Supplemental Digital Content is available in the text
Keywords: anti-vascular endothelial growth factor (VEGF), bevacizumab (Avastin), retinal arterial macroaneurysm
Abstract
Retinal arterial macroaneurysms (RAMs) develop as outpouchings of the arterial wall that is weakened by arteriosclerosis. The traditional treatment of RAMs comprises observation, focal laser photocoagulation, or surgery. Recently, intravitreal injection of anti-vascular endothelial growth factor (VEGF) drugs has been announced as an effective therapy for fovea-threatening RAMs and quickly improve visual acuity and central retinal thickness (CRT).
In the retrospective series, medical charts and ocular images of 24 patients diagnosed as having RAM between May 2011 and November 2018 in our facility were reviewed to delineate clinical manifestations and visual prognosis in RAM patients receiving different treatment modalities. Twenty-four patients (25 eyes; 11 men and 13 women) were enrolled, and one eye with comorbidity of branch retinal vein occlusion was excluded. The mean age of the patients was 69.00 ± 13.45 years. Fourteen patients (58.33%) had a history of hypertension, and 17 patients (70.83%) were aged > 60 years. Furthermore, patients with fovea-threatening RAMs presented with either hypertension or were aged > 60 years.
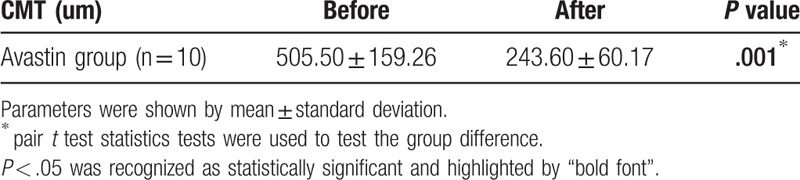
Eyes with fovea involvement (n = 18) were analyzed and separated into two groups according to their treatment modalities: those receiving anti-VEGF intravitreal injections (n = 13) and observation only (n = 5). The baseline visual acuity revealed no significant difference in the two groups. In patients receiving anti-VEGF intravitreal injections, a significantly better visual acuity was detected after anti-VEGF intravitreal injections than the baseline visual acuity (logMAR, 0.78 ± 0.51 vs 1.52 ± 0.48, P < .001), and CRT significantly improved (505.50 ± 159.26 μm vs 243.60 ± 60.17 μm, P = .001). Patients receiving anti-VEGF intravitreal injections also revealed better final visual acuity than those in the observation group (logMAR, 0.78 ± 0.51 vs 1.34 ± 0.48, P = .04).
A systematic work-up for hypertension and arteriosclerotic disease could be considered the recommended procedure once RAM has been diagnosed. With better final visual acuity, significant visual improvements, and fast reduction of CRT observed in patients with fovea-threatening RAMs receiving anti-VEGF intravitreal injections, intravitreal anti-VEGF was considered an effective therapy for complicated RAM. During the follow-up period, the majority of RAM eyes had good maintenance of visual function even with foveal complications.
1. Introduction
Retinal arterial macroaneurysms (RAMs) are acquired, focal dilatations of retinal arterial branches and develop as an outpouching of the arterial wall, which has been weakened by arteriosclerosis.[1] Associated systemic conditions include hypertension and arteriosclerotic disease, and elderly women were reported to be the most commonly affected group.[2,3] RAM is usually found in the temporal retina within the first three orders of bifurcation of the retinal arterial vasculature, and the size can range from 100 to 250 μm in diameter.[4,5] Associated findings of RAM include capillary telangiectasias, vascular remodeling, and retinal edema. In clinical practice, RAM can be classified as hemorrhagic or exudative according to the presence or absence of subretinal/intraretinal fluid, hard exudates, or hemorrhages.[3,5]
Diagnosis of RAM is based on clinical examination and via imaging modalities such as fluorescein angiography (FA) and spectral-domain optical coherence tomography (SD-OCT), and these special investigations can quantify the exudates in complicated RAM. [1,4,5] However, there are no approved guidelines for treating RAM.[6,7] Most RAMs resolve spontaneously, and observation alone is sufficient; however, the treatment of complicated RAMs with macular edema or hemorrhage is controversial. Therapeutic interventions other than observation for treating RAM and related complications include direct photocoagulation, pneumatic displacement with tissue plasminogen activator, surgical removal of hemorrhage with pars plana vitrectomy, and photodisruption of the internal limiting membrane (ILM) or the posterior hyaloid using neodymium:yttrium-aluminum-garnet (Nd:YAG) or argon laser to release the extravasated blood.[8–14]
Although visual prognosis is generally good in RAM, vision loss can occur from macular edema or hemorrhage due to rupture of the aneurysm.[4,5] Recently, the use of anti-VEGF intravitreal injections has been announced as an effective therapy for complicated RAM with macular involvement, quickly improving best-corrected visual acuity (BCVA) and increasing central retinal thickness (CRT).[4,7,15] Some case reports and studies have also reported encouraging results on intravitreal anti-VEGF agents for RAMs in recent years.[16–19]
As far as RAMs are concerned, there is still no consensus in terms of treatment, and the results of different modes of management vary. Although the use of intravitreal anti-VEGF agents is an available alternative option, only few case reports/series describe its benefits in patients with RAMs, and there are no standard protocols for their use. Hence, in this retrospective series, we aimed to delineate the clinical manifestations and visual prognosis of RAM patients treated with different modalities at our facility.
