Abstract
The treatments for early-onset scoliosis (EOS) remain great challenges for spine surgeons. This study aimed to comprehensively review the treatments for EOS, especially the advancements made in the last decade. Current studies on EOS were retrieved through a search on PubMed, UpToDate, the Web of Science Core Collection and Scopus were reviewed. The most pertinent information related to the current treatments for EOS was collected. The foci of treatments for EOS have included creating a well-developed thoracic cavity, improving lung volume, and improving pulmonary function. Conservative treatments include bracing, casting, halo-gravity traction, and physiotherapy. Serial casting is the most effective conservative treatment for EOS. Surgical treatments mainly include growth-friendly techniques, which are generally classified into three types according to the amount of correction force applied: distraction-based, compression-based, and growth-guided. The distraction-based systems include traditional or conventional growing rods, magnetically controlled growing rods, and vertical expandable prosthesis titanium ribs. The compression-based systems include vertebral body stapling and tethering. The growth-guided systems include the Shilla system and modern Luque trolley. In addition, some newer techniques have emerged in recent years, such as posterior dynamic deformity correction (ApiFix). For EOS patients presenting with sharp deformities in a long, congenital spinal deformity, a hybrid technique, one-stage posterior osteotomy with short segmental fusion and dual growing rods, may be a good choice. Hemivertebra resection is the gold standard for congenital scoliosis caused by single hemivertebra. Although the patient's growth potential is preserved in growth-friendly surgeries, a high complication rate should be expected, as well as a prolonged treatment duration and additional costs. Knowledge about EOS and its treatment options is rapidly expanding. Conservative treatments have specific limitations. For curves requiring a surgical intervention, surgical techniques may vary depending on the patients’ characteristics, the surgeon's experience, and the actual state of the country.
Keywords: Early-onset scoliosis, Techniques, Conservative treament, Hemivertebra resection, Fusionless, Growth-friendly
Introduction
The rapidly expanding volume of medical knowledge on early-onset scoliosis (EOS) has led to a significant evolution in the understanding and management of EOS. EOS refers to a spinal deformity that is present before the age of 10 years, and it is categorized into five types according to the etiology: idiopathic, congenital, thoracogenic, neuromuscular, and syndromic.[1]
Because of the various etiologies of EOS, there is no standardized or widely accepted classification system. Based on the etiology, magnitude of the major curve, kyphosis and the annual rate of progression, a new classification of EOS was recently designed by 15 experienced pediatric spine surgeons.[2] High levels of agreement and consistency of this classification have been reported.[2,3]
The treatment goals for EOS include minimizing the spinal deformity while maximizing the thoracic volume and pulmonary function.[4] Because there is a deep understanding of the relationship between the chest wall, lungs, and the spine, the foci of treatments have included creating a well-developed thoracic cavity, improving lung volume, and improving pulmonary function.[5,6] The purpose of this paper was to comprehensively review the treatments for EOS, especially the advancements made in the last decade.
Treatments
Treatments for EOS included conservative treatments and surgical interventions. Although conservative treatments should be considered first, they have certain limitations, and for patients for whom the limitations are numerous, surgical interventions are usually indicated.
Non-operative treatment
Non-operative treatment, including bracing, casting, halo-gravity traction (HGT) and physiotherapy, may achieve satisfactory correction in some patients, and most commonly, it helps delay surgery. HGT is commonly an adjunctive treatment and is usually accompanied by other treatments, such as surgeries.[7,8] It is indicated in patients with severe and rigid curves, those with scoliosis associated with kyphosis, and those with decreased pulmonary or nutritional status. Physiotherapy for idiopathic scoliosis in skeletally immature children remains controversial and requires further validation.[9] Casting and bracing are two major non-operative treatments for spinal deformity.
Casting
Serial casting is the most effective conservative treatment for EOS. It can preserve the growth potential and delay or even eliminate the need for surgery in some patients, especially in patients with idiopathic scoliosis.[10–14] The casting techniques vary between different reports. Commonly, patients undergo general anesthesia, and traction is applied. Padding is placed on bony prominences. Plaster is applied and molded with derotational forces to correct scoliosis. Casts are scheduled to be changed every 2 to 3 months, depending on the patient's growth.
Serial casting is generally indicated if the degree of curvature is more than 25°, with a minimum of 10° of documented progression, or the rib-vertebral angle difference >20°.[15] If indicated, serial casting should be initiated as early as possible because an older age and a large degree of curvature have been reported to be two risk factors for a failed casting treatment.[11,16]
Gussous et al[11] treated 74 consecutive patients (41 idiopathic and 33 non-idiopathic scoliosis patients, average age: 19 months, mean follow-up: 11 months) with the casting treatment. The Cobb angle was corrected from 47° to 27° in the idiopathic group and from 62° to 57° in the non-idiopathic group. The correction rate was higher in the idiopathic group than in the non-idiopathic group. At the last follow-up, there were 6 (8%) minor complications, including pressure sores, pyogenic granuloma, exacerbations of gastroesophageal reflux, and humeral fractures that the parents attributed to impaction on the cast. There were three (4%) major complications, including subclavian vein thrombosis, cardiac arrest on the induction of general anesthesia, and death attributed to an acute asthma attack. The spine of individuals with idiopathic scoliosis is usually flexible, and the degree of curvature is typically mild, which may explain why individuals with idiopathic scoliosis respond better to casting than non-idiopathic scoliosis. Cao et al[17] studied eight patients with congenital scoliosis (CS) and 15 patients with non-CS (average initial age: 3.25 years old). After an average follow-up time of 23.91 months, both the CS and non-CS groups showed a significant decrease in the Cobb angle after the first cast and at the last follow-up. The correction rate was significantly higher in the non-CS group than in the CS group (approximately 50% vs. 20%). At the last follow-up, there were two patients who underwent growing rod insertion, eight patients who weared a brace, and 13 patients who continued serial casting. Five patients had friction-induced ulcers, one had vomiting and one had continuous elevated airway pressure. The spine of individuals with CS is commonly rigid for structural defects, and the curvatures also have great potential for progression, so the effect of casting is limited. Mehta et al[18] prospectively enrolled 136 children (average age: 4 years old, average follow-up time: 9 years) with progressive infantile scoliosis who received serial corrective plaster jackets (Mehta plaster). The deformity was corrected in 94 children with younger ages at baseline (1 year and 7 months) and moderate curves (32°), and no additional intervention was needed. However, in 42 children with older ages at baseline (2 years and 6 months) and more severe curves (52°), the deformity did not resolve. Studies on Mehta plaster in individuals with CS are relatively rare. This treatment may delay the need for surgery, but the complication rate may be as high as those of other plaster techniques. Above all, the high complication rate associated with casting is a major concern. However, if young patients can tolerate the complications, casting remains an effective treatment option.
