Abstract
Background:
Baicalein has been shown to have anti-inflammatory and anti-tumor activities. However, the mechanisms underlying its anti-inflammatory effect on colitis remain unclear.
Methods:
A dextran sodium sulfate (DSS)-induced model of acute colitis was established in BALB/c mice (6–8 weeks old, weighing 18–22 g). Six groups of mice received: (1) water for 10 days (control), n = 6; (2) DSS 4% solution in the drinking water for 7 days, followed by normal water for 3 days, n = 7; (3), (4), and (5) as for group 2 plus baicalein (10, 20, 40 mg/kg) administered once daily starting on day 1, n = 6; and (6) as for (2) plus 5-aminosalicylic acid (50 mg/kg) administered once daily starting on day 1, n = 6. Body weights, stool consistency, and hematochezia were recorded, and the severity of colitis was evaluated using a disease activity index. On day 11, the mice were euthanized, and organs and blood were collected for analysis. Serum inflammatory factors were detected by enzyme-linked immunosorbent assay; CD11b-positive cells were analyzed by immunofluorescence microscopy; expression of retinoic-acid-receptor-related orphan nuclear receptor gamma, sphingosine kinase 1 (SPHK1), and phosphorylated signal transducer and activator of transcription 3 (p-STAT3) was detected by immunohistochemistry; and expression of nucleotide-binding oligomerization domain 2 (NOD2), SPHK1, sphingosine 1-phosphate receptor 1 (S1PR1), total STAT3, and p-STAT3 were detected by western blotting analysis. Inter-group differences were compared using Student's t test.
Results:
Baicalein treatment dose-dependently reduced DSS-induced weight loss (P < 0.01 or P < 0.05), splenomegaly (P < 0.01), and colonic damage, as reflected by amelioration of diarrhea, rectal bleeding, and colonic ulceration, congestion, edema (shown as colon length, P < 0.05 or P < 0.01), and inflammatory cell infiltration. Baicalein also significantly decreased the levels of inflammatory mediators in the serum (P < 0.01) and colon, and significantly inhibited expression of NOD2 SPHK1, S1PR1, and p-STAT3 in the colon (P < 0.05).
Conclusions:
Baicalein treatment ameliorated colitis in mice by inhibiting S1P-STAT3 signaling, suggesting that this flavonoid might be beneficial in the treatment of colitis.
Keywords: Baicalein, Colitis, STAT3, Sphingosine kinase 1
Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory disease of the gastrointestinal tract characterized by periods of remission and relapse. The most common forms of IBD are Crohn's disease and ulcerative colitis, which may affect the oral cavity, esophagus, stomach, intestine, and anus.[1] IBD has diverse causes, including immune-related, environmental, and genetic factors, and various symptoms, including diarrhea, abdominal pain, intestinal bleeding, and weight loss.[2,3] Thus, it has been difficult to establish the precise etiology of the disease and, consequently, its treatment. Administration of dextran sodium sulfate (DSS) is commonly used to model colitis in animals, because it evokes similar histopathological, clinical, and immunological responses to those seen in patients with IBD.[4–6]
Sphingosine-1-phosphate (S1P) is a pleiotropic bioactive sphingolipid metabolite involved in the regulation of several cellular processes and participates in mediating signal transduction inside and outside of the cell.[7,8] S1P, which is mainly produced via phosphorylation of sphingosine by the sphingosine kinases SPHK1 and SPHK2, activates intracellular signal transduction by binding to one of five cell surface sphingosine-1-phosphate receptors (S1PR1–5). Several studies have identified a close relationship between S1P and the development of IBD. For example, expression of SPHK1, SPHK2, S1PR1, S1PR2, and S1PR4 are significantly up-regulated in children with IBD,[8,9] and inhibition of SPHK1 reduces the expression of inflammatory markers and the infiltration of neutrophils in colonic tissue of mice with IBD.[9,10] Signal transducer and activator of transcription 3 (STAT3) is also over-expressed in the intestinal mucosa of patients with active and inactive IBD.[11] STAT3 mRNA and protein levels are abnormally high in patients with colorectal cancer, and STAT3 is thought to contribute to this cancer via an interleukin (IL)-22-STAT3 signaling pathway.[12,13] Inhibition of STAT3 and its associated pathways prevents the occurrence and progression of cancer and inflammatory diseases. The anti-tumor effects of dietary cocoa are mediated via inhibition of IL-6-STAT3 signaling.[14] Metformin reduces inflammation and the severity of IBD by inhibiting expression of phosphorylated STAT3 (p-STAT3) and IL-17.[15] IL-6 is generally considered to be a crucial factor in the activation of STAT3 signaling.[16] It has been shown that S1P can maintain STAT3 in an activated state, which contributes to the development of colitis-associated colon cancer.[17]
Baicalein (5,6,7-trihydroxypyrimone) is a major bioactive flavonoid isolated from the root of the plant Astragalus membranaceus. Among other effects, baicalein has been shown to have anti-inflammatory, anti-bacterial, anti-hypertensive, and anti-tumor activity, and it has also proven beneficial in the treatment of colitis.[5,18,19] Indeed, oral administration of baicalein to mice significantly ameliorates all inflammatory symptoms of colitis, including weight loss, hematochezia, rectal bleeding, and other tissue indicators.[19] In the rat DSS-induced colitis model, baicalein promotes the proliferation of colonic epithelial cells, down-regulates expression of STAT3 and STAT4 mRNA in the JAK-STAT signaling pathway in T cells, and regulates T-cell proliferation.[20] However, the mechanism of action of baicalein is complex, and many aspects of its ability to ameliorate colitis remain unclear. Therefore, in this study, we evaluated whether baicalein exerts its anti-colitic activity through effects on the S1P-STAT3 signaling pathway.
