Abstract
Objective:
To compare the effects of letrozole and human menopausal gonadotropin (HMG) in the treatment of patients with polycystic ovary syndrome (PCOS) resistant to clomiphene citrate (CC).
Methods:
A total of 96 clomiphene resistance polycystic ovary syndrome patients infertility were randomly divided into an LE group, and HMG group (n = 48). LE group orally received letrozole at 5.0 mg/d−1 on the 3rd–5th days of menstrual cycle for 5 consecutive days, and 75 U/d−1 HMG was given through intramuscular injection for 5 days starting from the third day of menstrual cycle in HMG group. Number of growing and mature follicles, serum E2 (pg/mL), serum P (ng/mL), endometrial thickness, occurrence of pregnancy and miscarriage were observed.
Results:
There was no significant difference in the number of ovulation cycles between the 2 groups (53.6% vs 64.7%, P > .05). The number of mature follicular cycles in the HMG group was higher than that of the letrozole group (P < .01). There were no significant differences in the clinical pregnancy rate (22.9% vs 27.1%, P > .05) and abortion rate (6.2% vs 10.4%, P > .05). There was no significant difference in the endometrial thickness between the 2 groups on the day of HCG injection [(9.1 ± 0.2) mm vs (10.7 ± 1.6) mm, P > .05]; the serum estradiol (E2) was lower in the letrozole group. The incidence of ovarian cysts was lower than that of HMG group (P < .05). There was2 ovarian hyperstimulation syndrome in the letrozole group; the incidence of ovarian hyperstimulation syndrome in the HMG group was 12.5%.
Conclusion:
Letrozole-induced ovulation can obtain ovulation rate and pregnancy rate similar to gonadotropin, but reduce the risk associated with treatment. It can be used as an effective ovulation option for patients with polycystic ovary syndrome who are resistant to clomiphene.
Keywords: aromatase inhibitor, clomiphene resistance, gonadotropin, induced ovulation, polycystic ovary syndrome
1. Introduction
Polycystic ovary syndrome (PCOS) is a common most common endocrine disorders for women of childbearing age, and induced to an ovulatory infertility. The traditional therapy took clomiphene citrate to induce ovulation in this condition. However, it has a certain impact on the endometrium and cervical mucus, and in some condition, it exist clomiphene resistance, as a result of failing to include ovulation.[1]
In recent years, Aromatase inhibitors (AIs), such as letrozole or anastrozole, have been introduced for treatment of PCOS women with CC-resistant anovulation. It has been postulated that blocking estrogen production by inhibiting aromatization in the ovary would release the hypothalamic-pituitary axis from estrogenic negative feedback. As a result, FSH secretion increases, stimulating the development of ovarian follicles, while reducing the gonadotropin-induced ovulation complication.[2] Human menopausal gonadotropin (HMG), which contains follicle stimulating hormone (FSH) and luteinizing hormone (LH), can secrete gonadotropin to promote follicle maturation, so as to stimulate ovulation and to accelerate the development of corpus luteum.[3,4]
Preliminary studies have reported that aromatase inhibitors and HMG were useful for inducing ovulation and in superovulation.[2,5,6] So far, there have been no studies comparing the effects of letrozole and HMG in the treatment of patients with polycystic ovary syndrome (PCOS) resistant to clomiphene citrate. In this study, 138 patients with PCOS resistant to CC who were admitted in our hospital were selected and given different drug therapies, aiming to compare the effects of letrozole and HMGon the ovulation induction and pregnancy rate of patients with polycystic ovary syndrome (PCOS) resistant to clomiphene citrate.
2. Materials and methods
2.1. Study design
The study recruited 96 women with clomiphene resistant PCOS among those attending the gynecology outpatient clinic in The Fifth Affiliated Hospital, Sun Yat-Sen University, China, setting in the period from December 2015 to December 2018. The diagnosis of PCOS based on the revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome.[7,8] At least two of the following three were met:
-
1)
ovulation abnormality (sporadic ovulation or no ovulation) occurred after continuous monitoring for two or more natural cycles;
-
2)
the results of B ultrasound showed polycystic ovary;
-
3)
patients had hyperandrogenism or showed clinical manifestations of androgen excess, and those with androgen excess caused by other diseases such as adrenal hyperplasia, Cushing syndrome and androgen-secreting tumors were excluded.