Bracing
Although bracing has been proven to be effective for adolescent idiopathic scoliosis,[19] there are few reports focusing on bracing for EOS. Thometz et al[20] applied an elongation bending derotation brace (EBDB) to 38 patients with infantile or juvenile scoliosis (average age: 6.2 years old, nine neuromuscular, one congenital, 28 idiopathic). Within a 12-month follow-up period, the juvenile group showed 25.7% correction and 42.9% stabilization, while the infantile group showed 50% correction and 32.1% stabilization. No patients required surgery within the follow-up period. However, the follow-up duration was relatively short, and the patients did not undergo a second growth spurt. Then, the authors focused on nine patients with infantile idiopathic scoliosis (average age: 11 months) receiving EBDB with a minimum follow-up period of 2 years. Four patients were fully corrected with serial bracing alone (final curve ≤10°). Five patients with more rigid curves showed improvement from a mean of 57° to a mean of 21°.[21] Moreau et al[22] studied 33 patients with early-onset idiopathic scoliosis (mean age: 50 months, median Cobb angle: 31°) who received detorsion night-time bracing. After a mean follow-up time of 102 months, the success rate (patients with a progression of 5° or less) was 67%, with a median Cobb angle reduction of 15°. Only one patient underwent surgery. In addition, the authors noted that the degree of curvature and age at brace initiation appeared to be important prognostic factors. Because of the high flexibility of idiopathic scoliosis spines, bracing may be beneficial. Few studies have compared the outcomes of casting and bracing. However, casting may achieve better results than bracing because once it is applied, the patient cannot take it off.
Operative treatment
Long spinal fusion in patients with EOS may endanger thoracic growth and pulmonary function and is associated with an increased risk of the crank shaft phenomenon or decompensation. There are a variety of growth-friendly techniques, and they are generally categorized into three types according to the amount of correction force applied: distraction-based, compression-based and growth-guided. In the distraction-based system, a mechanical distractive force is exerted on the spine segments, ribs and/or pelvis. It includes traditional or conventional growing rods (TGRs), magnetically controlled growing rods (MCGRs), and vertical expandable prosthesis titanium ribs (VEPTRs). In the compression-based system, a compressive force is applied to the convex side of the deformity, leading to growth inhibition on the ipsilateral side. Examples are vertebral body stapling (VBS) and vertebral body tethering (VBT). In the growth-guided system, the end and apical vertebrae are anchored, and the spine can slide along the rod. Examples of this system include the Shilla system and modern Luque trolley (MTR). In addition, some newer techniques have emerged in recent years, such as posterior dynamic deformity correction (ApiFix). Although the growth potential is preserved in growth-friendly surgeries, a high complication rate should be expected, as well as a prolonged treatment duration and additional costs. On the other hand, for CS caused by a single vertebra deformity (such as hemivertebra), osteotomy and short segment fusion are the gold standards.
TGRs
TGRs constitute the most commonly applied technique and are considered the gold standard for EOS with long curves[23]; TGRs were first introduced by Harrington in 1962.[24] After various modifications were made, the widely applied dual-rod growing rod technique was reported by Akbarnia et al.[25] Correction is achieved by distraction and maintained by proximal and distal instrumentation and fusion. Growth potential, especially lung growth, is preserved by unfused spine segments and lengthening procedures.
The most widely agreed upon indication for growing-rod surgery is the failure of treatment with bracing or casting, a degree of curvature of more than 60°, and ages younger than 10 years old.[26] However, theoretically, TGRs can be indicated for any patient with skeletal immaturity.
For the initial surgery, the correction rate has been reported to vary from 30% to 60%, and the lengthening interval has been shown to differ for different surgeons, mostly ranging from 6 to 12 months.[27–32] The complication rate of the growing rod technique has been reported to be as high as 50%,[29,30,33] and the complications have included dislodged implants, rod breakage, surgical site infections, wound healing problems, neurological impairment, proximal junctional kyphosis, and others. Several well-recognized risk factors for complications of growing rods are a younger age at the initial surgery, a single rod, longer lengthening times, thoracic hyperkyphosis, and subcutaneous rod placement.[29,30,33] Bess et al[33] reported 140 patients (mean age at the initial surgery: 6 years old, mean follow-up time: 5 years) undergoing a total of 897 growing-rod procedures. The mean degree of curvature was 75° at the time of insertion and 47° at the last follow-up. The mean number of surgical procedures per patient was 6.4, and the mean number of lengthening procedures per patient was 4.5, with an average lengthening interval of 10.4 months. The average age of the patients at the time of the final fusion was 12 years old. There were 177 complications in eighty-one patients (57.9%). The most concerning problem related to TGRs is the high complication rate. The risk for implant failure, infections, and wound healing problems are significantly increased as a consequence of the repeated lengthening procedures and the unfused spine. If rod breakage or screw displacement occurs, revision surgeries are indicated to change the rod or extend the instrumented segments. Additionally, repeated general anesthesia may pose a threat to mental health. Adequate informed consent and close follow-ups are necessary.