Methods
Reagents
The DSS (molecular weight 36,000–50,000 Da) was purchased from MP Biomedicals (Irvine, CA, USA). Mouse IL-6, IL-1β, and tumor necrosis factor (TNF)-α enzyme-linked immunosorbent assay (ELISA) kits were purchased from Feiya Biotech (Jiangsu, China). Primary antibodies against SPHK1, p-STAT3 (phospho-S727), S1PR1/EDG1, and retinoic-acid-receptor-related orphan nuclear receptor gamma (RORγt) were purchased from Abcam (Cambridge, MA, USA), anti-β-actin and anti-STAT3 were purchased from Cell Signaling Technology (Danvers, MA, USA), anti-lamin A was purchased from Bioworld Technology (St. Louis Park, MN, USA), and rabbit anti-mouse CD11b-fluorescein isothiocyanate (FITC) was purchased from eBioscience (San Diego, CA, USA).
Establishment of DSS-induced colitis in mice and experimental design
Female BALB/c mice (35–40 days of age, weighing 18–22 g) were obtained from Jinan Pengyue Experimental Animal Breeding Co. Ltd. (Jinan, China). Animals had access to food and water ad libitum and were maintained on a 12 h/12 h light/dark cycle at 21 ± 2°C with a relative humidity of 45 ± 10%. Animal care and surgery protocols were approved by Experimental Animal Ethics Committee of Jining Medical University. Acute colitis was induced in five groups of six mice by addition of 5% (w/v) DSS in the drinking water for 7 days, after which normal drinking water was provided for 3 days. The six groups were randomly assigned to receive water (n = 6), DSS (n = 7), baicalein (10, 20, and 40 mg/kg) (n = 6), or 5-aminosalicylic acid (5ASA, 50 mg/kg) (n = 6). Baicalein and 5ASA were administered intragastrically once daily from day 1 to 10. All mice were sacrificed on day 11.
Disease activity index
The disease activity index (DAI) scale ranged from 0 (healthy) to 12 (severe colitis) and was calculated from the combined scores of 0 to 4 for body weight loss, stool consistency, and rectal bleeding, as shown in Table 1.
Table 1.
The calculation method of disease activity index of all groups.

Collection of tissues and preparation of serum samples
Animals were sacrificed on day 11, and organs and blood were collected. The colon was opened longitudinally and washed with phosphate-buffered saline. Colon tissues from three mice were fixed in formalin for hematoxylin and eosin (H&E) and immunohistochemical (IHC) staining. The remaining colon tissues were stored in liquid nitrogen for western blot analysis. Blood was collected retroorbitally into a 1.5-mL tube and centrifuged at 3000 rpm for 5 min. Serum was removed and stored at 4°C until analysis.
Macroscopic assessment and histological analysis of colonic lesions
Paraffin-embedded colonic tissue blocks were cut into 4-μm-thick sections using a microtome and then stained with H&E using a standard protocol.
Immunohistochemistry
Expression of IL-6, IL-1β, TNF-α, RORγt, SPHK1, and p-STAT3 in colonic tissue sections was assessed by IHC as previously described.[21]
Immunofluorescence confocal microscopy
Colonic tissue sections were stained with FITC-conjugated anti-CD11b antibody at a dilution of 1:100 and then incubated with 4′6-diamidino-2-phenylindole to visualize nuclei. The sections were analyzed and images were captured using an Olympus FV1000 confocal microscope (Takachiho, Japan).
