Abstract
Objectives
To examine weight changes relative to surgical success in children with Down syndrome and obstructive sleep apnea (OSA).
Study design
Retrospective chart review of children with Down syndrome undergoing tonsillectomy from 2005 to 2016 for OSA at a tertiary care children’s hospital. Only patients with pre-and postoperative polysomnogram within 6 months of tonsillectomy were included. Demographics, weight, height, and polysomnogram data were collected. Body mass index (BMI), expressed as a percentage of the 95th percentile (%BMIp95), was calculated for 24 months prior to and following surgery. Pre-and postoperative OSA severity were also recorded. The postoperative obstructive/hypopnea index identified subjects with resolution of obstruction (obstructive/hypopnea index <2 events/hour) or persistent mild/moderate/severe obstructive apnea. Regression analyses were used to compare %BMIp95 pre- and post-tonsillectomy with %BMIp95 by OSA status following tonsillectomy.
Results
A total of 78 patients with Down syndrome whose mean age was 5.29 years at time of tonsillectomy were identified. There was no difference between best-fit curves of %BMI p95 pre-and post-tonsillectomy. There was no difference between best-fit curves of %BMI p95 in patients who saw resolution of OSA after tonsillectomy vs patients with residual OSA.
Conclusions
Tonsillectomy neither alters the BMI trajectory of children with Down syndrome, nor changes differentially the risk for obesity in children whose OSA did or did not resolve after surgery.
Down syndrome is associated with hypotonia, facial dysmorphology, feeding issues, congenital heart defects, visual and hearing impairments, cognitive impairment of varying severity, obstructive sleep apnea (OSA), thyroid abnormalities, and obesity.1–13
With an incidence of 20%−80%, OSA is a common disorder in children with Down syndrome.14–21 The factors contributing to OSA are multifactorial: craniofacial structure, neuromuscular tone, adenotonsillar hypertrophy, and obesity. Studies have shown increased body mass index (BMI) and percent body fat in children with Down syndrome when compared with children without Down syndrome. The factors causing obesity in Down syndrome are not completely understood. It is hypothesized that endocrinological factors, including hypothyroidism22–26 and leptin resistance,27–29 contribute to elevated weight status in Down syndrome. Other mechanisms that are thought to contribute to obesity in Down syndrome are resting metabolic rate,30–34 physical activity,35–37 and dietary patterns.38–40 In a retrospective study of 303 children with Down syndrome, the prevalence of obesity was calculated to be 48%, and 74% had polysomnogram confirming OSA.41
Adenotonsillectomy is the primary treatment for OSA.42–44 Previous work suggests that nonsyndromic children gain weight following their recovery from a tonsillectomy for either obstructive sleep disordered breathing or recurrent infections.45–56 Obesity is a known risk factor for persistent OSA, and the success of adenotonsillectomy is variable.57–64 In 2017, Ingram et al reported a surgical cure of only 21% for OSA in Down syndrome when cure was defined as obstructive apnea hypopnea index (OAHI) of <2 events/hour; 52% had moderate/severe residual OSA with an OAHI ≥5 events/hour.65 Because the surgical cure rate is worse in children with Down syndrome, who are more likely to be obese (BMI ≥95th percentile for age and sex) when compared with the general population, it is concerning that studies have shown tonsillectomy to accelerate weight in nonsyndromic children with OSA.46,48–58 Weight gain may increase risk for residual OSA, recurrence of OSA after initial success, and obesity-related morbidity. The primary goal of this study was to assess whether children with Down syndrome who saw resolution of their OSA following a tonsillectomy were at increased risk for becoming obese, compared with those who had residual OSA.
Methods
Colorado Multiple Institutional Review Board approval was obtained. The patient sample was identified using i2b2 software (HealthCare System, Boston, Massachusetts) to query the electronic health record (EHR) by The International Classification of Diseases, Ninth Revision (ICD-9) and Current Procedural Terminology (CPT) codes for children with Down syndrome who underwent tonsillectomy with or without concurrent adenoidectomy for OSA from 2003 to 2015 inclusive. A retrospective chart review was conducted on the identified dataset to confirm the following inclusion criteria: diagnosis of Down syndrome, polysomogram confirmed, and tonsillectomy performed for OSA. To ensure data integrity, strict inclusion and exclusion criteria were defined and implemented (Table I). Collected data included sex, ethnicity, age at time of tonsillectomy and polysomnograms, comorbidities, and tonsil and adenoid size assessed by otolaryngologist at time of surgery. Polysomnogram characteristics collected included sleep architecture, respiratory events, oxygenation, and carbon dioxide level. OSA was defined as OAHI ≥2 events/hour on polysomnogram. Preoperative OSA severity was categorized into mild (OAHI 2–4.9 events/hour), moderate (OAHI 5.0–9.9 events/hour), or severe (OAHI ≥10 events/hour). Postoperative OSA severity included a cure category defined by OAHI <2 events/hour.
Table I.
