Abstract
Background:
High levels of plasma homocysteine occur almost uniformly in patients with end-stage renal disease (ESRD). IgA nephropathy (IgAN) is the most common form of primary glomerulonephritis and a common cause of ESRD in young adults. Here, we aimed to detect whether homocysteine was elevated and associated with clinical-pathologic manifestations of IgAN patients and tested its causal effects using a two-sample Mendelian randomization (MR) approach.
Methods:
For observational analysis, 108 IgAN patients, 30 lupus nephritis (LN) patients, 50 minimal change disease (MCD) patients, and 206 healthy controls were recruited from April 2014 to April 2015. Their plasma homocysteine was measured and clinical-pathologic manifestations were collected from medical records. For MR analysis, we further included 1686 IgAN patients. The missense variant methylenetetrahydrofolate reductase C677T (rs1801133) was selected as an instrument, which was genotyped by TaqMan allele discrimination assays.
Results:
Majority of IgAN patients (93.52%, 101/108) showed elevated levels of plasma homocysteine (>10 μmol/L). Plasma homocysteine in IgAN patients was significantly higher than that in MCD patients (median: 18.32 vs. 11.15 μmol/L, Z = −5.29, P < 0.01) and in healthy controls (median: 18.32 vs. 10.00 μmol/L, Z = −8.76, P < 0.01), but comparable with those in LN patients (median: 18.32 L vs. 14.50 μmol/L, Z = −1.32, P = 0.19). Significant differences were observed in sub-groups of IgAN patients according to quartiles of plasma homocysteine for male ratio (22.22% vs. 51.85% vs. 70.37% vs. 70.37%, χ2 = 14.29, P < 0.01), serum creatinine (median: 77.00 vs. 100.00 vs. 129.00 vs. 150.00 μmol/L, χ2 = 34.06, P < 0.01), estimated glomerular filtration rate (median: 100.52 vs. 74.23 vs. 52.68 vs. 42.67 mL·min−1·1.73 m−2, χ2 = 21.75, P < 0.01), systolic blood pressure (median: 120.00 vs. 120.00 vs. 125.00 vs. 130.00 mmHg, χ2 = 2.97, P = 0.05), diastolic blood pressure (median 80.00 vs. 75.00 vs. 80.00 vs. 81.00 mmHg, χ2 = 11.47, P < 0.01), and pathologic tubular atrophy and interstitial fibrosis (T) (T0/T1/T2: 62.96%/33.33%/3.70% vs. 29.63%/40.74%/29.63% vs. 24.00%/48.00%/28.00% vs. 14.81%/37.04%/48.15%, χ2 = 17.66, P < 0.01). The coefficient of each rs1801133-T allele on homocysteine levels after controlling age and sex was 7.12 (P < 0.01). MR estimates showed causal positive effects of homocysteine on serum creatine (β = 0.76, P = 0.02), systolic blood pressure (β = 0.26, P = 0.02), diastolic blood pressure (β = 0.20, P = 0.01), and pathologic T lesion (β = 0.01, P = 0.01) in IgAN.
Conclusions:
By observational and MR analyses, consistent results were observed for associations of plasma homocysteine with serum creatinine, blood pressures, and pathologic T lesion in IgAN patients.
Keywords: Homocysteine, IgA nephropathy, Causality
Introduction
IgA nephropathy (IgAN) is the most common form of primary glomerulonephritis worldwide and the leading cause of the end-stage renal disease (ESRD) in young adults.[1] About 30% of IgAN patients progressed to ESRD in about 20 to 30 years. Therefore, it is critical to identify modifiable risk factors to slow down disease progression and improve the clinical outcomes of IgAN.
Elevated homocysteine is frequently observed in patients with chronic kidney disease (CKD) [2] and occurs almost uniformly in patients with ESRD.[3] Randomized controlled trial (RCT) suggested that folic acid therapy can significantly delay the progression of CKD among patients with mild-to-moderate CKD.[4] IgAN is the most common form of glomerulonephritis and the leading cause of ESRD. However, as far as we know, there is only one small retrospective study suggesting that elevated levels of homocysteine were associated with intrarenal arterial lesions and poor renal outcome in IgAN patients.[5] As potential bias is inherent to cohort or observational studies. It is a key challenge to detect whether the association between homocysteine and IgAN is reverse causal[6] or is confounded by other factors.[7,8] The answers to these questions have clinical importance, as it has been reported that supplementation with folate can reduce homocysteine levels by 25%, and that treatment with vitamin B12 can reduce homocysteine levels by an additional 7%.[9]
Normally, high quality and well-powered RCTs are required to establish a causal relationship. However, conventional RCTs are time-consuming and costly. In the absence of large and well-designed RCTs, the Mendelian randomization (MR) approach provides a timely opportunity to test the causal effects of homocysteine on clinical-pathologic manifestations of IgAN. In essence, MR exploits the random allocation of genetic variants at conception; therefore, less susceptible to confounding than traditional observational studies. It is at the interface of experimental and observational studies and can be used to obtain evidence in support of a potential causal effect or of potential targets of interventions.[10] The missense mutation C677T (rs1801133) in the human gene methylenetetrahydrofolate reductase (MTHFR), which encodes a key enzyme for homocysteine metabolism, was reported to have the most consistent effect on plasma homocysteine in Europeans.[11] It is predicted to substitute a valine residue for an alanine (A222V), which is likely to reduce the enzyme's activity by 50%, resulting in high plasma levels of homocysteine. Considering that the risk allele T of MTHFR C677T (rs1801133) is especially high in Chinese compared with Europeans,[12] the genetic variant MTHFR C677T (rs1801133) could be an ideal instrument for testing whether elevated plasma homocysteine is causally related to IgAN in Chinese.
