Abstract
The main challenge in the field of Helicobacter pylori (H. pylori) infection is antibiotic resistance, which influences the efficacy of eradication regimens. Bismuth-containing quadruple therapy has been confirmed as an effective regimen for eradicating H. pylori, especially in strains with antibiotic resistance. High-dose proton-pump inhibitor-amoxicillin dual therapy could decrease the use of unnecessary antibiotics, which is a promising alternative approach. Adjuvant therapy (specific probiotic or vitamin) also showed good results, although more evidence is needed. Novel anti-H. pylori drugs are needed, and the establishment of the H. pylori database is an effective way to acknowledge the real-time information of H. pylori management. This review provides the recent progress of H. pylori treatment, and further studies are needed to address the role of different regimens in improving H. pylori eradication rate, especially in strains with antibiotics resistance.
Keywords: Helicobacter pylori; Bismuth, dual therapy; Vonoprazan; Probiotics; Vitamin
Introduction
Helicobacter pylori (H. pylori) is a gastric Gram-negative, spiral-shaped microaerophilic pathogen closely associated with gastric and extra-gastric diseases (chronic gastritis, peptic ulcers, gastric mucosa-associated lymphoid tissue lymphoma, gastric cancer, iron deficiency anemia, etc).[1,2] Appropriately half of the global population is infected with H. pylori, and the prevalence of H. pylori varied among regions, which appeared to be explained by the differences in economic and social conditions.[3,4] Additionally, H. pylori recurrence should not be neglected. The global annual H. pylori recurrence rate was higher in the 2010s (4.8%) than in the 1990s (3.9%) and 2000s (4.4%), which might increase the number of the infected person.[5]H. pylori infection persists for a lifetime if not eradicated. Several consensus reports have been published in the past 5 years, mainly focusing on the management of H. pylori infection.[6–10]
Currently, the main challenge in the field of H. pylori infection is antibiotic resistance, which influences the efficacy of eradication regimens. The latest systematic review and meta-analysis reported that the primary and secondary resistance rates to clarithromycin, metronidazole, and levofloxacin exceeded 15% (alarming levels) in all the World Health Organization (WHO) regions.[11] In 2017, clarithromycin-resistant H. pylori was defined as a high-priority bacterium in the WHO priority list of antibiotic-resistant bacteria.[12] The traditional proton-pump inhibitor (PPI)-based triple therapy (PPI plus two antibiotics) has been used for eradicating H. pylori for more than 20 years. However, PPI-based triple therapy provides low treatment success (intention-to-treat [ITT] analysis below 80% in most studies),[13] which is defined as unacceptable according to the report card used to grade H. pylori therapy.[14] In 2012, the Maastricht IV/Florence Consensus Report[15] recommended that PPI-clarithromycin-containing triple therapy should be abandoned in areas with clarithromycin resistance rates above 15% to 20%. In addition, bismuth-containing quadruple therapy (BQT) is recommended as a first-line treatment for eradicating H. pylori in areas with high or low clarithromycin resistance because of its high efficiency, safety, and tolerance.[15] More recently, a number of studies were conducted to evaluate the efficiency of other regimens (eg, sequential, concomitant, hybrid therapy, high-dose PPI-amoxicillin dual therapy, vonoprazan [VPZ]-based triple therapy, probiotics supplemented triple therapy or combined with BQT) in H. pylori eradication. In this review, we summarize the recent progress in H. pylori eradication.
BQT
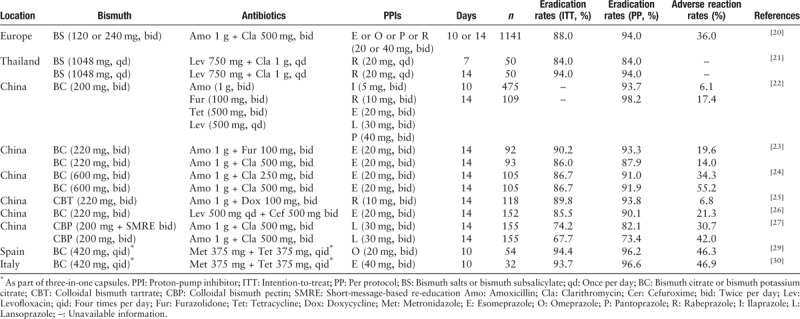
Bismuth, a chemical element with the symbol Bi (atomic number 83), has been used for treating syphilis, colitis, and wound infection for over three centuries.[16] The treatment success of bismuth alone in eradicating H. pylori was 16% to 20%, although it belongs to the non-antibiotic.[17] Additionally, the bismuth add-on triple therapy can improve an additional 30% to 40% success in resistant strains. Recently, Ko et al[18] conducted a systemic review and meta-analysis to evaluate the efficacy of non-antibiotic (bismuth) supplements as a first-line regimen for H. pylori eradication. In total, twenty-five randomized trials (3990 patients) were included for analysis. According to per protocol (PP) analysis, the H. pylori eradication rate was higher in the BQT group (85.8%) than in the non-BQT regimen group (74.2%), which was significantly different. Of the three trials evaluating the efficiency of bismuth add-ons in standard triple therapy, BQT showed superior to triple therapy (odds ratio [OR]: 3.55, 95% confidence interval [CI]: 2.33–5.41). In five trials conducted in areas with a clarithromycin resistance rate greater than 15%, BQT also showed a higher eradication rate than that of the control group (OR: 3.55, 95% CI: 1.07–2.39). Moreover, an in vitro study[19] revealed that the bacterium-host cell adhesion, oxidative stress defense ability, and pH buffering ability of H. pylori were reduced by treatment with bismuth, which might explain the sustainable anti-microbial activity of bismuth against H. pylori and the relatively low H. pylori resistance to bismuth. These advantages of bismuth make BQT achieve acceptable or good or excellent outcomes in most studies, even in areas with high resistance rates.