Cytokine quantification by enzyme-linked immunosorbent assay
IL-6, IL-1β, and TNF-α in sera were quantified using an ELISA kit according to the manufacturer's recommendations, as previously described.[22] The experiments were repeated three times.
Western blotting analysis
Tissues were lysed and analyzed by Western blotting as previously described.[21] Blots were probed with rabbit anti-SPHK1 (1:1000), rabbit anti-S1P1/EDG1 (1:1000), mouse anti-STAT3 (1:1000), and rabbit anti-p-STAT3 (phospho-S727) (1:2000) primary antibodies. β-Actin (antibody dilution 1:2000) was probed as an internal loading control.
Statistical analysis
Statistical analysis was performed using Prism 5 software (GraphPad Software, La Jolla, CA, USA). Values are presented as the mean ± standard deviation (SD) of three independent experiments for each individual treatment or dose. Group means were compared using Student's t-test (for normal distribution). A value of P < 0.05 was considered statistically significant.
Results
Baicalein attenuates DSS-induced colon injury and inflammatory symptoms in mice
To investigate the effects of baicalein on colitis in a mouse model, we provided DSS to the drinking water of groups of BALB/c mice for 7 days and treated them with water, baicalein (10–40 mg/kg), or 5ASA (50 mg/kg) for 10 days starting on day 1. A model control group received water without DSS. As shown in Figure 1A, mice in the DSS group showed significant weight loss compared with the control mice, but the baicalein group showed a dose-dependent and significant reduction in body weight loss compared with the DSS group. The mean colon length of DSS-treated mice was significantly shorter than that of the control mice (P < 0.01, t = 8.35), and baicalein treatment (10, 20, and 40 mg/kg) also reversed the colon shortening (P < 0.05, t = 2.51; P < 0.05, t = 2.62, and P < 0.01, t = 3.27, respectively) [Figure 1B and 1C]. In addition, baicalein at 20 and 40 mg/kg reduced the splenomegaly caused by DSS treatment (P < 0.01, t = 3.40 and P < 0.01, t = 4.10, respectively) [Figure 1D]. Pathological examination of colon sections indicated that DSS treatment induced severe mucosal necrosis accompanied by extensive inflammatory cell infiltration, congestion, and edema of the submucosa. This colonic damage was significantly reduced by baicalein treatment in a dose-dependent manner, with a concomitant reduction in inflammatory cell infiltration [Figure 1E]. The overall disease activity of DSS-treated mice, as measured by the DAI, was also reduced by baicalein treatment, consistent with the amelioration of colonic damage [Figure 1F].
Figure 1.

Baicalein attenuates dextran sodium sulfate (DSS)-induced colon injury and inflammation. (A) Body weights of the control, DSS, DSS + baicalein, and DSS + 5-aminosalicylic acid (5ASA) groups of mice. Macroscopic appearance (B) and length (C) of colons from each mouse group. (D) Changes in spleen weights during disease progression. (E) Hematoxylin and eosin staining of serial sections of the colon. Black arrows and red arrows indicate infiltration of inflammatory cells in the mucosa or submucosa. (F) Disease activity index. All results are presented as the mean ± standard deviation. ∗P < 0.05, †P < 0.01 compared with the control group; ‡P < 0.05, §P < 0.01 compared with the DSS group.
Baicalein reduces the production of inflammatory mediators in the serum and colon of mice with DSS-induced colitis
Compared with the water-treated mice, baicalein at 10, 20, and 40 mg/kg significantly reduced the DSS-induced increase in serum IL-6 and IL-1β levels (IL-6: P < 0.01, t = 17.90; P < 0.01, t = 5.78; and P < 0.01, t = 5.98, respectively; IL-1β: P < 0.01, t = 6.05; P < 0.01, t = 16.86; and P < 0.01, t = 17.13, respectively) [Figure 2A]. In addition, baicalein reduced the number of IL-6-positive, IL-1β-positive, and TNF-α-positive cells in the colonic mucosa of DSS-treated mice [Figure 2B].
Figure 2.

Baicalein reduces levels of inflammatory mediators in the serum and colon of mice with dextran sodium sulfate (DSS)-induced colitis. Enzyme-linked immunosorbent assay analysis of serum interleukin (IL)-6 (A) and IL-1β (B) concentrations. Results are presented as the mean ± standard deviation of triplicates. †P < 0.01 compared with the control group; ‡P < 0.05, §P < 0.01 compared with the DSS group. (C) Immunohistochemical staining of IL-1β, IL-6, and tumor necrosis factor (TNF)-α in colon sections (original magnification, ×40).