Inclusion criteria and exclusion criteria
| Inclusions | Exclusions |
|---|---|
|
|
|
|
|
|
|
|
Data Collection
Height and weight data for all available encounters 24 months prior to and following tonsillectomy were extracted from our EHR by Children’s Hospital Colorado Research Informatics team. Although BMI growth charts exist for children with Down syndrome in the US, a comparison of the Centers for Disease Control (CDC) BMI charts and Down syndrome-specific BMI charts revealed the CDC BMI charts to be a better indicator of excess adiposity with better sensitivity and specificity.66 As a result, our study used the CDC BMI charts rather than the Down syndrome-specific charts.
Age- and sex-specific BMI, expressed as a percentage of the 95th percentile (%BMIp95) was calculated for every available time point using height and weight. The %BMI p95 has advantages over other relative BMI metrics for validity of comparisons across age, sex, and severity of obesity.67 Demographic characteristics included comorbidities present at time of surgery and were summarized via descriptive statistics. Prematurity, cardiovascular, respiratory, endocrine/renal, gastrointestinal, metabolic, and hematologic/immunologic diseases coexisting at time of tonsillectomy were collected. Regression and correlation analysis were used to examine the relationship between tonsillectomy and %BMIp95, and OSA status and %BMI p95. Children under age 2 years at time points prior to tonsillectomy were included in the model if they had at least 2 post-tonsillectomy measurements after turning 2 years of age. Study data were collected and managed using REDCap (Research Electronic Data Capture) data capture tools hosted at University of Colorado. REDCap is a secure, web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources.68
Data Analyses
Piece-wise linear mixed effects regression models were used to model %BMIp95 over time for the pre- and post-tonsillectomy time periods, in which the correlation among the repeated measures on a patient was considered. Regression lines and corresponding equations were generated for pre-and postoperative %BMIp95 and stratified by OSA status. Regression equations were examined for differences in their constants and slope coefficients using hypothesis testing. %BMIp95 changes were not assessed for children 0–2 years of age as sensitivity and specificity of BMI in infancy remains unclear, and it is not an accepted estimate of adiposity in this population. The data analysis for this article was generated using SAS software v 9.4 (SAS Institute Inc, Cary, North Carolina). A P value of ≤.05 was considered statistically significant. Table II (available at www.jpeds.com) includes a detailed review of the statistical design.
Table II.
Description of statistical analysis: piece-wise linear mixed effects regression model
| Residual OSA groups | |
| Outcome | %BMIp95 |
| Predictors | Pre-/post-tonsillectomy status, age group >2 y, time |
| Repeated measure | Patient |
| OSA cure groups | |
| Outcome | %BMIp95 |
| Predictors | Pre-/post-tonsillectomy status, age group = 2 y, age group 2–7 y, age group >7 y, time |
| Repeated measure | Patient |
Results
Study Sample Characteristics
We identified 543 patients between 2003 and 2015 (inclusive) at Children’s Hospital Colorado with a diagnosis code for Down syndrome and procedure code for tonsillectomy; 78 patients met the inclusion criteria and were retained for analysis (Table I). All patients underwent adenoidectomy concurrently with tonsillectomy or prior to tonsillectomy. The cohort included 42 male children with mean age at tonsillectomy of 5.3 years ± 3.8 years and range of 6.1 months to 16.6 years (Table III; available at www.jpeds.com). Patients were stratified into 2 groups, obese (% BMIp95 ≥100) and nonobese (%BMIp95 < 100). The prevalence of overweight and obese at time of tonsillectomy were 21% and 24%, respectively. Mean OAHI prior to tonsillectomy was 21.6 ± 19.3 events/hour and range of 2.1 to 115.9 events/hour. Mean OAHI following tonsillectomy was 13.4 ± 14.2 events/hour and range of 0.2 to 81.8 events/hour. Sixteen patients saw a cure in their OSA with an OAHI <2 events/hour at postoperative polysomnogram. Mean BMI percentile for nonobese group was 59.71 ± 26.91 and range of 2.00–94.88 prior to tonsillectomy. Pre-tonsillectomy mean %BMIp95 for obese group was 113.48 ± 17.23 with a range of 95.69–155.21. A summary of BMI percentile and %BMIp95 changes stratified by obesity and OSA status are summarized in Table IV.
Table III.
Demographics sample characteristics
| All | |
|---|---|
| Total, N (%) | 78 (100) |
| Age at tonsillectomy, mean (SD) | 5.29 (3.79) |
| Sex | |
| Female | 36 (46.2) |
| Male | 42 (53.8) |
| Race/ethnicity | |
| White | 38 (48.7) |
| Hispanic | 31 (39.7) |
| Black | 3 (3.8) |
| Asian | 0 (0.0) |
| Other | 5 (6.4) |
| Comorbidities | |
| Prematurity | 21 (26.9) |
| Congenital heart anomalies | 62 (79.5) |
| Pulmonary hypertension | 8 (10.3) |
| Hypothyroidism | 18 (23.1) |
Table IV.
BMI % and %BMIp95 changes following tonsillectomy by obesity and OSA status
| Non-obese (BMI%) | Obese (%BMIp95) | |||
|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | |
| Cure | ||||
| Pre | 65.8 (21.5) | 36.1–91.7 | 99.6 (3.84) | 95.7–103.4 |
| Post | 71.0 (17.6) | 36.3–89.6 | 102.2 (1.9) | 100.2–104.0 |
| Residual | ||||
| Pre | 58.6 (27.9) | 2.0–94.9 | 116.7 (17.6) | 100.2–155.2 |
| Post | 65.8 (24.2) | 1.6–91.9 | 125.2 (19.6) | 104.2–173.0 |
BMI %, BMI percentile.
