Abstract
Background:
Although calcitonin gene-related peptide antagonists and botulinum toxin A have been shown efficacy in preventing chronic migraine, there is no direct evidence for their comparative effectiveness. This review is to assess the comparative effectiveness and safety of calcitonin gene-related peptide antagonists and botulinum toxin A for chronic migraine using network meta-analysis.
Methods:
OVID MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials will be searched for relevant randomized controlled trials from their inception to December 2019 without language restriction. We will include trials testing the effectiveness of calcitonin gene-related peptide antagonists or botulinum toxin A in patients with chronic migraine. The outcomes are mean change from baseline in the number of headache days, the mean change from baseline in the number of migraine days, the mean change from baseline in headache hours, responder rate, and adverse events rate. The methodological quality of the included randomized controlled trials will be evaluated using Cochrane Collaboration's risk of bias tool. Standardized mean difference will be used to synthesize continuous variables and risk ratio will be used to synthesize categorical variables. Pairwise and network meta-analysis will be performed using a frequentist method in netmeta package (R 3.5.0, www.r-project.org).
Results:
Ethical approval and informed consent are not required for this systematic review. The results will be submitted to a peer-reviewed journal and conference abstracts for publication.
Conclusion:
The result of the review will systematically provide suggestions for clinicians, patients, and policy makers in the treatment of chronic migraine.
PROSPERO registration number: CRD42018089201.
Keywords: botulinum toxin A, calcitonin gene-related peptide antagonists, chronic migraine, network meta-analysis, study protocol
1. Introduction
Migraine is a neurological disorder characterized by periodic attacks of headache and often accompanied by a series of reversible systemic symptoms such as photophobia, phonophobia, vertigo, nausea, and vomiting.[1] The occurrence of headache on more than 15 days per month, and that at least 8 days meet diagnostic criteria of migraine is defined as chronic migraine.[2] The population prevalence of chronic migraine ranged from 0.9% to 5.1%,[3] of which 3/4 is female.[4] Patients with chronic migraine often reported substantially impaired quality of life, decreased productivity, and rising costs of health-care.[5–7] Because of huge social and economic burden, chronic migraine ranks one of the most prevalent and disabling medical illnesses in the world.[8]
The primary goal of chronic migraine treatment is to reduce the impact of disease on patient's lives. So, it is necessary to decrease the frequency and duration of headache attacks and reduce migraine-related disability.[2,9] Traditional preventive medications, for example, antidepressants, β blockers, calcium-channel blockers, and anticonvulsants can reduce the frequency of attacks by about 50%; however, accompanied with intolerable side effects.[10]
Guidelines recommended botulinum toxin A (BoNT-A) as an effective and well-tolerated treatment prophylactic medication for chronic migraine.[11,12] The mechanisms of BoNT-A for chronic migraine may include modulation of neurotransmitter release, changes in surface expression of receptors and cytokines as well as enhancement of opioidergic transmission.[13] Peripheral injection 155 U195 U to 31–39 sites every 12-weeks has proved to be effective.[12]
Recent studies have shown that calcitonin gene-related peptide (CGRP) is a key factor in the pathogenesis of migraine.[14] CGRP antagonists can significantly improve the treatment effect of chronic migraine,[15] this may expected to attract 20% of migraine patients who do not respond to existing treatments.[16] Several pivotal trials have proved that anti-CGRP monoclonal antibodies to be well tolerated and efficacious for the prevention of chronic migraine. And erenumab-aoo, an anti-CGRP monoclonal antibodies, has also been approved by the US Food and Drug Administration as a preventive treatment for chronic migraine.[17–20]
Heretofore, there is no trial to directly compare BoNT-A with CGRP antagonists, so in order to provide clinicians and patients with the best treatment decisions, we will conduct a systematic review and network meta-analysis to indirectly compare the effectiveness of 2 medications and answer the following questions: Which is better and safer in reducing the attacks of headache?
2. Methods
2.1. Study design and registration
The systematic review and network meta-analysis will assess the comparative effectiveness and safety of CGRP antagonists and BoNT-A for migraine prophylaxis. We will conduct this network meta-analysis in accordance with preferred reporting items for systematic review and meta-analysis protocols.[21] The network meta-analysis has been registered in PROSPERO (https://www.crd.york.ac.uk/PROSPERO/, CRD), PROSPERO registration number is CRD42018089201.