2. Methods
Medical charts and ocular images of patients diagnosed with RAM who were treated with either anti-VEGF intravitreal injection or observation only between May 2011 and November 2018 in Chang Gung Memorial Hospital (CGMH), Kaohsiung, Taiwan were retrospectively reviewed. A total of 24 patients (25 RAMs) were enrolled in our study, and we excluded one eye with comorbidity of branch retinal vein occlusion. All procedures involving human participants adhered to the tenets of the Declaration of Helsinki. Institutional review board/ethics committee approval was obtained from the committee of medical ethics and human experiments of CGMH.
2.1. Diagnosis
Three experienced retina specialists (CWY, CCH, and KHK) made the diagnosis of RAM based on the fundus examination and the results of FA. Round or fusiform dilation of a retinal arteriole with either hemorrhages or exudates could be seen on the fundus examination, and the angiograms typically showed focal hyperfluorescent dilation of the arteriole in the early arterial phase, and staining of the vessel walls may be found in the later phase.[1,5]
2.2. Inclusion and exclusion criteria
Patients who met the following criteria were included in this study:
-
(1)
presence of RAM in either or both eyes confirmed by fundus examination and FA;
-
(2)
no history of intravitreal injections; and
-
(3)
a minimum follow-up period of 6 months, and the interval between the last injection and the last follow-up is at least 3 months.
Patients who met the following criteria were excluded:
-
(1)
history of other ocular or systemic comorbidity that may affect visual acuity and macular thickness;
-
(2)
complicated with a breakthrough vitreous hemorrhage;
-
(3)
administration of treatment other than intravitreal bevacizumab injection; or
-
(4)
previous vitreoretinal surgery. No limits on visual acuity were set for inclusion or exclusion criteria.
With patients with symptomatic RAMs (fovea involvement), treatment groups were defined according to the treatment modalities:
-
(1)
the anti-VEGF group, in which patients received anti-VEGF intravitreal injections, and
-
(2)
the observation group, in which patients underwent observation only and were regularly followed but not treated.
We furthered classified the RAMs as hemorrhagic or exudative following a protocol first used by Moosavi et al in their study of 34 patients with RAMs.[1,5]
2.3. Treatment modalities
Patients with macula-threatening RAMs were offered the treatment of intravitreal anti-VEGF injection. The treatment decision was made by the patient. In the observation group (patients with hesitation about the treatment), regular monthly follow-up was arranged. In the anti-VEGF group, the patients all received intravitreal bevacizumab injection (Avastin; 1.25 mg/0.05 mL; F. Hoffmann-La Roche Ltd., Wurmisweg, Kaiseraugst, Switzerland). The patients were informed about the potential risks, benefits, and the off-label nature of the drug. Informed consent was obtained from all patients before intravitreal injection of bevacizumab. If persistent macula-threatening subretinal fluid or hemorrhage was observed on OCT at least 1 month after the previous injection, retreatment with further intravitreal bevacizumab injection was considered an option.