Baicalein reduces the DSS-induced infiltration of inflammatory cells into the colon
To further evaluate the protective effect of baicalein on DSS-induced colitis, we measured inflammatory cell infiltration by IHC staining of colon sections for CD11b+, which is expressed on many leukocytes, including monocytes, neutrophils, natural killer cells, granulocytes, and macrophages. As shown in Figure 3, a large number of CD11b+ cells were observed in the lesional mucosa of colons from DSS/water-treated mice, but the cell abundance was dramatically reduced by baicalein treatment. Moreover, baicalein also attenuated the expression of the transcription factor RORγt, which is pivotal in the development of proinflammatory Th17 cells [Figure 4].
Figure 3.

Baicalein reduces inflammatory cell infiltration in the colons of mice with dextran sodium sulfate (DSS)-induced colitis. (A) Immunofluorescence microscopy of CD11b+ cells in colon sections. (B) Results are presented as the mean ± standard deviation. †P < 0.01 compared with the control group; §P < 0.01 compared with the DSS group.
Figure 4.

Baicalein reduces the expression of retinoic-acid-receptor-related orphan nuclear receptor gamma (RORγt) in colonic tissue from mice with DSS-induced colitis. Immunohistochemical staining of RORγt in colon sections.
Baicalein inhibits the expression of nucleotide-binding oligomerization domain 2 protein in colonic tissues from DSS-treated mice
Nucleotide-binding oligomerization domain 2 (NOD2) is an intracellular pattern recognition receptor expressed in both hematopoietic and non-hematopoietic cells in the intestinal epithelium and plays a key role in stimulating the host immune response.[23,24] Western blot analysis of colon extracts showed that NOD2 expression was increased by DSS treatment, while baicalein significantly inhibited its expression compared with the DSS/water group [Figure 5A and 5B].
Figure 5.

Baicalein reduces the expression of nucleotide-binding oligomerization domain 2 (NOD2) in colonic tissue from mice with dextran sodium sulfate (DSS)-induced colitis. (A, B) Western blotting analysis of NOD2 expression in extracts of colon tissues. †P < 0.01 compared with the control group; ‡P < 0.05 compared with the DSS group.
Baicalein ameliorates colitis by inhibiting the S1P–STAT3 signaling pathway
As noted, enzymes that control S1P levels (including SHPK1 and SPHK2) are consistently dysregulated in animal models of IBD and in human colonic tissues.[25] IHC analysis of colon sections from DSS-treated mice revealed large numbers of SPHK1- and p-STAT3-positive cells, but their abundance was significantly reduced by baicalein treatment at 40 mg/kg [Figure 6A]. In addition, western blot analysis confirmed that baicalein at 40 mg/kg significantly inhibited DSS-induced phosphorylation of STAT3 and reversed the over-expression of SPHK1 and S1PR1 [Figure 6B and 6C]. However, the expression of total STAT3 was not changed by baicalein treatment [Figure 6B]. These results indicate that baicalein exerts its anti-colitic effects through the inhibition of SPHK1 expression and STAT3 activation.
Figure 6.
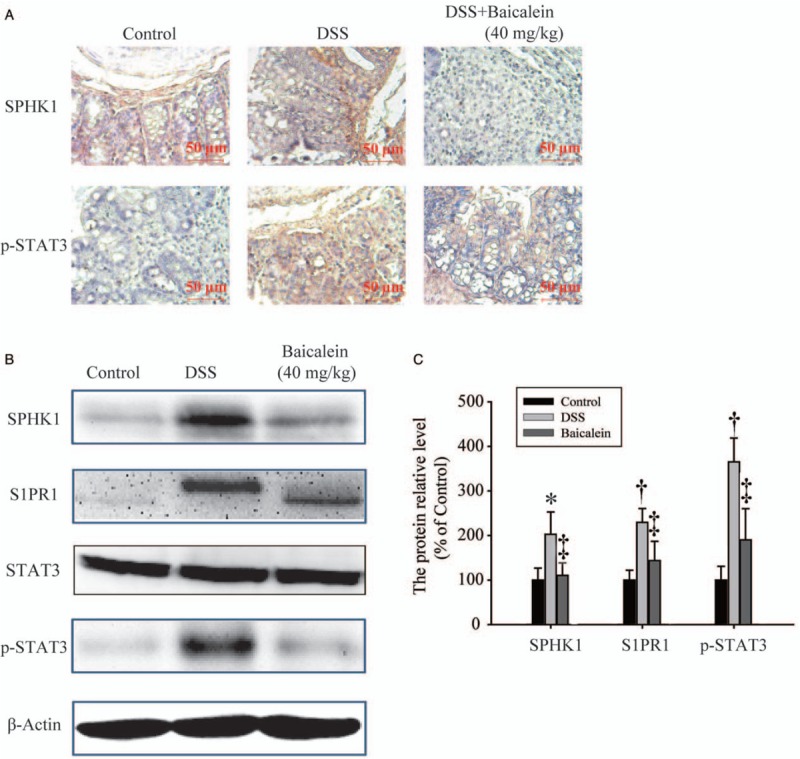
The anti-colitic effects of baicalein are mediated by inhibition of dextran sodium sulfate (DSS)-induced sphingosine-1-phosphate (S1P)–signal transducer and activator of transcription 3 (STAT3) signaling. (A) Immunohistochemical staining of sphingosine kinase 1 (SPHK1) and phosphorylated signal transducer and activator of transcription 3 (p-STAT3) in colonic tissues (original magnification, ×40). (B, C) Western blotting analysis of SPHK1, sphingosine 1-phosphate receptor 1 (S1PR1), STAT3, and p-STAT3 in colonic tissue extracts. ∗P < 0.05, †P < 0.01 compared with the control group; ‡P < 0.05 compared with the DSS group.
