Abstract
Background:
Patients with cancer are of a high level risk of venous thromboembolism (VTE). Low molecular weight heparin (LMWH) is recommended as the normal treatment for cancer-associated venous thrombosis. Recently, some studies suggest that patients with cancer-associated venous thrombosis can get a good efficacy and safety profile from treating with direct oral anticoagulants (DOACs) compared with other anticoagulants. However, when it comes to the efficacy of DAOCs in preventing VTE in patient with cancer, the data are limited. Thus, we performed such a meta-analysis to determine the efficacy and safety of DOACs in preventing VTE in patient with cancer compared with LMWHs.
Methods:
Medline/PubMed and CENTRAL (The Cochrane Central Register of Controlled Trials) were systematically searched for relevant studies. For each trial, data on VTE, major bleeding, or bleeding were extracted by 2 reviewers independently. Pooled risk ratios (RRs) were calculated by using Review Manager 5.3 software and the significance was determined by the Z test.
Results:
A total of 6 studies with 7185 patients were included in our meta-analysis. DOACs (RR = 0.55, 95% confidence interval [95%CI]: 0.34–0.90, I2 = 31%) had a similar prevention effect of VTE to LMWH (RR = 0.59, 95% CI: 0.37–0.95, I2 = 59%). DOACs (RR = 1.52, 95% CI: 0.99–2.33, I2 = 0%) yielded a similar bleeding occurrence rate compared with LMWH (RR = 1.35, 95% CI: 1.07–1.70, I2 = 35%). DOACs (RR = 1.95, 95% CI: 0.88–4.30, I2 = 0%) showed a sight higher major bleeding occurrence rate than LMWH (RR = 1.38, 95% CI: 0.88–2.14, I2 = 0%).
Conclusion:
DOACs show comparable efficacy to LMWH in cancer patients without VTE with a slightly higher major bleeding occurrence rate. DOACs are inclined to be an alternative thromboprophylaxis strategy in cancer patients as they have superiorities compared to traditional anticoagulation agents. Further studies are still demanded as exiting relevant researches are limited.
Keywords: oral anticoagulants, low molecular weight heparin, cancer, thrombosis, prevention
1. Introduction
It is well-known that cancer and its treatments are risk factors for venous thromboembolism (VTE), including deep-vein thrombosis (DVT) and pulmonary embolism (PE). Compared with the general population, the risk of VTE increased by 7-times and the risk is inclined to be increased further in patient with advanced stage of malignancy.[1,2] Furthermore, the risk is considerably associated with racial and gender differences of the patient, the histologic type and primary site of the cancer, duration of its treatment (e.g., chemotherapy and antiangiogenic agents).[3–5]
Low molecular weight heparin (LMWH) has been the primary treatment for VTE in patient diagnosed with cancer for many years. One classical randomized clinical trial conducted by Lee and Levine demonstrated a relative ratio (RR) reduction of recurrent VTE in patients treated with dalteparin without increasing the risk of bleeding compared with oral anticoagulants.[6] Then some guidelines recommended LMWHs as the preferred treatment for VTE in patient with malignancy.[7,8]
In recent years, the direct oral anticoagulants (DOACs), including the factor IIa (thrombin) inhibitor dabigatran and the factor Xa inhibitors (apixaban, rivaroxaban, and edoxaban), have been developed and are recommended to be applied in VTE patients without cancer.[8] Also, these new oral agents have been investigated for use in cancer patients and were attractive in these population, as they are utilized in a fixed dose and do not need to be adjusted for continuous laboratory monitoring index (international normalized ratio).[9] In the NCCN guideline, edoxaban and rivaroxban are advised to be preferred choices for patients with cancer who diagnosed with VTE.[10]
However, existing studies on the efficacy and safety profile of DOACs in preventing VTE in cancer patients without VTE are limited and some of their research conclusions lack consistency due to heterogeneity. Therefore, we conducted such a meta-analysis to compare DOACs vs LMWHs in preventing VTE in patient with cancer.
2. Methods
We performed the meta-analysis in agreement with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement.[11] An ethical approval is not necessary in our meta-analysis.
