Abstract
To assess the feasibility of using contrast-enhanced spectral mammography (CESM) for operative planning of patients with breast cancers who were initially diagnosed by sonographic guided biopsy.
With the approval of the Institutional Review Board of our hospital, we retrospectively reviewed the data on patients with breast cancers who underwent CESM and contrast-enhanced magnetic resonance imaging (CE-MRI) prior to operation and were followed up for at least 5 years postoperatively. The patients with breast cancer diagnosed by sonographic guided biopsy without mammography were included for analysis. The size and number of cancers on low-energy mammograms (LE-MG), recombined subtracted mammograms (RSM), and CE-MRI were recorded and compared with microscopic histopathologic data and at least 5 years of clinical follow-up data.
Fifty-one cancerous breasts of 46 patients were included in the analysis. All the principal cancers could be detected by RSM or CE-MRI; however, only 45 were by LE-MG. The Pearson correlation coefficients for the size on microscopy were 0.44 for LE-MG, 0.77 for RSM, and 0.84 for CE-MRI (all P-values ≤.001). Regarding the microscopic reports, RSM or CE-MRI had sensitivities of 100% and a positive predictive value of 63.6% for multicentric cancers. One breast cancer with partial mastectomy recurred after 3 years of follow-up.
CESM was feasible for assessing the cancer extension and multicentric cancers as secondary examination in patients with diagnosed breast cancers after sonographic biopsy.
Keywords: breast cancer, magnetic resonance imaging, mammography
Key Points:
CESM was feasible for assessing the patients with multiple suspected breast cancers prior to operation.
RSM or CE-MRI was more sensitive than LE-MG for cancer display.
RSM was comparable to CE-MRI for cancer extension and multicentric cancer.
1. Introduction
Breast cancer is one of the most common fatal malignancies in woman. Preoperative assessment when choosing between total and conservative mastectomy depends on the extent of the cancer and whether the cancer is multicentric. Therefore, a comprehensive preoperative imaging evaluation, such as first line imaging evaluation by mammography and sonography followed by CESM or CE-MRI is essential for patients suspected to have multiple breast cancers.[1] Conservative mastectomy should be considered when cancer could be completely removed. Accurate detection of cancer distribution, such as cancer size or multiplicity, is mandatory. Although no definite cancer size criterion for the choice of conservative mastectomy is available, quadrantectomy should be preferred to lumpectomy or wide excision when the cancer is >3 cm in the greatest diameter.[1] Otherwise, multiplicity of the cancers alters the operative decision for partial or total mastectomy, in which multifocal or multicentric breast cancers have been reported in 30% to 60% of mastectomy specimens.[2,3] The extent of mastectomy thus depends on the availability of precise preoperative information on cancer extent and coexisting multifocal cancer.
Both mammography and sonography are basic modalities for breast examinations. Mammography is a conventional modality of breast evaluation used for screening or clinical diagnosis of breast cancers. However, the detection sensitivity is affected by breast density. Small or isodense cancers may be obscured by superimposition of breast fibroglandular tissues; the false-negative rate is 20% to 30%.[4] The cancer detection sensitivity is only about 50% to 60% in dense breasts because of poor contrast between the cancer and the background.[5] Thus, the under-detection of cancer is possible. Sonography is commonly used for cancer detection, as well as for preoperative evaluation of cancer status due to the advantages of being handheld, convenient, and radiation-free. Particularly in dense breasts, sonography is more sensitive than mammography (by 8.5% for non-dense breasts and 15.9% for dense breasts).[6] However, supplementary sonography to mammography increased the false positive cancer rate by 2.4% and the positive predictive value decreased by 10.3%.[5] The accuracy of sonography for multifocal breast cancers is 83%.[7]
Recently, an emerging imaging technique of contrast-enhanced spectral mammography (CESM) provides both a low energy mammogram (LE-MG) and a recombined subtracted mammogram (RSM) after intravenously administering iodinated contrast medium images in the same session of examination. The computer recombines the dual-energy images (a pair of low and high energy images in the same positioning and breast compression) to balance out or remove the background breast glandular tissue and fat to accentuate tumor enhancement. The mechanism of cancer enhancement secondary to the neo-angiogenesis is similar to CE-MRI.[8–12] The sensitivity has been reported superior to that of mammography or sonography alone[13–18] with high inter-observer consistency between radiologists.[19,20]