MCGRs
MCGRs were first introduced as remote-controlled growing spinal instrumentation by Takaso et al in 1998.[34] In 2016, Cheung et al[35] first described the early clinical results of MCGRs. He stated that MCGRs did not require open lengthening like TGRs did. The system contains 1 or 2 sterile and titanium growth rods and an external remote control for lengthening. Each rod contains a telescopic distraction actuator. Each actuator has a potential lengthening of 28 or 48 mm. Lengthening can be achieved by an external remote control without repeated anesthesia and open surgical lengthening. No consensus on the lengthening intervals or distraction numbers has been reached. However, most surgeons lengthen the rods more frequently than the TGRs, in intervals of 3 to 6 months.[36,37] Theoretically, the indication for MCGRs should be similar to that for TGRs. However, MCGRs are still not officially approved in some areas.
A mean correction of 27° to 36° and complications similar to those associated with TGRs, except for wound healing problems, have been reported. Akbarnia et al[37] enrolled 14 patients (mean age: 8 years 10 months) with EOS who received MCGRs with an average follow-up time of 10 months. The correction rate after the initial surgery was 50% and was well maintained at the final follow-up. The spine height increased from 292 to 322 mm after surgery and to 338 mm at the final follow-up. The average distraction number per patient was 4.9. Complications included one superficial infection, one prominent implant, and three losses of initial distraction after index. La Rosa et al[36] reported similar results and stated that MCGRs can prevent surgical scarring, surgical site infections, and psychological distress, which occur in patients with TGRs due to the multiple surgeries. The decreased rate of infections and wound healing problems in patients who received MCGRs is of great benefit to patients and reduces medical costs. However, Aslan et al[38] used psychosocial tools to compare the mental status of patients receiving MCGRs and TGRs. He stated that if the patient noticed a benefit of the growing rods and did not experience major complications, the non-invasiveness of the lengthening procedures did not show an advantage on patients’ psychosocial status. In addition, Teoh et al[39] found that although MCGRs were associated with a lower rate of both deep and superficial infections compared to TGRs, they were associated with a significantly increased risk of metalwork problems and unplanned revisions. Studies with larger sample sizes and longer follow-up times are still needed to help better understand and utilize this relatively new technique.
VEPTRs
VEPTRs were developed by Dr. Robert Cambell and Melvin Smith; they are mainly indicated for patients with thoracic insufficiency syndrome (TIS),[40] but are sometimes indicated for individuals with EOS who are at risk for secondary TIS.[41] VEPTRs are titanium alloy longitudinal rod distraction devices. Anchors are placed at the ribs, and spine. After they are inserted, lengthening is performed every 4 to 6 months.[42] The complication rate can be as high as 100%,[42] which limits its applications.
VBT and VBS
VBT was first described by Crawford and Lenke in 2010.[43] VBT involves the use of a flexible spinal implant that has the potential to prevent progression of the deformity during growth spurts by stopping spinal growth on the convex side of a scoliotic spine. The patient is placed in the lateral decubitus position with the convex side facing upward. The procedure is performed through an endoscopic approach. Segmental vessels are coagulated, and screws are sequentially placed from the cephalad to caudal direction. The tether is placed, and correction is achieved by both the tensioning of the tether and the translation of the spine.[44]
The indications for VBT include thoracic curves between 30° and 70° and thoracolumbar or lumbar curves from 30° to 60° in skeletally immature patients.[23] Hyperkyphosis (>40°) in the thoracic region is a relative contraindication because of the use of anterior instrumentation.
Samdani et al[44] reported 32 patients (average age: 12 years old, average follow-up time: 12 months) receiving anterior VBT for thoracic idiopathic scoliosis. The major Cobb angle was corrected from 43° to 21° at the first erect and continued to decrease to 18° at the last follow-up. The non-instrumented lumbar curve demonstrated significant spontaneous correction from 25° to 18° at first follow-up and 13° at the final follow-up. The sagittal plane parameters in this series remained stable. One patient experienced prolonged atelectasis, which required bronchoscopy. No other major complications were observed.
VBS was first proposed in 1951 but showed poor results.[45,46] Guille et al[47] introduced the modern nitinol C-shaped staple to provide additional compression across the growth plate. VBS can inhibit spinal growth on the convex side while preserving the motion segments of the whole spine without fusion.[48,49] Thoracic curves were stapled via a thoracoscopic technique, and motion segments below the diaphragm (T12-L1 and below) were stapled using a mini-open retroperitoneal approach.[47]
The indications for VBS are strict: idiopathic scoliosis, a Risser sign 0–2, a degree of curvature of 25° to 40°, and failure of brace treatment.[50] Cahill et al[51] reviewed 63 patients who underwent VBS at a mean age of 10.78 years and had a mean follow-up time of 3.62 years. The mean thoracic Cobb angle was 29.5° before surgery and 21.8° at the last follow-up. Seventy-four percent of the patients who had thoracic VBS did not show progression and/or fusion. The mean lumbar Cobb angle was 31.1° before surgery and 21.6° at the final follow-up. Eighty-two percent of the patients who had lumbar VBS did not show progression and/or fusion. Five of 390 staples (1%) in five of 63 patients (8%) showed evidence of staple movement/loosening. Four of sixty-three patients (6%) had broken staples. There were no neuromonitoring changes or objective sensory or motor deficits. At the most recent follow-up, the complications included two cases of localized sympathetic dysfunction in the left foot, two cases of atelectasis, one case of mucous plug, two cases of superior mesenteric artery syndrome, one case of superficial incisional site infection, and four overcorrections of a stapled curve.