This study has been approved by the ethics committee of our hospital, and written consent has been obtained from all patients. Through salpingography or hydrotubation under transvaginal B ultrasound and other examinations, all cases were confirmed to have tubal patency on at least one side. The semen of male was normal.
Exclusion criteria:
-
1)
Infertility patients caused bynon-PCOS ovulatory disorder or other factors;
-
2)
patients with history of ovarian surgery or complication with endometriosis or pelvic adhesion;
-
3)
patients complicated with liver, kidney or thyroid dysfunction;
-
4)
patients who did not receive treatment after enrollment according to the established regimen or gave up in the midst of treatment.
The hormone (e.g., FSH, LH, estradiol (E2), testosterone (T), insulin and prolactin) levels in the venous blood of all patients were detected on the 2nd–4th days of menstruation. The patients with normal hormone levels were directly enrolled, and those with abnormalities were enrolled after neuroendocrine treatment. Patients were then randomly allocated using a computer-generated random table into two treatment groups: LE group and HMG group (n = 48). The study was approved by the hospital Research Ethics Committee, and all participants gave informed consent before inclusion in the trial.
2.2. Treatment
2.2.1. LE group
The patients orally took 5.0 mg/d−1 LE (trade name: Fu Rui, Gudangdong Hengrui Medicine Co., Ltd.) on the 3rd–7th days of menstrual cycle for 5 consecutive days.
2.2.2. HMG group
The patients received intramuscular injection of HMG (trade name: Lebaode, Livzon Group Livzon Pharmaceutical Co., Ltd.) 75 U/d−1 on the 3rd–7th days of menstrual cycle for 5 consecutive days.
2.3. Assessment of clinical efficacy
The 2 groups were expected to receive the treatment of ovulation induction for 4 to 6 cycles, and patients with poor outcomes were treated by assisted reproductive technologies.
Treatment monitoring: Starting from the 10th day of menstruation, the growth conditions of follicles and endometrium in the patients were monitored once every other day by transvaginal B ultrasound, and then daily when the average diameter of the follicles was ≥16 mm. When the average diameter of follicles was ≥18 mm, the endometrial thickness, number of mature follicles and diameter of the largest follicle were recorded, and 5000 to 10,000 U of human chorionic gonadotropin (HCG) was injected to induce ovulation. On the same day, the venous blood of patients was drawn to examine the LH, E2 and T levels, who were then guided to have sexual intercourse within 24 hour. B ultrasound examination was performed again 48 hour after HCG injection to observe follicle rupture and single follicle ovulation. If fetal heart beat was visible under transvaginal ultrasound on the 30th day after ovulation, the patients were diagnosed as clinical pregnancy.
The primary outcome measures were number of growing and mature follicles, serum E2 (pg/mL), serum P (ng/mL), and endometrial thickness (mm). Secondary outcome measures were the occurrence of pregnancy, ovarian hyperstimulation syndrome (OHSS) and miscarriage.
2.4. Statistical analysis
All data were analyzed by SPSS17.0. The categorical data were expressed as mean ± standard deviation. Two groups were compared by analysis of variance, and intergroup comparisons were performed by the SNK method. The numerical data were subjected to χ2 test for R × C table. When the theoretical frequency of at least one cell was lower than the expected value, the Fisher exact test was used. P value of ≤ .05 was considered statistically significant. SPSS 22.0 software (SPSS Inc. Chicago, IL) was used for the statistical analysis.
3. Result
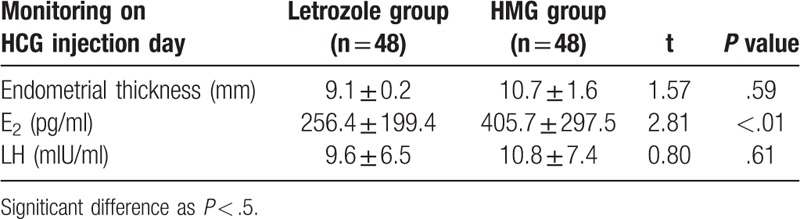
There were no statistical significant differences between the 2 groups regarding age, body weight, height, body mass index (BMI), or presenting symptoms and signs (Table 1). The letrozole group has 125 mature follicles, and there are 119 mature follicles in the HMG group. The result of ≥2 mature follicular cycle numbers in the letrozole group is 13 (10.4%), HMG group was 30 (25.2%), the difference between the 2 groups Statistically significant (P < .01), it was shown that letrozole ovulation induction was easier to obtain a single mature follicle (Table 2). Table 3 shows that the endometrial thickness and lh were measured on the day of HCG injection. There was no significant difference between the two groups (P > .05) (Table 3). The serum E2 concentration in HMG group was significantly higher than that in the letrozole group, the difference was statistically significant (P < .01).