Discussion
The results of this study demonstrated that mice treated with DSS exhibited weight loss, colon shortening, and colon tissue damage accompanied by extensive inflammatory cell infiltration. However, these effects were significantly reduced by treatment with baicalein in a dose-dependent manner. Importantly, the DSS-induced increase in serum IL-6 and IL-1β and colonic IL-6, IL-1β, and TNF-α were reduced by baicalein treatment, as was the expression of the Th17 cell transcription factor RORγt in the colon.
Studies to date have shown that baicalein exhibits potent anti-inflammatory activity with little to no toxicity in animals and humans.[26] Kim et al[27] demonstrated that baicalein inhibits the production of various cytokines, chemokines, and growth factors by the RAW264.7 macrophage cell line. In a mouse model, baicalein reduced acute pancreatitis-associated inflammation by inhibiting the NF-κB, MAPK, and STAT3 signaling pathways.[18] Although baicalein has been reported to have anti-colitic activity,[5,19] the precise anti-inflammatory mechanisms were not clear. Here, we employed the well-characterized DSS model with 5ASA, which has established efficacy in preventing colitis in animal models,[28] as a positive control to compare with the effects of baicalein.
NOD2 plays an important role in inflammation and associated carcinogenesis.[23,24,29] After binding of muramyl dipeptide, a molecule produced by all bacteria, NOD2 promotes the production of proinflammatory factors, such as cytokines and chemokines, by increasing expression of MAPK and NF-κB.[30] In the present study, we found that NOD2 was expressed at higher levels in the model control group (no DSS) than the DSS/water group, indicating that DSS suppresses NOD2 expression. However, baicalein further inhibited NOD2 expression, consistent with the inhibition of colitis symptoms.
S1P is known to play an important role in inflammatory diseases, especially IBD.[7] SPHK1 and SPHK2 are expressed at low levels in healthy colon tissues, but SPHK1 is up-regulated and activated during inflammation.[31] Expression of SPHK1 in the colonic epithelium is required for autocrine induction of cyclo-oxygenase 2 during DSS-induced colitis, and SPHK1 is also over-expressed in colitis-related cancers.[32] STAT3 has important functions in the induction and progression of IBD. Lovato et al[33] found that STAT3 is constitutively activated in intestinal T cells isolated from patients with Crohn's disease, but not from healthy volunteers. It has been demonstrated that p-STAT3 expression is restricted to areas of active inflammation in the gastrointestinal tract in patients with Crohn's disease and ulcerative colitis.[34,35] STAT3 mediates the transcription of numerous genes involved in colitis and inflammation-related cancers.[36] S1P mediates the activation of STAT3, which is crucial to the development of IBD-associated colorectal cancer.[37] Conversely, STAT3 can regulate the concentration of S1P levels to some extent.[16,36,37] These observations point to a reciprocal stimulatory relationship between S1P and STAT3 in promoting carcinogenesis.[36] In the present study, we showed that baicalein significantly inhibited the DSS-induced increase in SPHK1 and p-STAT3 expression in the colon, providing a potential mechanism for the protective effect of baicalein in DSS-induced colitis. Although we found that baicalein also reduced RORγt expression in the colon, we did not investigate the presence of Th17 cells. Such analyses are planned for future studies. We also plan to further explore the anti-inflammatory mechanism of baicalein using RNA interference-mediated inhibition of S1PR1 and STAT3 expression.