There have been cases of overcorrection with compression-based implants in which the curve corrects and then develops in the opposite direction. Therefore, some surgeons have insisted that this technique be reserved for patients with limited growth remaining, such as 9- to 10-year-old patients.[52] In addition to skeletal maturity, VBT and VBS are confined to mild to moderate deformity. Despite the strict indications and limited applications, the greatest advantage of VBT and VBS is that they can preserve the growth potential and mobility in the spinal segments. The major disadvantages of these two techniques include the pulmonary and bowel complications caused by anterior surgeries.
Shilla growth guidance system (SGGS)
The SGGS was first reported in 2014.[53] SGGS guides spinal growth toward a normal alignment. The Shilla technique first corrects the apical deformity toward a neutral alignment. Then, the upper and lower growth guidance portions extend into the distal and proximal areas of the curve using polyaxial screws. These special screws have locking caps that fix to the top of the screws (not the rod) and capture the rod, allowing it to slide with growth in a longitudinal direction. Multiple open lengthening surgeries are avoided, as in MCGRs. The indications for the SGGS include early-onset spinal deformity and coronal curves >50°.[53]
McCarthy et al[54] performed SGGS for 40 patients with EOS (nine idiopathic, one congenital, 16 neuromuscular, and 14 syndromic). The average age at the index surgery was 6 years and 11 months, and the average follow-up duration was 7 years. The average degree of curvature was 69° pre-operatively and 38.4° at the final follow-up. The complication rate was as high as 73%, and the complications included six secondary infections, eight alignment issues, and 24 implant-related problems. Wilkinson et al[55] noted that the apex of the fused primary curve shifted in approximately 62% of patients, and nearly all of these (92%) involved distal migration. Luhmann et al[56] found that the Shilla growth guidance system compared favorably with TGRs in terms of the degree of correction of the major curve, spinal length and growth, and maintenance of sagittal alignment. The >4-fold decrease in the number of additional surgeries makes the Shilla system an attractive alternative to minimize the occurrence of comorbidities associated with additional surgeries. Similar to MCGR and TGR, the SGGS is associated with a very high rate of implant-related complications, which usually results in revision surgery. For patients with great growth potential, the distal and proximal screws may slide off the rod, requiring the rods to be changed.
MTR
MTR is a newly developed growth guidance technique. According to the literature, MLT was first reported by Ouellet et al in 2011.[57] MLT does not involve sublaminar or cerclage wires, and it consists of gliding spinal anchors traveling along fixed, overlapping rods. It can prevent the progression of spinal deformities while still allowing relative normal spinal growth. The indications for MLT include a Cobb angle >40°, failed conservative treatment and considerable growth potential.[58]
Ouellet et al[57] enrolled five patients (average age: 4.5 years; average follow-up time: 3.6 years) with EOS who received MLT surgery. The primary curve was corrected from 60° to 21° and was maintained at 21° at a mean follow-up time of 4 years. Two of the five cases outgrew the construct requiring a lengthening of the rods. One patient had a gradual recurrence of the deformity that required revision surgery after 4 years. However, MLT is a new technique and has not been officially approved in most areas.
Posterior dynamic deformity correction
Posterior dynamic deformity correction (ApiFix Ltd, Misgav, Israel) is a new, less invasive device that involves relatively fewer instrumented segments and preserves growth potential without spinal fusion. It was first reported in clinical practice by Floman et al in 2015.[59] The device is inserted on the concave side of the curve, and it allows the elongation of an expandable rod. The rod includes a unidirectional ratchet that can elongate when the patient performs side-bending exercises toward the convexity. When the patient returns to standing upright, the rod cannot shorten because of the ratchet. Therefore, after surgery, dynamic correction is achieved by certain exercises. There are few studies on ApiFix.
Osteotomy and short fusion
Hemivertebra is the most common pathology of CS. It usually creates a wedge-shaped deformity, which progresses with spinal growth.[60] Due to the anticipated poor prognosis of most cases of hemivertebrae, early surgical intervention is indicated.[61] Since Royle first reported the use of hemivertebra resection, it has become a gold standard for CS caused by hemivertebra.[62] Wang and Zhang[61] reported 36 patients who had CS (mean age 59 months, mean follow-up time: 62.3 months) caused by hemivertebra and underwent hemivertebra resection and bisegmental fusion. The segmental scoliosis curves were corrected from 36.6° to 5.1°, and segmental kyphosis was corrected from 21.2° to 5.8° at the last follow-up. The complications included one case of delayed wound healing, two cases of pedicle fractures and one case of progressive deformity. These patients are usually very young with poor bone quality and thin soft tissue coverage. As a result, the risk for wound healing problems and screw displacements are higher in these patients than in adults. However, correction of the malformation and solid fusion can yield satisfactory correction and prognosis.
Hybrid technique – author's preferred technique for severe rigid EOS
TGRs are considered the gold standard for EOS and are widely applied worldwide. However, for EOS patients presenting sharp deformities in a long congenital spinal deformity, a hybrid technique, one-stage posterior osteotomy with short segmental fusion and dual growing rods, maybe a good choice; this technique was first reported by Zhang and Wang in 2014.[63] An osteotomy with short segmental fusion may help to improve the correction and decrease the incidence of implant failures of growing rods (GR), and the GR technique can achieve a good control of the whole long curve while allowing the spine to grow.[63] In the study by these authors, for EOS patients with a mean age of 5.9 years old, the Cobb angle was corrected from 81.4° to 40.1° after the initial surgery and was 41.0° at the 53.3-month follow-up. The average increase in T1–S1 length was 1.23 cm per year. The same technique was reported in 2019, and the outcome was similar.[64] Correction of a major deformity can not only increase the correction rate but also decrease the stress on the implant and consequently lower the implant failure rate, while lung growth is preserved by the growing rod. Prolonged operation duration may be life-threatening, so the hybrid technique requires both accuracy and efficiency.
