Supplemental Digital Content is available in the text.
Background.
Graft recipient weight ratios are lower in adult-to-adult living-donor liver transplantation than in adult-to-adult deceased-donor liver transplantation. Rapid liver regeneration is essential for increased recipient survival rates in adult-to-adult living-donor liver transplantation. However, the influence of biliary reconstruction methods, including choledocho-choledochostomy and choledocho-jejunostomy, on small partial liver grafts remains unknown. Herein, we investigate the impact of these biliary reconstruction methods on small partial liver grafts.
Methods.
Male Lewis rats underwent isogenic arterialized 30% partial liver transplantation with small partial grafts, either via choledocho-jejunostomy or choledocho-choledochostomy.
Results.
The 7-day survival rates of the choledocho-choledochostomy and choledocho-jejunostomy groups were 100% and 50%, respectively (P = 0.011). Choledocho-jejunostomy provoked reflux cholangitis, as confirmed by neutrophil infiltration around the bile ducts; suppressed and delayed liver regeneration in grafts, as confirmed by significant increases in intrahepatic interleukin-1β level, significant decreases in the graft weight increase ratios, hepatocyte proliferation, and intrahepatic mRNA expression of vascular endothelial growth factor; and induced graft dysfunction, as confirmed by the presence of massive ascites, significantly decreased bile production, and prolonged elevation of total bilirubin, aspartate aminotransferase, and alanine aminotransferase.
Conclusions.
Choledocho-jejunostomy predisposed grafts to cholangitis, impaired liver regeneration, and aggravated animal survival, suggesting that choledocho-choledochostomy may be preferable over choledocho-jejunostomy in adult-to-adult living-donor liver transplantation.
The living-donor liver transplantation (LDLT) program started with choledocho-jejunostomy (CJ) with a left lateral segment graft in a pediatric case.1 Thereafter, LDLT was applied to adult-to-adult cases.2 In adult-to-adult LDLT, graft recipient weight ratios (GRWRs) are usually lower than in adult-to-adult deceased-donor whole liver transplantation (LT), and using small-for-size grafts is, sometimes, inevitable.2,3 Delayed liver regeneration in small partial liver grafts often predisposes LDLT patients to graft dysfunction.4,5 Therefore, rapid liver regeneration is essential for increased recipient survival rates in adult-to-adult LDLT.6
Liver regeneration requires hepatocyte proliferation and the reconstruction of a complex network of sinusoidal endothelial cells, through which hepatic blood flows.7 Liver regeneration is a multistep process. Each step is characterized by the expression and secretion of various cytokines and growth factors.8 Interleukin (IL)-6 is an inflammatory cytokine that promotes liver regeneration,9 but excessive IL-6 inhibits liver regeneration.10 In addition, vascular endothelial growth factor (VEGF) is one of the most important liver regeneration factors. It is secreted by proliferating hepatocytes and is an important sinusoidal endothelial cell proliferation stimulator.11 Alternatively, IL-1β is a strong hepatocyte proliferation inhibitor.12 Liver regeneration requires the well-controlled regulation of inflammatory cytokines and growth factors. Biliary reconstruction in LDLT is usually performed via choledocho-choledochostomy (CC) or CJ.13,14 However, the preferable biliary reconstruction method for yielding better short- and long-term results is a controversial topic.13–19 Although there are various factors involved in liver regeneration, including recipient clinical status, graft ischemia-reperfusion injury, hepatic vascular hemodynamics, donor condition, and ABO incompatibility,20–24 the influence of CC and CJ on post-transplant liver regeneration remains unknown.
CJ has the specific complication of reflux cholangitis, which is not observed in CC.25 Cholangitis caused by CJ provokes excessive inflammation and disturbs inflammatory cytokine and growth factor regulation.26 Therefore, we hypothesized that CJ is inferior to CC in terms of liver regeneration in small partial grafts. Our study aimed to assess the impact of CC and CJ on small partial liver grafts in a rat orthotopic LT model.
MATERIALS AND METHODS
Animals
Male Lewis rats (300–400 g) (Charles River Laboratories Japan, Inc., Yokohama, Japan) were housed under specific pathogen-free conditions in a temperature-controlled and humidity-controlled environment with a 12-hour light-dark cycle and allowed free access to tap water and standard chow pellets. All experiments were conducted in accordance with the Animal Research Committee of Kyoto University, and all animals received humane care in accordance with the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH Publication No. 86-23, revised 1985).
Study Design
CJ in rats has been shown to induce reflux cholangitis.26–28 Using this procedure,26 we compared CJ with CC in an isogenic arterialized orthotopic LT model.
For the survival study of LT with small partial liver grafts, 10 rats that underwent arterialized 30% partial LT with CC (30% CC) and 10 that underwent arterialized 30% partial LT with CJ (30% CJ) were examined. To obtain blood, bile, and tissue samples, 5 rats per group were killed at 12, 24, 72, and 168 hours post-LT from the 30% CC and 30% CJ groups (Figure 1A). For the survival study of LT with sufficient liver volume, 5 rats that underwent arterialized whole LT with CC (100% CC) and 5 that underwent arterialized whole LT with CJ (100% CJ) were examined. To obtain blood, bile, and tissue samples, 3 rats per group were killed at 12, 24, 72, and 168 hours post-LT from the 100% CC and 100% CJ groups.
FIGURE 1.