IBD pathogenesis is caused by an imbalance between microflora and intestinal mucosal integrity and barrier function. Patients with this disease not only suffer seriously reduced quality of life but also have a greatly increased risk of developing colorectal cancer.[31,38] Standard IBD treatments, including steroids, other immunosuppressants, aminosalicylate, and antibiotics, are not always effective, and many refractory cases require surgery, albeit often with unsatisfactory efficacy and safety outcomes. In this study, we found that baicalein improved DSS-induced weight loss, colonic congestion and edema, splenomegaly, diarrhea, rectal bleeding, and the accompanying colonic and systemic inflammation, suggesting that this flavonoid might be beneficial for the treatment of colitis in humans. However, further studies will be necessary to evaluate the safety of baicalein in clinical applications and to optimize its beneficial effects in patients.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (Nos. 81603143, 21807041), Shandong Medical and Health Science and Technology Development Project (Nos. 2017WS646, 2017WS653), Teachers’ Research Support Fund of Jining Medical University (No. JYFC2018KJ037), and National Natural Science Foundation Breeding Programs of Jining Medical University (No. JYP201708).
Conflicts of interest
None.
Footnotes
How to cite this article: Yao J, Liu T, Chen RJ, Liang J, Li J, Wang CG. Sphingosine-1-phosphate signal transducer and activator of transcription 3 signaling pathway contributes to baicalein-mediated inhibition of dextran sulfate sodium-induced experimental colitis in mice. Chin Med J 2020;133:292–300. doi: 10.1097/CM9.0000000000000627
Jing Yao and Tao Liu contributed equally to the work.
References
- 1.Alavala S, Sangaraju R, Nalban N, Sahu BD, Jerald MK, Kilari EK, et al. Stevioside, a diterpenoid glycoside, shows anti-inflammatory property against dextran sulphate sodium-induced ulcerative colitis in mice. Eur J Pharmacol 2019; 855:192–201. doi: 10.1016/j.ejphar.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Zhao M, Burisch J. Impact of genes and the environment on the pathogenesis and disease course of inflammatory bowel disease. Dig Dis Sci 2019; 64:1759–1769. doi: 10.1007/s10620-019-05648-w. [DOI] [PubMed] [Google Scholar]
- 3.Liu XW, Wang CD. Melatonin alleviates circadian rhythm disruption exacerbating DSS-induced colitis by inhibiting the distribution of HMGB1 in intestinal tissues. Int Immunopharmacol 2019; 73:108–117. doi: 10.1016/j.intimp.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Yao J, Wang JY, Liu L, Li YX, Xun AY, Zeng WS, et al. Anti-oxidant effects of resveratrol on mice with DSS-induced ulcerative colitis. Arch Med Res 2010; 41:288–294. doi: 10.1016/j.arcmed.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Luo X, Yu Z, Deng C, Zhang J, Ren G, Sun A, et al. Baicalein ameliorates TNBS-induced colitis by suppressing TLR4/MyD88 signaling cascade and NLRP3 inflammasome activation in mice. Sci Rep 2017; 7:16374.doi: 10.1038/s41598-017-12562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang WY, Seo GS, Kim YC, Sohn DH, Lee SH. PF2405, standardized fraction of Scutellaria baicalensis, ameliorates colitis in vitro and in vivo. Arch Pharm Res 2015; 38:1127–1137. doi: 10.1007/s12272-015-0553-3. [DOI] [PubMed] [Google Scholar]
- 7.Yuza K, Nagahashi M, Shimada Y, Nakano M, Tajima Y, Kameyama H, et al. Upregulation of phosphorylated sphingosine kinase 1 expression in colitis-associated cancer. J Surg Res 2018; 231:323–330. doi: 10.1016/j.jss.2018.05.085. [DOI] [PubMed] [Google Scholar]
- 8.Han C, He X, Xia X, Guo J, Liu A, Liu X, et al. Sphk1/S1P/S1PR1 signaling is involved in the development of autoimmune thyroiditis in patients and NOD.H-2(h4) mice. Thyroid 2019; 29:700–713. doi: 10.1089/thy.2018.0065. [DOI] [PubMed] [Google Scholar]