Table 1.
Clinical characteristics of CC-resistant PCOS patients in letrozole group and in HMG group.
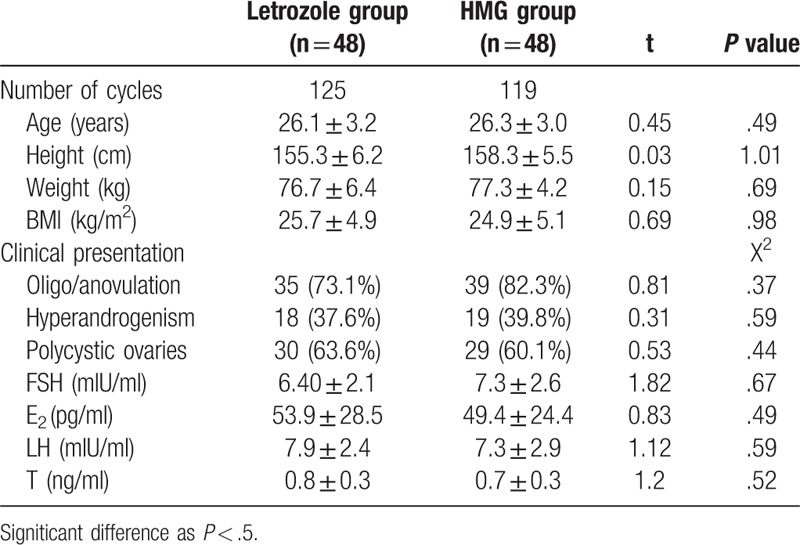
Table 2.
Effects of different regimes on ovulation induction.
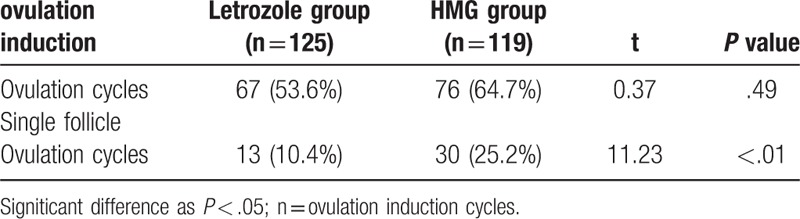
Table 3.
Endometrial thickness, E2 or LH at Day of HCG administration in 2 groups.
The incidence of ovarian cysts and the incidence of OHSS in the letrozole group were lower than those in HMG group, and the difference was statistically significant (P < .05). The cycle pregnancy rate of the 2 groups was similar (P > .05), and the ratio of abortion in the letrozole group was lower (2%) than HMG group, but the difference was not statistically significant (P > .05). The multiple pregnancy rate in HMG group was significantly higher than that in the letrozole group, and the difference was statistically significant (P < .05). The live birth of the 2 groups was similar (P > .05). The results are shown in Table 4. The pregnancy rate over period of cycles is shown in Figure 1.
Table 4.
Effects of different regimens on pregnancy.
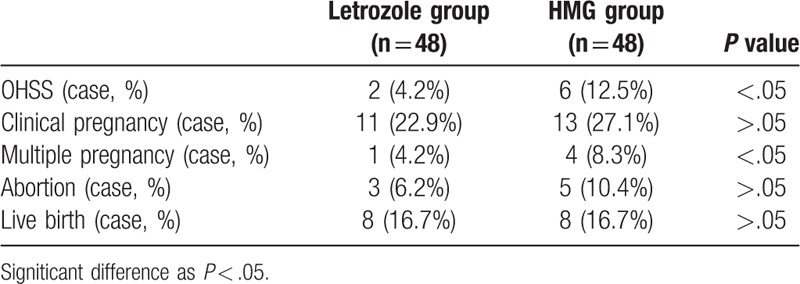
Figure 1.