2.4. Outcome measurements
Similar to a protocol first conducted by Pichi,[7] we included the mean change in the visual acuity and CRT from the first to the final visit as outcome measurements. Visual acuity were assessed using the Landolt C chart and recorded after conversion to logarithm of the minimal angle of resolution (logMAR) values for statistical analysis; CRT was measured using SD-OCT (Spectralis OCT; Heidelberg Engineering GmbH, Heidelberg, Germany). All SD-OCT scans were performed with a scan rate of 40,000 A-scans per second within a 4.5 × 6.0 mm area.[7,20] With the use of image alignment software, subsequent follow-up scans were aligned with the precise location of the original scan. The above outcome measurements were performed in a blind manner by examiners who did not know the treatment of the patient.
2.5. Statistical analysis
All statistical analyses were performed using SPSS version 22 and Microsoft Excel 2010. To determine the statistical differences for target parameters between groups, the Mann-Whitney U test was used to analyze the statistical differences in age (years) and visual acuity (logMAR), Chi-square test was applied on nonparametric variables such as sex (woman/man), lesion eye (OD/OS), location (ST/IT), and complication type (hemorrhagic/exudative). The paired-t test was used to test the statistical differences of visual acuity (logMAR) before and after the treatment on the same patient. Statistical significance was set at P < .05.
3. Results
A total of 24 patients (25 RAMs) were enrolled in our study, and we excluded 1 eye with comorbidity of branch retinal vein occlusion. The baseline characteristics of the 24 eyes in 24 patients (mean age 69.00 ± 13.45 years, 11 men/13 women) are presented in Table 1. Fourteen patients (58.33%) had a history of hypertension, and 17 patients (70.83%) were aged > 60 years. Only 3 patients had no history of hypertension and were aged < 60 years, but 2 of them had heart disease and one had breast cancer. Furthermore, all patients with fovea-threatening RAMs presented with either hypertension or were aged > 60 years. The RAMs were equally prevalent in the right or left eye, and they were all observed in the temporal half of the retina, with a higher distribution in the superotemporal (14/24, 58.33%) than in the inferotemporal arcades (10/24, 41.67%). Sixteen out of 24 eyes (66.67%) showed various hemorrhagic complications and the other 8 (33.33%) had minor hemorrhagic complications but showed extensive exudative changes. Eighteen out of 24 eyes (75%) involved the fovea.
Table 1.
Demographic data of the study patients with retinal arterial macroaneurysms.
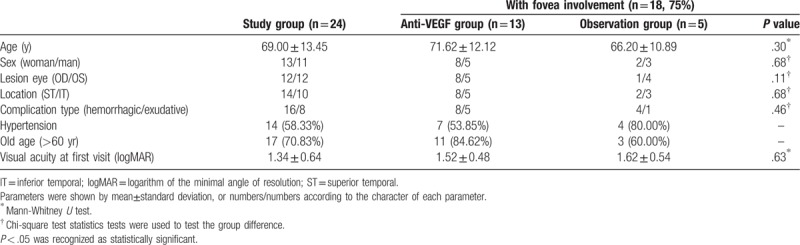
Patients with fovea involvement (n = 18) were analyzed and further separated into 2 groups according to their treatment modalities, those on anti-VEGF intravitreal injections (n = 13) and those on observation only (n = 5) (Tables 1 and 2). The demographic characteristics and the baseline visual acuity revealed no statistically significant difference between the 2 groups (logMAR, anti-VEGF group vs observation group, 1.52 ± 0.48 vs 1.62 ± 0.54, P = .63). The number of injections ranged from 1 to 4 and the mean number of injections was 2.08 ± 0.86 (shown as histogram in supplementary file 1). In patients receiving anti-VEGF intravitreal injections, a significantly better visual acuity was detected after anti-VEGF intravitreal injections than the baseline visual acuity (logMAR, baseline vs final, 1.52 ± 0.48 vs 0.78 ± 0.51, P = .00045), and CRT significantly improved (before vs after, 505.50 ± 159.26 μm vs 243.60 ± 60.17 μm, P = .001) (Tables 2 and 3). The final visual acuity was significantly better in patients receiving anti-VEGF intravitreal injections than in patients under observation only (logMAR, anti-VEGF group vs observation group, 0.78 ± 0.51 vs 1.34 ± 0.48, P = .04) (Table 2). There was no new retinal hemorrhage or an increase of the severity after the intravitreal injection of Avastin. All the RAMs showed spontaneous decrease in size and regressed as a fibrotic dot at the final fundus exam.
Table 2.
Comparisons of the visual acuity between the Anti-VEGF group and the observation group in patients with fovea involvement.