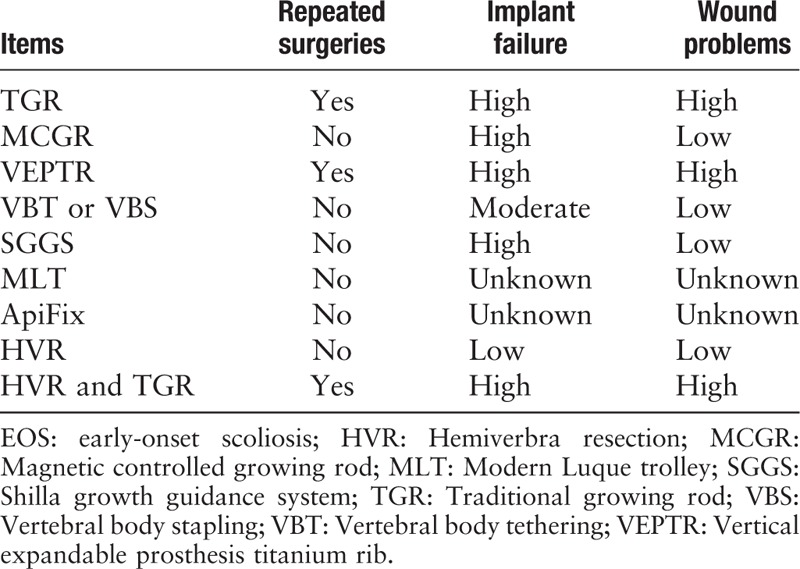
Repeated surgeries and complications are two major concerns in the management of EOS, and different techniques have different advantages and drawbacks [Table 1]. In addition to the treatments listed above, there are some other surgical treatments for EOS, such as convex epiphysiodesis, which is less commonly used in modern clinical practices.
Table 1.
Comparisons between different treatments on EOS.
Conclusions
Knowledge about EOS and its treatment options is rapidly expanding. Conservative treatment should always be considered first. For curves requiring a surgical intervention, surgical techniques may vary depending on the patients’ characteristics, the surgeon's experience, and the actual state of the country. The complication rate in the treatment of EOS is still high, which is inevitable because of the need for multiple surgeries, implant bulk, and the stresses on instrumentation in a mobile spine. Despite the challenges in the treatment of EOS, the evolution of techniques and knowledge presents hope for a better understanding and management in the future.
Funding
The study was granted by a grant from the National Natural Science Foundation of China (No. 81972037).
Conflicts of interest
None.
Footnotes
How to cite this article: Zhang YB, Zhang JG. Treatment of early-onset scoliosis: techniques, indications, and complications. Chin Med J 2020;133:351–357. doi: 10.1097/CM9.0000000000000614
References
- 1.El-Hawary R, Akbarnia BA. Early onset scoliosis - time for consensus. Spine Deform 2015; 3:105–106. doi: 10.1016/j.jspd.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Williams BA, Matsumoto H, McCalla DJ, Akbarnia BA, Blakemore LC, Betz RR, et al. Development and initial validation of the classification of early-onset scoliosis (C-EOS). J Bone Joint Surg Am 2014; 96:1359–1367. doi: 10.2106/JBJS.M.00253. [DOI] [PubMed] [Google Scholar]
- 3.Cyr M, Hilaire TS, Pan Z, Thompson GH, Vitale MG, Garg S. Classification of early onset scoliosis has excellent interobserver and intraobserver reliability. J Pediatr Orthop 2017; 37:e1–e3. doi: 10.1097/BPO.0000000000000688. [DOI] [PubMed] [Google Scholar]
- 4.Yang S, Andras LM, Redding GJ, Skaggs DL. Early-onset scoliosis: a review of history, current treatment, and future directions. Pediatrics 2016; 137.doi: 10.1542/peds.2015-0709. [Epub 2015 Dec 7]. [DOI] [PubMed] [Google Scholar]
- 5.Karol LA, Johnston C, Mladenov K, Schochet P, Walters P, Browne RH. Pulmonary function following early thoracic fusion in non-neuromuscular scoliosis. J Bone Joint Surg Am 2008; 90:1272–1281. doi: 10.2106/JBJS.G.00184. [DOI] [PubMed] [Google Scholar]
- 6.Canavese F, Dimeglio A. Normal and abnormal spine and thoracic cage development. World J Orthop 2013; 4:167–174. doi: 10.5312/wjo.v4.i4.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu E, Gao R, Jiang H, Lin T, Shao W, Zhou X. Combined halo gravity traction and dual growing rod technique for the treatment of early onset dystrophic scoliosis in neurofibromatosis type 1. World Neurosurg 2019; 126:e173–e180. doi: 10.1016/j.wneu.2019.01.290. [DOI] [PubMed] [Google Scholar]
- 8.Welborn MC, Krajbich JI, D’Amato C. Use of magnetic spinal growth rods (MCGR) with and without preoperative halo-gravity traction (HGT) for the treatment of severe early-onset scoliosis (EOS). J Pediatr Orthop 2019; 39:e293–e297. doi: 10.1097/BPO.0000000000001282. [DOI] [PubMed] [Google Scholar]
- 9.Iyer S, Duah HO, Wulff I, Osei TH, Mahmud R, Yankey KP, et al. The use of halo gravity traction in the treatment of severe early onset spinal deformity. Spine (Phila Pa 1976) 2019; 44:E841–E845. doi: 10.1097/BRS.0000000000002997. [DOI] [PubMed] [Google Scholar]
- 10.Baulesh DM, Huh J, Judkins T, Garg S, Miller NH, Erickson MA. The role of serial casting in early-onset scoliosis (EOS). J Pediatr Orthop 2012; 32:658–663. doi: 10.1097/BPO.0b013e318269c438. [DOI] [PubMed] [Google Scholar]
- 11.Gussous YM, Tarima S, Zhao S, Khan S, Caudill A, Sturm P, et al. Serial derotational casting in idiopathic and non-idiopathic progressive early-onset scoliosis. Spine Deform 2015; 3:233–238. doi: 10.1016/j.jspd.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Fedorak GT, Stasikelis PJ, Carpenter AM, Nielson AN, D’Astous JL. Optimization of casting in early-onset scoliosis. J Pediatr Orthop 2019; 39:e303–e307. doi: 10.1097/BPO.0000000000001288. [DOI] [PubMed] [Google Scholar]