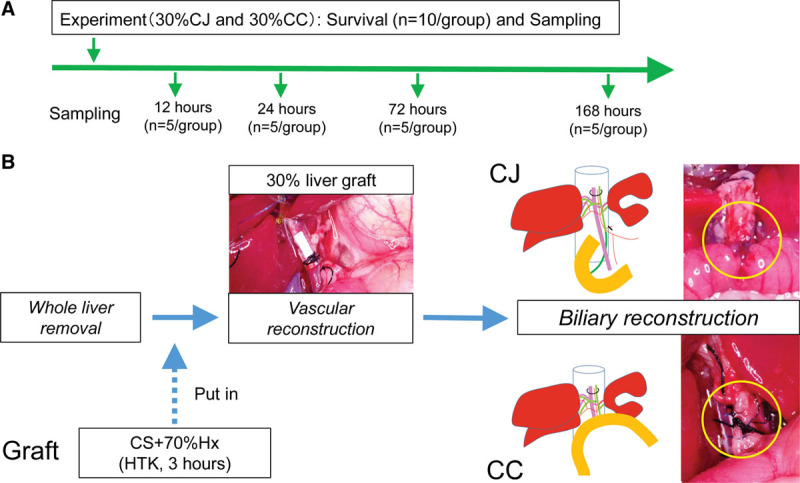
Experimental protocol. A, Experimental schedule. For the survival study, 10 rats per group were examined in the 30% CC and 30% CJ groups. To obtain blood, bile, and tissue samples, 5 rats per group were killed at 12, 24, 72, and 168 h post-LT in the 30% CC and 30% CJ groups. B, Schematic of 30% partial liver transplantation. 30% partial liver graft is put in the recipient abdomen 3 h after CS in HTK. After vascular reconstruction is completed, CC is performed in the 30% CC group or CJ is performed in the 30% CJ group. 30% CC, arterialized 30% partial liver transplantation with choledocho-choledocostomy; 30% CJ, arterialized 30% partial liver transplantation with choledocho-jejunostomy; CC, choledocho-choledochostomy; CJ, choledocho-jejunostomy; CS, cold storage; HTK, histidine-tryptophan-ketoglutarate solution; LT, liver transplantation.
Endpoints
The follow-up period for survival after LT was 7 days. We examined ascites; serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin (T-bil); bile production volume; lactate dehydrogenase (LDH) in bile; liver histology; and intrahepatic toll-like receptor (TLR) 4 and IL-6 mRNA expression to assess reflux cholangitis and liver damage. We defined “massive” ascites when we found overflow of peritoneal fluid at the time of laparotomy, while “mild” ascites was recorded when we recognized fluid in the peritoneal cavity without spillover. We examined graft weight increase ratios, the number of Ki-67–stained proliferative cells, and intrahepatic IL-6, IL-1β, and VEGF mRNA expressions to assess liver regeneration.
Donor Operation
Under anesthesia with isoflurane, the donor rat’s abdomen was opened by a bilateral subcostal incision. After the liver was mobilized, the infrahepatic inferior vena cava (IHIVC) was separated from the right adrenal vein. The rat was subsequently heparinized via the penile vein with 300 IU of heparin (Mochida Pharmaceutical Co., Ltd., Tokyo, Japan) in 1 mL of Ringer solution. The bile duct was then divided and cannulated with a 24-gauge polyethylene tube (TERUMO, Tokyo, Japan) for stenting. Next, the gastroduodenal artery was ligated and divided. The portal vein (PV) was isolated by transecting the pyloric and splenic vein. In situ liver perfusion from aortic bifurcation with 100 mL of cold histidine-tryptophan-ketoglutarate (HTK) solution was performed, just as clinical procurement. Next, the IHIVC, PV, common hepatic artery (CHA), and suprahepatic inferior vena cava (SHIVC) were divided and then the graft was immediately immersed in a basin filled with HTK solution at 4°C.
Ex Vivo Graft Preparation
The glissonean pedicle toward the median and left lateral lobe was ligated, and the median and left lobes were resected ex vivo (Video, SDC, http://links.lww.com/TXD/A236). This procedure was omitted in the 100% CC and 100% CJ groups. The right and caudate lobe of the liver was selected for the 30% partial liver graft. The cuff, made from a 14-gauge catheter, was attached to the PV. The SHIVC was then attached with two 7-0 polypropylene sutures. The graft was stored for 3 hours in HTK solution at 4°C.
Recipient Operation
Under anesthesia with isoflurane, the recipient rat’s abdomen was opened by a bilateral subcostal incision. The IHIVC was separated from the right adrenal vein after the mobilization of the liver. The bile duct and the proper hepatic artery (PHA) were ligated and divided at the hepatic hilum. Then, after injecting 2 mL of Ringer solution through the penile vein, the IHIVC, PV, and SHIVC were clamped. These vessels were divided and the recipient’s liver was subsequently removed (Video, SDC, http://links.lww.com/TXD/A236). The liver graft was then placed orthotopically in the abdominal cavity (Video, SDC, http://links.lww.com/TXD/A236). The SHIVC was reconstructed in an end-to-end fashion using continuous 7-0 polypropylene sutures (Video, SDC, http://links.lww.com/TXD/A236). The PV anastomosis was performed by pulling the recipient’s vein over the cuff and securing them with a circumferential suture (Video, SDC, http://links.lww.com/TXD/A236). The PV and SHIVC were unclamped for the recirculation of the liver (Video, SDC, http://links.lww.com/TXD/A236). Next, the IHIVC was anastomosed in an end-to-end fashion using continuous 8-0 polypropylene sutures (Video, SDC, http://links.lww.com/TXD/A236). Again, 2 mL of Ringer solution was injected through the penile vein. Afterward, the hepatic artery was reconstructed in the modified sleeve method (Video, SDC, http://links.lww.com/TXD/A236).29 The recipient’s PHA was inserted into the graft’s CHA and the stump of the CHA was sutured at the recipient’s PHA with 10-0 nylon (Video, SDC, http://links.lww.com/TXD/A236). In the 30% CC and 100% CC groups, CC was performed by tying the duct over a tube stent in the usual rat LT manner30 (Figure 1B). In the 30% CJ and 100% CJ groups, CJ was performed by inserting the stent into the jejunum by applying a purse-string suture with 7-0 polypropylene26 (Figure 1B, Video, SDC, http://links.lww.com/TXD/A236). Finally, the abdominal cavity was washed with 20 mL of saline, and the abdominal incision was closed with 2 layers of continuous sutures.
Sample Collection
Rats underwent relaparotomy at 12–168 hours postoperation. The bile duct was cannulated again via stent tube. Subsequently, bile was collected for 10 minutes. Afterward, blood was drawn via puncture of aortic bifurcation and collected in serum tubes to be centrifuged at 1500 rpm for 15 minutes. The transplanted graft livers were perfused with saline from aortic bifurcation, just as donor operation. Afterward, the transplanted graft livers were procured, weighed, and excised for tissue sample collection. Liver tissue samples were then fixed in formaldehyde or snap-frozen in liquid nitrogen to be preserved at −80°C until analysis.
