To the Editor: Talaromyces marneffei (T. marneffei), formerly known as Penicillium marneffei, was first isolated from the liver of a wild Chinese bamboo rat in Vietnam by Capponi et al in the Pasteur Institute in 1956. Disalvo et al reported the first case of human natural infection in 1973. The patient was a human immunodeficiency virus (HIV)-negative American missionary with Hodgkin disease. The main epidemic areas of the disease are Southeast Asia, including Thailand, Vietnam, and China. Guangdong and Guangxi are the most common areas in China.[1]T. marneffei is a conditional pathogenic fungus mostly found in people with low immunity to infections; HIV-positive patients are the most commonly affected. However, the acquired infection has been reported in a number of apparently immunocompetent individuals. In both of these groups, untreated T. marneffei infections are fatal.
A 33-year-old woman suffered from a headache and dizziness on February 19, 2018. The headache presented on bilateral temporal areas, which was not paid attention to. On February 23, her headache and dizziness aggravated, accompanied by blurred vision and vomiting. She was admitted to a local hospital. Lumbar puncture and cranial magnetic resonance imaging (MRI) were recommended. The cerebral spinal fluid (CSF) revealed elevated opening pressure of 240 mmH2O, white blood cell (WBC) count of 176 cells/μL (normal range: 0–8 cells/μL), decreased glucose level of 1.1 mmol/L (normal range: 2.8–4.5 mmol/L), and slightly increased protein level of 0.87 g/L (normal range: 0.15–0.45 g/L). Cranial MRI with contrast showed diffused meningeal enhancement and mild white matter high signals on the T2-weighted image. Tuberculosis (TB) meningoencephalitis was suspected, and anti-TB regimen of isoniazid 0.6 g qd, rifampicin 0.45 g qd, pyrazinamide 0.5 g tid, and ethambutol 0.75 g qd was initiated. However, her neurological status deteriorated with recurrent epilepsy and decreased consciousness. She also had a high fever of 39°C. She was transferred to the neurointensive unit of the Nanfang Hospital, Southern Medical University, for diagnosis and treatment. At admission, the neurological examination showed that the patient was in a coma with a Glasgow Coma scale (GCS) of 5 (E1V1M3) and neck stiffness. Lumbar puncture was repeated with a dramatically increased opening pressure of 330 mmH2O. The CSF WBC count (150 cells/μL) was elevated with lymphocyte predominance. The CSF chemistry showed that the glucose level dramatically decreased to 0.58 mmol/L (normal range: 2.8–4.0 mmol/L), and the protein level was within the normal range. Cranial MRI revealed multiple abnormal signals in the bilateral basal ganglia and bilateral paraventricular and frontotemporal lobes, complicated with acute cerebral ischemia, infarction hemorrhage, hydrocephalus, interstitial brain edema, and also abnormal enhancement of meninges with contrast [Figure 1]. Magnetic resonance angiography showed multiple intracranial vascular lumen thinning, wall thinning, and roughness due to vasculitis or brain edema [Figure 1]. Other laboratory screenings for autoimmune diseases, HIV infections, syphilis, and other pathogens (herpes simplex virus [HSV]-1, HSV-2, cytomegalovirus, influenza, TB, mycoplasma, and adenovirus legionella pneumonia) were all negative. The pathogenic smears and cultures of sputum, peripheral blood, bone marrow, and CSF were all negative. Serum 1,3-β-D glucan test and galactomannan were all negative. Chest computed tomography (CT) revealed bilateral inflammatory infiltrations of the apical and posterior segments of the right upper lobe and bilateral lower lobes. Abdominal CT revealed no abnormality.
Figure 1.