There are still multiple researches conducted in this year to evaluate the efficiency and side effects of different antibiotics doses, frequency, combinations in BQT. Moreover, the efficiency and safety of BQT in real-world practice were evaluated in China and Europe. In 2013, the European Helicobacter and Microbiota Study Group promoted an international multicenter prospective non-interventional registry regarding H. pylori management, which will last for more than 10 years. The interim analysis of data from this registry was performed to evaluate the efficiency of BQT in treating 1141 infected patients with no history of H. pylori eradication.[20] According to the ITT and PP analyses, the eradication rates of these regimens were 88% and 94%, respectively. The 14-day regimens showed a higher eradication rate than the 10-day regimens (ITT: 92% vs. 79%). In addition, good compliance and no serious adverse reactions were observed in this study.[20] Considering the drugs used in H. pylori eradication regimens should be given 2 to 4 times daily, which might influence the compliance in a subset of patients, Auttajaroon et al[21] conducted a prospective randomized pilot study to evaluate whether a once-daily dosing H. pylori eradication regimen (levofloxacin 750 mg, clarithromycin 1 g, rabeprazole 60 mg, and bismuth subsalicylate 1048 mg for 7 or 14 days) could provide a good compliance and eradication rate. It is noteworthy that a high dose of rabeprazole was used in this study, which would be expected to provide a median pH ≥4 for approximately 20 h/day and improve the efficiency of antibiotics. Fourteen-day regimens provided 94% treatment success in 50 subjects, and a high eradication rate was also achieved in cases with clarithromycin or levofloxacin resistance, which suggested that once-a-day tailored 14-day BQT could be the empiric therapy for eradicating H. pylori in Thailand.[21]
The prevalence of H. pylori primary resistance to clarithromycin, metronidazole, and levofloxacin was high and bismuth is available in China. In the Fifth Chinese National Consensus, seven BQT (different combinations of antibiotics) were recommended.[10] Our group conducted an observational study of furazolidone-containing BQT for eradicating 584 H. pylori-infected patients in real-world settings and found that 10-or 14-day regimens were efficient and safe.[22] A single-center, prospective, randomized, open-label study was conducted to compare the cost-efficacy of 14-day furazolidone-based and clarithromycin-based BQT as the first-line treatment for H. pylori infection in China, the results showed that no differences in the eradication rate and adverse reactions were observed in the two groups; however, furazolidone-based BQT was more cost-effective than clarithromycin-based BQT.[23] Considering that clarithromycin is expensive but available in most regions of China, Chen et al[24] investigated the efficiency and economy of half-dose or standard-dose clarithromycin-containing BQT in populations of China. The results indicated similar eradication frequencies in the two groups. However, the adverse reactions and cost were less in half-dose clarithromycin-containing BQT in comparison with standard-dose clarithromycin-containing BQT. In addition, the high efficiency and safety of different antibiotic combinations in BQT (eg, amoxicillin and doxycycline or cefuroxime and levofloxacin) were achieved in H. pylori clinical practice in China.[25,26] Interestingly, twice daily short-message-based re-education could improve compliance in the young population, leading to an increased eradication rate of BQT.[27] Improving the eradication rate of the first-line regimens is of great importance because it avoids the secondary resistance to antibiotics and reduces H. pylori prevalence. If the sensitive antibiotics are available, selecting two sensitive antibiotic combinations (amoxicillin + furazolidone or tetracycline + furazolidone or amoxicillin + tetracycline) in BQT is a good choice in clinical practice of China because the expected high eradication rate could be achieved. If not, the other antibiotic combinations (one sensitive and one resistant antibiotic) are also used.
A novel three-in-one single capsule (bismuth, metronidazole, and tetracycline; BMT) has been used in many countries. A meta-analysis of BMT plus PPI for H. pylori infection (studies were selected up to October 2018) showed that a 10-day treatment of this regimen provides approximately 90% success in the first-line and second-line treatment. In addition, this outcome was also achieved with clarithromycin- or metronidazole-resistant strains.[28] In the northwest of Spain, an area with a high clarithromycin resistance rate (22.4%), the eradication rates of BMT (four times a day) plus omeprazole (twice a day) for 10 days were 94.4% and 96.2% according to ITT and PP analysis.[29] In addition, this regimen was safe. This result was also confirmed by a recent pilot study.[30] Taken together, despite the different combinations of antibiotics and PPIs used in BQT [Table 1], this regimen is efficient, safe, and tolerated for eradicating H. pylori in an era of increasing antibiotic resistance.
Table 1.
Summary of bismuth-containing quadruple therapy studies conducted in 2019.
Sequential, hybrid, and concomitant therapy
Compared to the conventional regimens, drug administration sequences were modified in sequential, hybrid, and concomitant therapy. The sequential therapy was initially reported to achieve a high eradication rate and is widely used in Italy.[31] However, the efficiency of this regimen has decreased over time because it was influenced by clarithromycin and metronidazole resistance.[7,32] Therefore, sequential therapy was not recommended by the recent consensus reports.[7,8,33] Few sequential therapy articles were reported this year. Zullo et al[34] reported that 10- and 14-day sequential therapies (esomeprazole 40 mg and amoxicillin 1 g twice for 5 or 7 days followed by esomeprazole 40 mg, clarithromycin 500 mg, and tinidazole 500 mg twice for 5 or 7 days) achieved high treatment success (above 90% according to PP analysis) for first-line H. pylori eradication in clinical practice in Italy. However, the antibiotic resistance information was lacking in this study, and the efficiency of these regimens in the sub-groups of antibiotic resistance was unclear. In Korea, where the antibiotic resistance rates are relatively high, the efficiency of 10-day sequential therapy was reported as 76.3% and 85.0% according to ITT and PP analysis, which was defined as an unsatisfied outcome, although it showed superior to 7-day clarithromycin-containing triple therapy.[35]
Concomitant and hybrid therapy contain three antibiotics and PPI; the eradication rate or these regimens were not undermined by clarithromycin or metronidazole resistance, except for dual resistance. A prospective, open-label study conducted in Greece showed that the eradication rates of 10- and 14-day concomitant therapy were above 90% according to PP analysis, in addition, the regimens were tolerated, and 31.3% of treated patients presented side effects.[36] Consistent with this study, 10-day concomitant therapy also showed a markedly higher eradication rate than 7-day triple therapy (PP: 90.6% vs. 71.4%).[35] Moreover, the efficiency of 14-day concomitant therapy was similar with 10-day BQT.[29] Hybrid therapy has attracted widespread attention in recent years. A systemic review and meta-analysis (eight studies with 2516 subjects included)[37] showed that the mean cure rates of hybrid therapy were 88.5% (1207 patients) and 93.3% (1109 patients) according to ITT and PP analysis. No difference in eradication rate was observed between hybrid and concomitant therapy. Recently, Francesco et al[38] conducted a prospective, open-label pilot study showing that a novel hybrid therapy with a 5-day dual therapy followed by a 5-day BMT therapy achieved excellent outcome (ITT: 97.5%, PP: 100%) as the first-line therapy for H. pylori infection. Moreover, reverse hybrid therapy (PPI plus amoxicillin for 14 days and clarithromycin plus metronidazole for the initial 7 days) achieved a similar eradication rate in comparison with 14-day BQT in the total infected population and in cases with clarithromycin-resistant strains or metronidazole-resistant strains. Fewer adverse events were observed in reverse hybrid therapy (18.7%) than in BQT (47.7%).[39] Although comparable outcomes were achieved between concomitant, hybrid therapy, and BQT in some studies,[29,39] the drug administrations of concomitant and hybrid therapy were complex, which might influence the patient compliance. Moreover, the effect of concomitant and hybrid therapy on the short- or long-term gut microbiota remained uncertain and need to be further investigated.