- 9.Suh JH, Degagne E, Gleghorn EE, Setty M, Rodriguez A, Park KT, et al. Sphingosine-1-phosphate signaling and metabolism gene signature in pediatric inflammatory bowel disease: a matched-case control pilot study. Inflamm Bowel Dis 2018; 24:1321–1334. doi: 10.1093/ibd/izy007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pulkoski-Gross MJ, Uys JD, Orr-Gandy KA, Coant N, Bialkowska AB, Szulc ZM, et al. Novel sphingosine kinase-1 inhibitor, LCL351, reduces immune responses in murine DSS-induced colitis. Prost Other Lipid Mediat 2017; 130:47–56. doi: 10.1016/j.prostaglandins.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vavricka SR, Galvan JA, Dawson H, Soltermann A, Biedermann L, Scharl M, et al. Expression patterns of TNFalpha, MAdCAM1, and STAT3 in intestinal and skin manifestations of inflammatory bowel disease. J Crohns Colitis 2018; 12:347–354. doi: 10.1093/ecco-jcc/jjx158. [DOI] [PubMed] [Google Scholar]
- 12.Khare V, Paul G, Movadat O, Frick A, Jambrich M, Krnjic A, et al. IL10R2 overexpression promotes IL22/STAT3 signaling in colorectal carcinogenesis. Cancer Immunol Res 2015; 3:1227–1235. doi: 10.1158/2326-6066.cir-15-0031. [DOI] [PubMed] [Google Scholar]
- 13.Khare V, Paul G, Movadat O, Frick A, Jambrich M, Krnjic A, et al. IL10R2 overexpression promotes IL22/STAT3 signaling in colorectal carcinogenesis. Cancer Immunol Res 2015; 3:1227–1235. doi: 10.1158/2326-6066.CIR-15-0031. [DOI] [PubMed] [Google Scholar]
- 14.Saadatdoust Z, Pandurangan AK, Ananda Sadagopan SK, Mohd Esa N, Ismail A, Mustafa MR. Dietary cocoa inhibits colitis associated cancer: a crucial involvement of the IL-6/STAT3 pathway. J Nutr Biochem 2015; 26:1547–1558. doi: 10.1016/j.jnutbio.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Lee SY, Lee SH, Yang EJ, Kim EK, Kim JK, Shin DY, et al. Metformin ameliorates inflammatory bowel disease by suppression of the STAT3 signaling pathway and regulation of the between Th17/Treg balance. PLoS One 2015; 10:e0135858.doi: 10.1371/journal.pone.0135858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Deng J, Wang L, Lee H, Armstrong B, Scuto A, et al. S1PR1 is an effective target to block STAT3 signaling in activated B cell-like diffuse large B-cell lymphoma. Blood 2012; 120:1458–1465. doi: 10.1182/blood-2011-12-399030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang J, Nagahashi M, Kim Eugene Y, Harikumar Kuzhuvelil B, Yamada A, Huang W-C, et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell 2013; 23:107–120. doi: 10.1016/j.ccr.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pu WL, Bai RY, Zhou K, Peng YF, Zhang MY, Hottiger MO, et al. Baicalein attenuates pancreatic inflammatory injury through regulating MAPK, STAT 3 and NF-kappaB activation. Int Immunopharmacol 2019; 72:204–210. doi: 10.1016/j.intimp.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Hong T, Jin GB, Cho S, Cyong JC. Evaluation of the anti-inflammatory effect of baicalein on dextran sulfate sodium-induced colitis in mice. Planta Med 2002; 68:268–271. doi: 10.1055/s-2002-23143. [DOI] [PubMed] [Google Scholar]
- 20.Xu J, Liu J, Yue G, Sun M, Li J, Xiu X, et al. Therapeutic effect of the natural compounds baicalein and baicalin on autoimmune diseases. Mol Med Rep 2018; 18:1149–1154. doi: 10.3892/mmr.2018.9054. [DOI] [PubMed] [Google Scholar]
- 21.Yao J, Zhao L, Zhao Q, Zhao Y, Sun Y, Zhang Y, et al. NF-kappaB and Nrf2 signaling pathways contribute to wogonin-mediated inhibition of inflammation-associated colorectal carcinogenesis. Cell Death Dis 2014; 5:e1283.doi: 10.1038/cddis.2014.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao J, Pan D, Zhao Y, Zhao L, Sun J, Wang Y, et al. Wogonin prevents lipopolysaccharide-induced acute lung injury and inflammation in mice via peroxisome proliferator-activated receptor gamma-mediated attenuation of the nuclear factor-kappaB pathway. Immunology 2014; 143:241–257. doi: 10.1111/imm.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Udden SMN, Peng L, Gan JL, Shelton JM, Malter JS, Hooper LV, et al. NOD2 suppresses colorectal tumorigenesis via downregulation of the TLR pathways. Cell Rep 2017; 19:2756–2770. doi: 10.1016/j.celrep.2017.05.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalmasso G, Nguyen HT, Ingersoll SA, Ayyadurai S, Laroui H, Charania MA, et al. The PepT1-NOD2 signaling pathway aggravates induced colitis in mice. Gastroenterology 2011; 141:1334–1345. doi: 10.1053/j.gastro.2011.