- 13.Canavese F, Samba A, Dimeglio A, Mansour M, Rousset M. Serial elongation-derotation-flexion casting for children with early-onset scoliosis. World J Orthop 2015; 6:935–943. doi: 10.5312/wjo.v6.i11.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fletcher ND, McClung A, Rathjen KE, Denning JR, Browne R, Johnston CR. Serial casting as a delay tactic in the treatment of moderate-to-severe early-onset scoliosis. J Pediatr Orthop 2012; 32:664–671. doi: 10.1097/BPO.0b013e31824bdb55. [DOI] [PubMed] [Google Scholar]
- 15.Sanders JO, D’Astous J, Fitzgerald M, Khoury JG, Kishan S, Sturm PF. Derotational casting for progressive infantile scoliosis. J Pediatr Orthop 2009; 29:581–587. doi: 10.1097/BPO.0b013e3181b2f8df. [DOI] [PubMed] [Google Scholar]
- 16.Iorio J, Orlando G, Diefenbach C, Gaughan JP, Samdani AF, Pahys JM, et al. Serial casting for infantile idiopathic scoliosis: radiographic outcomes and factors associated with response to treatment. J Pediatr Orthop 2017; 37:311–316. doi: 10.1097/BPO.0000000000000654. [DOI] [PubMed] [Google Scholar]
- 17.Cao J, Zhang XJ, Sun N, Sun L, Guo D, Qi XY, et al. The therapeutic characteristics of serial casting on congenital scoliosis: a comparison with non-congenital cases from a single-center experience. J Orthop Surg Res 2017; 12:56.doi: 10.1186/s13018-017-0554-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta MH. Growth as a corrective force in the early treatment of progressive infantile scoliosis. J Bone Joint Surg Br 2005; 87:1237–1247. doi: 10.1302/0301-620X.87B9.16124. [DOI] [PubMed] [Google Scholar]
- 19.Weinstein SL, Dolan LA, Wright JG, Dobbs MB. Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med 2013; 369:1512–1521. doi: 10.1056/NEJMoa1307337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thometz J, Liu X, Rizza R, English I, Tarima S. Effect of an elongation bending derotation brace on the infantile or juvenile scoliosis. Scoliosis Spinal Disord 2018; 13:13.doi: 10.1186/s13013-018-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thometz J, Liu XC. Serial CAD/CAM bracing: an alternative to serial casting for early onset scoliosis. J Pediatr Orthop 2019; 39:e185–e189. doi: 10.1097/BPO.0000000000001287. [DOI] [PubMed] [Google Scholar]
- 22.Moreau S, Lonjon G, Mazda K, Ilharreborde B. Detorsion night-time bracing for the treatment of early onset idiopathic scoliosis. Orthop Traumatol Surg Res 2014; 100:935–939. doi: 10.1016/j.otsr.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 23.Hardesty CK, Huang RP, El-Hawary R, Samdani A, Hermida PB, Bas T, et al. Early-onset scoliosis: updated treatment techniques and results. Spine Deform 2018; 6:467–472. doi: 10.1016/j.jspd.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Harrington PR. Treatment of scoliosis. Correction and internal fixation by spine instrumentation. J Bone Joint Surg Am 1962; 44-A:591–610. doi:10.2106/00004623-200202000-00020. [PubMed] [Google Scholar]
- 25.Akbarnia BA, Marks DS, Boachie-Adjei O, Thompson AG, Asher MA. Dual growing rod technique for the treatment of progressive early-onset scoliosis: a multicenter study. Spine (Phila Pa 1976) 2005; 30:S46–S57. doi: 10.1097/01.brs.0000175190.08134.73. [DOI] [PubMed] [Google Scholar]
- 26.Yang JS, McElroy MJ, Akbarnia BA, Salari P, Oliveira D, Thompson GH, et al. Growing rods for spinal deformity: characterizing consensus and variation in current use. J Pediatr Orthop 2010; 30:264–270. doi: 10.1097/BPO.0b013e3181d40f94. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe K, Uno K, Suzuki T, Kawakami N, Tsuji T, Yanagida H, et al. Risk factors for proximal junctional kyphosis associated with dual-rod growing-rod surgery for early-onset scoliosis. Clin Spine Surg 2016; 29:E428–E433. doi: 10.1097/BSD.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 28.Dede O, Demirkiran G, Bekmez S, Sturm PF, Yazici M. Utilizing the “stable-to-be vertebra” saves motion segments in growing rods treatment for early-onset scoliosis. J Pediatr Orthop 2016; 36:336–342. doi: 10.1097/BPO.0000000000000467. [DOI] [PubMed] [Google Scholar]
- 29.Liang J, Li S, Xu D, Zhuang Q, Ren Z, Chen X, et al. Risk factors for predicting complications associated with growing rod surgery for early-onset scoliosis. Clin Neurol Neurosurg 2015; 136:15–19. doi: 10.1016/j.clineuro.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 30.Sun ZJ, Qiu GX, Zhao Y, Guo SG, Zhang JG, Shen JX, et al. Dual growing rod treatment in early onset scoliosis: the effect of repeated lengthening surgeries on thoracic growth and dimensions. Eur Spine J 2015; 24:1434–1440. doi: 10.1007/s00586-014-3668-1. [DOI] [PubMed] [Google Scholar]