The quality of LE-MG is not inferior to a full digital mammogram,[21] and the RSM exploits tumor iodine uptake.[9–13,16] One multi-reader evaluation found that the diagnostic performance of CESM was significantly superior to that of conventional mammography, and comparable to CE-MRI.[22] Other than the obtainable anatomical (mammogram) and physiological images (recombined subtracted image) in the same session of examination, the use of CESM can also solve the problems of CE-MRI, including limited availability, high cost, and resistance to the procedure from claustrophobic patients on clinical practice.[9,10]
Sonography is a convenient method to detect a symptomatic cancer and allows biopsy for pathological diagnosis.[23] In clinical practice, sonographic guided biopsy is often performed to diagnose suspicious mass lesions before ordering mammographic examinations. In order to analyze the feasibility of using CESM in assessing the patients who were initially diagnosed with breast cancers by sonographic guided biopsy without mammography, we retrospectively correlated the CESM and CE-MRI to the surgicopathological findings. We also reviewed the results of 5 years of clinical follow-up.
2. Materials and methods
With the approval of our Institutional Review Board, we retrospectively reviewed the cases of CESM from March 2012 to December 2014 that fulfilled the following criteria: breast cancers initially diagnosed by sonographic guided biopsy without conventional mammography; CE-MRI examination within 1 week after CESM; surgicopathologically proven breast cancers; and at least 5 years of postoperative clinical follow-up. All patients had no history of allergic reaction to iodinated contrast medium and had been checked for normal renal function by serum laboratory. After full communications to the patients, their written consents were obtained prior to the performance of CESM or CE-MRI in accordance to the clinical regulations.
We used a commercial platform (Senographe Essential CESM; GE Healthcare, Buc, France) as the model of CSM, delivering intermittent exposures of low- and high-energy at 1 to 2 seconds intervals during the same session of breast compression. After a single-bolus injection of non-ionic contrast medium (Omnipaque 350 mg/mL; GE Healthcare, Dublin, Ireland; rate of 3 mL/s to a total dose of 1.5 mL/kg body weight) via an intravenous catheter placed in the forearm, the CESM procedure was similar to conventional mammography, consecutively affording craniocaudal (CC) and mediolateral oblique (MLO) views of bilateral breasts within 2 to 6 minutes. All CC views were acquired within 2 to 3 minutes and MLO views within 3 to 6 minutes after contrast medium injection. All patients were requested to hold their breath during exposure to avoid motion artifacts. Noises (non-enhanced anatomical structures) on the low- and high-energy mammograms were immediately eliminated by the computer and the images were then recombined to yield RSM images. The area of hyperdensity indicated the iodine uptake. LE-MG and RSM obtained in a single view were acquired together for reading.
All CE-MRI examinations were performed using a 1.5-Tesla MR scanner (HDx Twin; GE, Milwaukee, WI) with patients in the prone position using a dedicated 8-channel breast coil to evaluate both breasts via standardized pulse sequences: T1-weighted imaging, T2-weighted short-inversion-time inversion-recovery, and dynamic CE-MRI (T1-weighted VIBRANT with a bolus injection of 0.1 mmol/kg gadopentetate dimeglumine [Magnevist; Bayer Schering Pharma, Berlin, Germany]) in the axial projection. The pulse sequence of VIBRANT was TR = 7.3 ms, TE = 3.3 ms, flip angle = 10°, field of view 34 × 34 cm, obtaining post-contrast acquisitions of 6 cycles approximately with 1 min/cycle. The slice thickness was routinely set to 1.5 mm, without an interval gap.
The sizes of principal cancers (the greatest diameters) measured on LE-MG, RSM, and CE-MRI, were individually correlated with sizes on microscopy by Bland-Altman plots and Pearson correlations. In order to obtain cancer sizes at an approximate time after contrast medium injection, the cancer sizes on RSM (CC view) taken 2 to 3 minutes after contrast medium injection and the axial projections of 3 cycles of dynamic CE-MRI were used to calculate the size correlations.