Blood and Bile Investigation
Serum AST, ALT, and T-bil were measured using the standard spectrophotometric method with an automated clinical analyzer (JCABM9030; JEOL Ltd., Tokyo, Japan). The volume of bile sample was measured. LDH in bile samples were measured as an index of biliary damage,31 by using the standard spectrophotometric method with an automated clinical analyzer (JCABM9030; JEOL Ltd., Tokyo, Japan).
Histological Study of Graft Livers
Liver tissue samples were fixed in 10% buffered formalin, dehydrated in a graded ethanol series, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Tissue sections from each rat were observed under the BZ-9000 microscope (Keyence, Osaka, Japan). Tissue sections were also stained with naphthol AS-D chloroacetate esterase to assess neutrophil infiltration32 and Ki-67 (monoclonal rabbit anti-Ki-67 antibody, Abcam, Cambridge, United Kingdom) to assess hepatocyte proliferation33 and quantify the number of proliferating hepatocytes. Ki-67–positive hepatocytes were counted in 5 randomly selected high power fields (×400) and calculated as an average number of positive cells.
Graft Weight Increase Ratio
Graft weights before and after LT were recorded for the 30% CJ and 30% CC groups. Graft weight increase ratios were calculated as follows: graft weight increase ratio (%) = 100 × (B − A)/A, in which A is pretransplant 30% partial graft weight and B is post-transplant graft weight at the time of sampling.5
Quantitative Reverse-Transcription Polymerase Chain Reaction
Total mRNA was extracted from the liver tissue at each time point using the NucleoSpin RNA Kit (MACHEREY-NAGEL GmbH & Co. Kg, Duren, Germany). Equal amounts of mRNA were adjusted with NanoDrop2000 (NanoDrop Technologies, Washington, DE). cDNA was reverse-transcribed using an Omniscript RT kit (Qiagen, Tokyo, Japan). Quantitative reverse-transcription polymerase chain reaction was performed with the following amplification conditions: 50°C for 2 seconds and 95°C for 10 seconds during the holding stage, followed by 45 cycles of 95°C for 0.15 seconds and 60°C for 1 second. Polymerase chain reaction products were analyzed with the StepOnePlus Real-Time PCR System (Applied Biosystems, Life Technologies Japan, Tokyo, Japan). Target gene expressions were calculated relative to the housekeeping gene, β-actin. TaqMan probes and primers for TLR4 (assay ID: Rn00569848_m1), IL-6 (assay ID: Rn01410330_m1), IL-1β (assay ID: Rn00580432_m1), VEGF (assay ID: Rn01511602_m1), and β-actin (assay ID: Rn00667869_m1) were obtained from TaqMan gene expression assays (Applied Biosystems, Tokyo, Japan).
Statistical Analysis
All statistical analyses were performed using Prism 6 (Graph Pad Software, Inc., San Diego, CA). All values are expressed as mean ± SD. For comparisons between groups with n ≥ 4, the Mann-Whitney U test (nonparametric test) was used. For comparisons between groups with n < 4, the Student t test (parametric test) was used. Animal survival was evaluated via the Kaplan-Meier method and log-rank test. A P value of <0.05 was considered significant.
RESULTS
CJ Aggravates Animal Survival in Small Partial LT
In the 30% CJ group, 5 rats (50%) survived for more than 7 days, whereas in the 30% CC group, all rats (100%) survived (P = 0.011, Figure 2A). Postmortem examinations of the 30% CJ group revealed massive ascites and no technical problems, including bile leakage.
FIGURE 2.
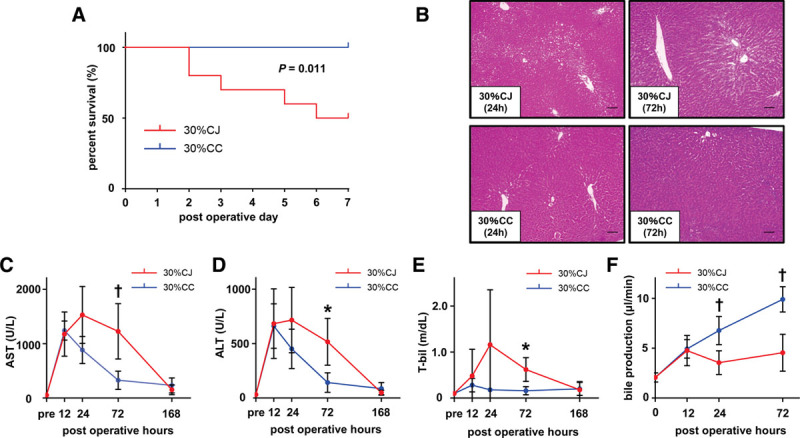
CJ exacerbates liver damage and aggravates animal survival in small partial liver graft. A, The 7-day survival rates in the 30% CC and 30% CJ groups were 100% (10/10) and 50% (5/10), respectively, with a significant difference (P = 0.011, log-rank test). B, Representative HE staining of the graft liver in the 30% CJ and 30% CC groups at 24 and 72 h post-LT. The 30% CJ group showed severe liver damage, as confirmed by moderate vacuolization and moderate necrosis at 24 h post-LT and moderate vacuolization and moderate necrosis at 72 h post-LT. The 30% CC group showed mild vacuolization and no necrosis at 24 h post-LT and minimal vacuolization and no necrosis at 72 h post-LT. The original magnification was ×100 for both images. The scale bar in each panel represents 100 μm. C and D, Highly elevated serum AST and ALT levels at 12 h post-LT were immediately improved at 24 h post-LT in the 30% CC group while elevated AST and ALT levels persisted until 72 h post-LT in the 30% CJ group. Serum AST and ALT levels at 72 h post-LT were significantly higher in the 30% CJ group than in the 30% CC group. *P < 0.05, †P < 0.01, Mann-Whitney U test. n = 5/group. E, Serum T-bil level was elevated only in the 30% CJ group. Serum T-bil level at 72 h post-LT was significantly higher in the 30% CJ group than in the 30% CC group. *P < 0.05, Mann-Whitney U test. n = 5/group. F, Bile production volume was significantly less in the 30% CJ group than in the 30% CC group at 24 and 72 h post-LT (24 h: P = 0.008, 72 h: P = 0.008). *P < 0.05, †P < 0.01, Mann-Whitney U test. n = 5/group. 30% CC, arterialized 30% partial liver transplantation with choledocho-choledochostomy; 30% CJ, arterialized 30% partial liver transplantation with choledocho-jejunostomy; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CJ, choledocho-jejunostomy; HE, hematoxylin-eosin; LT, liver transplantation; T-bil, total bilirubin.