BMI% and %BMIp95 were not calculated for children 0 to <2 years of age at time of height and weight collection.
Comparison of Regression Lines for %BMIp95 and OSA Status following Tonsillectomy
For patients with residual OSA on postoperative polysomnogram, the regression equation for %BMIp95 and age >2 years showed an equation constant (Yi) of 84.4 and slope coefficient (b) of 2.36 prior to tonsillectomy. Following tonsillectomy, the patients’ regression equation had a Yi of and 84.3 and b of 2.43. On hypothesis testing, the difference in the slopes was not significant with a P value of .99 (Figure 1).
Figure 1.
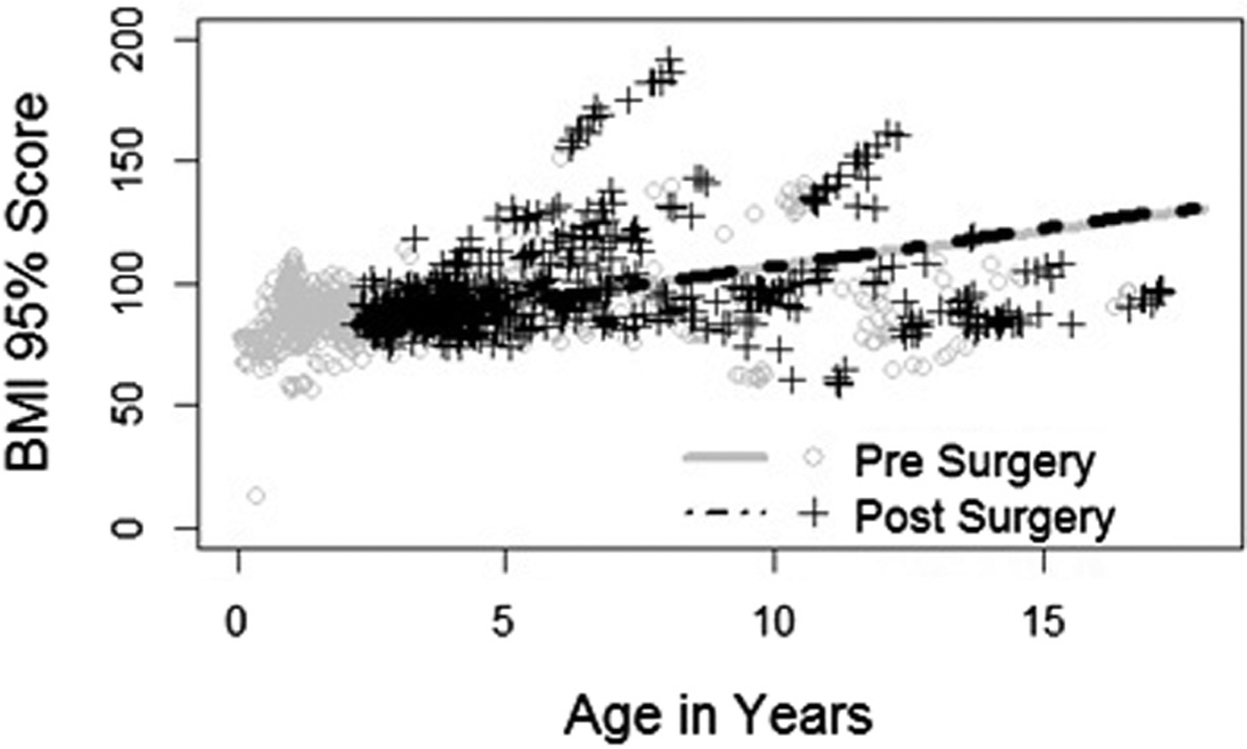
%BMIp95 vs age in years in children with Down syndrome with residual OSA.
For children without residual OSA, the model required several regression equations. The preoperative results are as follows: %BMIp95 and age = 2 years and age = 2–7 years (Yi = 91.0, b = 0.29); %BMIp95 and age >7 years (Yi = 95.7, b = 1.5). Following tonsillectomy, the regression equation for children without residual were as follows: %BMIp95 and age = 2 years and age = 2 – 7 (Yi = 96.7, b = 1.63); %BMIp95 and age >7 years (Yi = 90.7, b = 0.38). On hypothesis testing, the differences between pre-and postoperative regressions did not reach significance with a P value of .81 (Figure 2).
Figure 2.