Thus, the present study was designed to answer the following three questions: (1) Whether plasma homocysteine is elevated and associated with clinical-pathologic manifestations of patients with IgAN? (2) Whether MTHFR C677T (rs1801133) is associated with plasma homocysteine in IgAN patients? (3) Whether the associations of plasma homocysteine with any clinical-pathologic manifestations of IgAN patients are causal?
Methods
Ethical approval
The study and consent procedures were performed in accordance with the Declaration of Helsinki. This study was approved by the Ethics Committee of Peking University First Hospital (No. 2013[548]) and written informed consent was provided by all participants.
Participants and data collection
The study design is shown in Figure 1. For observational analysis, to compare the plasma levels of homocysteine between IgAN patients and controls, we recruited 108 IgAN patients (male 58/108, 53.70%; mean age 37.02 ± 11.04 years), 30 lupus nephritis (LN) patients (male 7/30, 23.33%; mean age 35.57 ± 14.17 years), 50 minimal change disease (MCD) patients (male 24/50, 48.00%; mean age 36.80 ± 16.36 years), and 206 healthy controls from April 2014 to April 2015. Their plasma homocysteine was detected and the clinical-pathologic manifestations of the patients were collected. For MR analysis, the relationship between MTHFR C677T (rs1801133) and homocysteine was estimated in the 108 IgAN patients. To detect the associations of MTHFR C677T (rs1801133) with clinical-pathologic manifestations, 1686 IgAN patients (male 888/1686, 52.67%; mean age 33.77 ± 11.77 years) [Table 1] were included.
Figure 1.
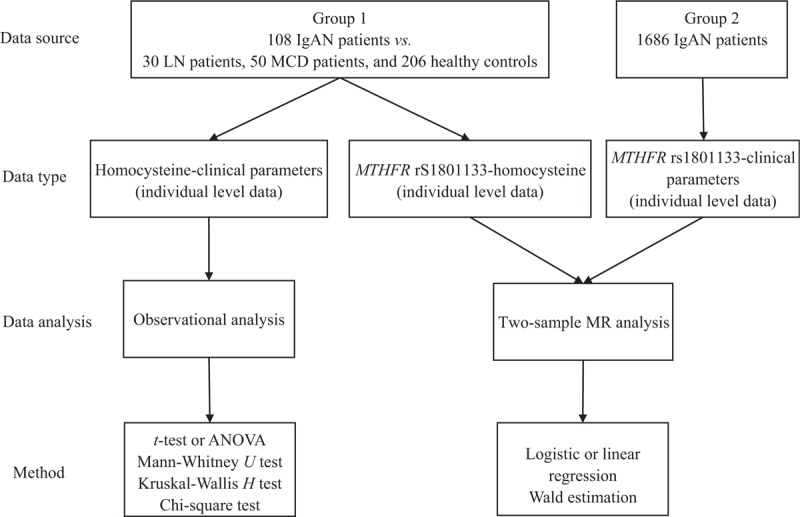
Study design of the work. For observational analysis, we recruited 108 IgAN patients, 30 LN patients, 50 MCD patients, and 206 healthy controls. Their plasma homocysteine was detected and the clinical-pathologic manifestations of the patients were collected. For MR analysis, the relationship between MTHFR C677T (rs1801133) and homocysteine was estimated in the 108 IgAN patients. To detect the associations of MTHFR C677T (rs1801133) with clinical-pathologic manifestations, 1686 IgAN patients were included. The effects of homocysteine on the outcomes were established using the “Wald estimation.” ANOVA: Analysis of variance; IgAN: IgA nephropathy; LN: Lupus nephritis; MCD: Minimal change disease; MR: Mendelian randomization; MTHFR: Methylenetetrahydrofolate reductase.
Table 1.
Baseline clinical-pathologic manifestations of the 1686 patients with IgA nephropathy.
