic involvement (B, arrow) by PET, respectively; hence, lymphadenopathy was diagnosed (C and D, red arrow).
Under gastroscopy (Fig. 2), all patients had gastric lesions in the stomach, including ulcers and hyperemia in 5 patients and multiple erosions in 1 patient. Multiple ulcers with irregular or oval shape and variable size from 0.5 to 1.5 cm were observed in 4 patients. Involvement of the gastric antrum could be seen in all patients. Esophageal and duodenal ulcers occurred in patients 3 and 2, respectively. For the histopathologic features (Fig. 3), inclusion bodies were observed in the biopsy samples of 4 patients. All (5/5) patients had positive immunohistochemistry staining using anti-CMV monoclonal antibodies.
Figure 2.
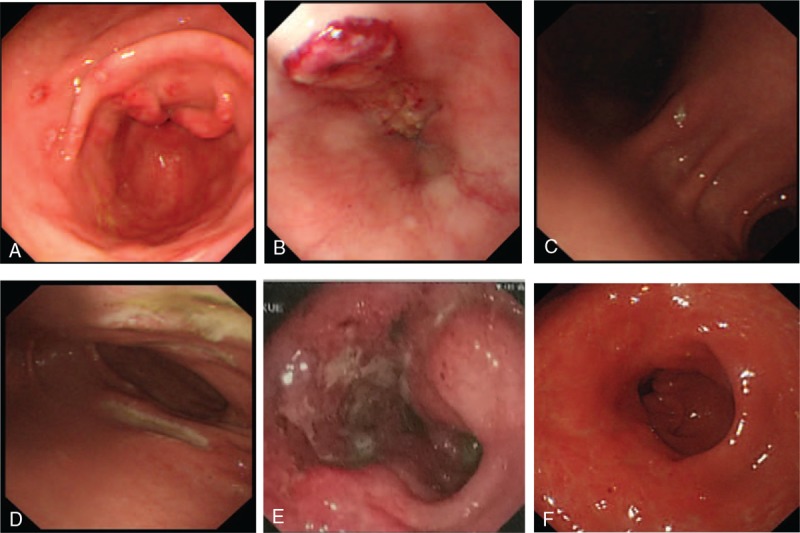
Illustrations of gastroscopic features. (A) Multiple superficial ulcers in the antrum 0.5 cm in size (Patient 3). (B) Irregular deep ulcer in the esophagus (Patient 3). (C) Oval superficial ulcer in the gastric angle (Patient 2). (D) Multiple large ulcers in the duodenum (Patient 2). (E) Large irregular deep ulcer in the gastric antrum (Patient 6). (F) Large ulcer in the gastric antrum and healing after antiviral therapy follow-up (Patient 6).
Figure 3.
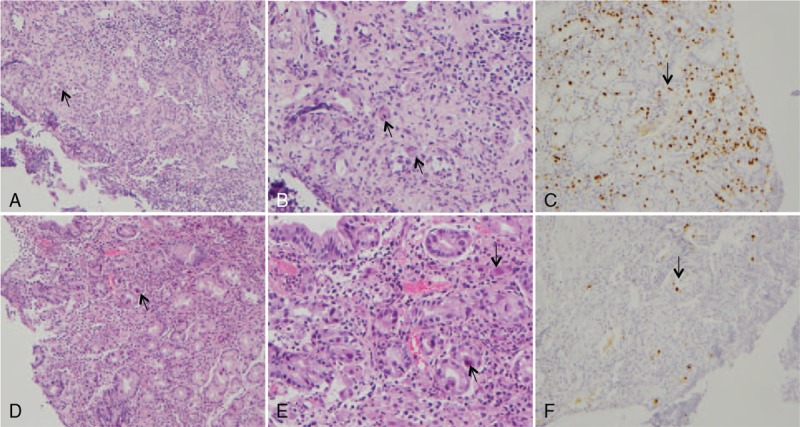
Histopathology of cytomegalovirus (CMV) gastritis in Patient 3. (A) Histological detection of CMV inclusion bodies (arrow), biopsy specimen of a gastric ulcer. Hematoxylin and eosin. ×100. (B) Histological detection of CMV inclusion bodies (arrow), biopsy specimen of a gastric ulcer. Hematoxylin and eosin. ×200. (C) Positive CMV immunohistochemistry (arrow). Biopsy specimen of a gastric ulcer. × 100. (D) Histological detection of CMV inclusion bodies (arrow), biopsy specimen of an esophageal ulcer. Hematoxylin and eosin. × 100. (E) Histological detection of CMV inclusion bodies (arrow), biopsy specimen of an esophageal ulcer. Hematoxylin and eosin. ×200. (F) Positive CMV immunohistochemistry (arrow), biopsy specimen of an esophageal ulcer. ×100.