High-dose PPI-amoxicillin dual therapy
High-dose dual therapy (HDDT) was defined as the combination of amoxicillin (eg, 1 g tid or 750 mg qid) and PPI (standard-dose tid or qid or double standard-dose bid) for 14 days. Previous studies[40,41] showed that HDDT achieved high treatment success as empirical first-line or rescue therapy for H. pylori infection. A recent systematic review and meta-analysis included four randomized clinical trials with 829 patients to compare the efficiency and side effects in the HDDT and BQT groups. Similar eradication rates and compliances were observed in both groups. However, the number of side effects was less in HDDT than BQT.[42] Three randomized trials were conducted this year to evaluate the efficiency of HDDT for first-line H. pylori therapy. Yang et al[43] randomly allocated H. pylori-infected, treatment-naive patients into two groups: modified HDDT (amoxicillin 750 mg and esomeprazole 20 mg, qid) and BQT (bismuth potassium citrate 1 g, amoxicillin 1 g, clarithromycin 500 mg, and esomeprazole 20 mg; bid), the results showed that modified HDDT showed a similar eradication rate as BQT, although the antibiotic resistance was higher in the HDDT group than in the BQT group, and the side effects and cost of medications were lower in the HDDT group than those in the BQT group. Moreover, variations in the CYP2C19, IL-1B-511, and H. pylori VacA genotypes were ameliorated by HDDT.[43] HDDT (esomeprazole 40 mg tid and amoxicillin 750 mg qid) was also confirmed to achieve a high eradication rate (ITT: 91.7%, PP: 95.7) as first-line H. pylori therapy in comparison with 7-day non-bismuth quadruple but with fewer side effects.[44] Considering the role of bismuth in improving the additional eradication rate in resistance strains,[45] Yu et al[46] conducted an open-label, randomized single-center study to address whether the addition of bismuth to HDDT could improve the eradication rate. The study indicated that the eradication rate was higher in the HDDT group than in the HDDT with bismuth group (ITT: 92.5% vs. 88.8%, PP: 96.1% vs. 93.3%), although there was no significant difference. The additional effect of bismuth on HDDT was only observed among infected smokers. High-dose PPI-amoxicillin dual therapy could achieve acceptable outcomes and decrease the use of unnecessary antibiotics, which is a promising alternative approach. Considering the concomitant medication and the side effects were common in elderly populations, it is of great interests to investigate the efficiency and safety of HDDT in elderly populations.
VPZ-based therapy
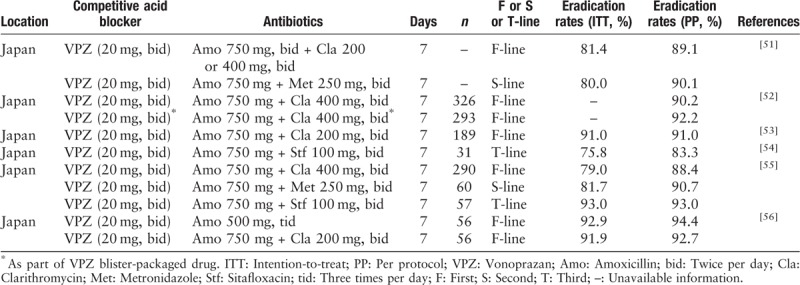
VPZ is a new, potent acid-inhibitory drug, which became clinically available in Japan in 2015. Compared to conventional PPI, gastric acid was inhibited by VPZ more rapidly, more strongly and for a longer duration. Moreover, it does not require pharmacological activation and has a longer half-life.[47] A study[48] reported that VPZ (20 mg bid) potently suppressed acid for 24 h irrespective of CYP2C19 genotype, with intra-gastric pH 4 and 5 holding time rates as high as 100% and 99%, respectively. In this regard, the advantages of VPZ were considered to improve the efficiency of H. pylori eradication regimens. A recent systematic review with meta-analysis reported that VPZ-based triple therapy showed superior efficacy for H. pylori eradication in comparison with PPI-based triple therapy, which was attributed to the increased eradication rate among the clarithromycin-resistant H. pylori strains.[49,50]
The Tokyo H. pylori Study Group conducted a retrospective analysis to examine the trend of the eradication rate of H. pylori from 2013 to 2018 and examined factors concerning successful eradication,[51] in total, 4097 and 3572 patients (from the metropolitan area) in the first- and second-line eradication therapies were included in the study, the percentages of lansoprazole in the first-line and second-line regimens dramatically decreased from 81.8% in 2013 to only 3.4% in 2018 and 65.7% in 2013 to only 1.6% in 2018. A similar trend was also observed in rabeprazole. In contrast, the percentage of VPZ in the first- and second-line eradication therapies rapidly increased to 95.5% and 90.2% in 2018, respectively. The eradication rate of the first-line therapy was relatively low (<80%) in 2013 and 2014. However, the eradication rate of the first-line therapy increased from 2015 to 2018 because of the increased use of VPZ in the regimens. Univariate logistic regression analysis revealed that VPZ and age over 75 years were independent factors for successful eradication. Compliance is an important factor influencing successful H. pylori eradication.[51] The VONOSAP pack (available in Japan since 2016) is a triple-drug blister pack containing VPZ as packaged medicine, which may be able to increase drug adherence of H. pylori eradication. Nishida et al[52] found that the overall successful eradication rates were above 90% in the VPZ-based therapy group and the VONOSAP group. In the sub-group analysis of patients older than 75 years, the VONOSAP group showed a better treatment benefit than the VPZ group. In addition, smoking, drinking, and lifestyle-related factors had no effects on the failure of H. pylori eradication using VPZ-based therapy.[53]
Sitafloxacin is the major antibiotic used in the third-line treatment of H. pylori in Japan. The VPZ group (VPZ 20 mg, amoxicillin 750 mg, and sitafloxacin 100 mg, bid) showed a significant difference compared to the PPI group in the third-line treatment of H. pylori.[54] Moreover, the H. pylori eradication rate in the third-line treatment was higher in VPZ-based therapy than in PPI-based therapy, even in the sitafloxacin-resistant H. pylori strains.[55] Considering the high eradication rate of VPZ-based triple therapy in the resistant H. pylori strains, VPZ with amoxicillin dosed in tid or qid manners is theoretically expected to achieve a sufficient eradication outcome. Recently, Furuta et al[56] confirmed that dual therapy with VPZ and amoxicillin was not inferior to VPZ-based therapy as the first-line therapy for H. pylori. Overall, VPZ-based triple therapy showed a high efficiency in the first or second or third-line treatment of H. pylori in Japan. Moreover, VPZ-based dual therapy showed a similar result with VPZ-triple therapy in the first-line H. pylori treatment [Table 2]. The emergence of VPZ shed a new light in eradicating H. pylori especially in an era of increasing antibiotic resistance.