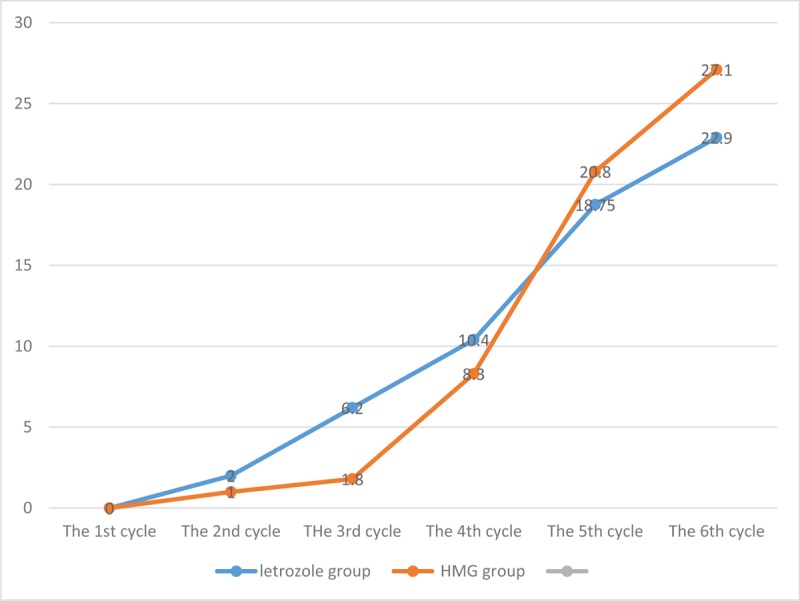
The pregnancy rate over period of cycles.
4. Discussion
Currently, CC and LE are used to treat PCOS. CC remains the first-line drug for ovulation induction, with ovulation rates of 75% to 80%.[9] Cc is similar in structure to estrogen. By blocking the estrogen receptor in the hypothalamus, it blocks the negative feedback of circulating estrogen on the hypothalamic-pituitary axis, and promotes the release of FSH from the pituitary gland to induce follicular development. However, Cc also has peripheral antiestrogen effects, such as endometrium and cervix, which partly explains the contradiction of high ovulation rate and low pregnancy rate and high abortion rate. There are about 20% to 25% of patients Cc resistance causes ovulation treatment failure.[10] For this group of patients, the traditional choice is laparoscopic ovarian surgery and administration of gonadotropins such as HMG, recombinant follicle stimulating hormone and other drugs to promote ovulation.[11,12] However, both of these options have problems of high price and high risk, especially gonadotropin-induced ovulation may lead to excessive ovarian stimulation and multiple pregnancy.
In accordance with the this study, we took into account the effect and avoid adverse reactions, using 5 mg d1≥ 2 mature follicular cycle numbers in the letrozole group is 13 (10.4%), HMG group was 30 (25.2%), the difference between the two groups Statistically significant (P < .01), it was shown that letrozole ovulation induction was easier to obtain a single mature follicle; the total pregnancy rate reached 23 (25%), and the abortion rate was 7 (7.2%), there was no statistically significant difference in ovulation rate, pregnancy rate, and abortion rate compared with HMG group, moreover, The incidence of ovarian cysts and the incidence of OHSS in the letrozole group were lower than those in the HMG group, confirming that letrozole ovulation induction therapy can achieve similar therapeutic effects as gonadotropin. Ganesh et al[13] conducted a large randomized, single-blind clinical trial comparing the effects of letrozole, CC with recombinant FSH, and recombinant FSH alone in the treatment of CC-resistant PCOS patients with letrozole 5 mg/d, obtaining ovulation rate 79.3% (295/372), the cycle pregnancy rate was 23.39% (87/372), The ovulation rate was better in the letrozole group than in the CC-recombinant FSH, but in the single-use recombinant FSH group. There was no significant difference in pregnancy rate and abortion rate between the 2 groups.
One clear weakness of our current report is the small number of enrolled patients, which is explained by our choice of strict inclusion /exclusion criteria and rigid procedure. On the other hand, although our results are promising, limited by the self-control designed study, particularly, observation period wasn’t long. These interpretations prompted the need for a larger, perspective cohort study to evaluate the efficacy of letrozole and human menopausal gonadotropin (HMG) in the treatment of patients with polycystic ovary syndrome (PCOS) resistant to clomiphene citrate (CC).
In summary, the regimen using LE had a satisfactory effect on ovulation, medication cycle and clinical pregnancy rate, which provides a promising option for the treatment of patients with PCOS resistant to clomiphene citrate. Because the pregnancy rate is related to the number of antral follicles, anti-Mullerian hormone, estrogen levels, and patient selection, patients with polycystic ovary syndrome have significant heterogeneity, and a fixed program is difficult. Furthermore, A large sample-size and multi-center research is required to confirm the application value of the regimen in patients with PCOS resistant to clomiphene citrate.