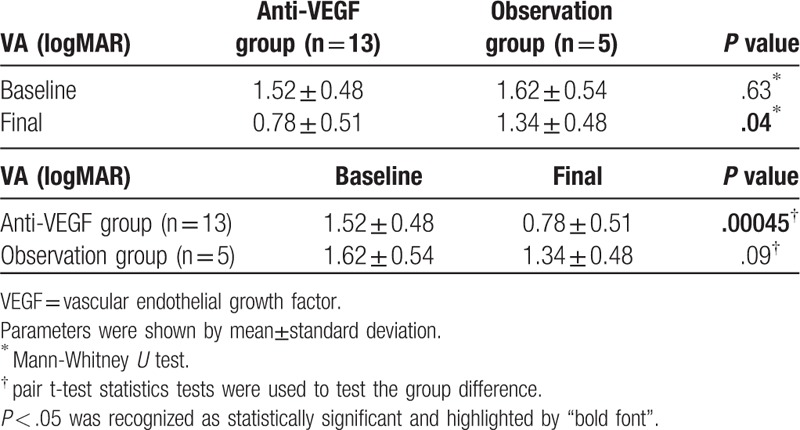
Table 3.
Comparisons of the central macular thickness (CMT) before and after intravitreal injections of anti-VEGF agents in patients with fovea involvement.
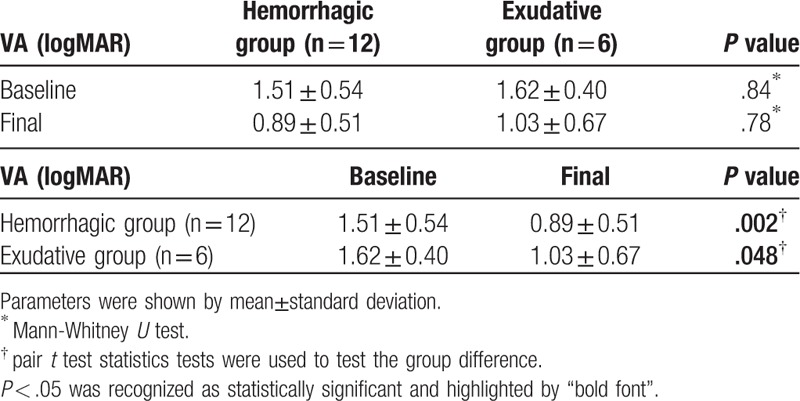
Patients with either hemorrhagic (n = 12) (Fig. 1) or exudative (n = 6) (Fig. 2) foveal complications had similar baseline and final visual acuity (logMAR, 1.51 ± 0.54 vs 1.62 ± 0.40, P = .84; 0.89 ± 0.51 vs 1.03 ± 0.67, P = .78, respectively), and the final visual acuity was both significantly better than the baseline visual acuity in the two groups (logMAR, 0.89 ± 0.51 vs 1.51 ± 0.54, P = .002; 1.03 ± 0.67 vs 1.62 ± 0.40, P = .048, respectively) (Table 4).
Figure 1.
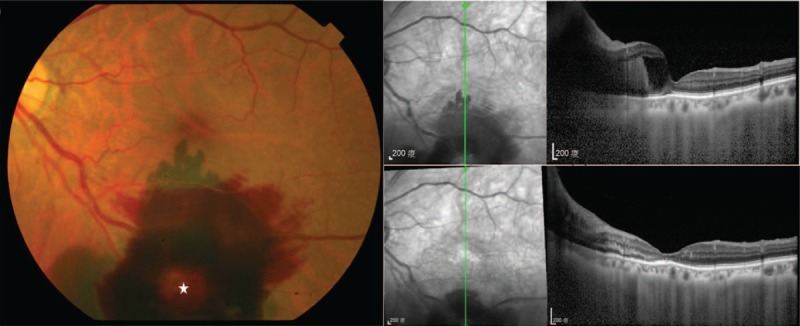
Clinical and imaging appearance of a hemorrhagic retinal macroaneurysms. (Left) Fundus photograph of a 78-year-old female with hypertension revealed a RAM (star) over the inferior-temporal arcade with pre-retinal and sub-retinal hemorrhages. Initial visual acuity was 0.03. (Top right) Initial spectral-domain optical coherence tomography (SD-OCT) image showed pre-retinal and sub-retinal hemorrhages with central macular edema with an elevated fovea. (Bottom right) SD-OCT image which was performed 1 month after the second intravitreal bevacizumab injection showed resolved subretinal hemorrhage and flattened macula, and visual acuity was 0.2.