Table 2.
Summary of vonoprazan-based studies conducted in 2019.
Susceptibility-guided therapy or tailored therapy
Theoretically, susceptibility-guided therapy is considered to achieve a high success rate because it is based on the results of antibiotic resistance and avoids misused antibiotics. Studies also indicated that susceptibility-guided therapy was as effective as the first-line or second-line treatment for H. pylori eradication, especially in regions with high antibiotic resistance.[57,58] However, Chen et al[59] found that susceptibility-guided therapy was not superior to local empiric therapy (esomeprazole 20 mg, bismuth 220 mg bid, amoxicillin 1 g, and metronidazole 400 mg tid) in Shanghai, China. Considering that susceptibility-guided therapy is time consuming and expensive, the clinical choice should depend on the availability of susceptibility testing and the efficiency of local empiric therapy. Recently, dual priming oligonucleotide-based multiplex polymerase chain reaction (DPO-PCR) has been introduced, which can detect H. pylori infection and clarithromycin resistance based on the A2142G and A2143G mutations of 23S rRNA. A tailored therapy based on DPO-PCR showed a high efficiency and cost-effectiveness as the first-line treatment of H. pylori infection in Korea.[60–62] Moreover, the DPO-PCR method is well correlated with the culture-based susceptibility test.[60] GenoType HelicoDR is a molecular test that detects H. pylori infection and clarithromycin and levofloxacin resistance. A multi-center prospective open-label randomized study (526 patients included) conducted in France demonstrated that the eradication rate of tailored therapy based on the GenoType HelicoDR test was higher than empirical triple therapy and that tailored therapy in routine practice might be a means to limit the emergence of antibiotic resistance.[63] The emergence of PCR method provides a quick, convenient way to guide tailored therapy in clinical practice of H. pylori. However, the clinical application of this method should be elucidated using a large-scale prospective study.
Probiotics
The effect of probiotic supplementation on the increased H. pylori eradication rate and reduced adverse reactions might be strain-specific. Several potential mechanisms included inhibiting colonization and adhesion of H. pylori, alleviating inflammation induced by H. pylori, regulating immune responses of H. pylori, reducing the incidence of adverse effects and subsequently improving compliance.[64,65] Zhou et al[66] conducted an updated systematic review and meta-analysis with trial sequential analysis to explore the effects of Saccharomyces boulardii as an adjuvant therapy on H. pylori eradication rates and adverse effects. The results showed that a significantly increased eradication rate and reduced side effects were observed in triple therapy with the S. boulardii group compared with the triple therapy group. Lactobacillus, as an adjunct to triple therapy, can also increase the efficiency of triple therapy and reduce the incidence of therapy-related diarrhea in adults and children.[67,68] Multiple shifts and alterations in the intestinal microbiota can be caused by H. pylori eradication.[69] However, supplementation with Bacillus subtilis and Enterococcus faecium with triple therapy could protect and restore the intestinal microbiota.[70]
Vitamins
Vitamins (eg, vitamin C and E) could decrease oxidation reactions, eliminate reactive oxygen species and decrease N-nitrosamine in gastric fluid and exert an anti-oxidant effect, which may act as a protected role in gastric carcinogenesis. Li et al[71] conducted a blinded randomized placebo-controlled trial (3365 residents of high-risk region for gastric cancer included) to evaluate the effect of H. pylori treatment and vitamin supplementation on the incidence and mortality of gastric cancer. A total of 1130 H. pylori-infected participants received H. pylori eradication, and 1677 participants received one capsule of 250 mg of vitamin C, 100 U of vitamin E, 37.5 μg of selenium for 7.3 years and 7.5 mg of β-carotene for 6 months. After 22 years post-intervention, the results indicated that H. pylori treatment for 2 weeks and vitamin supplementation for 7.3 years could reduce the incidence and mortality of gastric cancer. In addition, vitamin C and E are reported to have an inhibitory effect on H. pylori intensity and neutrophilic activity. The levels of vitamin C and E were decreased in H. pylori-positive patients and increased after H. pylori eradication. Our previous systematic review with meta-analysis showed that the addition of vitamin C and E at a high dose to eradication regimens could improve the efficiency.[72]
A multi-center observational prospective cohort study was conducted in China to explore whether serum vitamin D levels affected H. pylori infection and eradication rates. The level of vitamin D was significantly lower in 496 H. pylori-positive patients (17.0 ± 6.9 ng/mL) than in 257 H. pylori-negative patients (19.2 ± 8.0 ng/mL). Moreover, the H. pylori eradication rate was higher in patients with high serum vitamin D levels (≥10 ng/mL) than in patients with low serum vitamin D levels (<10 ng/mL).[73] Another study also reported the associations between vitamin D deficiency and increased risk of H. pylori infection.[74] A recent meta-analysis regarding the effect of vitamin D on H. pylori infection and eradication demonstrated that the average vitamin D level was lower in H. pylori-positive patients than in H. pylori-negative patients, and a lower H. pylori eradicate rate was observed in individuals with vitamin D deficiency.[75] Although the recent studies showed a correlation between vitamin D levels and eradication rates, a large-scale prospective study need to be conducted to address whether the addition of vitamin D could improve the eradication rate.