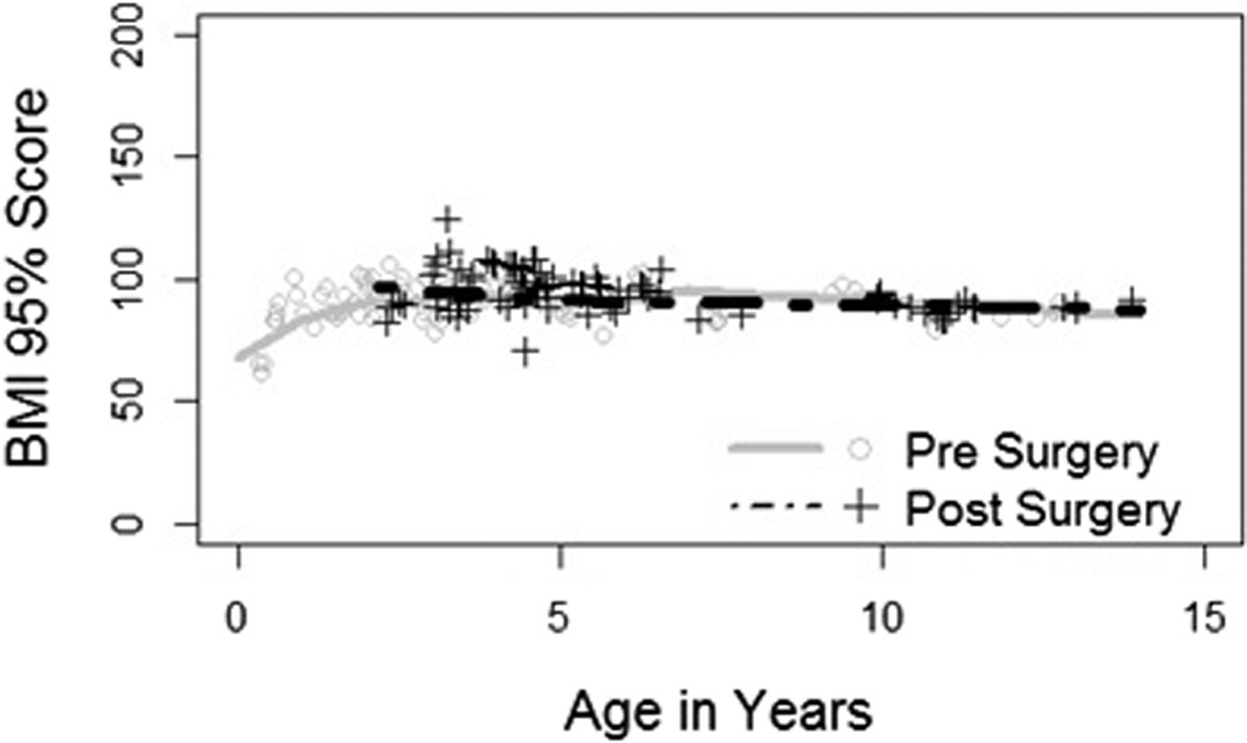
%BMIp95 vs age in years in children with Down syndrome without residual OSA.
Children with residual OSA on postoperative polysomnogram showed slightly higher preoperative and postoperative %BMIp95 and tended to be older when compared with those without residual OSA post-tonsillectomy. Children without residual OSA on post-tonsillectomy polysomnogram were younger and had slightly lower pre- and post-tonsillectomy %BMIp95 when compared with children with residual disease.
Discussion
Tonsillectomy remains the first-line treatment for OSA for both children with Down syndrome and the general population.42–44 However, tonsillectomy success for curing OSA is substantially lower among obese children and children with Down syndrome.42,43,62,65 The only randomized control trial for tonsillectomy showed that only 67% of obese children who underwent surgery had an OAHI <2 events/hour.63 Increased risk for obesity following tonsillectomy has been reported in the literature. Thus, the interaction between obesity, OSA, and tonsillectomy is of great importance when considering management of OSA in children with Down syndrome who have a preexisting susceptibility to obesity, perioperative complications, and lower cure rates.
Because children’s growth rate is dependent upon both their age and sex, the CDC recommends using BMI-for-age to define and assess obesity. However, recent literature has shown the limitations of using BMI-for-age and BMI z score for obese children.67 BMI z scores are limited by an upper limit, and above the 97th percentile are difficult to estimate resulting in use of the BMI ≥120% of the 95th percentile to define severe obesity instead of assigning percentiles greater than the 95th. As we approach the upper limit for BMI-for-age, BMIs are compressed resulting in a wide range of BMIs mapping to similar z scores. Our study examines weight changes following tonsillectomy for OSA in children with Down syndrome using %BMIp95, a metric more appropriate than BMI z score in a population with high rates of obesity. The initial analysis did use the BMI z score and demonstrated no difference; however, the %BMIp95 analysis was performed out of concern that the z score findings may be spurious.
For nonsyndromic children, the literature suggests that children gain weight following their recovery from a tonsillectomy.45–56 Weight gain occurred for children who had either obstructive sleep disordered breathing or recurrent infections.45–56 Previous investigations suggest that younger children are the ones more likely to gain weight.50,51,54 The exact mechanism for increased growth velocity is unknown, but several hypotheses exist. A few studies have found increases in growth hormone secretion following tonsillectomy, resulting in weight gain.45,46,56 Investigations have also implicated dietary habits, reporting that children who underwent tonsillectomy for sleep disordered breathing ate more sugar products and edible fats postoperatively.69 For a cohort of children with moderate OSA (OAHI >5 events/hour), a decrease in C-reactive protein levels following a tonsillectomy correlated with an increase in body weight for boys. Another hypothesis is that OSA resolution results in decreased work of nighttime breathing and energy expenditure. Roemmich et al reported in a cohort of children with resolved OSA (OAHI <1 event/hour) that there was both a reduction in motor activity and hyperactivity scores and subsequently an increase percentage overweight.55
Growth patterns prior to tonsillectomy should be used to assess potential determinants of post-tonsillectomy weight changes. For example, when examining increased weight in younger children, as has been seen in the literature, normal growth trajectory of prepubertal children should be considered and models should be adjusted to account for normal developmental growth patterns. Children with poor weight gain prior to tonsillectomy may benefit from an acceleration in growth post-tonsillectomy as long as the accelerated growth pattern does not continue long term. However, if a tonsillectomy directly increases a child’s risk of obesity or makes them more obese postoperatively, surgeons will need to carefully assess the risks and benefits of performing a tonsillectomy for children who are less likely to achieve surgical cure.