Figure 2.
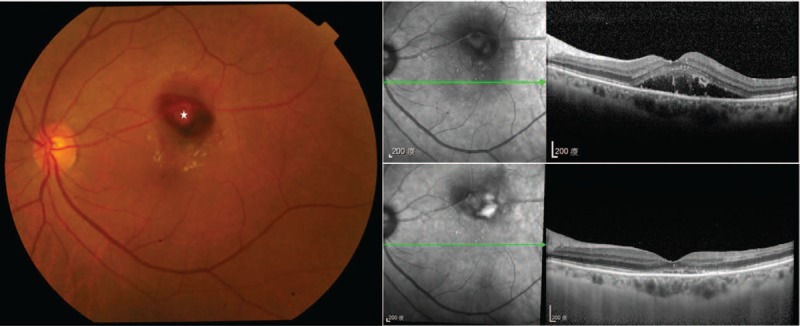
Clinical and imaging appearance of an exudative retinal macroaneurysms. (Left) Fundus photograph of a 75-year-old female without known underlying diseases revealed a RAM (star) over superior parafoveal area with macular edema. Initial visual acuity was 0.1. (Middle) Initial spectral-domain optical coherence tomography (SD-OCT) image showed exudations and central macular edema. (Right) SD-OCT image which was performed 1 month after the first intravitreal bevacizumab injection showed almost dry and flattened macula, and visual acuity was 0.3.
Table 4.
Comparisons of the visual acuity between the hemorrhagic group and the exudative group in patients with fovea involvement.
4. Discussion
RAM is associated with hypertension and age-related arteriosclerotic changes in vessel walls.[3] Previous series have pointed out that RAM is more commonly found in elderly women and easily develops in patients with poorly controlled hypertension or arteriosclerosis;[14] abnormal lipid levels were also mentioned to be relevant to RAM in a few articles,[2,21,22] as is predictable from the role of adverse lipid profiles in atherosclerosis. Our retrospective study showed similar results; we found female gender, history of hypertension and old age was recorded in the majority of patients in our sample (54.17%, 58.33%, and 70.83%, respectively); in addition, patients with complicated RAM all had either hypertension or were elderly (more than 60 years old). Consequently, in patients diagnosed with RAM, it is important to pursue a systematic work-up for hypertension and general arteriosclerotic disease to detect and treat associated systemic diseases.
Although spontaneous resolution of RAM with recovery of visual function is not uncommon, persistent macular exudates or hemorrhages can still lead to photoreceptor deterioration with functional impairment.[6,14] There are currently no approved guidelines for the management of complicated RAMs, and previous treatment options such as direct laser photocoagulation have a variable visual prognosis.[13,16,23,24] Moreover, laser photocoagulation may lead to subretinal fibrosis, laser scar enlargement, and even choroidal neovascularization.[25–27] Previous studies have also reported vitreous hemorrhage, RAM recurrence, branch retinal artery occlusion, increased retinal exudation, and scarring with possible retinal traction following laser photocoagulation.[15,24,28,29]
Because of its critical role in angiogenesis, VEGF is an important target of anticancer therapy because tumor growth and metastasis are angiogenesis-dependent events.[30] Bevacizumab, an anti-angiogenic agent, is a humanized anti-VEGF monoclonal IgG1 antibody, which has been approved by the US Food and Drug Administration to treat metastatic breast cancer, advanced colorectal cancer, advanced non-small cell lung cancer, and advanced renal cell cancer along with chemotherapy.[30–34] Bevacizumab is also a proven treatment modality and has gained widespread use as an intravitreal injection agent in ophthalmology for treating neovascular conditions or macular edema originating from various underlying diseases, such as age-related macular degeneration, central or branch retinal vein occlusion, and diabetic macular edema.[35] RAMs are considered as focal embolic damage to arterial walls which may subsequently lead to localized ischemia.[2,3,14] VEGF upregulation is then activated and stimulates endothelial production of nitric oxide, resulting in retinal artery dilation and increased permeability.[7] As VEGF inhibition plays a critical role in blocking the angiogenic and vasopermeability effects of VEGF and reduces nitric oxide production to initiate vasoconstriction, it may reduce leakage and central retinal thickness and consequently improve visual acuity in various neovascular and exudative ocular diseases.[36,37] VEGF inhibition may also disturb the balance between the coagulation and fibrinolysis processes, enabling the clearance of the hemorrhages in different retinal layers. And these mechanisms are believed to be helpful in managing hemorrhagic or exudative complications in RAMs with foveal involvement.[7,19]