All patients received ganciclovir IV as the first line anti-CMV therapy. The dosage is 5 mg/kg every 12 hours for patients with normal renal function and 2.5 mg/kg every 12 hours for patients with renal insufficiency. The duration of anti CMV medication varied from 3 to 16 weeks. Patient 2 also received foscarnet as the combination medication. IVIG was administered to 2 patients. All patients achieved complete (4/6) or partial remission (2/6) and ulcer healing was observed in 4 out of 5 patients during the follow-up gastroscopies.
4. Discussion
Cytomegalovirus (CMV), a β herpes virus with double-stranded DNA, had a high seroprevalence of 40% to 100% in various populations.[1] CMV gastritis is a rare and under-diagnosed disease, usually presenting as a co-comorbidity of disseminated CMV infection. The prevalence of CMV infection of the upper gastrointestinal tract has not been determined. To improve the recognition and early diagnosis of CMV gastritis in Chinese patients, this study retrospectively enrolled 6 patients with CMV gastritis. We found that CMV gastritis could be an aspect of disseminated CMV infection and our patients shared many features in common with those of previous studies, including middle-aged onset, male predominance, abdominal pain as the most common symptom, the antrum of stomach as the most frequently involved site, unspecific multiple ulcers in stomach, and resolution following at least 3 weeks of treatment with ganciclovir.[1,6,10–12]
It is well known that aberrant cell-mediated immunity contributes to the reactivation of CMV infection.[13,14] Immunosuppressive diseases (acquired immune deficiency syndrome, lymphoma, organ transplantation, or rheumatoid arthritis) or exposure to immunosuppressive agents are the most important triggers for the CMV reactivation. The monitoring of lymphocyte subsets, specifically the number of CD4+ T lymphocytes, is crucial for the evaluation of cell-mediated immunity. The consensus on solid organ transplantation recommended that prophylaxis with oral valganciclovir be advocated in HIV-infected patients with a CD4+ T cell count <50/mm3 for immunocompromised patients.[15,16] Beyond the number of CD4+ T cells, the increased percentage of activated CD38+CD8+ T lymphocytes in the total CD8+ T lymphocyte population also suggests virus infection. In this study, 3 patients had a decreased number of CD4+ T lymphocytes and B lymphocytes and also had an increased percentage of activated CD8+ T lymphocytes.
At the same time, CMV gastritis can occur in apparently immunocompetent patients and there is no consensus on the time-point for starting prophylaxis. In one case series study, 4 out of 30 patients with CMV infection with upper gastrointestinal tract involvement were diagnosed as harboring malignant lesions of various organs approximately 5 to 16 months after the histological detection of CMV infection,[6] which might indicate a potential immune disturbance in these patients. However, the correlation between CMV infection and malignancy is still undetermined.[17] CMV colitis is known to cause disease flare-ups, increasing the risk of steroid-refractory colitis, which suggests that CMV infection can exacerbate the symptoms or endoscopic appearances of underlying diseases.[18,19] CMV gastritis could mimic and overlap with gastric malignancy in this study, as shown in patients 1 and 6. Patient 6 was an immunocompetent patient with a large ulcer in the antrum, obviously thickened wall observed under CT scan, and hypermetabolic involvement under PET/CT. EMR and repeated biopsies revealed no malignant cells. The complete remission of symptoms and ulcer healing in the antrum further supported the diagnosis of CMV gastritis without malignancy. Patient 1 experienced a partial improved of symptoms and gastroscopic findings after treatment with ganciclovir; however, repeated biopsies lead to a final diagnosis of non-Hodgkin lymphoma. Therefore, long term follow-ups of immunocompetent patients with CMV gastritis are required to determine whether there is underlying disease, regardless of apparent recovery.
The diagnosis of CMV gastritis should be taken into consideration when immunocompromised patients suffer from epigastric pain and nausea, with diagnosis dependent on gastroscopy and taking biopsies. The diagnostic potential of serum CMV-IgM antibody, antigen, and CMV viral load for CMV gastritis was relatively limited. One Japanese study reported that only 50% of patients with rheumatoid arthritis and CMV infection of the upper gastrointestinal tract had positive CMV antigenemia.[20] Another cohort study also reported that the sensitivity of the CMV antigenemia assay for the diagnosis of CMV gastrointestinal disease was 54% with 95% confidence interval (41%–68%).[21] Our study revealed no positive CMV antigenemia in 6 patients, only 1 out of 5 patients with positive CMV-IgM, and 2 out of 5 patients with positive CMV viral load. Thus, CMV gastritis must not be ignored or excluded where patients have no positive CMV antigenemia, IgM antibody, or increased CMV viral load.