Acknowledgments
The authors thank all patients who participated in this study.
Author contributions
Investigation: Fang Fang Jiang.
Methodology: Yuan Zhuang, Le Chen.
Resources: Ting Hong.
Supervision: Xiao Ling Huang.
Writing – original draft: Shaoquan Shi.
Writing – review & editing: Shaoquan Shi.
Footnotes
Abbreviations: BMI = body mass index, CC = clomiphene citrate, E2 = estradiol, FSH = follicle stimulating hormone, HMG = human menopausal gonadotropin, LH = luteinizing hormone, OHSS = ovarian hyperstimulation syndrome, PCOS = polycystic ovary syndrome.
How to cite this article: Shi S, Hong T, Jiang F, Zhuang Y, Chen L, Huang X. Letrozole and human menopausal gonadotropin for ovulation induction in clomiphene resistance polycystic ovary syndrome patients: A randomized controlled study. Medicine. 2020;99:4(e18383).
SS and TH contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Azziz R, Woods KS, Reyna R, et al. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004;89:2745–9. [DOI] [PubMed] [Google Scholar]
- [2].Badawy A, Mosbah A, Tharwat A, et al. Extended letrozole therapy for ovulation induction in clomiphene-resistant women with polycystic ovary syndrome: a novel protocol. Fertil Steril 2009;92:200–39. [DOI] [PubMed] [Google Scholar]
- [3].Xiang X, Shankun L, Yongkang Y, et al. Use of letrozole and clomiphene citrate combined with gonadotropins in clomiphene-resistant infertile women with polycystic ovary syndrome: a prospective study. Drug Des Dev Therap 2015;6001–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bo HY, Chon SJ, Park JH, et al. Minimal stimulation using gonadotropin combined with clomiphene citrate or letrozole for intrauterine insemination. Yonsei Med J 2015;56:490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Badawy AM, Metwally M, Fawzy M. Randomized controlled trial of three doses of letrozole for ovulation induction in patients with unexplained infertility. Reprod Biomed Online 2007;14:559–62. [DOI] [PubMed] [Google Scholar]
- [6].Zhihua C, Mengzhen Z, Yuhuan Q, et al. Effects of letrozole in combination with low-dose intramuscularinjection of human menopausal gonadotropin on ovulation and pregnancy of 156 patients with polycystic ovary syndrome. Pak J Med Sci 2016;32:1434–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Group ES PCW. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Hum Reprod 2004;81:19–25. [DOI] [PubMed] [Google Scholar]
- [8].Kousta E, Tolis G, Franks S. Polycystic ovary syndrome. Revised diagnostic criteria and long-term health consequences. Hormones 2005;4:133–6. [DOI] [PubMed] [Google Scholar]
- [9].Budinetz TH, Benadiva CA, Griffin DW, et al. Ovulation rate and cycle characteristics in a subsequent clomiphene citrate cycle after stair-step protocol. Fertil Steril 2015;103:675–9. [DOI] [PubMed] [Google Scholar]
- [10].Elnashar A, Fouad H, Eldosoky M, et al. Letrozole induction of ovulation in women with clomiphene citrate-resistant polycystic ovary syndrome may not depend on the period of infertility, the body mass index, or the luteinizing hormone/follicle-stimulating hormone ratio. Digest of the World Core Medical Journals (Obstetrics/Gynecology) 2006;85:511–3. [DOI] [PubMed] [Google Scholar]
- [11].Fauser BC, Tarlatzis BC, Rebar RW, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril 2012;97: 28-38.e25. [DOI] [PubMed] [Google Scholar]
- [12].Badawy A, Mosbah A, Tharwat A, et al. Extended letrozole therapy for ovulation induction in clomiphene-resistant women with polycystic ovary syndrome: a novel protocol. Fertil Steril 2009;92:0–239. [DOI] [PubMed] [Google Scholar]
- [13].Ganesh A, Goswami SK, Chattopadhyay R, et al. Comparison of letrozole with continuous gonadotropins and clomiphene-gonadotropin combination for ovulation induction in 1387 PCOS women after clomiphene citrate failure: a randomized prospective clinical trial. J Assist Reprod Genet 2009;26:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]