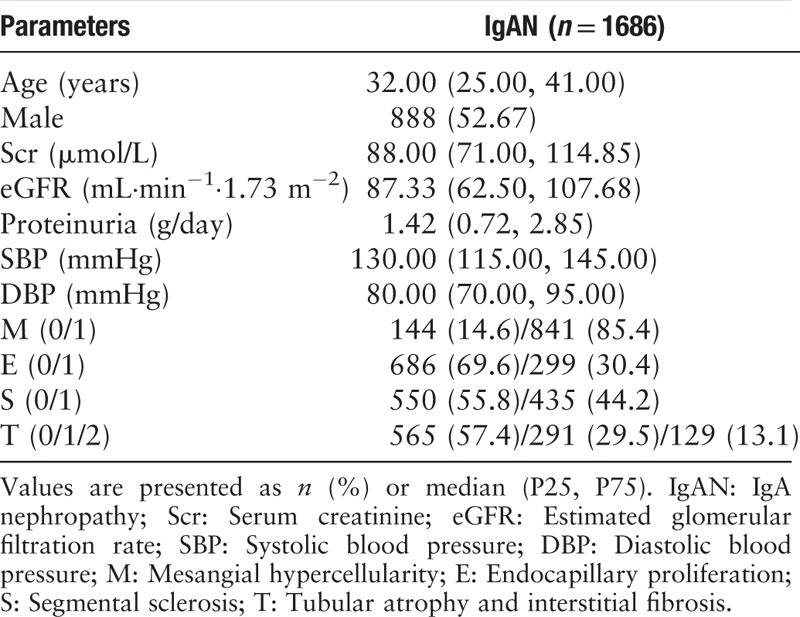
Patients with IgAN, LN, and MCD were diagnosed by renal biopsy. All renal-biopsy specimens were reviewed and graded by an independent pathologist who was blinded to patient information. Clinical-pathologic manifestations of the patients, including age, sex, serum creatinine, estimated glomerular filtration rate (eGFR, calculated using the Chronic Kidney Disease-Epidemiology Collaboration equation), urinary protein excretion, systolic/diastolic blood pressure, serum IgA, serum IgG, serum IgM, serum C3, IgA deposition, and C3 deposition were collected. The histological lesions for IgAN were also classified as proposed by the Oxford classification system, including mesangial hypercellularity (M), endocapillary proliferation (E), segmental sclerosis (S), and tubular atrophy and interstitial fibrosis (T).
Plasma homocysteine detection and grouping
Plasma homocysteine was measured using the ARCHITECT Homocysteine Reagent Kit (The Technology Park, Dundee, UK). It was divided into two groups by the cut off value of 10 μmol/L and into four equal groups according to the quartiles (Group 1: <13.01 μmol/L; Group 2: 13.01–18.31 μmol/L; Group 3: 18.32–25.87 μmol/L; and Group 4: >25.87 μmol/L).
Genetic instrument selection and genotyping
According to previous studies, we selected the missense variant MTHFR C677T (rs1801133) with the most consistent effect on plasma homocysteine as the genetic instrument.[11] It was genotyped using TaqMan allele discrimination assays (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions.
Statistical analyses
For observational analysis, continuous variables were tested for normal distribution using the Kolmogorov-Smirnov test. Variables with a normal distribution were expressed as means ± standard deviations, the independent-samples t-test (two groups) or analysis of variance (analysis of variance, multiple groups) was used for analyses. Non-normally distributed variables were expressed as medians and interquartile range and were analyzed using the Mann-Whitney U test (two groups) or the Kruskal-Wallis H test (multiple groups). Categorical variables were presented as n (%) and were analyzed using the Chi-square test.
For two-sample MR analysis, we first assessed the association of MTHFR C677T (rs1801133) with plasma homocysteine levels using linear regression by adjusting for age and sex, assuming a linear effect of MTHFR C677T (rs1801133) per additional allele-T in the 108 IgAN patients. The strength of the association was assessed by using Cragg-Donald F statistics; values greater than 10 were regarded as useful for MR analysis.[13] Then, the associations of MTHFR C677T (rs1801133) with clinical-pathologic manifestations of IgAN were analyzed using logistic or linear regression by adjusting for age and sex, assuming a linear effect of MTHFR C677T (rs1801133) per additional allele-T in the 1686 IgAN patients. Finally, the effects of homocysteine on the clinical-pathologic manifestations of IgAN patients were estimated using the “Wald estimation.”[14]
All analyses were performed using STATA software, version 13.1 (STATA Corporation, College Station, TX, USA). A two-tailed P < 0.05 was considered statistically significant.
Results
Baseline clinical and pathologic characteristics
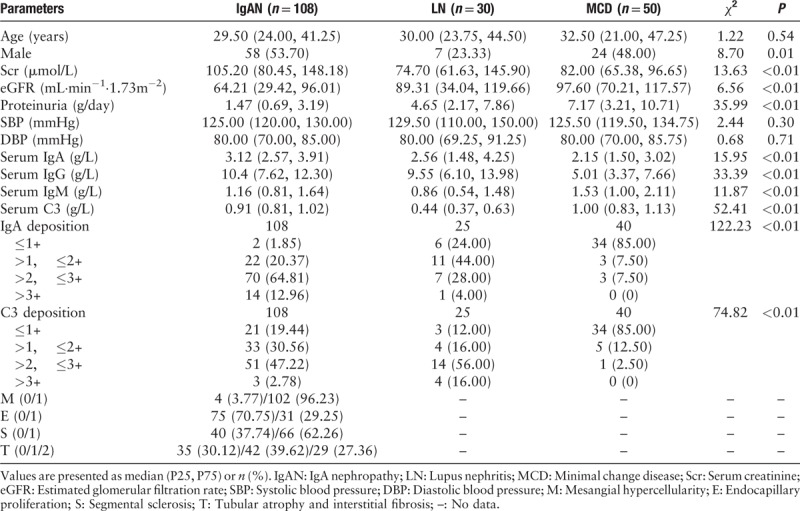
In total, 108 IgAN patients, 30 LN patients, 50 MCD patients, and 206 healthy controls were recruited. Except for age and blood pressure, all the other parameters varied across three groups of patients, representing their disease characteristics. There was a lower male rate in LN patients (53.70% in IgAN vs. 23.33% in LN vs. 48.00% in MCD, χ2 = 8.70, P = 0.01), higher levels of proteinuria in patients with LN and MCD (median: 1.47 g/d in IgAN vs. 4.65 g/d in LN vs. 7.17g/d in MCD, χ2 = 35.99, P < 0.01), higher serum levels of IgA in IgAN (median: 3.12 g/L in IgAN vs. 2.56 g/L in LN vs. 2.15 g/L in MCD, χ2 = 15.95, P < 0.01), and more intensive IgA deposition in IgAN (87.77% >2+ in IgAN vs. 32.00% >2+ in LN vs. 7.5% >2+, χ2 = 122.23, P < 0.01). Oxford M1, E1, S1, T1/2 were found in 96.23%, 29.25%, 62.26%, and 66.98% of IgAN patients, respectively [Table 2].