There are some limitations of the current study. First, due to the rarity and underestimation, only 6 cases were collected and the small sample size greatly limited the strength of our study, for example, in evaluating the value of serum CMV tests, the epidemiology data, and the risk factors of CMV gastritis. Second, all patients being recruited from 1 center definitely caused a selection bias. Third, some studies involving solid organ transplantation recipients reported that monitoring of CMV specific CD4+ T and CD8+ T cells might help to identify patients at higher risk of developing CMV reactivation.[22,23] Unfortunately, we were unable to conduct this test during the study period. Last, CMV gastritis could mimic or overlap with malignant gastric ulcers, but our study failed to reveal further indicators of use in differential diagnosis, limited by the small sample size. Therefore, multicenter prospective studies with larger numbers of CMV gastritis patients would be helpful for clinicians during the differential diagnosis and treatment process in the future.
In clinical practice, CMV gastritis might be an aspect of disseminated CMV infection, and also could be the sole involved site of CMV infection. It might be underestimated or mis-diagnosed, especially in compromised patients with abdominal pain and other gastrointestinal symptoms. Although gastroscopy and taking necessary biopsies are invasive procedures, they are still the major diagnostic methods for CMV gastritis up to now. The establishment of novel markers for the monitoring of the immunity status in immunocompromised patients is key for its early diagnosis and prophylaxis.
Author contributions
Conceptualization: Dan Chen, Ruijie Zhao, Wei Cao, Weixun Zhou, Ying Jiang, Shangzhu Zhang, Yang Chen, Guijun Fei, Ji Li, Jiaming Qian.
Data curation: Dan Chen, Ruijie Zhao, Wei Cao, Weixun Zhou, Ying Jiang, Shangzhu Zhang, Yang Chen, Guijun Fei, Ji Li, Jiaming Qian.
Formal analysis: Ji Li.
Investigation: Dan Chen, Ruijie Zhao, Ji Li, Jiaming Qian.
Methodology: Dan Chen, Ruijie Zhao, Wei Cao, Weixun Zhou, Ying Jiang, Shangzhu Zhang, Yang Chen, Guijun Fei, Ji Li, Jiaming Qian.
Project administration: Ji Li, Jiaming Qian.
Supervision: Ji Li, Jiaming Qian.
Writing – original draft: Dan Chen, Ruijie Zhao, Shangzhu Zhang, Yang Chen, Ji Li.
Writing – review & editing: Wei Cao, Weixun Zhou, Ying Jiang, Guijun Fei, Ji Li, Jiaming Qian.
Footnotes
Abbreviations: CA72–4 = cancer antigen 72–4, CMV = cytomegalovirus, CMV-pp65 = cytomegalovirus pp65 antigenemia, CRP = C-reactive protein, CTX = cyclophosphamide, CVID = combined variable immune deficiency, DM = dermatomyositis, EMR = endoscopic mucosal resection, ESR = erythrocyte sedimentation rate, Ig = immunoglobulin, IHC = immunohistochemistry, IV = intravenous injection, IVIG = intravenous immunoglobulin, LDH = lactate dehydrogenase, MPGN = membranous proliferative glomerulonephritis, NA = not available, NHL = non-Hodgkin lymphoma, PCR = polymerase chain reaction, PET/CT = positron emission tomography/computed tomography, PSC = primary sclerosing cholangitis, SD = standard deviation, WBC = white blood cell.
How to cite this article: Chen D, Zhao R, Cao W, Zhou W, Jiang Y, Zhang S, Chen Y, Fei G, Li J, Qian J. Clinical characteristics of cytomegalovirus gastritis: A retrospective study from a tertiary medical center. Medicine. 2020;99:5(e18927).
JQ and JL and DC and RZ have contributed equally for this paper.