It is unclear why our Down syndrome sample behaved differently than nonsyndromic children. In a retrospective review by D’Esposito et al, children with Down syndrome who underwent adenotonsillectomy for sleep disordered breathing showed no difference in height, weight, BMI, and z score data when compared with the control children with Down syndrome who did not undergo adenotonsillectomy and did not have sleep disordered breathing at time of data collection.47 Of note, trending z score over time in the group that underwent adenotonsillectomy were reported to stabilize long term, and the z score of the control group continued to increase.
Fortunately, our study demonstrated that children with Down syndrome are not predisposed to becoming heavier following a tonsillectomy with no significant difference between the %BMIp95 regression line pre- vs post-tonsillectomy in children with residual OSA and without residual OSA. Besides using a more accurate metric to measure growth trajectory (%BMIp95), another strength of our investigation is that the cohort included not only children who achieved surgical cure (OAHI <2 events/hour) but also those who had moderate/severe persistent OSA (OAHI ≥5 events/hour). By requiring that the preoperative polysomnogram was performed within 6 months of surgery, it was less likely that severity of OSA worsened prior to surgery. Because the %BMIp95 is a more accurate metric to assess for longitudinal changes in weight, we have higher confidence that the children are not becoming more obese due to the plateau effect seen when utilizing the BMI% for age to calculate a z score.
A potential limitation of our investigation is the short follow-up period. However, collecting all available data points for 2 years prior and following tonsillectomy provided repeated measures per each patient and sufficient data for the creation of growth curves to assess for any changes. Another potential limitation is that children who had either no OSA or successful surgery developed OSA during the study period. Most of these children are followed closely in our center for Down syndrome so a repeat polysomnogram would have been performed on any symptomatic child. Although a useful method when researching vulnerable populations like children with Down syndrome, a retrospective chart review has limitations, too. These include use of a convenience sample, potential errors in patient charts and data abstraction errors, especially prior to the launch of our fully integrated electronic health record in 2009. Retrospective chart reviews are also more susceptible to systematic and random error when compared with more rigorous study designs such as randomized controlled trials. Although the advantages of randomized controlled trials are well-known, designing and conducting trials with surgical interventions are logistically and ethically difficult. As a result, it is critical that retrospective studies are replicated to improve validity and reliability when randomized controlled trials are not feasible.
Because tonsillectomy cure rates are lower for children with Down syndrome, the risk/benefit ratio or therapeutic index is narrower and all variables associated with surgery should be critically analyzed for potential risk factors. Our investigation did not find an increase in BMI growth trajectories among children with Down syndrome following a tonsillectomy, regardless of post-tonsillectomy OSA status. These findings may provide valuable information to be included as an aid in the shared decision-making between parent and provider when considering tonsillectomy as a management option for OSA.
Acknowledgments
NCRR Colorado CTSI REDCap service was supported by National Institutes of Health (NIH)/National Center for Research Resources (NCRR) Colorado CTSI Grant Number UL1 RR025780. The contents of this publication are the authors’ sole responsibility and do not necessarily represent official NIH views. D.I. served on a medical advisory board and serves on the speakers’ bureau for Jazz Pharmaceuticals. N.F. is a member of the American Board of Internal Medicine (ABIM) Board of Directors and of the ABIM Internal Medicine Exam Committee. To protect the integrity of Board Certification, ABIM strictly enforces the confidentiality and its ownership of ABIM examination content, and N.F. has agreed to keep ABIM examination content confidential. No ABIM examination content is shared or otherwise disclosed in this article. The other authors declare no conflicts of interest
Glossary
- %BMIp95
Body mass index expressed as a percentage of the 95th percentile
- b
slope coefficient
- BMI
Body mass index
- CDC
Centers for Disease C’ontrol
- OAHI
Obstructive apnea hypopnea index
- OSA
Obstructive sleep apnea
- Yi
Equation constant