Table 2.
Baseline clinical-pathologic manifestations of patients with IgA nephropathy, lupus nephritis, and minimal change disease.
Plasma levels of homocysteine in IgAN patients and controls
Approximately 93.52% (101/108) patients with IgAN showed elevated plasma homocysteine (>10 μmol/L). Plasma homocysteine in IgAN patients were significantly higher than those in patients with MCD (median: 18.32 vs. 11.15 μmol/L, Z = −5.29, P < 0.01) and in healthy controls (median: 18.32 vs. 10.00 μmol/L, Z = −8.76, P < 0.01), but comparable with those in LN patients (median: 18.32 vs. 14.50 μmol/L, Z = −1.32, P = 0.19) [Figure 2].
Figure 2.
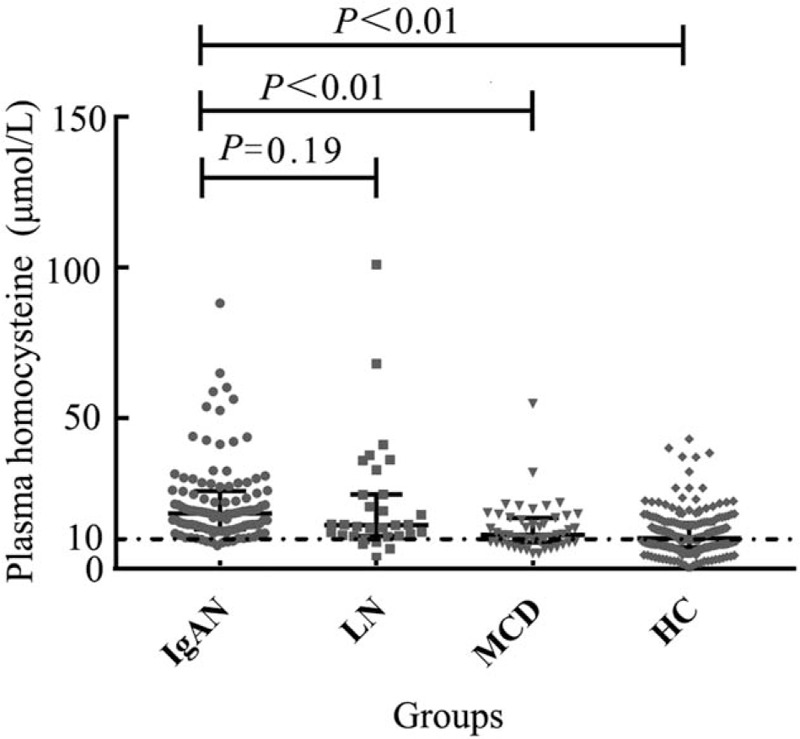
Comparison of plasma homocysteine in IgAN patients, LN patients, MCD patients, and healthy controls. The majority of patients with IgAN showed elevated plasma homocysteine (>10 μmol/L). Plasma homocysteine in IgAN patients was significantly higher than those in patients with MCD and in healthy controls but comparable with those in LN patients. IgAN: IgA nephropathy; LN: Lupus nephritis; MCD: Minimal change disease.
Observational analysis
Associations of plasma homocysteine with clinical-pathologic manifestations of IgAN patients
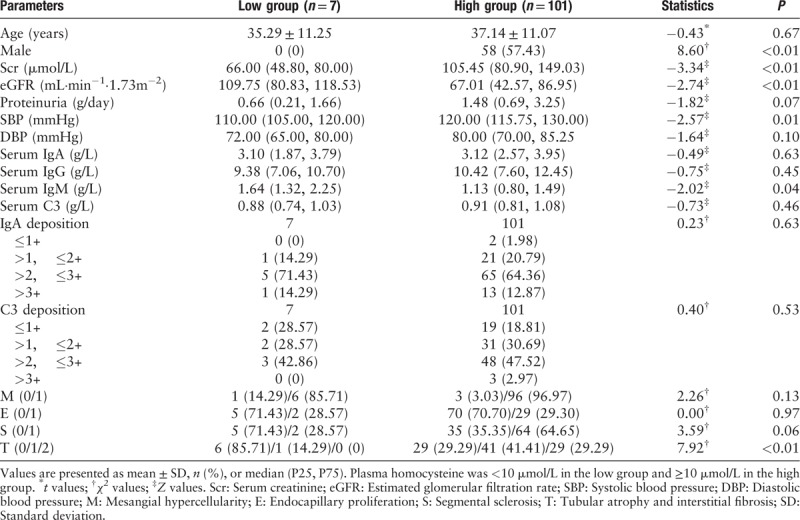
When the IgAN patients were divided into four groups by quartiles of plasma homocysteine, plasma homocysteine was positively associated with serum creatinine (P < 0.01), systolic blood pressure (P < 0.01), diastolic blood pressure (P ≤ 0.01), pathologic T lesion (P < 0.01), and negatively associated with eGFR (P < 0.01) [Table 3]. Similar association results were observed when the patients were divided by 10 μmol/L [Table 4]. Thus we further detect whether plasma homocysteine was causally associated with these clinical-pathologic manifestations.