Anti-H. pylori compounds
The search for new anti-H. pylori compounds can be a valuable route for drug discovery, which is an effective way to mitigate the challenges of anti-microbial resistance. A total of 1120 US Food and Drug Administration-approved drugs for molecules that bind to HP1043 (also named HsrA, involved in cell viability, transcriptional activator, and crucial cellular functions of H. pylori) were screened and identified to explore the bactericidal activities against H. pylori, and only four of them (apigenin, chrysin, kaempferol, and hesperetin) showed high bactericidal activities against H. pylori. Notably, chrysin exhibited synergy function with clarithromycin or metronidazole, and hesperetin had an additive or synergetic function with clarithromycin or metronidazole, supporting their potential role as adjuvant in regimens, especially in the treatment of H. pylori strains with multidrug-resistance.[76]H. pylori flavodoxins, a small acidic redox protein, is essential for H. pylori survival and maybe a suitable drug target for H. pylori. An in vivo and vitro study showed that a family of nitrobenzoxadiazole-based flavodoxin inhibitors had low toxicity and were effective against metronidazole-, clarithromycin-, and rifampicin-resistant H. pylori strains. In addition, these inhibitors could reduce the gastric colonization rate of H. pylori and were able to eradicate H. pylori in 60% of infected mice.[77]
H. pylori databases
The European Helicobacter and Microbiota Study Group promoted an international multicenter prospective non-interventional registry to include clinical, diagnostic, treatment, eradication confirmation, and outcome data of H. pylori in routine clinical practice of Europe. This project will last more than 10 years.[78] All study data are collected and managed using REDCap Electronic Data Capture tools. The database will allow researchers to acknowledge the real-time information regarding the management of H. pylori infection and perform specific sub-analyses after approval by the scientific committee of the study. To date, an interim analysis of the effectiveness and safety of BQT in eradication of H. pylori infection has been conducted using this database.[20]
Conclusions
In 2019, multiple clinical trials or meta-analyses have been published to evaluate the efficiency and safety of different regimens for eradicating H. pylori, especially for strains with antibiotic resistance. In conclusion, BQT could still achieve high efficiency, although the resistance rate of H. pylori has increased. Hybrid and concomitant therapy could be an option if the dual resistance rate of H. pylori was not high. HDDT and VPZ-based therapy showed good outcomes and need to be further evaluated in more populations. The clinical choice should depend on the availability of susceptibility testing and the efficiency of local empiric therapy, although susceptibility-guided therapy is considered to achieve a high success rate. The effect of probiotics might be strain-specific, and the dose and frequency of vitamins in the eradication regimens should be explored in more studies. The discovery of novel anti-H. pylori drugs and the establishment of H. pylori databases in different regions are warranted in the future.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81460116, 81470832, 81670507, and 81870395), and the Graduate Innovation Fund of Jiangxi Province (No. YC2016-B025).
Conflicts of interest
None.
Footnotes
How to cite this article: Hu Y, Zhu Y, Lu NH. Recent progress in Helicobacter pylori treatment. Chin Med J 2020;133:335–343. doi: 10.1097/CM9.0000000000000618
References
- 1.Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology 2015; 148:719–731. doi: 10.1053/j.gastro.2015.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crowe SE. Helicobacter pylori Infection. N Engl J Med 2019; 380:1158–1165. doi: 10.1056/NEJMcp1710945. [DOI] [PubMed] [Google Scholar]
- 3.Hooi J, Lai WY, Ng WK, Suen M, Underwood FE, Tanyingoh D, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 2017; 153:420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Zamani M, Ebrahimtabar F, Zamani V, Miller WH, Alizadeh-Navaei R, Shokri-Shirvani J, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther 2018; 47:868–876. doi: 10.1111/apt.14561. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y, Wan JH, Li XY, Zhu Y, Graham DY, Lu NH. Systematic review with meta-analysis: the global recurrence rate of Helicobacter pylori. Aliment Pharmacol Ther 2017; 46:773–779. doi: 10.1111/apt.14319. [DOI] [PubMed] [Google Scholar]
- 6.Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015; 64:1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017; 66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 8.Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol 2017; 112:212–239. doi: 10.1038/ajg.2016.563. [DOI] [PubMed] [Google Scholar]
- 9.El-Serag HB, Kao JY, Kanwal F, Gilger M, LoVecchio F, Moss SF, et al. Houston consensus conference on testing for Helicobacter pylori infection in the United States. Clin Gastroenterol Hepatol 2018; 16:992–1002. doi: 10.1016/j.cgh.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu WZ, Xie Y, Lu H, Cheng H, Zeng ZR, Zhou LY, et al. Fifth Chinese National Consensus report on the management of Helicobacter pylori infection. Helicobacter 2018; 23:e12475.doi: 10.1111/hel.12475. [DOI] [PubMed] [Google Scholar]
- 11.Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization Regions. Gastroenterology 2018; 155:1372–1382. doi: 10.1053/j.gastro.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018; 18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 13.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010; 59:1143–1153. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 14.Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter 2007; 12:275–278. doi: 10.1111/j.1523-5378.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 15.Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, et al. Management of Helicobacter pylori infection--the Maastricht IV/Florence Consensus Report. Gut 2012; 61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 16.Sun H. Biological Chemistry of Arsenic, Antimony and Bismuth. Chichester, U.K: Wiley; 2011. [Google Scholar]