The CC and MLO views of CESM were used to assess cancer multiplicity. A satellite cancer was defined as a tumor in proximity and separated from the main tumor,[24] which was required to be located at least 1 cm from the principal cancer. Multicentric cancer was defined as cancers in >1 quadrant, while multifocal cancer was defined as ≥2 tumors in the same quadrant.[24] When the differentiation of multifocal from multicentric cancer was difficult on images or in excised breast samples, multicentric cancer was considered where the satellite cancer was >4 cm apart from the principal cancers.[25] Two radiologists read the CESM and CE-MRI and reached a consensual agreement. We also reviewed the 5-year clinical follow-up data in terms of local recurrence.
3. Results
3.1. Patient data
In total, 46 women (aged 30–72 years; average age, 49.1 years) with 51 cancerous breasts (41 unilateral breast cancers and 5 bilateral breast cancers) were included in the analysis. Conservative mastectomy was performed for 36 breasts and total mastectomy for 15. The breast cancers were invasive ductal carcinoma in 40, invasive lobular carcinoma in 5, ductal carcinoma in situ in 3, metastatic carcinoma in 2, and a cystic adenoid carcinoma in 1 (Table 1).
Table 1.
Histological diagnoses of the 51 principal cancers in 46 patients.

Of the 51 principal cancers, 45 were detected on LE-MG while 6 (11.76%) were not. However, all were evident on RSM and CE-MRI. The average sizes were 2 cm (range: 0–4.5 cm; 95% CI = –2.513 to 2.353) on LE-MG; 2.43 cm (range: 0.9–5.9 cm; 95% CI = –1.485 to 1.803) on RSM; 2.3 cm (range: 1–4.6 cm; 95% CI = –1.292 to 1.347) on CE-MRI; and 2.27 cm (range: 0.8–5.1 cm) by microscopy. The Pearson correlation coefficients were all statistically significant for microscopic size, including 0.44 for LE-MG, 0.77 for RSM, and 0.84 for CE-MRI (P-values ≤.001, Fig. 1A, B).
Figure 1.
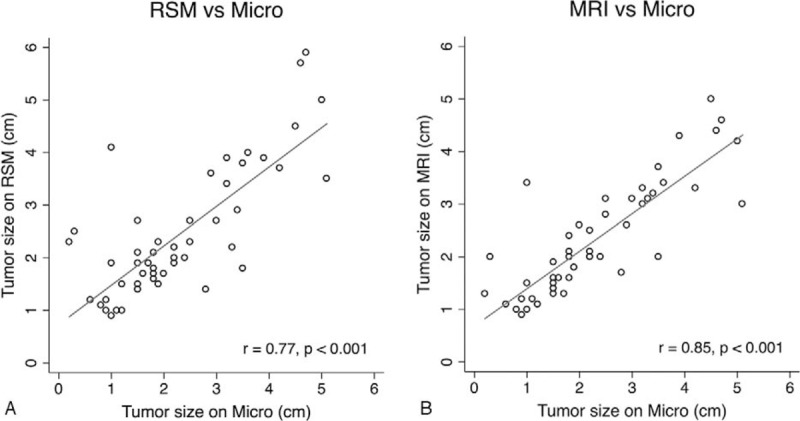
Pearson correlations of index cancer sizes on (A) recombined subtracted mammography (RSM); and (B) CE-MRI versus microscopy (micro). CE-MRI = contrast-enhanced magnetic resonance imaging.