Table 3.
Baseline clinical-pathologic manifestations of IgA nephropathy patients in four groups defined by quartiles of plasma homocysteine.
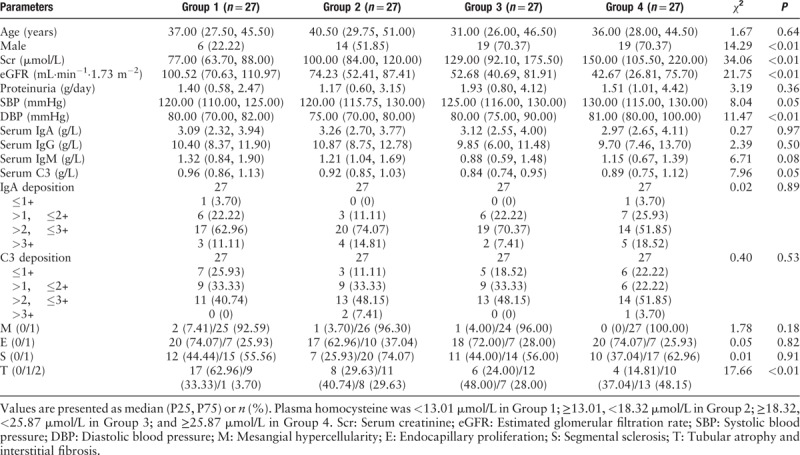
Table 4.
Baseline clinical-pathologic manifestations of IgA nephropathy patients in sub-groups defined by plasma homocysteine.
MR analysis
Association of MTHFR C677T (rs1801133) with plasma homocysteine of IgAN patients
The patients with genotype TT of MTHFR C677T (rs1801133) showed higher plasma levels of homocysteine than patients with genotypes of CT and CC (median: 25.93 vs. 17.07 vs. 18.17 μmol/L, χ2 = 8.86, P = 0.01) [Figure 3]. The effect of each MTHFR C677T (rs1801133) T allele on homocysteine levels after controlling age and sex was β = 7.12 (P < 0.01) [Table 5].
Figure 3.
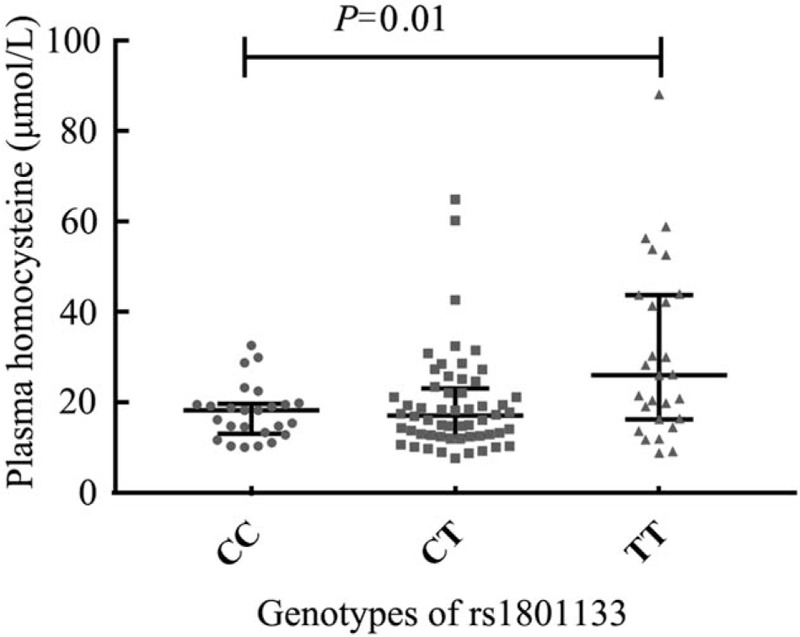
Comparison of plasma homocysteine according to rs1801133 genotypes in IgA nephropathy patients. The patients with the risk genotype TT of MTHFR C677T (rs1801133) showed higher plasma levels of homocysteine than patients with genotypes of CT and CC. MTHFR: Methylenetetrahydrofolate reductase.
Table 5.
Mendelian randomization analysis using rs1801133 as instrument.