- 17.Axon AT. Helicobacter pylori therapy: effect on peptic ulcer disease. J Gastroenterol Hepatol 1991; 6:131–137. doi: 10.1111/j.1440-1746.1991.tb01452.x. [DOI] [PubMed] [Google Scholar]
- 18.Ko SW, Kim YJ, Chung WC, Lee SJ. Bismuth supplements as the first-line regimen for Helicobacter pylori eradication therapy: systemic review and meta-analysis. Helicobacter 2019; 24:e12565.doi: 10.1111/hel.12565. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Wang R, Sun H. Systems approaches for unveiling the mechanism of action of bismuth drugs: new medicinal applications beyond Helicobacter Pylori infection. Acc Chem Res 2019; 52:216–227. doi: 10.1021/acs.accounts.8b00439. [DOI] [PubMed] [Google Scholar]
- 20.McNicholl AG, Bordin DS, Lucendo A, Fadeenko G, Fernandez MC, Voynovan I, et al. Combination of bismuth and standard triple therapy eradicates Helicobacter pylori infection in more than 90% of patients. Clin Gastroenterol Hepatol 2019; [Epub ahead of print] doi: 10.1016/j.cgh.2019.03.048. [DOI] [PubMed] [Google Scholar]
- 21.Auttajaroon J, Vilaichone RK, Chotivitayatarakorn P, Mahachai V. Once-daily rabeprazole, levofloxacin, clarithromycin-MR, and bismuth for Helicobacter pylori eradication: a randomized study of 7 or 14 days (ONCE study). Helicobacter 2019; 24:e12615.doi: 10.1111/hel.12615. [DOI] [PubMed] [Google Scholar]
- 22.Song C, Qian X, Zhu Y, Shu X, Song Y, Xiong Z, et al. Effectiveness and safety of furazolidone-containing quadruple regimens in patients with Helicobacter pylori infection in real-world practice. Helicobacter 2019; 24:e12591.doi: 10.1111/hel.12591.31111641 [Google Scholar]
- 23.Yi DM, Yang TT, Chao SH, Li YX, Zhou YL, Zhang HH, et al. Comparison the cost-efficacy of furazolidone-based versus clarithromycin-based quadruple therapy in initial treatment of Helicobacter pylori infection in a variable clarithromycin drug-resistant region, a single-center, prospective, randomized, open-label study. Medicine (Baltimore) 2019; 98:e14408.doi: 10.1097/MD.0000000000014408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu B, Wang J, Li J, Liu L, Chen Y. Half-dose clarithromycin-containing bismuth quadruple therapy is effective and economical in treating Helicobacter pylori infection: a single-center, open-label, randomized trial. Helicobacter 2019; 24:e12566.doi: 10.1111/hel.12566. [DOI] [PubMed] [Google Scholar]
- 25.Gu L, Li S, He Y, Chen Y, Jiang Y, Peng Y, et al. Bismuth, rabeprazole, amoxicillin, and doxycycline as first-line Helicobacter pylori therapy in clinical practice: a pilot study. Helicobacter 2019; 24:e12594.doi: 10.1111/hel.12594. [DOI] [PubMed] [Google Scholar]
- 26.Song Z, Fu W, Zhou L. Cefuroxime, levofloxacin, esomeprazole, and bismuth as first-line therapy for eradicating Helicobacter pylori in patients allergic to penicillin. BMC Gastroenterol 2019; 19:132.doi: 10.1186/s12876-019-1056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang T, Yang X, Li Y, Li L, Liu J, Ji C, et al. Twice daily short-message-based re-education could improve Helicobacter pylori eradication rate in young population: a prospective randomized controlled study. Helicobacter 2019; 24:e12569.doi: 10.1111/hel.12569. [DOI] [PubMed] [Google Scholar]
- 28.Nyssen OP, McNicholl AG, Gisbert JP. Meta-analysis of three-in-one single capsule bismuth-containing quadruple therapy for the eradication of Helicobacter pylori. Helicobacter 2019; 24:e12570.doi: 10.1111/hel.12570. [DOI] [PubMed] [Google Scholar]
- 29.Macias-Garcia F, Baston-Rey I, de la Iglesia-Garcia D, Calvino-Suarez C, Nieto-Garcia L, Dominguez-Munoz JE. Bismuth-containing quadruple therapy versus concomitant quadruple therapy as first-line treatment for Helicobacter Pylori infection in an area of high resistance to clarithromycin: a prospective, cross-sectional, comparative, open trial. Helicobacter 2019; 24:e12546.doi: 10.1111/hel.12546. [DOI] [PubMed] [Google Scholar]
- 30.Ciccaglione AF, Cellini L, Marzio L. Pylera(R) plus ranitidine vs Pylera(R) plus esomeprazole in first-line treatment of Helicobacter pylori infection: two pilot studies. Helicobacter 2019; 24:e12606.doi: 10.1111/hel.12606. [DOI] [PubMed] [Google Scholar]
- 31.Zullo A, Vaira D, Vakil N, Hassan C, Gatta L, Ricci C, et al. High eradication rates of Helicobacter pylori with a new sequential treatment. Aliment Pharmacol Ther 2003; 17:719–726. doi: 10.1046/j.1365-2036.2003.01461.x. [DOI] [PubMed] [Google Scholar]
- 32.Nyssen OP, McNicholl AG, Megraud F, Savarino V, Oderda G, Fallone CA, et al. Sequential versus standard triple first-line therapy for Helicobacter pylori eradication. Cochrane Database Syst Rev 2016; 28:CD9034.doi: 10.1002/14651858.CD009034.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH, et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 2016; 151:51–69. doi: 10.1053/j.gastro.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Zullo A, Fiorini G, Scaccianoce G, Portincasa P, De Francesco V, Vassallo R, et al. Sequential therapy for first-line Helicobacter pylori eradication: 10- or 14-day regimen? J Gastrointest Liver Dis 2019; 28:11–14. doi: 10.15403/jgld.2014.1121.281.hpy. [DOI] [PubMed] [Google Scholar]
- 35.Kim BJ, Lee H, Lee YC, Jeon SW, Kim GH, Kim HS, et al. Ten-day concomitant, 10-day sequential, and 7-day triple therapy as first-line treatment for Helicobacter pylori infection: a nationwide randomized trial in Korea. Gut and Liver 2019; 13:531–540. doi: 10.5009/gnl19136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kapizioni C, Koutoufaris G, Ntouli V, Makris K, Milioni K, Kourkoulis P, et al. Optimal duration of concomitant nonbismuth quadruple therapy as first-line therapy for Helicobacter pylori: a prospective, open-label, comparative study. Eur J Gastroenterol Hepatol 2019; 31:1206–1210. doi: 10.1097/MEG.0000000000001517. [DOI] [PubMed] [Google Scholar]