Multifocal cancers were reported in 5 breasts by LE-MG, 9 by RSM, 7 by CE-MRI, and 7 by microscopy; multicentric cancers were seem in 1 breast by LE-MG, 11 by RSM, 11 by CE-MRI, and 7 by microscopy. The above information is summarized in a flow diagram (Fig. 2). All 7 microscopically proven multicentric cancers were correctly diagnosed by RSM or CE-MRI, and only 1 (14.28%) by LE-MG. The positive predictive value of multicentric cancer by RSM and CE-MRI was 7 of 11 cases (63.6%). The false positive lesions (4 of 11 cases, 27.27%) were finally histologically diagnosed as an atypical ductal hyperplasia, a fibroadenoma, an intraductal papilloma (Fig. 3A–C), and a sclerosing adenosis. All the patients were treated and followed-up in our hospital. Only 1 patient developed a local recurrence (Fig. 4A–C). Although abnormal multicentric enhancement was revealed on both RSM and CE-MRI, the patient instead requested conservative mastectomy due to negative sonography in the second visit. It was, thus, a controversial false negative outcome.
Figure 2.
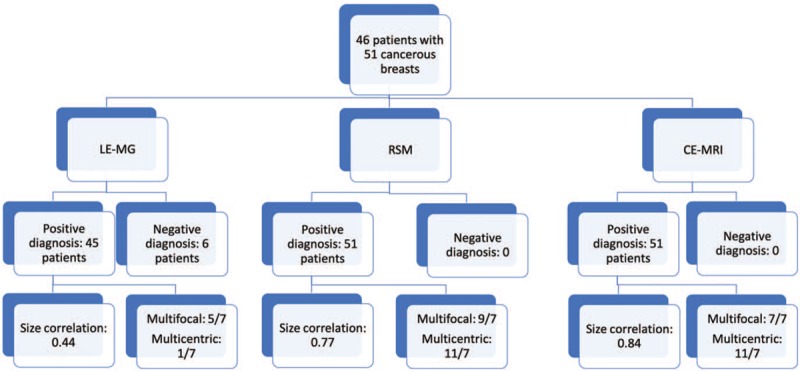
Flow diagram of this study. Demonstrating the diagnostic accuracy comparing low energy mammogram (LE-MG), recombined subtracted mammography (RSM), and contrast-enhanced magnetic resonance imaging (CE-MRI) with microscopy results. The size correlation signifies the Pearson correlation coefficients as compared with microscopy according to size. The multicentric or multifocal diagnosis of cancer is denoted by index study/microscopy reference.
Figure 3.
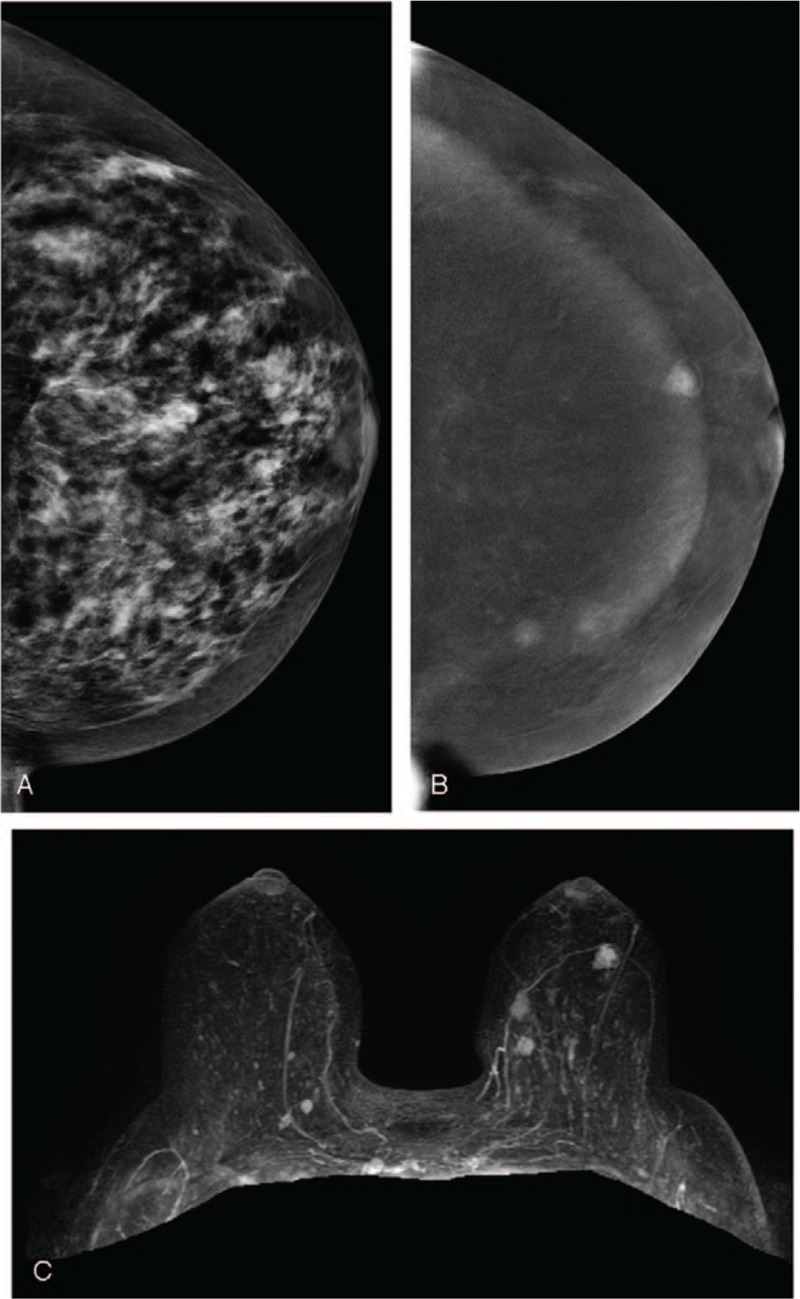