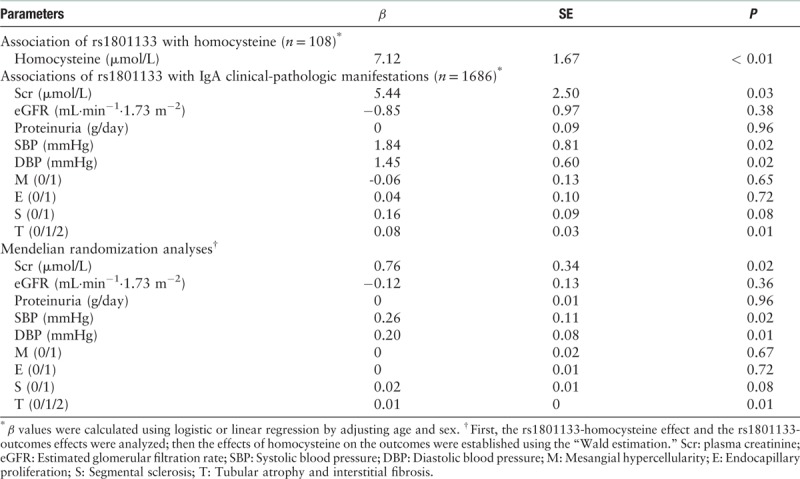
Associations of MTHFR C677T (rs1801133) with clinical-pathologic manifestations of IgAN patients
The associations of MTHFR C677T (rs1801133) with clinical-pathologic characteristics, including serum creatinine, eGFR, proteinuria, systolic blood pressure, diastolic blood pressure, and Oxford MEST score, were shown in Table 5. Regression analysis after adjusting for age and sex suggested that patients with the risk allele T tended to have higher serum creatinine (β = 5.44, P = 0.03), higher systolic blood pressure (β = 1.84, P = 0.02), higher diastolic blood pressure (β = 1.45, P = 0.02), and a higher rate of T lesion (β = 0.08, P = 0.01) [Table 5].
Causal effect of plasma homocysteine with clinical-pathologic manifestations of IgAN patients
After combining two estimates of gene-homocysteine and gene-disease phenotype, our MR estimates showed causal effects of homocysteine on serum creatine (β = 0.76, P = 2.40 × 10−2), higher systolic blood pressure (β = 0.26, P = 0.02), higher diastolic blood pressure (β = 0.20, P = 0.01), and higher proportion of T lesion (β = 0.01, P = 0.01) in IgAN.
Discussion
In this very first observational and MR study, we detected the associations between plasma homocysteine with clinical-pathologic parameters of IgAN and tested whether these associations were causal using two-sample MR analysis. We found that plasma homocysteine is commonly elevated in IgAN (>90% patients). It was significantly associated with sex, serum creatinine, eGFR, blood pressure, and T lesion, but not associated with neither serum IgA nor IgA deposition in renal biopsy. The missense variant MTHFR C677T (rs1801133) is associated with plasma homocysteine in Chinese. Using the two-sample MR method, the causal effects of plasma homocysteine and serum creatinine, blood pressures, and T lesion were confirmed, suggesting that plasma homocysteine would be a candidate modifiable risk factor for IgAN.
Numbers of studies have suggested the vascular damage effects of homocysteine and its association with cardiovascular disease in ESRD.[15,16] Homocysteine has a detrimental effect on the vascular wall by inducing the expression of chemokines (interleukin [IL]-8 and/or monocyte chemotactic protein 1 [MCP-1]) and their receptors in vascular cells and monocytes.[17,18] Moreover, it could also stimulate the production of many other cytokines and pro-inflammatory molecules, including IL-1β,[19] IL-12,[19] IL-6,[19–21] C-reactive protein,[20] IL-1 receptor antagonist,[21] IL-18,[22] adhesion molecules (P-selectin, E-selectin, intercellular adhesion molecules 1),[23] and metalloproteinases.[24] These immuno- and inflammatory responses caused by homocysteine could induce a series of pathological processes, including blood pressure elevation and blood vessel proliferation.[25] In the current study, our observational analysis suggested the associations between homocysteine and sex, systolic blood pressure, diastolic blood pressure, serum creatinine, eGFR, and T lesion. Since homocysteine is reported reversely affected by eGFR levels and other confounders, such as sex, baseline folate, and smoking status.[26] We use the missense variant MTHFR C677T (rs1801133), which showed consistent effects on homocysteine levels in genome-wide association studies and experimental studies,[11] as the genetic instrument to detect the causal role of homocysteine on clinical-pathologic manifestations of IgAN patients using MR method. We first validated the significant association of MTHFR C677T (rs1801133) with plasma homocysteine in Chinese. Then, using the two-sample MR method, the positive associations between homocysteine and systolic blood pressure, diastolic blood pressure, serum creatinine, and T lesion were confirmed. Although the genetically elevated homocysteine is negatively associated with eGFR levels, no statistical significance was observed, which might due to the relatively small sample size in our study. Overall, our results suggested that renal insufficiency could result in elevated homocysteine, which in turn deteriorate renal function through elevating blood pressure and inducing T lesion in IgAN.