References
- 1.Roper RJ, Reeves RH. Understanding the basis for Down syndrome phenotypes. PLoS Genet 2006;2:e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sforza C, Dellavia C, Zanotti G, Tartaglia GM, Ferrario VF. Soft tissue facial areas and volumes in subjects with Down syndrome. Am J Med Genet A 2004;130A:234–9. [DOI] [PubMed] [Google Scholar]
- 3.Sforza C, Elamin F, Dellavia C, Rosati R, Lodetti G, Mapelli A, Ferrario VF. Morphometry of the orbital region soft tissues in Down syndrome. J Craniofac Surg 2012;23:198–202. [DOI] [PubMed] [Google Scholar]
- 4.Dey A, Bhowmik K, Chatterjee A, Chakrabarty PB, Sinha S, Mukhopadhyay K. Down syndrome Related Muscle Hypotonia: Association with COL6A3 Functional SNP rs2270669. Front Genet 2013;4:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starbuck JM, Cole TM III, Reeves RH, Richtsmeier JT. The influence of trisomy 21 on facial form and variability. Am J Med Genet A 2017;173: 2861–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schieve LA, Boulet SL, Boyle C, Rasmussen SA, Schendel D. Health of children 3 to 17 years of age with Down syndrome in the 1997–2005 national health interview survey. Pediatrics 2009;123:e253–60. [DOI] [PubMed] [Google Scholar]
- 7.Pfitzer C, Helm PC, Rosenthal LM, Berger F, Bauer UMM, Schmitt KR. Dynamics in prevalence of Down syndrome in children with congenital heart disease. Eur J Pediatr 2018;177:107. [DOI] [PubMed] [Google Scholar]
- 8.Bergström S, Carr H, Petersson G, Stephansson O, Bonamy AK, Dahlström A, et al. Trends in congenital heart defects in infants with Down syndrome. Pediatrics 2016;138. [DOI] [PubMed] [Google Scholar]
- 9.Stoll C, Dott B, Alembik Y, Roth MP. Associated congenital anomalies among cases with Down syndrome. Eur J Med Genet 2015;58:674–80. [DOI] [PubMed] [Google Scholar]
- 10.Jackson A, Maybee J, Moran MK, Wolter-Warmerdam K, Hickey F. Clinical characteristics of dysphagia in children with Down syndrome. Dysphagia 2016;31:663–71. [DOI] [PubMed] [Google Scholar]
- 11.Glasson EJ, Dye DE, Bittles AH. The triple challenges associated with age-related comorbidities in Down syndrome. J Intellect Disabil Res 2014;58:393–8. [DOI] [PubMed] [Google Scholar]
- 12.Bull MJ, Committee on genetics. Health supervision for children with Down syndrome. Pediatrics 2011;128:393–406. [DOI] [PubMed] [Google Scholar]
- 13.Uppal H, Chandran S, Potluri R. Risk factors for mortality in down syndrome. J Intellect Disabil Res 2015;59:873–81. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmire CA, Magyar CI, Connolly HV, Fernandez ID, van Wijngaarden E. High prevalence of sleep disorders and associated comorbidities in a community sample of children with down syndrome. J Clin Sleep Med 2014;10:411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassell JL, Phan H, Leu R, Kronk R, Visootsak J. Sleep profiles in children with Down syndrome. Am J Med Genet A 2015;167A: 1830–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcus CL, Keens TG, Bautista DB, von Pechmann WS, Ward SL. Obstructive sleep apnea in children with Down syndrome. Pediatrics 1991;88:132–9. [PubMed] [Google Scholar]
- 17.Ramia M, Musharrafieh U, Khaddage W, Sabri A. Revisiting Down syndrome from the ENT perspective: review of literature and recommendations. Eur Arch Otorhinolaryngol 2014;271:863–9. [DOI] [PubMed] [Google Scholar]
- 18.Pravit J Bronchoscopic findings in Down syndrome children with respiratory problems. J Med Assoc Thai 2014;97(Suppl 6):S159–63. [PubMed] [Google Scholar]
- 19.Bertrand P, Navarro H, Caussade S, Holmgren N, Sánchez I. Air way anomalies in children with Down syndrome: endoscopic findings. Pediatr Pulmonol 2003;36:137–41. [DOI] [PubMed] [Google Scholar]
- 20.Verstegen RH, van Hout RW, de Vries E. Epidemiology of respiratory symptoms in children with Down syndrome: a nationwide prospective web-based parent-reported study. BMC Pediatr 2014;14:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maris M, Verhulst S, Wojciechowski M, Van de Heyning P, Boudewyns A. Prevalence of obstructive sleep apnea in children with Down syndrome. Sleep 2016;39:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iughetti L, Predieri B, Bruzzi P, Predieri F, Vellani G, Madeo SF, et al. Ten-year longitudinal study of thyroid function in children with Down’s syndrome. Horm Res Paediatr 2014;82:113–21. [DOI] [PubMed] [Google Scholar]
- 23.Graber E, Chacko E, Regelmann MO, Costin G, Rapaport R. Down syndrome and thyroid function. Endocrinol Metab Clin North Am 2012;41: 735–45. [DOI] [PubMed] [Google Scholar]
- 24.Purdy IB, Singh N, Brown WL, Vangala S, Devaskar UP. Revisiting early hypothyroidism screening in infants with Down syndrome. J Perinatol 2014;34:936–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myrelid A, Jonsson B, Guthenberg C, von Döbeln U, Annerén G, Gustafsson J. Increased neonatal thyrotropin in Down syndrome. Acta Paediatr 2009;98:1010–3. [DOI] [PubMed] [Google Scholar]