A 50-year-old woman. (A) LE-MG (CC view) showed dense breasts without obvious breast nodules. (B) RSM (CC view) revealed three irregular enhanced masses in the inner region and subareolar region of the left breast. (C) CE-MRI with three-dimensional reconstruction displayed three enhanced masses corresponding to RSM. Finally, pathology confirmed that the two masses at the inner region were cancers and the mass at the subareolar region was a benign papillary tumor. CE-MRI = contrast-enhanced magnetic resonance imaging, LE-MG = low-energy mammography, RSM = recombine subtracted mammography.
Figure 4.
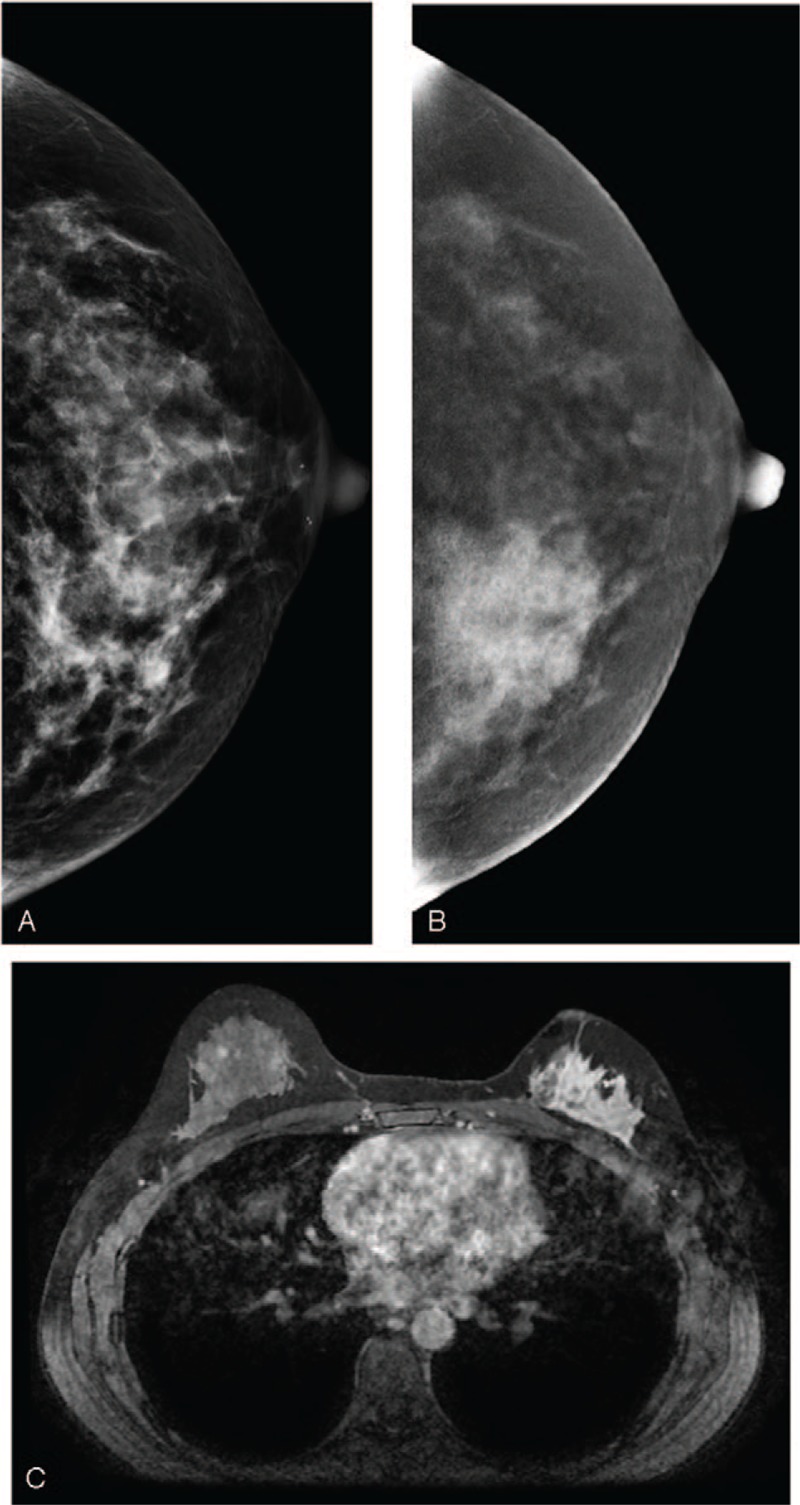
A 38-year-old woman. (A) LE-MG (CC view) showed dense breasts with an irregular hyperdense patch of tissue distortion in the inner region of the left breast. (B) RSM (CC view) revealed a remarkable segmental enhancement in the inner region and multiple nodular enhancement in the outer region of the left breast. The patient requested partial mastectomy due to impalpable, negative sonography, and conventional mammography. The cancer was confirmed to be invasive ductal carcinoma. (C) CE-MRI 3 years after treatment demonstrated a non-mass enhanced recurrent cancer in the outer region of the left breast. CE-MRI = contrast-enhanced magnetic resonance imaging, LE-MG = low-energy mammography, RSM = recombine subtracted mammography.
4. Discussion
When planning the extent of conservative mastectomy, cancer extension (particularly the size of principal cancer) and any additional satellite cancers (multicentric cancers) are of paramount concern. A previous study correlated cancer size to