Six criteria used in the MR approach must be considered.[27] (1) Suitable genetic variants are required to study modifiable exposures of interest. The missense variant MTHFR C677T (rs1801133) affected enzyme activity was considered to have the most consistent effect on plasma homocysteine concentrations.[11] (2) Reliable genotype-intermediate-phenotype and genotype-disease associations can be established. Here, we showed that the risk allele T of MTHFR C677T (rs1801133) was positively associated with plasma homocysteine and clinical-pathologic manifestations of IgAN patients. (3) There is no confounding of these relationships. Only the variant with the most consistent effect on plasma homocysteine was chosen in this study. (4) There are no pleiotropic effects of the genetic variants of interest. Currently, we are not aware of any association between MTHFR C677T (rs1801133) and a phenotype besides changes in homocysteine level that could influence the risk of IgAN. (5) There is no compensation from other genes during development. Although this sort of compensation is genetically difficult to assess, many polymorphisms modifying the metabolism of folate or vitamin B12 (which can affect homocysteine plasma concentrations) have been identified. However, MTHFR C677T (rs1801133) was regarded to have the most consistent effect while the others showed no major effect on homocysteine concentrations.[11] (6) Subject population does not differ between cases and controls. The present study was conducted using a large, ethnically homogenous patients of Chinese Han descent, and effectively can exclude admixture effects. Thus, the classical limitations of MR do not appear to be a major problem in the study.
However, there are still limitations in the present study. Notably, this study is limited to the single instrument (MTHFR C677T) of homocysteine, even it was reported to have the most consistent effect on plasma homocysteine and was widely used in RCTs [28] and MR studies.[29,30] In addition, as in most epidemiological studies, MR assumes a linear relation between homocysteine and clinical manifestations, which might not invariably be the case. In the future, RCTs are required to detect the benefits of homocysteine-lowering in IgAN patients.
In summary, plasma homocysteine is commonly elevated in patients with IgAN. By observational and MR analysis, consistent results were detected for the associations between homocysteine and serum creatinine, blood pressures, and pathogenic T lesion in IgAN. Since high levels of homocysteine is commonly seen in IgAN patients and folic acid is an inexpensive and important contributor to lowering blood homocysteine, our results provide clues for targeting homocysteine in preventing the disease progression of IgAN.
Funding
This work was supported by grants from the Beijing Natural Science Foundation (No. 7184253), and the National Natural Science Foundation of China (No. 81800636).
Conflicts of interest
None.
Footnotes
How to cite this article: Zhang YM, Zhou XJ, Shi SF, Liu LJ, Lyu JC, Zhang H. Homocysteine and IgA nephropathy: observational and Mendelian randomization analyses. Chin Med J 2020;133:277–284. doi: 10.1097/CM9.0000000000000613
References
- 1.Nair R, Walker PD. Is IgA nephropathy the commonest primary glomerulopathy among young adults in the USA? Kidney Int 2006; 69:1455–1458. doi: 10.1038/sj.ki.5000292. [DOI] [PubMed] [Google Scholar]
- 2.Cohen E, Margalit I, Shochat T, Goldberg E, Krause I. The relationship between the concentration of plasma homocysteine and chronic kidney disease: a cross sectional study of a large cohort. J Nephrol 2019; 32:783–789. doi: 10.1007/s40620-019-00618-x. [DOI] [PubMed] [Google Scholar]
- 3.Bostom AG, Culleton BF. Hyperhomocysteinemia in chronic renal disease. J Am Soc Nephrol 1999; 10:891–900. [DOI] [PubMed] [Google Scholar]
- 4.Xu X, Qin X, Li Y, Sun D, Wang J, Liang M, et al. Efficacy of folic acid therapy on the progression of chronic kidney disease: the renal substudy of the china stroke primary prevention trial. JAMA Intern Med 2016; 176:1443–1450. doi: 10.1001/jamainternmed.2016.4687. [DOI] [PubMed] [Google Scholar]
- 5.Duan S, Liu S, Sun X, Zheng Y, Liu L, Yao F, et al. Potential association of hyperhomocysteinemia with the progression of IgA nephropathy: a retrospective study. Chin Med J 2014; 127:1849–1852. doi: 10.3760/cma.j.issn.0366-6999.20140391. [PubMed] [Google Scholar]
- 6.Robinson K, Gupta A, Dennis V, Arheart K, Chaudhary D, Green R, et al. Hyperhomocysteinemia confers an independent increased risk of atherosclerosis in end-stage renal disease and is closely linked to plasma folate and pyridoxine concentrations. Circulation 1996; 94:2743–2748. doi: 10.1161/01.cir.94.11.2743. [DOI] [PubMed] [Google Scholar]
- 7.Vychytil A, Fodinger M, Wolfl G, Enzenberger B, Auinger M, Prischl F, et al. Major determinants of hyperhomocysteinemia in peritoneal dialysis patients. Kidney Int 1998; 53:1775–1782. doi: 10.1046/j.1523-1755.1998.00918.x. [DOI] [PubMed] [Google Scholar]
- 8.Selhub J, Jacques PF, Wilson PWF, Rush D, Rosernberg IH. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA 1993; 270:2693–2698. doi: 10.1001/jama.1993.03510220049033. [DOI] [PubMed] [Google Scholar]