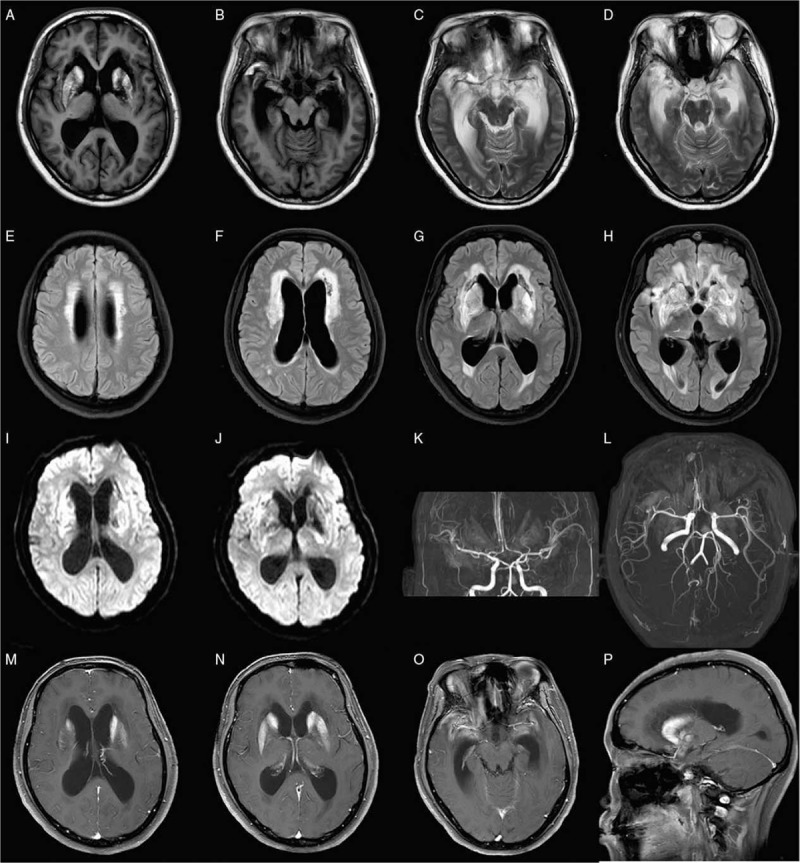
Cranial MRI images of the patient. (A and B) T1 images revealed hemorrhage of bilateral basal ganglia and temporal lobes. (C and D) Cranial MRI of T2, (E–H) FLAIR, and (I and J) DWI images revealed multiple abnormal signals in the bilateral basal ganglia and bilateral paraventricular and frontotemporal lobes, complicated with acute cerebral ischemia, infarction hemorrhage, hydrocephalus, and interstitial brain edema. Magnetic resonance angiography showed multiple intracranial vascular lumen thinning, wall thinning, and roughness. T1 images with contrast revealed abnormal enhancement of meninges (M–P). DWI: Diffused weighted image; FLAIR: Fluid-attenuated inversion recovery; MRI: Magnetic resonance image.
Tuberculous meningoencephalitis was considered, and anti-TB therapy was continued. For the screening of pathogens by next-generation sequencing (NGS), the CSF sample was snap-frozen and stored at −20°C. DNA was extracted from 300 μL of the CSF sample using the TIANamp Micro DNA Kit (DP316, Tiangen Biotech, Beijing, China) according to manufacturer's protocols. For samples with a suspected fungal infection, CSF was pre-treated with glass beads before DNA preparation (Z250465, Sigma, MO, USA). Then, 300 μL of the supernatant was collected for DNA extraction as described earlier.[2] On the seventh day of admission, the NGS (Beijing Genome Institute) of CSF revealed T. marneffei infection (49 reads); voriconazole was prescribed promptly, and anti-TB therapy was discontinued. With the anti-fungal therapy for 3 weeks, another CSF examination showed the opening pressure of 230 mmH2O, normal WBC count of 7 cells/μL, and normal glucose level of 2.86 mmol/L. Lumbar puncture and NGS were also repeated 1 month later and confirmed T. marneffei infection (six reads). Two and a half months later, the patient's neurological status was stable with a GCS score of eight (E4VTM3), and she was transferred to the rehabilitation department.
T. marneffei proliferates in macrophages and disseminates via the reticuloendothelial system. The clinical features are characterized by fungal invasions of multiple organs, especially blood, bone marrow, skin, lungs, and reticuloendothelial tissues.
T. marneffei infections are now widely reported in South China, predominately in immunocompromised patients.[2] A retrospective review of T. marneffei infections in the mainland of China showed that 99.4% of the cases were reported in the southern part of China with 42.8% from Guangxi and 40.6% from Guangdong province. The patient in the present case was also a resident of Guangdong province. The route of transmission of this pathogen to patients is still a mystery. The habit of hunting and eating bamboo rats from the natural environment is considered to be a risk factor, despite the lack of evidence confirming this hypothesis.[2] However, the patient in the present case had no history of contact with bamboo rats.