pathology; the Pearson correlations were 0.6 for LE-MG, 0.73 for RSM, and 0.65 for CE-MRI,[9] while another study by Fallenberg et al[22] showed 0.61 for mammography, 0.69 for CESM, and 0.79 for MRI. In our series, the Pearson correlations were 0.44 for LE-MG, 0.77 for RSM, and 0.84 for CE-MRI. The findings from our study were similar to the reported result from Fallenberg et al,[22] which may be due to similarity of study population, such as age (mean age of 49 years in our study and 53 years in Fallenberg study) and the different breast compositions that could influence the Pearson correlations for LE-MG. In contrast, the higher correlations of cancer size for RSM and CE-MRI compared with LE-MG could be explained by enhanced cancers. The better correlation of CE-MRI than RSM could be attributed to the absence of the spreading effect caused by breast compression during CESM.[26,27]
Multicentric breast cancer is the predominant indication for total mastectomy. For the multifocal cancer within the same quadrant of principal cancer, conservative mastectomy can still be planned. However, the presence of multicentric cancer in different quadrants essentially requires more extensive mastectomy. From our study, both RSM and CE-MRI could detect 6 additional cases (11.76%) of principle tumor as compared with LE-MG, and RSM comparatively displayed better cancer extension, including the sizes and multicentricity, to CE-MRI. This could be related to better detection of tumor presence and boundaries for accurate size measurements using contrast-enhanced imaging from both RSM and CE-MRI as opposed to LE-MG.