- 37.Hsu PI, Lin PC, Graham DY. Hybrid therapy for Helicobacter pylori infection: a systemic review and meta-analysis. World J Gastroenterol 2015; 21:12954–12962. doi: 10.3748/wjg.v21.i45.12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Francesco V. A novel hybrid first-line therapy for H. pylori eradication: results of a pilot study. J Gastrointestin Liver Dis 2019; 28:129–130. doi: 10.15403/jgld.2014.1121.281.hyb. [DOI] [PubMed] [Google Scholar]
- 39.Hsu PI, Tsay FW, Graham DY, Tsai TJ, Tsai KW, Kao JY, et al. Equivalent efficacies of reverse hybrid and bismuth quadruple therapies in eradication of Helicobacter pylori infection in a randomized controlled trial. Clin Gastroenterol Hepatol 2018; 16:1427–1433. doi: 10.1016/j.cgh.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 40.Yang JC, Lin CJ, Wang HL, Chen JD, Kao JY, Shun CT, et al. High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clin Gastroenterol Hepatol 2015; 13:895–905. doi: 10.1016/j.cgh.2014.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sapmaz F, Kalkan IH, Atasoy P, Basyigit S, Guliter S. A non-inferiority study: modified dual therapy consisting higher doses of rabeprazole is as successful as standard quadruple therapy in eradication of Helicobacter pylori. Am J Ther 2017; 24:e393–e398. doi: 10.1097/MJT.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 42.Yang X, Wang JX, Han SX, Gao CP. High dose dual therapy versus bismuth quadruple therapy for Helicobacter pylori eradication treatment: a systematic review and meta-analysis. Medicine (Baltimore) 2019; 98:e14396.doi: 10.1097/MD.0000000000014396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang J, Zhang Y, Fan L, Zhu YJ, Wang TY, Wang XW, et al. Eradication efficacy of modified dual therapy compared with bismuth-containing quadruple therapy as a first-line treatment of Helicobacter pylori. Am J Gastroenterol 2019; 114:437–445. doi: 10.14309/ajg.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 44.Tai WC, Liang CM, Kuo CM, Huang PY, Wu CK, Yang SC, et al. A 14 day esomeprazole- and amoxicillin-containing high-dose dual therapy regimen achieves a high eradication rate as first-line anti-Helicobacter pylori treatment in Taiwan: a prospective randomized trial. J Antimicrob Chemother 2019; 74:1718–1724. doi: 10.1093/jac/dkz046. [DOI] [PubMed] [Google Scholar]
- 45.Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut 2016; 65:870–878. doi: 10.1136/gutjnl-2015-311019. [DOI] [PubMed] [Google Scholar]
- 46.Yu L, Luo L, Long X, Liang X, Ji Y, Graham DY, et al. High-dose PPI-amoxicillin dual therapy with or without bismuth for first-line Helicobacter pylori therapy: a randomized trial. Helicobacter 2019; 24:e12596.doi: 10.1111/hel.12596. [DOI] [PubMed] [Google Scholar]
- 47.Sugimoto M, Yamaoka Y. Role of vonoprazan in Helicobacter pylori eradication therapy in Japan. Front Pharmacol 2018; 9:1560.doi: 10.3389/fphar.2018.01560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kagami T, Sahara S, Ichikawa H, Uotani T, Yamade M, Sugimoto M, et al. Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to CYP2C19 genotype. Aliment Pharmacol Ther 2016; 43:1048–1059. doi: 10.1111/apt.13588. [DOI] [PubMed] [Google Scholar]
- 49.Jung YS, Kim EH, Park CH. Systematic review with meta-analysis: the efficacy of vonoprazan-based triple therapy on Helicobacter pylori eradication. Aliment Pharmacol Ther 2017; 46:106–114. doi: 10.1111/apt.14130. [DOI] [PubMed] [Google Scholar]
- 50.Li M, Oshima T, Horikawa T, Tozawa K, Tomita T, Fukui H, et al. Systematic review with meta-analysis: vonoprazan, a potent acid blocker, is superior to proton-pump inhibitors for eradication of clarithromycin-resistant strains of Helicobacter pylori. Helicobacter 2018; 23:e12495.doi: 10.1111/hel.12495. [DOI] [PubMed] [Google Scholar]
- 51.Mori H, Suzuki H, Omata F, Masaoka T, Asaoka D, Kawakami K, et al. Current status of first- and second-line Helicobacter pylori eradication therapy in the metropolitan area: a multicenter study with a large number of patients. Therap Adv Gastroenterol 2019; 12:321919921.doi: 10.1177/1756284819858511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nishida T, Tsujii Y, Okamoto A, Tomita R, Higaki Y, Osugi N, et al. A triple-drug blister-packaged drug with vonoprazan improves first-line eradication of Helicobacter pylori in elderly patients: a retrospective propensity score-matched cohort study. Digestion 2019; [Epub ahead of print] doi: 10.1159/000501608. [DOI] [PubMed] [Google Scholar]
- 53.Takara Y, Endo H, Nakano R, Kawachi K, Hidaka H, Matsunaga T, et al. Smoking and drinking did not increase the failure of therapeutic Helicobacter pylori eradication by vonoprazan, clarithromycin, and amoxicillin. Digestion 2019; 99:172–178. doi: 10.1159/000490889. [DOI] [PubMed] [Google Scholar]
- 54.Sue S, Shibata W, Sasaki T, Kaneko H, Irie K, Kondo M, et al. Randomized trial of vonoprazan-based versus proton-pump inhibitor-based third-line triple therapy with sitafloxacin for Helicobacter pylori. J Gastroenterol Hepatol 2019; 34:686–692. doi: 10.1111/jgh.14456. [DOI] [PubMed] [Google Scholar]
- 55.Saito Y, Konno K, Sato M, Nakano M, Kato Y, Saito H, et al. Vonoprazan-based third-line therapy has a higher eradication rate against sitafloxacin-resistant Helicobacter pylori. Cancers (Basel) 2019; 11:E116.doi: 10.3390/cancers11010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Furuta T, Yamade M, Kagami T, Uotani T, Suzuki T, Higuchi T, et al. Dual therapy with vonoprazan and amoxicillin is as effective as triple therapy with vonoprazan, amoxicillin and clarithromycin for eradication of Helicobacter pylori. Digestion 2019; [Epub ahead of print] doi: 10.1159/000502287. [DOI] [PubMed] [Google Scholar]