Before 2013, there were few case reports discussing the treatment outcomes of intravitreal anti-VEGF agents for RAMs though they led to satisfactory results.[16–18] Cho et al retrospectively reviewed 23 patients (23 eyes) with symptomatic RAM divided into an intravitreal bevacizumab-treated group and an untreated group. BCVA improved from baseline after 1 month and 3 months in the bevacizumab-treated group, but there was no significant difference in BCVA improvement or central macular thickness improvement achieved between the 2 groups at the final visit.[19] Pichi et al conducted an interventional prospective nonrandomized study including 38 RAMs of 37 patients with foveal complications. With 3 monthly injections of bevacizumab 1.25 mg/0.05 mL, both BCVA and CRT significantly improved during the follow-up visits.[7] In our study, the follow-up period ranged from 6 to 24 months. To provide a reliable evaluation that could really reflect the final condition after the disease had stabilized, we only included patients with a minimum follow-up period of 6 months, and the interval between the last injection and the last follow-up was at least 3 months. Our results suggested that patients receiving anti-VEGF intravitreal injections not only gained significant CRT improvement and better visual acuity than the baseline but also showed better final visual acuity than the observation group. Moreover, there were no intravitreal injection-associated complications, such as new injection-induced hemorrhage, traumatic lens injury, retinal detachment or endophthalmitis. And no systemic adverse events were recorded for patients during follow-up period.
Limitations of the present study include the small sample size (stemming from the low incidence of clinically overt RAMs), and the retrospective nature of the design. To clarify the timing and the number of injections required to attain ideal results, further additional prospective randomized studies are warranted to establish the frequency and timing of injections that provide an optimal response.
5. Conclusions
In view of the strong association of RAM with systemic diseases, good clinical practice should include a systematic appraisal of blood pressure and ischemic heart disease whenever a diagnosis of RAM is made. In patients with fovea-threatening RAM, anti-VEGF intravitreal injections appeared as an effective therapy to gain better final visual acuity, visual improvements, and fast improvement of CRT. Most RAM eyes turned out to have satisfactory visual improvement even with foveal complications during the follow-up period.
Author contributions
Conceptualization: Hsi-Kung Kuo.
Data curation: Wei-Yu Chiang, Chih-Hsin Chen.
Formal analysis: Wan-Hua Cho, Wei-Yu Chiang.
Writing – original draft: Wan-Hua Cho.
Writing – review & editing: Hsi-Kung Kuo.
Wan-Hua Cho orcid: 0000-0003-2951-1077.
Supplementary Material
Footnotes
Abbreviations: BCVA = best-corrected visual acuity, CRT = central retinal thickness, FA = fluorescein angiography, ILM = internal limiting membrane, logMAR = logarithm of the minimal angle of resolution, Nd:YAG = neodymium: yttrium-aluminum-garnet, RAM = retinal arterial macroaneurysm, SD-OCT = spectral-domain optical coherence tomography, VEGF = vascular endothelial growth factor.
How to cite this article: Cho WH, Chiang WY, Chen CH, Kuo HK. To treat or not to treat: a clinical series of retinal arterial macroaneurysms: A single-center retrospective study. Medicine. 2020;99:5(e19077).
A part of our result has been presented in the 54th Local Academic Conference of the Ophthalmological Society of Taiwan in April 2019.
All procedures involving human subjects adhered to the Declaration of Helsinki. Institutional Review Board (IRB)/Ethics Committee approval was obtained from the Committee of Medical Ethics and Human Experiments of Chang Gung Memorial Hospital (CGMH, Taiwan).