CJ Exacerbates Liver Damage in Small Partial LT
At sacrifice, massive ascites was identified at 24 and 72 hours post-LT in the 30% CJ group, whereas mild ascites was identified at the same time points in the 30% CC group. We also observed more severe hepatocyte vacuolization and necrosis at 24 and 72 hours post-LT in the 30% CJ group than in the 30% CC group (Figure 2B). Serum AST, ALT, and T-bil levels at 72 hours post-LT were significantly higher in the 30% CJ group than in the 30% CC group (AST: 1104 ± 616 versus 327 ± 168 U/L, P = 0.032; ALT: 517 ± 216 versus 142 ± 90 U/L, P = 0.032; T-bil: 0.58 ± 0.31 versus 0.16 ± 0.09 mg/dL, P = 0.032; Figure 2C–E). At 12 hours post-LT, serum AST and ALT levels were highly elevated due to operative liver injury in both 2 groups (Figure 2C and D). Highly elevated serum AST and ALT levels at 12 hours post-LT immediately improved at 24 hours post-LT in the 30% CC group, while elevated serum AST and ALT levels persisted until 72 hours after LT in the 30% CJ group (Figure 2C and D). Serum T-bil level was elevated only in the 30% CJ group (Figure 2E). Bile production volume was significantly lower in the 30% CJ group than in the 30% CC group at 24 and 72 hours post-LT (24 hours: 3.55 ± 1.19 versus 6.77 ± 1.42 μL/min, P = 0.008; 72 hours: 4.55 ± 1.85 versus 9.90 ± 1.27 μL/min, P = 0.008; Figure 2F).
CJ Induces Neutrophil Infiltration Around Bile Ducts in Small Partial LT
In the 30% CJ group, we observed edematous change and the infiltration of inflammatory cells, including neutrophils, around bile ducts at 24 hours post-LT (Figure 3A). At 72 hours post-LT, neutrophil infiltration was aggravated around bile ducts and observable, even among biliary epithelial cells (Figure 3A and B). At 72 hours post-LT, biliary epithelial cells were injured and their nuclei were swollen (Figure 3A). However, biliary epithelial cells injury recovered and lamellar periductal fibrosis was observed at 168 hours post-LT (Figure 3A). Contrastingly, none of these findings were observed in the 30% CC group (Figure 3A and B).
FIGURE 3.
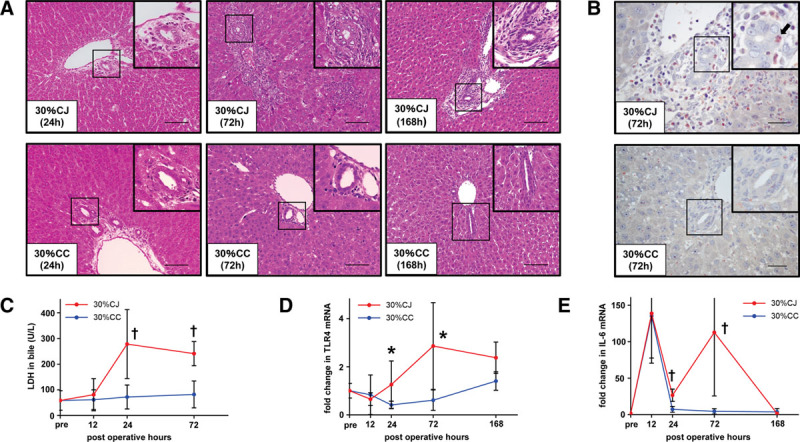
CJ provokes cholangitis in small partial liver graft. A, Representative HE staining of the graft liver in the 30% CJ and 30% CC groups at 24, 72, and 168 h post-LT. In the 30% CJ group, we observed edematous change and the infiltration of inflammatory cells, including neutrophils, around bile ducts at 24 h post-LT. We observed neutrophil infiltration not only around but also among biliary epithelial cells at 72 h post-LT in the 30% CJ group. Biliary epithelial cells were injured, and their nuclei were swollen at 72 h post-LT in the 30% CJ group. Biliary epithelial cells injury recovered and lamellar periductal fibrosis was observed at 168 h post-LT in the 30% CJ group. In the 30% CC group, neutrophil infiltration was not observed around bile ducts at 24, 72, and 168 h post-LT. The original magnification was ×200 for all images. The scale bar in each panel represents 100 μm. B, Representative liver sections stained by naphthol AS-D chloroacetate esterase at 72 h post-LT in the 30% CJ and 30% CC groups. Many neutrophils were observed around bile ducts in the 30% CJ group. A neutrophil (black arrow) was clearly observed among biliary epithelial cells in the 30% CJ group. Neutrophil infiltration was not observed around bile ducts in the 30% CC group. The original magnification was ×400 for all images. The scale bar in each panel represents 40 μm. C, LDH level in bile, reflecting biliary damage, was significantly higher in the 30% CJ group than in the 30% CC group at 24 and 72 h post-LT. †P < 0.01, Mann-Whitney U test. n = 5/group. D, Intrahepatic mRNA expression of TLR4, a representative endotoxin receptor, was significantly higher in the 30% CJ group than in the 30% CC group at 72 and 168 h post-LT. Intrahepatic TLR4 mRNA expression started to increase at 24 h post-LT in the 30% CJ group, which was not observed in the 30% CC group. *P < 0.05, †P < 0.01, Mann-Whitney U test. n = 5/group. E, Intrahepatic IL-6 mRNA expression was significantly higher in the 30% CJ group than in the 30% CC group at 24 and 72 h post-LT. Re-elevation of intrahepatic IL-6 mRNA expression was observed in the 30% CJ group, which was not observed in the 30% CC group. *P < 0.05, †P < 0.01, Mann-Whitney U test. n = 5/group. 30% CC, arterialized 30% partial liver transplantation with choledocho-choledochostomy; 30% CJ, arterialized 30% partial liver transplantation with choledocho-jejunostomy; CC, choledocho-choledochostomy; CJ, choledocho-jejunostomy; HE, hematoxylin-eosin; IL, interleukin; LDH, Lactate dehydrogenase; LT, liver transplantation; TLR, toll-like receptor.