- 57.Lee JW, Kim N, Nam RH, Lee SM, Kwon YH, Sohn SD, et al. Favorable outcomes of culture-based Helicobacter pylori eradication therapy in a region with high antimicrobial resistance. Helicobacter 2019; 24:e12561.doi: 10.1111/hel.12561. [DOI] [PubMed] [Google Scholar]
- 58.Yu L, Luo L, Long X, Liang X, Ji Y, Chen Q, et al. Susceptibility-guided therapy for Helicobacter pylori infection treatment failures. Therap Adv Gastroenterol 2019; 12:321903510.doi: 10.1177/1756284819874922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Q, Long X, Ji Y, Liang X, Li D, Gao H, et al. Randomised controlled trial: susceptibility-guided therapy versus empiric bismuth quadruple therapy for first-line Helicobacter pylori treatment. Aliment Pharmacol Ther 2019; 49:1385–1394. doi: 10.1111/apt.15273. [DOI] [PubMed] [Google Scholar]
- 60.Kwon YH, Jeon SW, Nam SY, Lee HS, Park JH. Efficacy of tailored therapy for Helicobacter pylori eradication based on clarithromycin resistance and survey of previous antibiotic exposure: a single-center prospective pilot study. Helicobacter 2019; 24:e12585.doi: 10.1111/hel.12585. [DOI] [PubMed] [Google Scholar]
- 61.Ong S, Kim SE, Kim JH, Yi NH, Kim TY, Jung K, et al. Helicobacter pylori eradication rates with concomitant and tailored therapy based on 23S rRNA point mutation: a multicenter randomized controlled trial. Helicobacter 2019; 24:e12654.doi: 10.1111/hel.12654. [DOI] [PubMed] [Google Scholar]
- 62.Cho JH, Jeon SR, Kim HG, Jin SY, Park S. Cost-effectiveness of a tailored Helicobacter pylori eradication strategy based on the presence of a 23S ribosomal RNA point mutation that causes clarithromycin resistance in Korean patients. J Gastroenterol Hepatol 2019; 34:700–706. doi: 10.1111/jgh.14383. [DOI] [PubMed] [Google Scholar]
- 63.Delchier JC, Bastuji-Garin S, Raymond J, Megraud F, Amiot A, Cambau E, et al. Efficacy of a tailored PCR-guided triple therapy in the treatment of Helicobacter pylori infection. Med Mal Infect 2019; [Epub ahead of print] doi: 10.1016/j.medmal.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 64.Qureshi N, Li P, Gu Q. Probiotic therapy in Helicobacter pylori infection: a potential strategy against a serious pathogen? Appl Microbiol Biotechnol 2019; 103:1573–1588. doi: 10.1007/s00253-018-09580-3. [DOI] [PubMed] [Google Scholar]
- 65.Chen YH, Tsai WH, Wu HY, Chen CY, Yeh WL, Chen YH, et al. Probiotic lactobacillus spp. act against Helicobacter pylori-induced inflammation. J Clin Med 2019; 8:E90.doi: 10.3390/jcm8010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou BG, Chen LX, Li B, Wan LY, Ai YW. Saccharomyces boulardii as an adjuvant therapy for Helicobacter pylori eradication: a systematic review and meta-analysis with trial sequential analysis. Helicobacter 2019; 24:e12651.doi: 10.1111/hel.12651. [DOI] [PubMed] [Google Scholar]
- 67.Shi X, Zhang J, Mo L, Shi J, Qin M, Huang X. Efficacy and safety of probiotics in eradicating Helicobacter pylori: a network meta-analysis. Medicine (Baltimore) 2019; 98:e15180.doi: 10.1097/MD.0000000000015180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fang HR, Zhang GQ, Cheng JY, Li ZY. Efficacy of Lactobacillus-supplemented triple therapy for Helicobacter pylori infection in children: a meta-analysis of randomized controlled trials. Eur J Pediatr 2019; 178:7–16. doi: 10.1007/s00431-018-3282-z. [DOI] [PubMed] [Google Scholar]
- 69.Olekhnovich EI, Manolov AI, Samoilov AE, Prianichnikov NA, Malakhova MV, Tyakht AV, et al. Shifts in the human gut microbiota structure caused by quadruple Helicobacter pylori eradication therapy. Front Microbiol 2019; 10:1902.doi: 10.3389/fmicb.2019.01902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu L, Wang Z, Sun G, Peng L, Lu Z, Yan B, et al. Effects of anti-H. pylori triple therapy and a probiotic complex on intestinal microbiota in duodenal ulcer. Sci Rep 2019; 9:12874.doi: 10.1038/s41598-019-49415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li WQ, Zhang JY, Ma JL, Li ZX, Zhang L, Zhang Y, et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow-up of a randomized intervention trial. BMJ 2019; 366:l5016.doi: 10.1136/bmj.l5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang-Ou YB, Hu Y, Zhu Y, Lu NH. The effect of antioxidants on Helicobacter pylori eradication: a systematic review with meta-analysis. Helicobacter 2018; 23:e12535.doi: 10.1111/hel.12535. [DOI] [PubMed] [Google Scholar]
- 73.Han C, Ni Z, Yuan T, Zhang J, Wang C, Wang X, et al. Influence of serum vitamin D level on Helicobacter pylori eradication: a multi-center, observational, prospective and cohort study. J Dig Dis 2019; 20:421–426. doi: 10.1111/1751-298012793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mut SD, Surmeli ZG, Bahsi R, Turgut T, Selvi OH, Atmis V, et al. Vitamin D deficiency and risk of Helicobacter pylori infection in older adults: a cross-sectional study. Aging Clin Exp Res 2019; 31:985–991. doi: 10.1007/s40520-018-1039-1. [DOI] [PubMed] [Google Scholar]
- 75.Yang L, He X, Li L, Lu C. Effect of vitamin D on Helicobacter pylori infection and eradication: a meta-analysis. Helicobacter 2019; 24:e12655.doi: 10.1111/hel.12655. [DOI] [PubMed] [Google Scholar]
- 76.Gonzalez A, Salillas S, Velazquez-Campoy A, Espinosa AV, Fillat MF, Sancho J, et al. Identifying potential novel drugs against Helicobacter pylori by targeting the essential response regulator HsrA. Sci Rep 2019; 9:11294.doi: 10.1038/s41598-019-47746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salillas S, Alias M, Michel V, Mahia A, Lucia A, Rodrigues L, et al. Design, synthesis, and efficacy testing of nitroethylene- and 7-nitrobenzoxadiazol-based flavodoxin inhibitors against Helicobacter pylori drug-resistant clinical strains and in Helicobacter pylori-infected mice. J Med Chem 2019; 62:6102–6115. doi: 10.1021/acs.jmedchem.9b00355. [DOI] [PubMed] [Google Scholar]
- 78.McNicholl AG, O’Morain CA, Megraud F, Gisbert JP. Protocol of the European Registry on the management of Helicobacter pylori infection (Hp-EuReg). Helicobacter 2019; 24:e12630.doi: 10.1111/hel.12630. [DOI] [PubMed] [Google Scholar]