2.2. Information sources
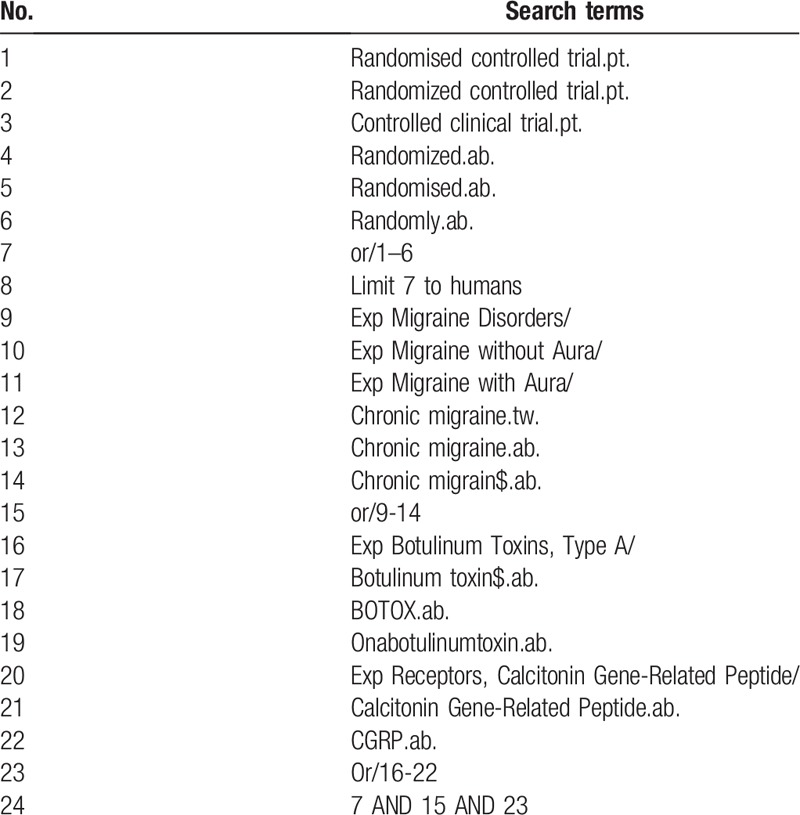
We will search OVID MEDLINE, EMBASE, the Cochrane Central Register of Clinical Trials from inception to December 2019 without language restriction, which examine the effectiveness of the CGRP antagonists and BOTOX interventions for chronic migraine prophylaxis. To find out randomized controlled trials (RCTs) that examined the effectiveness of the CGRP antagonists and BoNT-A for chronic migraine prophylaxis, we will develop a search strategy by using a combination of terms of medical subject headings (MeSH) and keywords. MeSH and keywords contain “chronic migraine,” “randomized controlled trials,” and synonymous words. Details of the search strategy are provided in Table 1. Systematic reviews examining the effect of CGRP antagonists and BoNT-A on chronic migraine will be retrieved, the reference of which will be screened for relevant RCTs.
Table 1.
Search strategy.
2.3. Inclusion and exclusion criteria
2.3.1. Studies design
We will include trials with randomized controlled design and exclude specific design of randomized controlled trials like the N-of-1 design and the cross-over design. Besides, the cohort studies, case reports, case series and experimental studies that focus on treatment mechanism will be excluded.
2.3.2. Participants
We will include males or females who meet the following conditions: with chronic migraine diagnosed according to the International Classification of Headache Disorders (ICHD 2nd edition or ICHD 3rd edition); with headache attacks for at least 15 days per month and migraine attacks for at least 8 days per month. Patients with suspected headaches due to trauma, elevated blood pressure, or other organic diseases will be excluded.
2.3.3. Interventions
We will include trials testing the effectiveness of CGRP antagonists or BoNT-A. To ensure the comparability between CGRP antagonists and BoNT-A, we will include trials using injections only. Moreover, we will include trials comparing CGRP antagonists with placebo and trials comparing BoNT-A with placebo. Trials comparing CGRP antagonists or BoNT-A with positive drug control will be excluded.
2.3.4. Outcome measures
The primary outcome was the mean change from baseline in the number of headache days during a 4-week assessment period (week 9–12). Secondary outcomes include the mean change from baseline in the number of migraine days, the mean change from baseline in headache-hours, the responder rate, and the rate of adverse events at week 4, 8, and 12. According to the IHS guideline,[22] if the headache lasts more than 4 hours a day, it is defined as a migraine day. The responder rates have been defined as a ≥50% reduction from baseline in number of migraine days.