This study waives informed consents according to our IRB regulation. The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Lavin MJ, Marsh RJ, Peart S, et al. Retinal arterial macroaneurysms: a retrospective study of 40 patients. Br J Ophthalmol 1987;71:817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Panton RW, Goldberg MF, Farber MD. Retinal arterial macroaneurysms: risk factors and natural history. Br J Ophthalmol 1990;74:595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pitkanen L, Tommila P, Kaarniranta K, et al. Retinal arterial macroaneurysms. Acta Ophthalmol 2014;92:101–4. [DOI] [PubMed] [Google Scholar]
- [4].Lee EK, Woo SJ, Ahn J, et al. Morphologic characteristics of retinal arterial macroaneurysm and its regression pattern on spectral-domain optical coherence tomography. Retina (Philadelphia, PA) 2011;31:2095–101. [DOI] [PubMed] [Google Scholar]
- [5].Moosavi RA, Fong KC, Chopdar A. Retinal artery macroaneurysms: clinical and fluorescein angiographic features in 34 patients. Eye (London, England) 2006;20:1011–20. [DOI] [PubMed] [Google Scholar]
- [6].Tsujikawa A, Sakamoto A, Ota M, et al. Retinal structural changes associated with retinal arterial macroaneurysm examined with optical coherence tomography. Retina (Philadelphia, PA) 2009;29:782–92. [DOI] [PubMed] [Google Scholar]
- [7].Pichi F, Morara M, Torrazza C, et al. Intravitreal bevacizumab for macular complications from retinal arterial macroaneurysms. Am J Ophthalmol 2013;155:287–94.e281. [DOI] [PubMed] [Google Scholar]
- [8].Gedik S, Gur S, Yilmaz G, et al. Retinal arterial macroaneurysm rupture following fundus fluorescein angiography and treatment with Nd:YAG laser membranectomy. Ophthalmic Surg Lasers Imaging 2007;38:154–6. [DOI] [PubMed] [Google Scholar]
- [9].Tan CS, Au Eong KG. Surgical drainage of submacular haemorrhage from ruptured retinal arterial macroaneurysm. Acta Ophthalmol Scand 2005;83:240–1. [DOI] [PubMed] [Google Scholar]
- [10].de Jong JH, van Zeeburg EJ, Cereda MG, et al. Intravitreal versus subretinal administration of recombinant tissue plasminogen activator combined with gas for acute submacular hemorrhages due to age-related macular degeneration: an exploratory prospective study. Retina 2016;36:914–25. [DOI] [PubMed] [Google Scholar]
- [11].Hillenkamp J, Surguch V, Framme C, et al. Management of submacular hemorrhage with intravitreal versus subretinal injection of recombinant tissue plasminogen activator. Graefes Arch Clin Exp Ophthalmol 2010;248:5–11. [DOI] [PubMed] [Google Scholar]
- [12].Wu TT, Sheu SJ. Intravitreal tissue plasminogen activator and pneumatic displacement of submacular hemorrhage secondary to retinal artery macroaneurysm. J Ocul Pharmacol Ther 2005;21:62–7. [DOI] [PubMed] [Google Scholar]
- [13].Parodi MB, Iacono P, Ravalico G, et al. Subthreshold laser treatment for retinal arterial macroaneurysm. Br J Ophthalmol 2011;95:534–8. [DOI] [PubMed] [Google Scholar]
- [14].Rabb MF, Gagliano DA, Teske MP. Retinal arterial macroaneurysms. Surv Ophthalmol 1988;33:73–96. [DOI] [PubMed] [Google Scholar]
- [15].Leung EH, Reddy AK, Vedula AS, et al. Serial bevacizumab injections and laser photocoagulation for macular edema associated with a retinal artery macroaneurysm. Clin Ophthalmol 2015;9:601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Javey G, Moshfeghi AN, Moshfeghi AA. Management of ruptured retinal arterial macroaneurysm with intravitreal bevacizumab. Ophthal Surg Lasers Imag 2010;41:1–5. [PubMed] [Google Scholar]
- [17].Golan S, Goldenberg D, Goldstein M. Long-term follow-up of intravitreal bevacizumab in retinal arterial macroaneurysm: a case report. Case Rep Ophthalmol 2011;2:387–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tsakpinis D, Nasr MB, Tranos P, et al. The use of bevacizumab in a multilevel retinal hemorrhage secondary to retinal macroaneurysm: a 39-month follow-up case report. Clin Ophthalmol 2011;5:1475–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cho HJ, Rhee TK, Kim HS, et al. Intravitreal bevacizumab for symptomatic retinal arterial macroaneurysm. Am J Ophthalmol 2013;155:898–904. [DOI] [PubMed] [Google Scholar]