- 26.Pueschel SM, Jackson IM, Giesswein P, Dean MK, Pezzullo JC. Thyroid function in Down syndrome. Res Dev Disabil 1991;12:287–96. [DOI] [PubMed] [Google Scholar]
- 27.Magge SN, O’Neill KL, Shults J, Stallings VA, Stettler N. Leptin levels among prepubertal children with Down syndrome compared with their siblings. J Pediatr 2008;152:321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magni P, Ruscica M, Dozio E, Roti E, Licastro F, Motta M, Corsi MM. Free and bound leptin in prepubertal children with Down’s syndrome and different degrees of adiposity. Eur J Clin Nutr 2004;58:1547–9. [DOI] [PubMed] [Google Scholar]
- 29.Yahia S, El-Farahaty RM, El-Hawary AK, El-Hussiny MA, Abdel-Maseih H, El-Dahtory F, et al. Leptin, insulin and thyroid hormones in a cohort of Egyptian obese Down syndrome children: a comparative study. BMC Endocr Disord 2012;12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allison DB, Gomez JE, Heshka S, Babbitt RL, Geliebter A, Kreibich K, et al. Decreased resting metabolic rate among persons with Down syndrome. Int J Obes Relat Metab Disord 1995;19:858–61. [PubMed] [Google Scholar]
- 31.Hill DL, Parks EP, Zemel BS, Shults J, Stallings VA, Stettler N. Resting energy expenditure and adiposity accretion among children with Down syndrome: a 3-year prospective study. Eur J Clin Nutr 2013;67: 1087–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luke A1, Roizen NJ, Sutton M, Schoeller DA. Energy expenditure in children with Down syndrome: correcting metabolic rate for movement. J Pediatr 1994;125(5 Pt 1):829–38. [DOI] [PubMed] [Google Scholar]
- 33.Pogribna M, Melnyk S, Pogribny I, Chango A, Yi P, James SJ. Homocysteine metabolism in children with Down syndrome: in vitro modulation. Am J Hum Genet 2001;69:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bauer J, Teufel U, Doege C, Hans-Juergen G, Beedgen B, Linderkamp O. Energy expenditure in neonates with Down syndrome. J Pediatr 2003;143:264–6. [DOI] [PubMed] [Google Scholar]
- 35.Sayers Menear K Parents’ perceptions of health and physical activity needs of children with Down syndrome. Downs Syndr Res Pract 2007;12:60–8. [DOI] [PubMed] [Google Scholar]
- 36.Segal M, Eliasziw M, Phillips S, Bandini L, Curtin C, Kral TV, et al. Intellectual disability is associated with increased risk for obesity in a nationally representative sample of U.S. children. Disabil Health J 2016;9:392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esposito PE, MacDonald M, Hornyak JE, Ulrich DA. Physical activity patterns of youth with Down syndrome. Intellect Dev Disabil 2012;50: 109–19. [DOI] [PubMed] [Google Scholar]
- 38.Luke A, Sutton M, Schoeller DA, Roizen NJ. Nutrient intake and obesity in prepubescent children with Down syndrome. J Am Diet Assoc 1996;96:1262–7. [DOI] [PubMed] [Google Scholar]
- 39.O’Neill KL, Shults J, Stallings VA, Stettler N. Child-feeding practices in children with down syndrome and their siblings. J Pediatr 2005;146: 234–8. [DOI] [PubMed] [Google Scholar]
- 40.Soler Marín A, Xandri Graupera JM. Nutritional status of intellectual disabled persons with Down syndrome. Nutr Hosp 2011;26: 1059–66. [DOI] [PubMed] [Google Scholar]
- 41.Basil JS, Santoro SL, Martin LJ, Healy KW, Chini BA, Saal HM. Retrospective study of obesity in children with Down syndrome. J Pediatr 2016;173:143–8. [DOI] [PubMed] [Google Scholar]
- 42.Shete MM, Stocks RM, Sebelik ME, Schoumacher RA. Effects of adeno-tonsillectomy on polysomnography patterns in Down syndrome children with obstructive sleep apnea: a comparative study with children without Down syndrome. Int J Pediatr Otorhinolaryngol 2010;74:241–4. [DOI] [PubMed] [Google Scholar]
- 43.Thottam PJ, Choi S, Simons JP, Kitsko DJ. Effect of adenotonsillectomy on central and obstructive sleep apnea in children with Down syndrome. Otolaryngol Head Neck Surg 2015;153:644–8. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell RB. Adenotonsillectomy for obstructive sleep apnea in children: outcome evaluated by pre-and postoperative polysomnography. Laryngoscope 2007;117:1844–54. [DOI] [PubMed] [Google Scholar]
- 45.Yılmaz MD, Hosal AS, Oguz H, Yordam N, Kaya S. The effects of tonsillectomy and adenoidectomy on serum IGFI and IGFBP3 levels in children. Laryngoscope 2002;112:922–5. [DOI] [PubMed] [Google Scholar]
- 46.Kiris M, Muderris TO, Celebi S, Cankaya H, Bercin S. Changes in serum IGF-1 and IGFBP-3 levels and growth in children following adenoidectomy, tonsillectomy or adenotonsillectomy. Int J Pediatr Otorhinolaryngol 2010;74:528–31. [DOI] [PubMed] [Google Scholar]