5. Conclusion
CESM was feasible for assessing the cancer extension and multicentric cancers as a secondary examination in patients with diagnosed breast cancers after sonographic biopsy.
Author contributions
Conceptualization: Yun-Chung Cheung.
Data curation: Yun-Chung Cheung, Yu-Hsiang Juan, Yung-Feng Lo, Yu-Ching Lin, Chih-Hua Yeh, Shir-Hwa Ueng.
Formal analysis: Yun-Chung Cheung, Yu-Hsiang Juan, Yung-Feng Lo, Chih-Hua Yeh, Shir-Hwa Ueng.
Investigation: Yun-Chung Cheung, Yu-Hsiang Juan, Yung-Feng Lo, Yu-Ching Lin.
Methodology: Yun-Chung Cheung, Yu-Hsiang Juan, Yu-Ching Lin.
Project administration: Yun-Chung Cheung.
Supervision: Yun-Chung Cheung, Yung-Feng Lo.
Validation: Yu-Hsiang Juan, Yu-Chin Lin, Chih-Hua Yeh, Shir-Hwa Ueng.
Visualization: Yun-Chung Cheung, Yung-Feng Lo, Yu-Ching Lin.
Writing – original draft: Yun-Chung Cheung.
Writing – review & editing: Yun-Chung Cheung, Yu-Hsiang Juan, Yung-Feng Lo, Yu-Ching Lin, Chih-Hua Yeh, Shir-Hwa Ueng.
Footnotes
Abbreviations: CC = craniocaudal, CE-MRI = contrast-enhanced magnetic resonance imaging, CESM = contrast-enhanced spectral mammography, LE-MG = low-energy mammography, MLO = mediolateral oblique, RSM = recombine subtracted mammography.
How to cite this article: Cheung YC, Juan YH, Lo YF, Lin YC, Yeh CH, Ueng SH. Preoperative assessment of contrast-enhanced spectral mammography of diagnosed breast cancers after sonographic biopsy: correlation to contrast-enhanced magnetic resonance imaging and 5-year postoperative follow-up. Medicine. 2020;99:5(e19024).
The authors have no conflicts of interest to disclose.
References
- [1].Jatoi I, Proschan MA. Randomized trials of breast-conserving therapy versus mastectomy for primary breast cancer: a pooled analysis of updated results. Am J Clin Oncol 2005;28:289–94. [DOI] [PubMed] [Google Scholar]
- [2].Anastassiades O, Iakovou E, Stavridou N, et al. Multicentricity in breast cancer. A study of 366 cases. Am J Clin Pathol 1993;99:238–43. [DOI] [PubMed] [Google Scholar]
- [3].Vaidya JS, Vyas JJ, Chinoy RF, et al. Multicentricity of breast cancer: whole-organ analysis and clinical implications. Br J Cancer 1996;74:820–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med 2005;353:1773–83. [DOI] [PubMed] [Google Scholar]
- [5].Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology 2002;225:165–75. [DOI] [PubMed] [Google Scholar]
- [6].Schaefer FK, Waldmann A, Katalinic A, et al. Influence of additional breast ultrasound on cancer detection in a cohort study for quality assurance in breast diagnosis–analysis of 102,577 diagnostic procedures. Eur Radiol 2010;20:1085–92. [DOI] [PubMed] [Google Scholar]
- [7].Park JM, Yoon GS, Kim SM, et al. Sonographic detection of multifocality in breast carcinoma. J Clin Ultrasound 2003;31:293–8. [DOI] [PubMed] [Google Scholar]
- [8].Patel BK, Lobbes MBI, Lewin J. Contrast enhanced spectral mammography: a review. Semin Ultrasound CT MR 2018;39:70–9. [DOI] [PubMed] [Google Scholar]
- [9].Fallenberg EM, Dromain C, Diekmann F, et al. Contrast-enhanced spectral mammography versus MRI: Initial results in the detection of breast cancer and assessment of tumour size. Eur Radiol 2014;24:256–64. [DOI] [PubMed] [Google Scholar]
- [10].Jochelson MS, Dershaw DD, Sung JS, et al. Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology 2013;266:743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lee-Felker SA, Tekchandani L, Thomas M, et al. Newly diagnosed breast cancer: comparison of contrast-enhanced spectral mammography and breast mr imaging in the evaluation of extent of disease. Radiology 2017;285:389–400. [DOI] [PubMed] [Google Scholar]