Another important finding was that the history of the patient was clean, and no immunocompromised condition was found with a thorough evaluation. As high as 91.5% of T. marneffei infections are reported in patients comorbid with HIV infections or other immunocompromised diseases.[2] Chan et al reviewed the T. marneffei infection in 119 non-HIV-infected patients. They found that the common clinical features of these patients included fever, malaise, weight loss, skin and soft tissue lesions, hepatosplenomegaly, lymphadenopathy, cough, and dyspnea, while neurological symptoms, including seizures and confusion, accounted for only 5%.[3] In non-HIV-infected patients with T. marneffei, most of the patients had various comorbidities, including autoimmune diseases, organ transplantation, hematological malignancy, novel anti-cancer targeted therapy, and other rare conditions.[3]
The central nervous system infection of T. marneffei has rarely been reported in non-HIV or HIV-infected patients.[4,5] Le et al reported 21 HIV-infected patients with definite central nervous system infections of T. marneffei, of which two had disseminated infections.[4] Disseminated T. marneffei infection diagnosed by NGS of multifarious specimens in an HIV-negative patient was also reported. This was a reported case of isolated T. marneffei meningoencephalitis in an immunocompetent patient.
NGS is a new diagnostic technology with more than 3000 kinds of pathogens tested at the same time. The diagnosis is very convenient and takes only 24 h. Another important point is that the laboratory tests in most hospitals are restricted to commonly known and suspected pathogens; hence, the rare infections are misdiagnosed easily. Currently, the diagnosis of the infection relies on the isolation of definite pathogens and thus has a quite low positive rate. The diagnosis of T. marneffei is commonly delayed, and it is underdiagnosed because of the limitations of the traditional laboratory tests. Moreover, these patients are easily misdiagnosed with TB because of similar clinical features and unspecific laboratory results. In the present case, NGS helped in making a precise and rapid diagnosis, thereby saving the patient.
Declaration of patient consent
Written informed consents were obtained from the patient and her family members for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Acknowledgements
The authors would like to extend their thanks to the patient and her family members for their generosity and cooperation.
Conflicts of interest
None.
Footnotes
How to cite this article: Wang DM, Ma HL, Tan MQ, Wu YM, Wang SN. Next-generation sequencing confirmed the diagnosis of isolated central nervous system infection caused by Talaromyces marneffei in an immunocompetent patient. Chin Med J 2020;133:374–376. doi: 10.1097/CM9.0000000000000593
Dong-Mei Wang and Hong-Ling Ma contributed equally to this study.
References
- 1.Hu Y, Zhang J, Li X, Yang Y, Zhang Y, Ma J, et al. Penicillium marneffei infection: an emerging disease in mainland China. Mycopathologia 2013; 175:57–67. doi: 10.1007/s11046-012-9577-0. [DOI] [PubMed] [Google Scholar]
- 2.Wang S, Chen Y, Wang D, Wu Y, Zhao D, Zhang J, et al. The feasibility of metagenomic next-generation sequencing to identify pathogens causing tuberculous meningitis in cerebrospinal fluid. Front Microbiol 2019; 10:1993.doi: 10.3389/fmicb.2019.01993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan JF, Lau SK, Yuen KY, Woo PC. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect 2016; 5:e19.doi: 10.1038/emi.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le T, Huu Chi N, Kim Cuc Ngo T, Manh Sieu Tran P, Shikuma Cecilia M, Farrar J, et al. AIDS-associated Penicillium marneffei infection of the central nervous system. Clin Infect Dis 2010; 51:1458–1462. doi: 10.1086/657400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye F, Luo Q, Zhou Y, Xie J, Zeng Q, Chen G, et al. Disseminated Penicilliosis marneffei in immunocompetent patients: a report of two cases. Indian J Med Microbiol 2015; 33:161–165. doi: 10.4103/0255-0857.148433. [DOI] [PubMed] [Google Scholar]