- 47.D’Esposito CF, Farhood Z, Baker AB, Nguyen SA, LaRosa AC, Lal C, White DR. Assessment of weight gain following adenotonsillectomy in children with Down syndrome. Int J Pediatr Otorhinolaryngol 2017;100:103–6. [DOI] [PubMed] [Google Scholar]
- 48.Soultan Z, Wadowski S, Rao M, Kravath RE. Effect of treating obstructive sleep apnea by tonsillectomy and/or adenoidectomy on obesity in children. Arch Pediatr Adolesc Med 1999;153:33–7. [DOI] [PubMed] [Google Scholar]
- 49.Lewis TL, Johnson RF, Choi J, Mitchell RB. Weight gain after adenotonsillectomy: a case control study. Otolaryngol Head Neck Surg 2015;152: 734–9. [DOI] [PubMed] [Google Scholar]
- 50.Nachalon Y, Lowenthal N, Greenberg-Dotan S, Goldbart AD. Inflammation and growth in young children with obstructive sleep apnea syndrome before and after adenotonsillectomy. Mediators Inflamm 2014;2014:146893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith DF, Vikani AR, Benke JR, Boss EF, Ishman SL. Weight gain after adenotonsillectomy is more common in young children. Otolaryngol Head Neck Surg 2013;148:488–93. [DOI] [PubMed] [Google Scholar]
- 52.Conlon BJ, Donnelly MJ, McShane DP. Tonsillitis, tonsillectomy and weight disturbance. Int J Pediatr Otorhinolaryngol 1997;42:17–23. [DOI] [PubMed] [Google Scholar]
- 53.Levi J, Leoniak S, Schmidt R. Evaluating tonsillectomy as a risk factor for childhood obesity. Arch Otolaryngol Head Neck Surg 2012;138: 897–901. [DOI] [PubMed] [Google Scholar]
- 54.Czechowicz JA, Chang KW. Analysis of growth curves in children after adenotonsillectomy. JAMA Otolaryngol Head Neck Surg 2014;140: 491–6. [DOI] [PubMed] [Google Scholar]
- 55.Roemmich JN, Barkley JE, D’Andrea L, Nikova M, Rogol AD, Carskadon MA, et al. Increases in overweight after adenotonsillectomy in overweight children with obstructive sleep-disordered breathing are associated with decreases in motor activity and hyperactivity. Pediatrics 2006;117:e200–8. [DOI] [PubMed] [Google Scholar]
- 56.Kang JM, Auo HJ, Yoo YH, Cho JH, Kim BG. Changes in serum levels of IGF-1 and in growth following adenotonsillectomy in children. Int J Pediatr Otorhinolaryngol 2008;72:1065–9. [DOI] [PubMed] [Google Scholar]
- 57.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000;284:3015–21. [DOI] [PubMed] [Google Scholar]
- 58.Verhulst SL, Schrauwen N, Haentjens D, Suys B, Rooman RP, Van Gaal L, De Backer WA, Desager KN. Sleep-disordered breathing in overweight and obese children and adolescents: prevalence, characteristics and the role of fat distribution. Arch Dis Child 2007;92:205–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chirinos JA, Gurubhagavatula I, Teff K, Rader DJ, Wadden TA, Townsend R, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med 2014;370:2265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhushan B, Maddalozzo J, Sheldon SH, Haymond S, Rychlik K, Lales GC, et al. Metabolic alterations in children with obstructive sleep apnea. Int J Pediatr Otorhinolaryngol 2014;78:854–9. [DOI] [PubMed] [Google Scholar]
- 61.Dayyat E, Kheirandish-Gozal L, Sans Capdevila O, Maarafeya MMA, Gozal D. Obstructive sleep apnea in children: relative contributions of body mass index and adenotonsillar hypertrophy. Chest 2009;136:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lennon CJ, Wang RY, Wallace A, Chinnadurai S. Risk of failure of adenotonsillectomy for obstructive sleep apnea in obese pediatric patients. Int J Pediatr Otorhinolaryngol 2017;92:7–10. [DOI] [PubMed] [Google Scholar]
- 63.Marcus CL1 Moore RH, Rosen CL, Giordani B, Garetz SL, Taylor HG, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med 2013;368:2366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, Mitchell RB, Promchiarak J, Simakajornboon N, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med 2010;182:676–83. [DOI] [PubMed] [Google Scholar]
- 65.Ingram DG, Ruiz AG, Gao D, Friedman NR. Success of tonsillectomy for obstructive sleep apnea in children with Down syndrome. J Clin Sleep Med 2017;13:975–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hatch-Stein JA, Zemel BS, Prasad D, Kalkwarf HJ, Pipan M, Magge SN, Kelly A. Body composition and BMI growth charts in children with Down syndrome. Pediatrics 2016;138:e20160541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freedman DS, Butte NF, Taveras EM, Lundeen EA, Blanck HM, Goodman AB, et al. BMI z-Scores are a poor indicator of adiposity among 2- to 19-year-olds with very high BMIs, NHANES 1999–2000 to 2013–2014. Obesity (Silver Spring) 2017;25:739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gkouskou KK, Vlastos IM, Hajiioannou I, Hatzaki I, Houlakis M, Fragkiadakis GA. Dietary habits of preschool aged children with tonsillar hypertrophy, pre- and post-operatively. Eur Rev Med Pharmacol Sci 2010;14:1025–30. [PubMed] [Google Scholar]