- 31.Shah SA, Karatas AF, Dhawale AA, Dede O, Mundis GJ, Holmes LJ, et al. The effect of serial growing rod lengthening on the sagittal profile and pelvic parameters in early-onset scoliosis. Spine (Phila Pa 1976) 2014; 39:E1311–E1317. doi: 10.1097/BRS.0000000000000565. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe K, Uno K, Suzuki T, Kawakami N, Tsuji T, Yanagida H, et al. Risk factors for complications associated with growing-rod surgery for early-onset scoliosis. Spine (Phila Pa 1976) 2013; 38:E464–E468. doi: 10.1097/BRS.0b013e318288671a. [DOI] [PubMed] [Google Scholar]
- 33.Bess S, Akbarnia BA, Thompson GH, Sponseller PD, Shah SA, El SH, et al. Complications of growing-rod treatment for early-onset scoliosis: analysis of one hundred and forty patients. J Bone Joint Surg Am 2010; 92:2533–2543. doi: 10.2106/JBJS.I.01471. [DOI] [PubMed] [Google Scholar]
- 34.Takaso M, Moriya H, Kitahara H, Minami S, Takahashi K, Isobe K, et al. New remote-controlled growing-rod spinal instrumentation possibly applicable for scoliosis in young children. J Orthop Sci 1998; 3:336–340. doi: 10.1007/s007760050062. [DOI] [PubMed] [Google Scholar]
- 35.Cheung JP, Bow C, Samartzis D, Kwan K, Cheung KM. Frequent small distractions with a magnetically controlled growing rod for early-onset scoliosis and avoidance of the law of diminishing returns. J Orthop Surg (Hong Kong) 2016; 24:332–337. doi: 10.1177/1602400312. [DOI] [PubMed] [Google Scholar]
- 36.La Rosa G, Oggiano L, Ruzzini L. Magnetically controlled growing rods for the management of early-onset scoliosis: a preliminary report. J Pediatr Orthop 2017; 37:79–85. doi: 10.1097/BPO.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 37.Akbarnia BA, Cheung K, Noordeen H, Elsebaie H, Yazici M, Dannawi Z, et al. Next generation of growth-sparing techniques: preliminary clinical results of a magnetically controlled growing rod in 14 patients with early-onset scoliosis. Spine (Phila Pa 1976) 2013; 38:665–670. doi:10.1097/BRS.0b013e3182773560. [DOI] [PubMed] [Google Scholar]
- 38.Aslan C, Olgun ZD, Ayik G, Karaokur R, Ozusta S, Demirkiran GH, et al. Does decreased surgical stress really improve the psychosocial health of early-onset scoliosis patients? A comparison of traditional growing rods and magnetically-controlled growing rods patients reveals disappointing results. Spine (Phila Pa 1976) 2019; 44:E656–E663. doi: 10.1097/BRS.0000000000002938. [DOI] [PubMed] [Google Scholar]
- 39.Teoh KH, Winson DM, James SH, Jones A, Howes J, Davies PR, et al. Do magnetic growing rods have lower complication rates compared with conventional growing rods? Spine J 2016; 16:S40–S44. doi: 10.1016/j.spinee.2015.12.099. [DOI] [PubMed] [Google Scholar]
- 40.Campbell RJ, Smith MD, Hell-Vocke AK. Expansion thoracoplasty: the surgical technique of opening-wedge thoracostomy. Surgical technique. J Bone Joint Surg Am 2004; 86-A Suppl 1:51–64. doi:10.2106/00004623-200400001-00008. [PubMed] [Google Scholar]
- 41.Yazici M, Emans J. Fusionless instrumentation systems for congenital scoliosis: expandable spinal rods and vertical expandable prosthetic titanium rib in the management of congenital spine deformities in the growing child. Spine (Phila Pa 1976) 2009; 34:1800–1807. doi: 10.1097/BRS.0b013e3181978ec9. [DOI] [PubMed] [Google Scholar]
- 42.Waldhausen JH, Redding G, White K, Song K. Complications in using the vertical expandable prosthetic titanium rib (VEPTR) in children. J Pediatr Surg 2016; 51:1747–1750. doi: 10.1016/j.jpedsurg.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 43.Crawford CR, Lenke LG. Growth modulation by means of anterior tethering resulting in progressive correction of juvenile idiopathic scoliosis: a case report. J Bone Joint Surg Am 2010; 92:202–209. doi: 10.2106/JBJS.H.01728. [DOI] [PubMed] [Google Scholar]
- 44.Samdani AF, Ames RJ, Kimball JS, Pahys JM, Grewal H, Pelletier GJ, et al. Anterior vertebral body tethering for immature adolescent idiopathic scoliosis: one-year results on the first 32 patients. Eur Spine J 2015; 24:1533–1539. doi: 10.1007/s00586-014-3706-z. [DOI] [PubMed] [Google Scholar]
- 45.Nachlas IW, Borden JN. The cure of experimental scoliosis by directed growth control. J Bone Joint Surg Am 1951; 33 A:24–34. [PubMed] [Google Scholar]
- 46.Smith AD, Von Lackum WH, Wylie R. An operation for stapling vertebral bodies in congenital scoliosis. J Bone Joint Surg Am 1954; 36:342–348. [PubMed] [Google Scholar]
- 47.Guille JT, D’Andrea LP, Betz RR. Fusionless treatment of scoliosis. Orthop Clin North Am 2007; 38:541–545. doi: 10.1016/j.ocl.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Trobisch PD, Samdani A, Cahill P, Betz RR. Vertebral body stapling as an alternative in the treatment of idiopathic scoliosis. Oper Orthop Traumatol 2011; 23:227–231. doi: 10.1007/s00064-011-0032-z. [DOI] [PubMed] [Google Scholar]