- [20].Hassenstein A, Meyer CH. Clinical use and research applications of Heidelberg retinal angiography and spectral-domain optical coherence tomography - a review. Clin Exp Ophthalmol 2009;37:130–43. [DOI] [PubMed] [Google Scholar]
- [21].Adamczyk DT, Olivares GE, Petito GT. Retinal arterial macroaneurysm: a longitudinal case study. J Am Optom Assoc 1989;60:840–5. [PubMed] [Google Scholar]
- [22].Cahuzac A, Scemama C, Mauget-Faysse M, et al. Retinal arterial macroaneurysms: clinical, angiographic, and tomographic description and therapeutic management of a series of 14 cases. Eur J Ophthalmol 2016;26:36–43. [DOI] [PubMed] [Google Scholar]
- [23].Verheul HM, Pinedo HM. Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nature reviews. Cancer 2007;7:475–85. [DOI] [PubMed] [Google Scholar]
- [24].Battaglia Parodi M, Iacono P, Pierro L, et al. Subthreshold laser treatment versus threshold laser treatment for symptomatic retinal arterial macroaneurysm. Invest Ophthalmol Vis Sci 2012;53:1783–6. [DOI] [PubMed] [Google Scholar]
- [25].Schatz H, Madeira D, McDonald HR, et al. Progressive enlargement of laser scars following grid laser photocoagulation for diffuse diabetic macular edema. Arch Ophthalmol 1991;109:1549–51. [DOI] [PubMed] [Google Scholar]
- [26].Han DP, Mieler WF, Burton TC. Submacular fibrosis after photocoagulation for diabetic macular edema. Am J Ophthalmol 1992;113:513–21. [DOI] [PubMed] [Google Scholar]
- [27].Shah GK. Photodynamic therapy for choroidal neovascularization after thermal laser photocoagulation for diabetic macular edema. Am J Ophthalmol 2003;135:114–6. [DOI] [PubMed] [Google Scholar]
- [28].Chen YY, Lin LY, Chang PY, et al. Laser and Anti-Vascular Endothelial Growth Factor Agent Treatments for Retinal Arterial Macroaneurysm. Asia Pac J Ophthalmol 2017;6:444–9. [DOI] [PubMed] [Google Scholar]
- [29].Chen Y, Chen SDM, Chen FK. Branch retinal vein occlusion secondary to a retinal arteriolar macroaneurysm: a novel mechanism supported by multimodal imaging. Retin Cases Brief Rep 2019;13:10–4. [DOI] [PubMed] [Google Scholar]
- [30].Kazazi-Hyseni F, Beijnen JH, Schellens JH. Bevacizumab. Oncologist 2010;15:819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mery B, Rowinski E, Vallard A, et al. Advocacy for a new oncology research paradigm: the model of bevacizumab in triple-negative breast cancer in a french cohort study. Oncology 2019;97:1–6. [DOI] [PubMed] [Google Scholar]
- [32].Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA 2017;317:2392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Proto C, Ferrara R, Signorelli D, et al. Choosing wisely first line immunotherapy in non-small cell lung cancer (NSCLC): what to add and what to leave out. Cancer Treatm Rev 2019;75:39–51. [DOI] [PubMed] [Google Scholar]
- [34].Choi Y, Keam B, Kim M, et al. Bevacizumab Plus Erlotinib Combination Therapy for Advanced Hereditary Leiomyomatosis and Renal Cell Carcinoma-Associated Renal Cell Carcinoma: A Multicenter Retrospective Analysis in Korean Patients. Cancer Res Treat 2019;51:1549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Low A, Faridi A, Bhavsar KV, et al. Comparative effectiveness and harms of intravitreal antivascular endothelial growth factor agents for three retinal conditions: a systematic review and meta-analysis. Br J Ophthalmol 2019;103:442–51. [DOI] [PubMed] [Google Scholar]
- [36].Campochiaro PA, Hafiz G, Shah SM, et al. Ranibizumab for macular edema due to retinal vein occlusions: implication of VEGF as a critical stimulator. Mol Ther 2008;16:791–9. [DOI] [PubMed] [Google Scholar]
- [37].Scheppke L, Aguilar E, Gariano RF, et al. Retinal vascular permeability suppression by topical application of a novel VEGFR2/Src kinase inhibitor in mice and rabbits. J Clin Invest 2008;118:2337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