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karuppuchamy T, Behrens EH, Gonzalez-Cabrera P, Sarkisyan G, Gima L, Boyer JD, et al. Sphingosine-1-phosphate receptor-1 (S1P1) is expressed by lymphocytes, dendritic cells, and endothelium and modulated during inflammatory bowel disease. Mucosal Immunol 2017; 10:162–171. doi: 10.1038/mi.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim DH, Hossain MA, Kang YJ, Jang JY, Lee YJ, Im E, et al. Baicalein, an active component of Scutellaria baicalensis Georgi, induces apoptosis in human colon cancer cells and prevents AOM/DSS-induced colon cancer in mice. Int J Oncol 2013; 43:1652–1658. doi: 10.3892/ijo.2013.2086. [DOI] [PubMed] [Google Scholar]
- 27.Kim YJ, Kim HJ, Lee JY, Kim DH, Kang MS, Park W. Anti-inflammatory effect of baicalein on polyinosinic(-)polycytidylic acid-induced RAW 264.7 mouse macrophages. Viruses 2018; 10:224.doi: 10.3390/v10050224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakashima T, Maeda T, Nagamoto H, Kumakura T, Takai M, Mori T. Rebamipide enema is effective for treatment of experimental dextran sulfate sodium induced colitis in rats. Dig Dis Sci 2005; 50: Suppl 1: S124–S131. doi: 10.1007/s10620-005-2817-0. [DOI] [PubMed] [Google Scholar]
- 29.Zheng B, Morgan ME, van de Kant HJG, Garssen J, Folkerts G, Kraneveld AD. Transcriptional modulation of pattern recognition receptors in chronic colitis in mice is accompanied with Th1 and Th17 response. Biochem Biophys Rep 2017; 12:29–39. doi: 10.1016/j.bbrep.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrand A, Al Nabhani Z, Tapias NS, Mas E, Hugot JP, Barreau F. NOD2 expression in intestinal epithelial cells protects toward the development of inflammation and associated carcinogenesis. Cell Mol Gastroenterol Hepatol 2019; 7:357–369. doi: 10.1016/j.jcmgh.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suh JH, Saba JD. Sphingosine-1-phosphate in inflammatory bowel disease and colitis-associated colon cancer: the fat's in the fire. Transl Cancer Res 2015; 4:469–483. doi: 10.3978/j.issn.2218-676X.2015.10.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snider AJ, Ali WH, Sticca JA, Coant N, Ghaleb AM, Kawamori T, et al. Distinct roles for hematopoietic and extra-hematopoietic sphingosine kinase-1 in inflammatory bowel disease. PLoS One 2014; 9:e113998.doi: 10.1371/journal.pone.0113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lovato P, Brender C, Agnholt J, Kelsen J, Kaltoft K, Svejgaard A, et al. Constitutive STAT3 activation in intestinal T cells from patients with Crohn's disease. J Biol Chem 2003; 278:16777–16781. doi: 10.1074/jbc.M207999200. [DOI] [PubMed] [Google Scholar]
- 34.Musso A, Dentelli P, Carlino A, Chiusa L, Repici A, Sturm A, et al. Signal transducers and activators of transcription 3 signaling pathway: an essential mediator of inflammatory bowel disease and other forms of intestinal inflammation. Inflamm Bowel Dis 2005; 11:91–98. doi: 10.1097/00054725-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Xu ZS, Zhang HX, Li WW, Ran Y, Liu TT, Xiong MG, et al. FAM64A positively regulates STAT3 activity to promote Th17 differentiation and colitis-associated carcinogenesis. Proc Natl Acad Sci U S A 2019; 116:10447–10452. doi: 10.1073/pnas.1814336116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee H, Deng J, Kujawski M, Yang C, Liu Y, Herrmann A, et al. STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat Med 2010; 16:1421–1428. doi: 10.1038/nm.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang J, Nagahashi M, Kim EY, Harikumar KB, Yamada A, Huang WC, et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell 2013; 23:107–120. doi: 10.1016/j.ccr.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim DH, Sung B, Chung HY, Kim ND. Modulation of colitis-associated colon tumorigenesis by baicalein and betaine. J Cancer Prev 2014; 19:153–160. doi: 10.15430/JCP.2014.19.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]