CJ Provokes Biliary Damage in Small Partial LT
LDH levels in bile reflect biliary damage,31 and they were significantly higher in the 30% CJ group than in the 30% CC group at 24 and 72 hours post-LT (24 h: 278 ± 134 versus 72 ± 47 U/L, P = 0.008; 72 h: 241 ± 47 versus 82 ± 53 U/L, P = 0.008; Figure 3C). LDH levels in bile were not elevated in the 30% CC group during 72 hours post-LT (Figure 3C).
CJ Enhances TLR4 mRNA Expression and Provokes Re-elevation of IL-6 mRNA Expression in Small Partial Liver Graft
Intrahepatic mRNA expression of TLR4, a representative of endotoxin receptor, was significantly higher in the 30% CJ group than in the 30% CC group at 72 and 168 hours post-LT (72 hours: 2.86 ± 1.80 versus 0.61 ± 0.42, P = 0.016; 168 hours: 2.37 ± 0.65 versus 1.40 ± 0.38, P = 0.016; Figure 3D). Intrahepatic TLR4 mRNA expression started to increase at 24 hours post-LT in the 30% CJ group, but not in the 30% CC group (Figure 3D). Intrahepatic IL-6 mRNA expression was significantly higher in the 30% CJ group than in the 30% CC group at 24 and 72 hours post-LT (24 h: 8.06 ± 2.56 versus 1.64 ± 1.11, P = 0.008; 72 h: 38.63 ± 42.11 versus 1.48 ± 1.02, P = 0.008; Figure 3E). At 12 hours post-LT, intrahepatic IL-6 mRNA expression was highly elevated due to operative inflammation in both groups (Figure 3E), but this immediately improved at 24 hours post-LT in the 30% CC group, while this did not completely improve in the 30% CJ group (Figure 3E). The re-elevation of intrahepatic IL-6 mRNA expression was observed in the 30% CJ group but not in the 30% CC group (Figure 3E).
CJ Suppresses Graft Weight Increase and Hepatocyte Proliferation in Small Partial LT
To assess liver regeneration in small partial grafts with CJ and CC, we compared graft weight increase ratios and the number of Ki-67–positive cells between the 30% CJ and 30% CC groups. Graft weight increase ratios were significantly lower in the 30% CJ group than in the 30% CC group at 24, 72, and 168 hours post-LT (24 hours: 5.7 ± 6.0 versus 28.9 ± 16.6%, P = 0.032; 72 hours: 91.1 ± 3.4 versus 125.3 ± 15.0%, P = 0.008; 168 hours: 158.7 ± 23.9 versus 214.3 ± 39.1%, P = 0.008; Figure 4A). The number of Ki-67–positive cells was significantly lower in the 30% CJ group than in the 30% CC group at 24 hours post-LT (1.00 ± 0.91 versus 18.4 ± 15.8, P = 0.008, Figure 4B). The number of Ki-67–positive cells was also lower in the 30% CJ group than in the 30% CC group at 72 and 168 hours post-LT, but the difference was not significant (Figure 4B).
FIGURE 4.
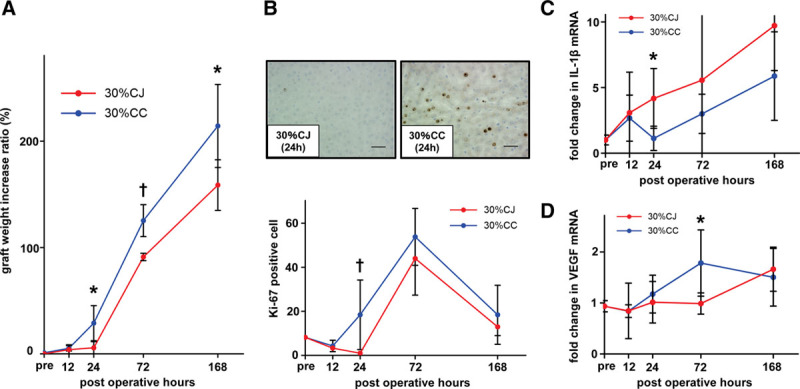
CJ suppresses and delays liver regeneration in small partial liver graft. A, Graft weight increase ratio was significantly lower in the 30% CJ group than in the 30% CC group at 24, 72, and 168 h post-LT. *P < 0.05, †P < 0.01, Mann-Whitney U test. n = 5/group. B, The number of Ki-67–positive cells was significantly lower in the 30% CJ group than in the 30% CC group at 24 h post-LT. The number of Ki-67–positive cells was lower in the 30% CJ group than in the 30% CC group at 72 and 168 h post-LT although the difference was not significant. *P < 0.05, Mann-Whitney U test. n = 5/group. C, We observed early elevation of intrahepatic mRNA expression of IL-1β, a strong inhibitor of hepatocyte proliferation, in the 30% CJ group at 24 h post-LT. Intrahepatic IL-1β mRNA expression was significantly higher in the 30% CJ group than in the 30% CC group at 72 h post-LT. *P < 0.05, Mann-Whitney U test. n = 5/group. D, The increase in intrahepatic VEGF mRNA expression was delayed in 30% CJ and intrahepatic VEGF mRNA expression was significantly lower in the 30% CJ group than in the 30% CC group at 72 h post-LT. *P < 0.05, Mann-Whitney U test. n = 5/group. 30% CC, arterialized 30% partial liver transplantation with choledocho-choledochostomy; 30% CJ, arterialized 30% partial liver transplantation with choledocho-jejunostomy; CC, choledocho-choledochostomy; CJ, choledocho-jejunostomy; IL, interleukin; LT, liver transplantation; VEGF, vascular endothelial growth factor.