- [12].Wang Q, Li K, Wang L, et al. Preclinical study of diagnostic performances of contrast-enhanced spectral mammography versus MRI for breast diseases in China. Springerplus 2016;5:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dromain C, Thibault F, Muller S, et al. Dual-energy contrast-enhanced digital mammography: initial clinical results. Eur Radiol 2011;21:565–74. [DOI] [PubMed] [Google Scholar]
- [14].Luczynska E, Heinze S, Adamczyk A, et al. Comparison of the mammography, contrast-enhanced spectral mammography and ultrasonography in a group of 116 patients. Anticancer Res 2016;36:4359–66. [PubMed] [Google Scholar]
- [15].Tagliafico AS, Bignotti B, Rossi F, et al. Diagnostic performance of contrast-enhanced spectral mammography: systematic review and meta-analysis. Breast 2016;28:13–9. [DOI] [PubMed] [Google Scholar]
- [16].Tennant SL, James JJ, Cornford EJ, et al. Contrast-enhanced spectral mammography improves diagnostic accuracy in the symptomatic setting. Clin Radiol 2016;71:1148–55. [DOI] [PubMed] [Google Scholar]
- [17].Mori M, Akashi-Tanaka S, Suzuki S, et al. Diagnostic accuracy of contrast-enhanced spectral mammography in comparison to conventional full-field digital mammography in a population of women with dense breasts. Breast Cancer 2017;24:104–10. [DOI] [PubMed] [Google Scholar]
- [18].Diekmann F, Freyer M, Diekmann S, et al. Evaluation of contrast-enhanced digital mammography. Eur J Radiol 2011;78:112–21. [DOI] [PubMed] [Google Scholar]
- [19].Cheung YC, Lin YC, Wan YL, et al. Diagnostic performance of dual-energy contrast-enhanced subtracted mammography in dense breasts compared to mammography alone: interobserver blind-reading analysis. Eur Radiol 2014;24:2394–403. [DOI] [PubMed] [Google Scholar]
- [20].Dromain C, Thibault F, Diekmann F, et al. Dual-energy contrast-enhanced digital mammography: initial clinical results of a multireader, multicase study. Breast Cancer Res 2012;14:R94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lalji UC, Jeukens CR, Houben I, et al. Evaluation of low-energy contrast-enhanced spectral mammography images by comparing them to full-field digital mammography using EUREF image quality criteria. Eur Radiol 2015;25:2813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fallenberg EM, Schmitzberger FF, Amer H, et al. Contrast-enhanced spectral mammography vs. mammography and MRI - clinical performance in a multi-reader evaluation. Eur Radiol 2017;27:2752–64. [DOI] [PubMed] [Google Scholar]
- [23].Brennan SB, D’Alessio D, Kaplan J, et al. Positive predictive value of biopsy of palpable masses following mastectomy. Breast J 2018;24:789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Giuliano AE, Connolly JL, Edge SB, et al. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:290–303. [DOI] [PubMed] [Google Scholar]
- [25].Bozzini A, Renne G, Meneghetti L, et al. Sensitivity of imaging for multifocal-multicentric breast carcinoma. BMC Cancer 2008;8:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Iotti V, Ravaioli S, Vacondio R, et al. Contrast-enhanced spectral mammography in neoadjuvant chemotherapy monitoring: a comparison with breast magnetic resonance imaging. Breast Cancer Res 2017;19:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Holland K, Sechopoulos I, Mann RM, et al. Influence of breast compression pressure on the performance of population-based mammography screening. Breast Cancer Res 2017;19:126. [DOI] [PMC free article] [PubMed] [Google Scholar]