2.1. Literature search and eligibility
Databases including Medline/PubMed and CENTRAL (The Cochrane Central Register of Controlled Trials) were systematically searched up until March 2019 for relevant papers. Search terms included: oral anticoagulants, rivaroxaban, apixaban endoxaban, low-molecular-weight-heparin, enoxaparin, dalteparin, tinzaparin, pulmonary embolism, deep vein thrombosis, venous thromboembolism, cancer. Dabigatran was removed from the search terms as its mechanism is different from other DOACs and the search results were limited to clinical trials. Also, we further reviewed the references of included studies and recently published reviews for relevant studies.
The abstract of studies generated by above searching strategy were independently screened by 2 reviewers (HC and JY), then they retrieved relevant studies and determined which ones were eligible. Included studies had to fit in following criteria: randomized controlled trials (RCTs); cancer patients without a concurrent VTE; treatments comparing LMWH or DOACs with placebo or no intervention; and outcomes: VTE (DVT or PE), bleeding, major bleeding. Case reports, review articles, guidelines, and textbook chapters were excluded from our analysis.
2.2. Data extraction and quality assessment
Two reviewers independently reviewed the full-text articles of relevant studies and extracted interested data. Any discrepancies were resolved through discussion with a 3rd reviewer to come to an agreement. The following data were extracted from each included study: publication information; the sample size of experimental group and control group; intervention (type of anticoagulants, doses, and duration of anticoagulation treatment).
The quality of the included studies was assessed by using the Jadad score which evaluates the study quality based on the randomization, binding, and description of study withdrawals to acquire a score of 0 to 5.[12] Studies were regarded to be of high quality if the Jadad score was ≥3 and of low quality if the score was ≤2.
2.3. Statistical analysis
The occurrence of VTE, bleeding, and major bleeding for the different anticoagulation treatment was used to calculate a separate pooled RR for each included study. The amount of statistical heterogeneity was quantified by the I2 statistic. I2 of more than 25%, 50%, and 75% were associated with low heterogeneity, moderate heterogeneity, and high heterogeneity, respectively. A fixed-effects model was used if I2 < 50% or a random-effects if I2 > 50%. The Z test was used to determine the significance of the pooled RR. A funnel plot was used to assess publication bias statistically and statistically significant was considered if a P-value <.05, while the Review Manager 5.3 software was used for meta-analysis.
3. Results
3.1. Study characteristics
The initial literature search yielded a total of 492 trials, from which 114 removed due to duplication. Another 355 articles were excluded after title and abstract screening because they were not in accordance with our inclusion criteria. After evaluating full-text of remaining articles, 17 trials were excluded for following reasons: extension of our included articles; not including the outcomes of our research; retrospective articles; others (limited sample size or not placebo/no intervention control trials). Finally, 6 eligible RCTs of 7185 patients with cancer were included in the meta-analysis.[13–18] The evaluation of the articles is shown in the flow diagram (Fig. 1). Table 1 shows some important details of the studies included in our meta-analysis. The patients of all included studies had a different type of malignancy. Two trials evaluated the use of DOACs in cancer patients without VTE.[17,18] And 4 trials evaluated the use of LMWH in these patients.[13–16] The concomitant treatments contained chemotherapy, radiotherapy, surgery, or hormone therapy.
Figure 1.

Flow diagram of article selection.
Table 1.
Characteristics of studies included in the meta-analysis.

3.2. VTE occurrence
All included trials were available to examine the efficacy of DOACs or LMWH on prevention VTE occurrence in patients with cancer. A pool analysis showed that low statistical heterogeneity was found for this outcome. According to the random-effects model, the use of anticoagulants significantly reduced the VTE occurrence rate (RR = 0.57, 95% confidence interval [95% CI]: 0.41–0.78, I2 = 43%) (Fig. 2).
Figure 2.

Forest plot showing the effect of anticoagulant treatment on venous thromboembolism occurrence in patients with cancer. CI = confidence interval, LMWH = low molecular weight heparin; NOAC = novel oral anticoagulant.
For prevention of VTE occurrence in subgroup analysis, DOACs (RR = 0.55, 95% CI: 0.34–0.90, I2 = 31%) showed a similar prevention effect compared with LMWH (RR = 0.59, 95% CI: 0.37–0.95, I2 = 59%). Totally, these trials suggested that 37 of 708 patients experienced VTE in the DOAC group vs 65 of 696 patients in the control group; 70 of 3063 patients experienced VTE in the LMWH group vs 114 of 2685 patients in the control group. And the funnel plot for VTE occurrence showed no evidence of publication bias (Fig. 3).