CJ Enhances IL-1β mRNA Expression and Delays the Increase in VEGF mRNA Expression in Small Liver Graft
To elucidate the underlying mechanisms of liver regeneration in small partial grafts with CJ and CC, we assessed the changes in intrahepatic mRNA expression of IL-1β, a strong inhibitor of hepatocyte proliferation, and intrahepatic mRNA expression of VEGF, an important stimulator of sinusoidal endothelial cell proliferation, in addition to intrahepatic IL-6 mRNA expression. We observed significantly higher intrahepatic IL-1β mRNA expression in the 30% CJ group than in the 30% CC group at 24 hours post-LT (4.46 ± 2.44 versus 1.21 ± 0.99, P = 0.032; Figure 4C). Intrahepatic VEGF mRNA expression was also significantly lower in the 30% CJ group than in the 30% CC group at 72 hours post-LT (0.99 ± 0.21 versus 1.72 ± 0.65, P = 0.032; Figure 4D). The expected increase in intrahepatic VEGF mRNA expression was delayed in the 30% CJ group (Figure 4D).
CJ Versus CC in Whole LT
Five rats (100%) survived in both the 100% CJ and 100% CC groups. No ascites were identified in either group throughout the 168-hour sacrifice period. The changes of serum AST, ALT, T-bil, and bile production in the 100% CJ group were similar to those in the 100% CC group (Figure S1A–D, SDC, http://links.lww.com/TXD/A234). We observed mild neutrophil infiltration around bile ducts in the 100% CJ group, but this was not observed in the 100% CC group (Figure S1E, SDC, http://links.lww.com/TXD/A234). LDH levels in bile were elevated in the 100% CJ group at 72 hours post-LT (Figure S1F, SDC, http://links.lww.com/TXD/A234). Intrahepatic TLR4 mRNA expression started to increase at 24 hours post-LT in the 100% CJ group, but this was not observed in the 100% CC group (Figure S1G, SDC, http://links.lww.com/TXD/A234). At 168 hours post-LT, intrahepatic TLR4 mRNA expression was significantly higher in the 100% CJ group than in the 100% CC group (1.96 ± 0.42 versus 0.90 ± 0.37, P = 0.032, Figure S1G, SDC, http://links.lww.com/TXD/A234). Intrahepatic IL-6 mRNA expression did not completely improve at 24 and 72 hours post-LT in the 100% CJ group (Figure S1H, SDC, http://links.lww.com/TXD/A234).
DISCUSSION
The pretransplant GRWR is one of the established factors to predict graft prognosis in LDLT,3,6 whereas several preclinical studies have shown the importance of post-transplant liver regeneration to improve recipient outcomes.4,5 In our current study, the 30% CC group had comparable GRWR to the 30% CJ group (0.985 ± 0.032 versus 0.950 ± 0.038%; P = 0.343), while exhibiting inhibited graft inflammation, showing increased liver regeneration, and experiencing significantly better posttransplant survival as compared with the 30% CJ group. Those indicate that augmented reflux cholangitis in small partial liver grafts with CJ anastomosis impaired liver regeneration, leading to aggravated animal survival. Interestingly, superiority of CC over CJ seems apparent in 30% LT but not in 100% LT (Figure S2, SDC, http://links.lww.com/TXD/A235). Unfavorable influence of cholangitis in CJ anastomosis (T-bil, bile production, LDH in bile, mRNA levels for TLR4/IL-6, etc) might be compensated when liver graft retained sufficient volume to resolve inflammation.
In our study, CJ provoked reflux cholangitis in small partial liver grafts, impaired liver regeneration, exacerbated liver damage, and aggravated animal survival in the 30% CJ group. CJ also induced dysfunction in small partial grafts, as confirmed by the presence of massive ascites, significantly decreased bile production, and prolonged elevation of T-bil, AST, and ALT. In whole LT with sufficient liver volume, CJ provoked mild cholangitis and slight inflammation in liver grafts, but scarcely exacerbated liver damage and did not aggravate animal survival.
Several clinical and basic studies have confirmed that CJ provokes reflux cholangitis.26–28,34,35 In such studies that used animal models, reflux cholangitis was confirmed chiefly by inflammatory cell infiltration around bile ducts, increased TLR4 mRNA expression, and increased inflammatory cytokine expression.26–28 In our study, these findings, as well as elevated LDH levels in bile, were observed in the 30% CJ group (Figure 3A–E), unlike in the 30% CC (Figure 3A–E) and 100% CC groups (Figure S1E–I, SDC, http://links.lww.com/TXD/A234). The lack of hepatic artery reconstruction in animal LT model was reported to result in high incidence of biliary complications.36,37 Contrastingly, we performed hepatic artery anastomosis in the current study and confirmed patency of arteries at the time of animal sacrifice in both CC/CJ cases. Therefore, we expect that ischemic change of biliary reconstruction was unlikely to influence animal outcomes.
In recent clinical practice, CC has been performed more often than CJ in adult-to-adult LDLT due to the expectations of shorter operation times, fewer septic complications, better physiologic enteric function, and easier endoscopic access to the biliary tract for future needs,13,38 but some transplant surgeons prefer CJ to CC.15 The preferable biliary reconstruction method for better short- and long-term results remains controversial.13–19 In our study, we revealed that CJ suppressed and delayed liver regeneration in small liver grafts (Figure 4A–D). Although various factors are involved in liver regeneration,20–24 biliary reconstruction method is one of the few factors that can be managed by transplant surgeons.
It has also been demonstrated that liver regeneration is suppressed secondary to cholangitis development in a 70% hepatectomy with CJ rat model.26 Due to the ampulla of Vater preventing reflux cholangitis,34,39 we did not observe cholangitis in the 30% CC and 100% CC groups (Figure 2A–E, Figure S1E–I, SDC, http://links.lww.com/TXD/A234). Therefore, it may be reasonable to assume that liver regeneration was suppressed in the 30% CJ group.
The regulation of the expression and secretion of transcription factors and cytokines is necessary for liver regeneration.7 In 70% hepatectomy rat models that show good liver regeneration,40–46 intrahepatic IL-6 mRNA expression reaches its peak at 2–12 hours postoperation and peaks out at 24 hours postoperation,44,45 and intrahepatic VEGF mRNA expression reaches its peak at 72 hours postoperation.46 The changes of intrahepatic IL-6 and VEGF mRNA expression in the 30% CC group (Figures 3E and 4D) were similar to those in previous reports regarding 70% hepatectomy rat models.44–46 Furthermore, IL-1β has antiproliferative effects on hepatocyte proliferation, and intrahepatic IL-1β mRNA expression elevates at 48 hours posthepatectomy.47 Intrahepatic IL-1β mRNA expression was already elevated at 24 hours post-LT in the 30% CJ group (Figure 4C). Reflux cholangitis caused by CJ gradually starts at 12–24 hours post-LT, thereby inducing excessive inflammatory responses and possibly disturbing cytokine production regulation, which is necessary for liver regeneration.