2.4. Study selection and data extraction
The process of study selection and data extraction will be performed and cross-checked by 2 independent reviewers. First, studies will be selected by reviewing the titles and abstracts according to the included criteria mentioned above. Any discrepancy in this procedure will be settled by team discussion or being arbitrated by a third reviewer. Then, possible candidates will be searched and downloaded for full-text copies for further evaluation and to determine the final included studies. At last, necessary information will be extracted from the included RCTs using a standard form, which is developed by a consensus of all the reviewers. The form covers the following domains: study ID, settings, baseline characteristics of each trial (sample size, age, sex ratio), number of study centers and groups, allocation ratio, the name of intervention and control, treatment duration, treatment frequency, and outcome assessments (primary outcome and secondary outcomes, measurement time point, the rate of adverse events). For trials with the missing data, we will contact the original authors for more information about data by email or phone calls. After all the data is extracted from the included RCTs, a third reviewer will check the completeness and correctness of the data, to ensure an accurate result of this study.
2.5. Risk of bias assessment
Cochrane Collaboration's risk of bias tool[23] will be used to evaluate the methodological quality of the included RCTs. The risk of bias tool focuses on 6 domains: sequence generation, allocation concealment, blinding, incomplete data, selective reporting, and other bias. Two independent investigators will use the risk of bias tool to independently evaluate the quality of RCTs. Disagreements in this procedure will be settled by discussion or be judged by a third reviewer.
2.6. Data synthesis
We will qualitatively summarize included trials, describing direct and indirect comparisons, listing the trial design and characteristics. Before performing meta-analysis, we will assess whether the heterogeneity is significant between trials by using a cut-off point of I2 = 50%. Missing values that cannot be acquired from the authors will be calculated through the available coefficients in reference to the Cochrane handbook.[24] The potential impact of these missing data on the results of the network meta-analysis will be tested in sensitivity analysis.
We will perform conventional pairwise comparisons and calculate the effect sizes and related 95% confidence intervals. We will calculate the effect sizes of continuous data with standardized mean difference (SMD), and categorical data with risk ratio. SMDs are recognized as small, median, and large effect size by using 0.2, 0.5, and 0.8 as cut-off points, respectively.[25] RCTs containing a treatment with zero event will be excluded from the meta-analysis.
We will perform a network meta-analysis to compare CGRP antagonists with BoNT-A by using a frequentist method in netmeta package (R 3.5.0, www.r-project.org). Consistency of the network will be assessed by using the Cochran Q test, and the source of inconsistency will be investigated by a design-by-treatment decomposition method.
Subgroup analysis will be performed to each individual of CGRP antagonists. We will perform meta-regression to determine source of heterogeneity like age, duration of migraine headaches, medication overuse, and types of migraine (with or without aura).
We will perform sensitivity analysis to the factors leading to significant heterogeneity. We will exclude studies with high or unclear risk of bias to check if the results were consistent with the primary analysis; we will also exclude studies with small sample size (n < 100 per group) to check if the results were consistent.
3. Discussion
We hope that we can integrate direct and indirect evidence about the effectiveness of CGRP antagonists and BoNT-A for chronic migraine prophylaxis and provide a ranking by using network meta-analysis. We expect that the results will help the physicians and chronic migraine patients to choose their most appropriate and best method according to their preferences and conditions. Of course, we also hope that the results will be of interest and adoption to the policy makers, so as to the best method would be covered by health insurance.
Author contributions
Conceptualization: Tianwei She, Hui Zheng.
Data curation: Min Chen, Hui Zheng.
Formal analysis: Min Chen, Hui Zheng.
Investigation: Yaoyao Chen, Taichun Tang
Methodology: Tianwei She, Hui Zheng.
Writing – original draft: Tianwei She, Yaoyao Chen, Min Chen, Hui Zheng.
Writing – review & editing: Tianwei She, Yaoyao Chen, Min Chen, Hui Zheng.
Hui Zheng orcid: 0000-0002-0494-1217.
Footnotes
How to cite this article: She T, Chen Y, Tang T, Chen M, Zheng H. Calcitonin gene-related peptide antagonists versus botulinum toxin A for the preventive treatment of chronic migraine protocol of a systematic review and network meta-analysis: a protocol for systematic review. Medicine. 2020;99:5(e18929).
Abbreviations: BoNT-A = botulinum toxin A, CGRP = calcitonin gene-related peptide, MeSH = medical subject headings, RCTs = randomized controlled trials, SMD = standardized mean difference.