Figure 3.

Funnel plot for venous thromboembolism occurrence. LMWH = low molecular weight heparin, NOAC = novel oral anticoagulant, RR = risk ratio.
3.3. Bleeding occurrence
All the trials assessed bleeding (including major bleeding and clinically relevant nonmajor bleeding) due to anticoagulant treatment in patients with cancer. A pool analysis showed that no significant statistical heterogeneity was found for this outcome. According to the fixed-effects model, the use of anticoagulants slightly increased the bleeding occurrence rate (RR = 1.39, 95% CI: 1.13–1.70, I2 = 0%) (Fig. 4).
Figure 4.

Forest plot showing the effect of anticoagulant treatment on bleeding occurrence in patients with cancer. CI = confidence interval, LMWH = low molecular weight heparin.
For bleeding occurrence in subgroup analysis, DOACs (RR = 1.52, 95% CI: 0.99–2.33, I2 = 0%) showed a similar bleeding occurrence rate compared with LMWH (RR = 1.35, 95% CI: 1.07–1.70, I2 = 35%). Totally, these trials suggested that bleeding occurred in 50 of 693 patients in the DOACs group vs 32 of 679 patients in the control group; bleeding occurred in 176 of 3049 patients in the LMWH group vs 106 of 2674 patients in the control group. And the funnel plot for VTE occurrence showed no evidence of publication bias (Fig. 5).
Figure 5.

Funnel plot for bleeding occurrence. LMWH = low molecular weight heparin, NOAC = novel oral anticoagulant, RR = risk ratio.
3.4. Major bleeding occurrence
Major bleeding occurrence was assessed in all the included trials to evaluate the safety of thromboprophylaxis of VTE in cancer patients. A pool analysis showed that no significant statistical heterogeneity was found for this outcome. According to the fixed-effects model, the use of anticoagulants increased the major bleeding occurrence rate (RR = 1.50, 95% CI: 1.02–2.21, I2 = 0%) (Fig. 6).
Figure 6.
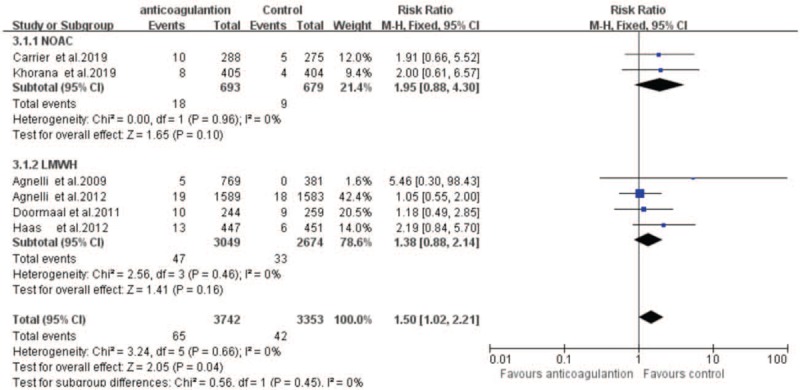
Forest plot showing the effect of anticoagulant treatment on major bleeding occurrence in patients with cancer. CI = confidence interval, LMWH = low molecular weight heparin, NOAC = novel oral anticoagulant.
For major bleeding occurrence in subgroup analysis, DOACs (RR = 1.95, 95% CI: 0.88–4.30, I2 = 0%) showed a higher bleeding occurrence rate than LMWH (RR = 1.38, 95% CI: 0.88–2.14, I2 = 0%). Totally, these studies suggested that major bleeding occurred in 18 of 693 patients in the DOACs group vs 9 of 679 patients in the control group; major bleeding occurred in 47 of 3049 patients in the LMWH group vs 33 of 2674 patients in the control group. And the funnel plot for VTE occurrence showed no evidence of publication bias (Fig. 7).
Figure 7.