We are aware of several limitations of our current study in terms of the gap between animal model and clinical LT cases.48 First, we did not use immunosuppressant or prophylactic antibiotics in our isogenic LT model which likely to influence the occurrence of ascending cholangitis. Second, in our CJ model, we use the short-length internal stent and did not apply the Roux-en Y method, which may enhance the ascending cholangitis or obstruction of bile flow. Third, despite we observed hepatic inflammatory markers, this study lacks the direct evidence of infection such as the result of culture of bile juice. Fourth, it remains unclear whether the 30% graft in our study corresponds to clinical cases of small-for-size graft. Further studies more similar to clinical situation are needed to elucidate the effect of biliary reconstruction methods in LT outcomes.
In conclusion, CJ predisposed small liver grafts to cholangitis, impaired the post-transplant regeneration of small grafts, and aggravated animal survival in the 30% CJ group. However, CJ has no critical influence on whole LT. These results may suggest that CC is preferable over CJ in adult-to-adult LDLT, if applicable.
ACKNOWLEDGMENTS
The authors are grateful for the support of the Center for Anatomical, Pathological, and Forensic Medical Research, Graduate School of Medicine, Kyoto University.
Supplementary Material
Footnotes
Published online 13 January, 2020.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
Source of funding was Kyoto University Grants.
The authors declare no conflicts of interest.
J.Y. mainly performed the experiments and assays and wrote the draft. K.H. designed the study protocol and participated in performing the research. K.N. participated in data analyses and writing the draft. Y.O. participated in performing the experiments and assays. S.U. obtained the grant, supervised the research, and participated in data analyses and writing the draft.
REFERENCES
- 1.Broelsch CE, Whitington PF, Emond JC, et al. Liver transplantation in children from living related donors. Surgical techniques and results. Ann Surg. 1991; 214428–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashikura Y, Makuuchi M, Kawasaki S, et al. Successful living-related partial liver transplantation to an adult patient. Lancet. 1994; 3431233–1234 [DOI] [PubMed] [Google Scholar]
- 3.Kiuchi T, Kasahara M, Uryuhara K, et al. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation. 1999; 67321–327 [DOI] [PubMed] [Google Scholar]
- 4.Pan N, Lv X, Liang R, et al. Suppression of graft regeneration, not ischemia/reperfusion injury, is the primary cause of small-for-size syndrome after partial liver transplantation in mice. PLoS One. 2014; 9e93636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu WY, Yan JQ, Shi MM, et al. Suppression of liver regeneration affects hepatic graft survival in small-for-size liver transplantation in rats. Hepatol Res. 2013; 43300–310 [DOI] [PubMed] [Google Scholar]
- 6.Yagi S, Uemoto S. Small-for-size syndrome in living donor liver transplantation. Hepatobiliary Pancreat Dis Int. 2012; 11570–576 [DOI] [PubMed] [Google Scholar]
- 7.Yoshida D, Akahoshi T, Kawanaka H, et al. Roles of vascular endothelial growth factor and endothelial nitric oxide synthase during revascularization and regeneration after partial hepatectomy in a rat model. Surg Today. 2011; 411622–1629 [DOI] [PubMed] [Google Scholar]
- 8.Kang LI, Mars WM, Michalopoulos GK. Signals and cells involved in regulating liver regeneration. Cells. 2012; 11261–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clavien PA. IL-6, a key cytokine in liver regeneration. Hepatology. 1997; 251294–1296 [DOI] [PubMed] [Google Scholar]
- 10.Tsutsumi R, Kamohara Y, Eguchi S, et al. Selective suppression of initial cytokine response facilitates liver regeneration after extensive hepatectomy in rats. Hepatogastroenterology. 2004; 51701–704 [PubMed] [Google Scholar]
- 11.Shimizu H, Miyazaki M, Wakabayashi Y, et al. Vascular endothelial growth factor secreted by replicating hepatocytes induces sinusoidal endothelial cell proliferation during regeneration after partial hepatectomy in rats. J Hepatol. 2001; 34683–689 [DOI] [PubMed] [Google Scholar]
- 12.Furutani M, Arii S, Monden K, et al. Immunologic activation of hepatic macrophages in septic rats: a possible mechanism of sepsis-associated liver injury. J Lab Clin Med. 1994; 123430–436 [PubMed] [Google Scholar]
- 13.Chok KS, Lo CM. Systematic review and meta-analysis of studies of biliary reconstruction in adult living donor liver transplantation. ANZ J Surg. 2017; 87121–125 [DOI] [PubMed] [Google Scholar]
- 14.Kasahara M, Egawa H, Takada Y, et al. Biliary reconstruction in right lobe living-donor liver transplantation: comparison of different techniques in 321 recipients. Ann Surg. 2006; 243559–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yi NJ, Suh KS, Cho JY, et al. In adult-to-adult living donor liver transplantation hepaticojejunostomy shows a better long-term outcome than duct-to-duct anastomosis. Transpl Int. 2005; 181240–1247 [DOI] [PubMed] [Google Scholar]
- 16.Marubashi S, Dono K, Nagano H, et al. Biliary reconstruction in living donor liver transplantation: technical invention and risk factor analysis for anastomotic stricture. Transplantation. 2009; 881123–1130 [DOI] [PubMed] [Google Scholar]
- 17.Hwang S, Lee SG, Sung KB, et al. Long-term incidence, risk factors, and management of biliary complications after adult living donor liver transplantation. Liver Transpl. 2006; 12831–838 [DOI] [PubMed] [Google Scholar]
- 18.Seo JK, Ryu JK, Lee SH, et al. Endoscopic treatment for biliary stricture after adult living donor liver transplantation. Liver Transpl. 2009; 15369–380 [DOI] [PubMed] [Google Scholar]
- 19.Kyoden Y, Tamura S, Sugawara Y, et al. Incidence and management of biliary complications after adult-to-adult living donor liver transplantation. Clin Transplant. 2010; 24535–542 [DOI] [PubMed] [Google Scholar]