- 9.Homocysteine Lowering Trialists’ Collaboration. Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr 2005; 82:806–812. doi: 10.1093/ajcn/82.4.806. [DOI] [PubMed] [Google Scholar]
- 10.Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 2018; 362:k601.doi: 10.1136/bmj.k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sunder-Plassmann G, Fodinger M. Genetic determinants of the homocysteine level. Kidney Int Suppl 2003; 63:S141–S144. doi: 10.1046/j.1523-1755.63.s84.52.x. [DOI] [PubMed] [Google Scholar]
- 12.Botto LD, Yang Q. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epidemiol 2000; 151:862–877. doi: 10.1093/oxfordjournals.aje.a010290. [DOI] [PubMed] [Google Scholar]
- 13.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 2013; 37:658–665. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greco MFD, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med 2015; 34:2926–2940. doi: 10.1002/sim.6522. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann MA, Lalla E, Lu Y, Ryu Gleason M, Wolf BM, Tanji N, et al. Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J Clin Invest 2001; 107:675–683. doi: 10.1172/JCI10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holven KB, Aukrust P, Holm T, Ose L, Nenseter MS. Folic acid treatment reduces chemokine release from peripheral blood mononuclear cell in hyperhomocysteinemic subjects. Arterioscler Thromb Vasc Biol 2002; 22:699–703. doi: 10.1161/01.atv.0000013288.35930.90. [DOI] [PubMed] [Google Scholar]
- 17.Desai A, Lankford HA, Warren JS. Homocysteine augments cytokine-induced chemokine expression in human vascular smooth muscle cells: implications for atherogenesis. Inflammation 2001; 25:179–186. doi: 10.1023/a:1011088431191. [DOI] [PubMed] [Google Scholar]
- 18.Siow YL, Au-Yeung KK, Woo CW, O K. Homocysteine stimulates the expression of monocyte chemoattractant protein-1 receptor (CCR2) in human monocytes: possible involvement of oxygen free radical. Biochem J 2006; 398:73–82. doi: 10.1042/BJ20051810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su SJ, Huang LW, Pai LS, Liu HW, Chang KL. Homocysteine at pathophysiological concentrations activates human monocyte and induces cytokine expression and inhibits macrophage migration inhibitory factor expression. Nutrition 2005; 21:994–1002. doi: 10.1016/j.nut.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Holven KB, Aukrust P, Retterstol K, Hagve TA, Morkrid L, Ose L, et al. Increased levels of C-reactive proteins and interleukin-6 in hyperhomocysteinemic subjects. Scand J Clin Lab Invest 2006; 66:45–54. doi: 10.1080/00335510500429821. [DOI] [PubMed] [Google Scholar]
- 21.Gori AM, Corsi AM, Fedi S, Gazzini A, Sofi F, Bartali B, et al. A pro-inflammatory state is associated with hyperhomocysteinemia in the elderly. Am J Clin Nutr 2005; 82:335–341. doi: 10.1093/ajcn.82.2.335. [DOI] [PubMed] [Google Scholar]
- 22.Tso TK, Huang WN, Huang HY, Chang CK. Relationship of plasma interleukin-18 concentrations to traditional and nontraditional cardiovascular factors in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2006; 45:1148–1153. doi: 10.1093/rheumatology/kel082. [DOI] [PubMed] [Google Scholar]
- 23.Mansoor MA, Seljeflot I, Arnesen H, Knudsen A, Bates CJ, Mishra G, et al. Endothelial cell adhesion molecules in healthy adults during acute hyperhomocysteinemia and mild hypertriglyceridemia. Clin Biochem 2004; 37:408–414. doi: 10.1016/j.clinbiochem.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Holven KB, Halvorsen B, Schultz H, Aukrust P, Ose L, Nenseter MS. Expression of matrix metalloproteinases-9 in mononuclear cells of hyperhomocystaeinemic subjects. Eur J Clin Invest 2003; 33:555–560. doi: 10.1046/j.1365-2362.2003.01189.x. [DOI] [PubMed] [Google Scholar]
- 25.Antoniades C, Antonopoulos AS, Tousoulis D, Marinou K, Stefanadis C. Homocysteine and coronary atherosclerosis: from folate fortification to the recent clinical trials. Eur Heart J 2009; 30:6–15. doi: 10.1093/eurheartj/ehn515. [DOI] [PubMed] [Google Scholar]
- 26.Wang B, Wu H, Li Y, Ban Q, Huang X, Chen L, et al. Effect of long-term low-dose folic acid supplementation on degree of total homocysteine-lowering: major effect modifiers. Br J Nutr 2018; 120:1122–1130. doi: 10.1017/S0007114518002477. [DOI] [PubMed] [Google Scholar]
- 27.Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 2003; 32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 28.Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA 2015; 313:1325–1335. doi: 10.1001/jama.2015.2274. [DOI] [PubMed] [Google Scholar]
- 29.Fu L, Li YN, Luo D, Deng S, Hu YQ. Plausible relationship between homocysteine and obesity risk via MTHFR gene: a meta-analysis of 38,317 individuals implementing Mendelian randomization. Diabetes Metab Syndr Obes 2019; 12:1201–1212. doi: 10.2147/DMSO.S205379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borges MC, Hartwig FP, Oliveira IO, Horta BL. Is there a causal role for homocysteine concentration in blood pressure? A Mendelian randomization study. Am J Clin Nutr 2016; 103:39–49. doi: 10.3945/ajcn.115.116038. [DOI] [PMC free article] [PubMed] [Google Scholar]