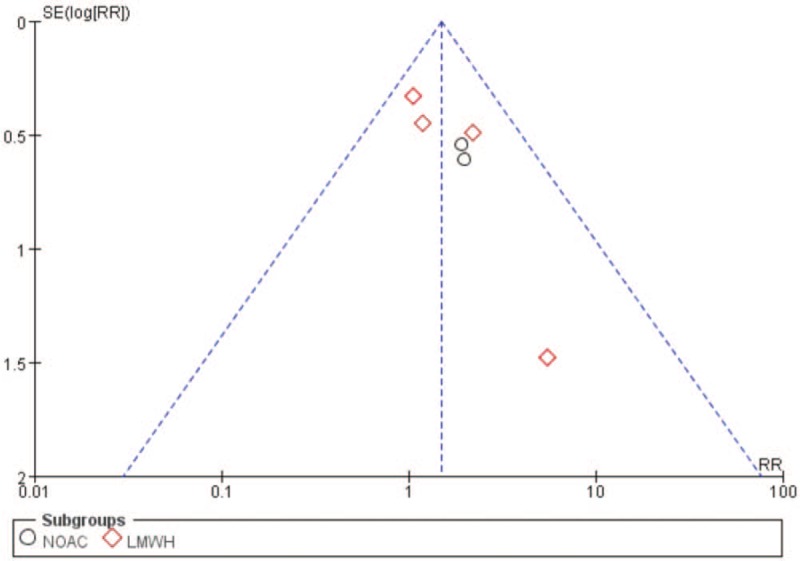
Funnel plot for major bleeding occurrence. LMWH = low molecular weight heparin, NOAC = novel oral anticoagulant, RR = risk ratio.
4. Discussion
The objective of the meta-analysis is to evaluate DOACs vs LMWH for the thromboprophylaxis of VTE in cancer patients who had no VTE by pooling data from all the available clinical trials. Many authors have studied the role of LMWH in patients with cancer. A relevant meta-analysis conducted by Che et al revealed that LMWH is effective in preventing VTE (RR = 0.53 95% CI: 0.42–0.67, I2 = 5.9%) and increases bleeding occurrence (RR = 1.32, 95% CI: 1.08–1.62, I2 = 40.4%) in cancer patients without VTE, there is no significant effect on major bleeding occurrence (RR = 1.22, 95% CI: 0.87–1.71, I2 = 9.2%).[19] LMWH reduces the incidence of symptomatic VTE by about half (RR = 0.54, 95% CI: 0.38–0.75, I2 = 0%) with a significantly 3-fold higher risk of clinically relevant bleeding (RR = 3.40, 95% CI: 1.20–9.63, I2 = 78%) compared to no intervention in a meta-analysis by Di Nisio et al.[20] And the upper bound of CI suggests that heparin treatment is associated with double risk of major bleeding (RR = 1.44, 95% CI: 0.98–2.11, I2 = 0%) while the CI is wide but close to statistical significance. Another Cochrane systematic review conducted by Akl EA and colleagues found that parenteral anticoagulation (with either unfractionated heparin or LMWH) reduces VTE occurrence (RR = 0.56, 95% CI: 0.47–0.68, I2 = 0%) but increases major bleeding risk (RR = 1.30, 95% CI: 0.94–1.79, I2 = 0%).[21] Some meta-analyses evaluated the efficacy and safety of LMWH in the lung cancer patients without VTE. Thein et al. found that LMWH prevent VTE without increasing major bleeding events but with increasing clinically relevant bleeding occurrence.[22] Another meta-analysis conducted by Fuentes et al demonstrated that VTE prophylaxis with LMWH reduces the VTE episodes without an apparent increase bleeding risk among ambulatory patients with lung cancer.[23]
Additionally, treatment benefits of LMWH are different when applied to patients with different histological types of cancer. The efficacy and safety of VTE prophylaxis in pancreatic and lung cancer subgroup meta-analyses suggested that LMWN treatment benefits are specific to these populations.[24,25]
In recent years, DOACs have been a novel treatment choice for VTE with following advantages: lack of need for routine laboratory monitoring, oral route of administration, and limited drug–drug or food–drug interactions. Prior review and meta-analysis studies showed that DOACs seems to be more effective and safer than LMWH in treatment of VTE in patients with cancer.[26,27] However, the benefits of prevention VTE with DOACs in cancer patients without VTE are unclear and relevant studies are limited. Two recent trials evaluated the use of DOACs as thromboprophylaxis in patients with cancer.[17,18] So, we conducted this meta-analysis.