- 20.Yagi S, Iida T, Taniguchi K, et al. Impact of portal venous pressure on regeneration and graft damage after living-donor liver transplantation. Liver Transpl. 2005; 1168–75 [DOI] [PubMed] [Google Scholar]
- 21.Hilmi I, Horton CN, Planinsic RM, et al. The impact of postreperfusion syndrome on short-term patient and liver allograft outcome in patients undergoing orthotopic liver transplantation. Liver Transpl. 2008; 14504–508 [DOI] [PubMed] [Google Scholar]
- 22.Olthoff KM, Emond JC, Shearon TH, et al. Liver regeneration after living donor transplantation: adult-to-adult living donor liver transplantation cohort study. Liver Transpl. 2015; 2179–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcos A, Fisher RA, Ham JM, et al. Liver regeneration and function in donor and recipient after right lobe adult to adult living donor liver transplantation. Transplantation. 2000; 691375–1379 [DOI] [PubMed] [Google Scholar]
- 24.Chae MS, Lee N, Choi HJ, et al. Comparison of liver graft regeneration between ABO-compatible and ABO-incompatible living donor liver transplantation: A propensity score matching analysis. Ann Transplant. 2018; 23507–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao S, Yagi S, Nagao M, et al. Etiologies, risk factors, and outcomes of bacterial cholangitis after living donor liver transplantation. Eur J Clin Microbiol Infect Dis. 2018; 371973–1982 [DOI] [PubMed] [Google Scholar]
- 26.Takagi T, Yokoyama Y, Kokuryo T, et al. Liver regeneration following experimental major hepatectomy with choledochojejunostomy. Br J Surg. 2015; 1021410–1417 [DOI] [PubMed] [Google Scholar]
- 27.Klaus A, Hinder RA, Nguyen JH, et al. Small bowel transit and gastric emptying after biliodigestive anastomosis using the uncut jejunal loop. Am J Surg. 2003; 186747–751 [DOI] [PubMed] [Google Scholar]
- 28.Hsieh CS, Huang CC, Wu JJ, et al. Ascending cholangitis provokes IL-8 and MCP-1 expression and promotes inflammatory cell infiltration in the cholestatic rat liver. J Pediatr Surg. 2001; 361623–1628 [DOI] [PubMed] [Google Scholar]
- 29.Howden B, Jablonski P, Grossman H, et al. The importance of the hepatic artery in rat liver transplantation. Transplantation. 1989; 47428–431 [DOI] [PubMed] [Google Scholar]
- 30.Nagai K, Yagi S, Uemoto S, et al. Surgical procedures for a rat model of partial orthotopic liver transplantation with hepatic arterial reconstruction. J Vis Exp. 2013; 73e4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vajdová K, Smreková R, Kukan M, et al. Bile analysis as a tool for assessing integrity of biliary epithelial cells after cold ischemia–reperfusion of rat livers. Cryobiology. 2000; 41145–152 [DOI] [PubMed] [Google Scholar]
- 32.Al Safadi L, Köhler G, Schaefer HE. Classification of acute myeloid leukaemia in trephine biopsies with special reference to lactoferrin. Anticancer Res. 1998; 183677–3684 [PubMed] [Google Scholar]
- 33.Scholzen T, Gerdes J. The ki-67 protein: from the known and the unknown. J Cell Physiol. 2000; 182311–322 [DOI] [PubMed] [Google Scholar]
- 34.Tanaka K, Shirahase I, Utsunomiya H, et al. A valved hepatic portoduodenal intestinal conduit for biliary atresia. Ann Surg. 1991; 213230–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto M, Tahara H, Hamaoka M, et al. Utility of hepatobiliary scintigraphy for recurrent reflux cholangitis following choledochojejunostomy: a case report. Int J Surg Case Rep. 2018; 42104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li GL, Lin HM, Long TZ, et al. High incidence of biliary complications in rat liver transplantation: can we avoid it? World J Gastroenterol. 2011; 173140–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hori T, Gardner LB, Chen F, et al. Impact of hepatic arterial reconstruction on orthotopic liver transplantation in the rat. J Invest Surg. 2012; 25242–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wachs ME, Bak TE, Karrer FM, et al. Adult living donor liver transplantation using a right hepatic lobe. Transplantation. 1998; 661313–1316 [DOI] [PubMed] [Google Scholar]
- 39.De Palma GD. Endoscopic papillectomy: indications, techniques, and results. World J Gastroenterol. 2014; 201537–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higgins GM, Anderson RE, Higgins GM, et al. Experimental pathology of the liver. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931; 12186–192 [Google Scholar]
- 41.Forbes SJ, Newsome PN. Liver regeneration - mechanisms and models to clinical application. Nat Rev Gastroenterol Hepatol. 2016; 13473–485 [DOI] [PubMed] [Google Scholar]
- 42.Meier M, Andersen KJ, Knudsen AR, et al. Liver regeneration is dependent on the extent of hepatectomy. J Surg Res. 2016; 20576–84 [DOI] [PubMed] [Google Scholar]
- 43.Meier M, Knudsen AR, Andersen KJ, et al. Gene expression in the liver remnant is significantly affected by the size of partial hepatectomy: an experimental rat study. Gene Expr. 2017; 17289–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Higashitsuji H, Arii S, Furutani M, et al. Expression of cytokine genes during liver regeneration after partial hepatectomy in rats. J Surg Res. 1995; 58267–274 [DOI] [PubMed] [Google Scholar]
- 45.Sowa JP, Best J, Benko T, et al. Extent of liver resection modulates the activation of transcription factors and the production of cytokines involved in liver regeneration. World J Gastroenterol. 2008; 147093–7100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mochida S, Ishikawa K, Inao M, et al. Increased expressions of vascular endothelial growth factor and its receptors, flt-1 and KDR/flk-1, in regenerating rat liver. Biochem Biophys Res Commun. 1996; 226176–179 [DOI] [PubMed] [Google Scholar]
- 47.García-Pérez R, Revilla-Nuin B, Martínez CM, et al. Associated liver partition and portal vein ligation (ALPPS) vs selective portal vein ligation (PVL) for staged hepatectomy in a rat model. Similar regenerative response? PLoS One. 2015; 10e0144096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamamoto M, Takakura S, Iinuma Y, et al. Changes in surgical site infections after living donor liver transplantation. PLoS One. 2015; 10e0136559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