- 49.Betz RR, Ranade A, Samdani AF, Chafetz R, D’Andrea LP, Gaughan JP, et al. Vertebral body stapling: a fusionless treatment option for a growing child with moderate idiopathic scoliosis. Spine (Phila Pa 1976) 2010; 35:169–176. doi: 10.1097/BRS.0b013e3181c6dff5. [DOI] [PubMed] [Google Scholar]
- 50.Bumpass DB, Fuhrhop SK, Schootman M, Smith JC, Luhmann SJ. Vertebral body stapling for moderate juvenile and early adolescent idiopathic scoliosis: cautions and patient selection criteria. Spine (Phila Pa 1976) 2015; 40:E1305–E1314. doi: 10.1097/BRS.0000000000001135. [DOI] [PubMed] [Google Scholar]
- 51.Cahill PJ, Auriemma M, Dakwar E, Gaughan JP, Samdani AF, Pahys JM, et al. Factors predictive of outcomes in vertebral body stapling for idiopathic scoliosis. Spine Deform 2018; 6:28–37. doi: 10.1016/j.jspd.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Yang S, Andras LM, Redding GJ, Skaggs DL. Early-onset scoliosis: a review of history, current treatment, and future directions. Pediatrics 2016; 137:137.doi: 10.1542/peds.2015-0709. [DOI] [PubMed] [Google Scholar]
- 53.McCarthy RE, Luhmann S, Lenke L, McCullough FL. The Shilla growth guidance technique for early-onset spinal deformities at 2-year follow-up: a preliminary report. J Pediatr Orthop 2014; 34:1–7. doi: 10.1097/BPO.0b013e31829f92dc. [DOI] [PubMed] [Google Scholar]
- 54.McCarthy RE, McCullough FL. Shilla growth guidance for early-onset scoliosis: results after a minimum of five years of follow-up. J Bone Joint Surg Am 2015; 97:1578–1584. doi: 10.2106/JBJS.N.01083. [DOI] [PubMed] [Google Scholar]
- 55.Wilkinson JT, Songy CE, Bumpass DB, McCullough FL, McCarthy RE. Curve modulation and apex migration using shilla growth guidance rods for early-onset scoliosis at 5-year follow-up. J Pediatr Orthop 2017; 37:e567–e574. doi: 10.1097/BPO.0000000000000983. [DOI] [PubMed] [Google Scholar]
- 56.Luhmann SJ, McCarthy RE. A comparison of Shilla growth guidance system and growing rods in the treatment of spinal deformity in children less than 10 years of age. J Pediatr Orthop 2017; 37:e567–e574. doi: 10.1097/BPO.0000000000000751. [DOI] [PubMed] [Google Scholar]
- 57.Ouellet J. Surgical technique: modern Luque trolley, a self-growing rod technique. Clin Orthop Relat Res 2011; 469:1356–1367. doi: 10.1007/s11999-011-1783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alkhalife YI, Padhye KP, El-Hawary R. New technologies in pediatric spine surgery. Orthop Clin North Am 2019; 50:57–76. doi: 10.1016/j.ocl.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 59.Floman Y, Burnei G, Gavriliu S, Anekstein Y, Straticiuc S, Tunyogi-Csapo M, et al. Surgical management of moderate adolescent idiopathic scoliosis with ApiFix(R): a short peri-apical fixation followed by post-operative curve reduction with exercises. Scoliosis 2015; 10:4.doi: 10.1186/s13013-015-0028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McMaster MJ, Ohtsuka K. The natural history of congenital scoliosis. A study of two hundred and fifty-one patients. J Bone Joint Surg Am 1982; 64:1128–1147. doi:10.2106/00004623-198264080-00003. [PubMed] [Google Scholar]
- 61.Wang S, Zhang J, Qiu G, Li S, Zhang Y, Yang Y, et al. Posterior-only hemivertebra resection with anterior structural reconstruction with titanium mesh cage and short segmental fusion for the treatment of congenital scoliokyphosis: the indications and preliminary results. Spine (Phila Pa 1976) 2017; 42:1687–1692. doi: 10.1097/BRS.0000000000002197. [DOI] [PubMed] [Google Scholar]
- 62.Piantoni L, Francheri WI, Tello CA, Noel MA, Galaretto E, Remondino RG, et al. Hemivertebra resection with instrumented fusion by posterior approach in children. Spine Deform 2015; 3:541–548. doi: 10.1016/j.jspd.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 63.Wang S, Zhang J, Qiu G, Wang Y, Weng X, Guo J. One-stage posterior osteotomy with short segmental fusion and dual growing rod technique for severe rigid congenital scoliosis: the preliminary clinical outcomes of a hybrid technique. Spine (Phila Pa 1976) 2014; 39:E294–E299. doi: 10.1097/BRS.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 64.Sun X, Xu L, Chen Z, Shi B, Chen X, Li S, et al. Hybrid growing rod technique of osteotomy with short fusion and spinal distraction: an alternative solution for long-spanned congenital scoliosis. Spine (Phila Pa 1976) 2019; 44:707–714. doi: 10.1097/BRS.0000000000002933. [DOI] [PubMed] [Google Scholar]