In our meta-analysis, thromboprophylaxis with anticoagulant agents (including DOACs and LMWH) yielded a significant reduction of VTE in patients with cancer with a slight increase in bleeding. When compared with LMWH, DOACs had a similar prevention effect of VTE, a similar bleeding occurrence rate and a slightly higher major bleeding occurrence rate. It is noteworthy that 95% CI of RR was wide with regard to the effect of DOACs on major bleeding occurrence. In the CASSINI trial, rates of the VTE or VTE-related death (6.0% vs 8.8%, P = .10) and of major bleeding (2.0% vs 1.0%, P = .27) were similar between rivaroxaban and placebo.[18] In AVERT trial, Apixaban significantly reduced VTE occurrence (4.2% vs 10.2%, P < .001), but significantly increased rates of major bleeding (3.5% vs 1.8%, P = .046) compared with placebo.[17] So the problem whether DOACs significantly increases major bleeding risk cannot reach a unanimous conclusion and further studies are needed.
At the same time, patients’ satisfaction and quality of life influenced by anticoagulants should also be concerned. Cancer patients who need longer duration of anticoagulant treatment seem to have poor compliance of treatment due daily subcutaneous injection of LMWH.[28] Some published studies demonstrated that patients with cancer were more satisfied with DOACs (rivaroxban) than LMWH during anticoagulant treatment.[29]
Hence, DOACs are inclined to attract growing attention as an alternative strategy for prevention of venous thromboembolism in cancer patients without VTE. And further relevant studies are still demanded to provide future direction and evidence for the role of DOACs in these patients because existing studies are limited.
Several limitations should be concerned in our meta-analysis: not all the relevant RCTs which probed the efficacy and safety of LMWH in preventing VTE in patients with cancer were included in our meta-analysis, and relevant studies examined DOACs are limited; optimal duration of anticoagulation therapy was not evaluated to come to an agreement, although most of included studies evaluated a 6-month of anticoagulation; patients with cancer of different types or stages and different prevention agents (e.g., medicine, dosing, and duration) were included in selected studies, a significant heterogeneity should not be ignored in our meta-analysis.
5. Conclusion
The DOACs show comparable efficacy to LMWH in cancer patients without VTE. And a slightly higher risk of major bleeding with DOACs was observed, but 95% CI was wide. DOACs are inclined to be an alternative strategy for prevention of VTE in cancer patients without VTE. Future studies are required to assess the efficacy and safety of DOACs for the prevention of VTE in this population due to limited available studies.
Author contributions
Conceptualization: Hailong Chen, Rui Tao, Jin Yang.
Data curation: Rui Tao, Hui Zhao, Jianjun Jiang.
Formal analysis: Hailong Chen, Jin Yang.
Funding acquisition: Jin Yang.
Investigation: Hailong Chen, Hui Zhao, Jin Yang.
Methodology: Rui Tao, Jianjun Jiang.
Software: Hailong Chen, Jianjun Jiang.
Supervision: Rui Tao, Hui Zhao.
Validation: Jin Yang.
Writing – original draft: Hailong Chen.
Writing – review & editing: Hailong Chen, Jin Yang.
Hailong Chen orcid: 0000-0001-6572-8535.
Footnotes
Abbreviations: DOACs = direct oral anticoagulants, LMWH = low molecular weight heparin, VTE = venous thromboembolism.
How to cite this article: Chen H, Tao R, Zhao H, Jiang J, Yang J. Prevention of venous thromboembolism in patients with cancer with direct oral anticoagulants: a systematic review and meta-analysis. Medicine. 2020;99:5(e19000).