TS and YC contributed equally to this work.
This review was supported by the National Natural Science Foundation of China (No. 81774321 and No. 81473777). The sponsor was not involved in the process of developing the protocol.
The authors have no conflicts of interest to disclose.
References
- [1].Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38:1–211. [DOI] [PubMed] [Google Scholar]
- [2].Schwedt TJ. Chronic migraine. BMJ 2014;348:g1416. [DOI] [PubMed] [Google Scholar]
- [3].Natoli JL, Manack A, Dean B, et al. Global prevalence of chronic migraine: a systematic review. Cephalalgia 2010;30:599–609. [DOI] [PubMed] [Google Scholar]
- [4].Herd CP, Tomlinson CL, Rick C, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database Syst Rev 2018;6:CD011616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vo P, Fang J, Bilitou A, et al. Patients’ perspective on the burden of migraine in Europe: a cross-sectional analysis of survey data in France, Germany, Italy, Spain, and the United Kingdom. J Headache Pain 2018;19:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Buse DC, Lipton RB. Global perspectives on the burden of episodic and chronic migraine. Cephalalgia 2013;33:885–90. [DOI] [PubMed] [Google Scholar]
- [7].Wang S-J, Wang P-J, Fuh J-L, et al. Comparisons of disability, quality of life, and resource use between chronic and episodic migraineurs: a clinic-based study in Taiwan. Cephalalgia 2013;33:171–81. [DOI] [PubMed] [Google Scholar]
- [8].Dodick DW. Migraine. Lancet (London, England) 2018;391:1315–30. [DOI] [PubMed] [Google Scholar]
- [9].Escher CM, Paracka L, Dressler D, et al. Botulinum toxin in the management of chronic migraine: clinical evidence and experience. Therap Adv Neurol Disord 2017;10:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Goadsby PJ, Lipton RB, Ferrari MD. Migraine–current understanding and treatment. N Engl J Med 2002;346:257–70. [DOI] [PubMed] [Google Scholar]
- [11].Simpson DM, Hallett M, Ashman EJ, et al. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the guideline development subcommittee of the American Academy of Neurology. Neurology 2016;86:1818–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bendtsen L, Sacco S, Ashina M, et al. Guideline on the use of onabotulinumtoxin A in chronic migraine: a consensus statement from the European Headache Federation. J Headache Pain 2018;19:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Do TP, Hvedstrup J, Schytz HW. Botulinum toxin: a review of the mode of action in migraine. Acta Neurol Scand 2018;137:442–51. [DOI] [PubMed] [Google Scholar]
- [14].Iyengar S, Johnson KW, Ossipov MH, et al. CGRP and the trigeminal system in migraine. Headache 2019;59:659–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Urits I, Jones MR, Gress K, et al. CGRP antagonists for the treatment of chronic migraines: a comprehensive review. Curr Pain Headache Rep 2019;23:29. [DOI] [PubMed] [Google Scholar]
- [16].Reinke T. Aimovig for migraine prevention: the new kid may have trouble fitting in. Manag Care 2018;27:10–1. [PubMed] [Google Scholar]
- [17].Tepper SJ. Anti-calcitonin gene-related peptide (CGRP) therapies: update on a previous review after the American Headache Society 60th Scientific Meeting, San Francisco, June 2018. Headache 2018;58 Suppl 3:276–90. [DOI] [PubMed] [Google Scholar]
- [18].Traynor K. FDA approves licensing of erenumab-aooe to prevent migraine. Am J Health Syst Pharm 2018;75:929–30. [DOI] [PubMed] [Google Scholar]
- [19].Ashina M, Tepper S, Brandes JL, et al. Efficacy and safety of erenumab (AMG334) in chronic migraine patients with prior preventive treatment failure: a subgroup analysis of a randomized, double-blind, placebo-controlled study. Cephalalgia 2018;38:1611–21. [DOI] [PubMed] [Google Scholar]
- [20].Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 2017;16:425–34. [DOI] [PubMed] [Google Scholar]
- [21].Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- [22].Tassorelli C, Diener H-C, Dodick DW, et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia 2018;38:815–32. [DOI] [PubMed] [Google Scholar]
- [23].Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Higgins JP. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011[M]. The Cochrane Collaboration. [Google Scholar]
- [25].Cohen J. A power primer. Psychol Bull 1992;112:155–9. [DOI] [PubMed] [Google Scholar]