This work was supported by grants from Health Research and Special Projects (No. 201002020) and Education Reform Projects of Peking Union Medical College (No. 2017zlgc0110). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Rafailidis PI, Mourtzoukou EG, Varbobitis IC, et al. Severe cytomegalovirus infection in apparently immunocompetent patients: a systematic review. Virol J 2008;5:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].You DM, Johnson MD. Cytomegalovirus infection and the gastrointestinal tract. Curr Gastroenterol Rep 2012;14:334–42. [DOI] [PubMed] [Google Scholar]
- [3].Bernard S, Germi R, Lupo J, et al. Symptomatic cytomegalovirus gastrointestinal infection with positive quantitative real-time PCR findings in apparently immunocompetent patients: a case series. Clin Microbiol Infect 2015;21:1121.e1–7. [DOI] [PubMed] [Google Scholar]
- [4].Yi F, Zhao J, Luckheeram RV, et al. The prevalence and risk factors of cytomegalovirus infection in inflammatory bowel disease in Wuhan, Central China. Virol J 2013;10:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gravito-Soares E, Almeida N. Cytomegalovirus disease of the upper gastrointestinal tract: an emerging infection in immunocompetent hosts. GE Port J Gastroenterol 2017;24:259–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bonetti LR, Losi L, Gregorio CD, et al. Cytomegalovirus infection of the upper gastrointestinal tract: a clinical and pathological study of 30 cases. Scand J Gastroenterol 2011;46:1228–35. [DOI] [PubMed] [Google Scholar]
- [7].Marques S, Carmo J, Pinto D, et al. Cytomegalovirus disease of the upper gastrointestinal tract: a 10-year retrospective study. GE Port J Gastroenterol 2017;24:262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Aqel NM, Tanner P, Drury A, et al. Cytomegalovirus gastritis with perforation and gastrocolic fistula formation. Histopathology 1991;18:165–8. [DOI] [PubMed] [Google Scholar]
- [9].Howaizi M, Abboura M, Sbai-Idrissi MS, et al. Case report: cytomegalovirus-associated perforated gastric ulcer healing under antiviral therapy. Dig Dis Sci 2002;47:2380–2. [DOI] [PubMed] [Google Scholar]
- [10].Lin WR, Su MY, Hsu CM, et al. Clinical and endoscopic features for alimentary tract cytomegalovirus disease: report of 20 cases with gastrointestinal cytomegalovirus disease. Chang Gung Med J 2005;28:476–84. [PubMed] [Google Scholar]
- [11].Himoto T, Goda F, Okuyama H, et al. Cytomegalovirus-associated acute gastric mucosal lesion in an immunocompetent host. Intern Med 2009;48:1521–4. [DOI] [PubMed] [Google Scholar]
- [12].Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med 1993;119:924–35. [DOI] [PubMed] [Google Scholar]
- [13].Terrazzini N, Kern F. Cell-mediated immunity to human CMV infection: a brief overview. F1000Prime Rep 2014;6:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang ECY, Pjechova M, Nightingale K, et al. Suppression of costimulation by human cytomegalovirus promotes evasion of cellular immune defenses. Proc Natl Acad Sci USA 2018;115:4998–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kotton CN, Kumar D, Caliendo AM, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2013;96:333–60. [DOI] [PubMed] [Google Scholar]
- [16].Nasa M, Sharma Z, Sud R, et al. Cytomegalovirus infection of gastrointestinal tract. Community Acquir Infect 2016;3:4–9. [Google Scholar]
- [17].Cinatl J, Jr, Vogel JU, Kotchetkov R, et al. Oncomodulatory signals by regulatory proteins encoded by human cytomegalovirus: a novel role for viral infection in tumor progression. FEMS Microbiol Rev 2004;28:59–77. [DOI] [PubMed] [Google Scholar]
- [18].Nakase H, Honzawa Y, Toyonaga T, et al. Diagnosis and treatment of ulcerative colitis with cytomegalovirus infection: importance of controlling mucosal inflammation to prevent cytomegalovirus reactivation. Intest Res 2014;12:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Roblin X, Pillet S, Oussalah A, et al. Cytomegalovirus load in inflamed intestinal tissue is predictive of resistance to immunosuppressive therapy in ulcerative colitis. Am J Gastroenterol 2011;106:2001–8. [DOI] [PubMed] [Google Scholar]
- [20].Ozaki T, Yamashita H, Kaneko S, et al. Cytomegalovirus disease of the upper gastrointestinal tract in patients with rheumatic diseases: a case series and literature review. Clin Rheumatol 2013;32:1683–90. [DOI] [PubMed] [Google Scholar]
- [21].Jang EY, Park SY, Lee EJ, et al. Diagnostic performance of the cytomegalovirus (CMV) antigenemia assay in patients with CMV gastrointestinal disease. Clin Infect Dis 2009;48:e121–4. [DOI] [PubMed] [Google Scholar]
- [22].Chiereghin A, Potena L, Borgese L, et al. Monitoring of cytomegalovirus (CMV)-specific cell-mediated immunity in heart transplant recipients: clinical utility of the QuantiFERON-CMV assay for management of posttransplant CMV infection. J Clin Microbiol 2018;56:e01040–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fernández-Ruiz M, Giménez E, Vinuesa V, et al. Regular monitoring of cytomegalovirus (CMV)-specific cell-mediated immunity in intermediate-risk kidney transplant recipients: predictive value of the immediate post-transplant assessment. Clin Microbiol Infect 2019;25:381.e1–0. [DOI] [PubMed] [Google Scholar]