References
- [1].Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation 2003;107:I17–21. [DOI] [PubMed] [Google Scholar]
- [2].Blom JW, Doggen CJ, Osanto S, et al. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005;293:715–22. [DOI] [PubMed] [Google Scholar]
- [3].White RH, Dager WE, Zhou H, et al. Racial and gender differences in the incidence of recurrent venous thromboembolism. Thromb Haemost 2006;96:267–73. [DOI] [PubMed] [Google Scholar]
- [4].Khorana AA, Dalal M, Lin J, et al. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 2013;119:648–55. [DOI] [PubMed] [Google Scholar]
- [5].Nalluri SR, Chu D, Keresztes R, et al. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA 2008;300:2277–85. [DOI] [PubMed] [Google Scholar]
- [6].Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146–53. [DOI] [PubMed] [Google Scholar]
- [7].Lyman GH, Bohlke K, Khorana AA, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline update 2014. J Clin Oncol 2015;33:654–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- [9].Levine MN. New antithrombotic drugs: potential for use in oncology. J Clin Oncol 2009;27:4912–8. [DOI] [PubMed] [Google Scholar]
- [10].Streiff MB, Holmstrom B, Angelini D, et al. NCCN guidelines insights: cancer-associated venous thromboembolic disease, version 2.2018. J Natl Compr Canc Netw 2018;16:1289–303. [DOI] [PubMed] [Google Scholar]
- [11].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [13].Agnelli G, Gussoni G, Bianchini C, et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol 2009;10:943–9. [DOI] [PubMed] [Google Scholar]
- [14].Agnelli G, George DJ, Kakkar AK, et al. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med 2012;366:601–9. [DOI] [PubMed] [Google Scholar]
- [15].Van Doormaal FF, Di Nisio M, Otten HM, et al. Randomized trial of the effect of the low molecular weight heparin nadroparin on survival in patients with cancer. J Clin Oncol 2011;29:2071–6. [DOI] [PubMed] [Google Scholar]
- [16].Haas SK, Freund M, Heigener D, et al. Low-molecular-weight heparin versus placebo for the prevention of venous thromboembolism in metastatic breast cancer or stage III/IV lung cancer. Clin Appl Thromb Hemost 2012;18:159–65. [DOI] [PubMed] [Google Scholar]
- [17].Carrier M, Abou-Nassar K, Mallick R, et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med 2019;380:711–9. [DOI] [PubMed] [Google Scholar]
- [18].Khorana AA, Soff GA, Kakkar AK, et al. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med 2019;380:720–8. [DOI] [PubMed] [Google Scholar]
- [19].Che DH, Cao JY, Shang LH, et al. The efficacy and safety of low-molecular-weight heparin use for cancer treatment: a meta-analysis. Eur J Intern Med 2013;24:433–9. [DOI] [PubMed] [Google Scholar]
- [20].Di Nisio M, Porreca E, Candeloro M, et al. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev 2016;12:CD008500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Akl EA, Kahale LA, Hakoum MB, et al. Parenteral anticoagulation in ambulatory patients with cancer. Cochrane Database Syst Rev 2017;9:CD006652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Thein KZ, Yeung SJ, Oo TH. Primary thromboprophylaxis (PTP) in ambulatory patients with lung cancer receiving chemotherapy: a systematic review and meta-analysis of randomized controlled trials (RCTs). Asia Pac J Clin Oncol 2018;14:210–6. [DOI] [PubMed] [Google Scholar]
- [23].Fuentes HE, Oramas DM, Paz LH, et al. Meta-analysis on anticoagulation and prevention of thrombosis and mortality among patients with lung cancer. Thromb Res 2017;154:28–34. [DOI] [PubMed] [Google Scholar]
- [24].Ben-Aharon I, Stemmer SM, Leibovici L, et al. Low molecular weight heparin (LMWH) for primary thrombo-prophylaxis in patients with solid malignancies - systematic review and meta-analysis. Acta Oncol 2014;53:1230–7. [DOI] [PubMed] [Google Scholar]
- [25].Phan M, John S, Casanegra AI, et al. Primary venous thromboembolism prophylaxis in patients with solid tumors: a meta-analysis. J Thromb Thrombolysis 2014;38:241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kahale LA, Hakoum MB, Tsolakian IG, et al. Anticoagulation for the long-term treatment of venous thromboembolism in people with cancer. Cochrane Database Syst Rev 2018;6:CD006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li A, Garcia DA, Lyman GH, et al. Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): a systematic review and meta-analysis. Thromb Res 2019;173:158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Van der Wall SJ, Klok FA, Den Exter PL, et al. Continuation of low-molecular-weight heparin treatment for cancer-related venous thromboembolism: a prospective cohort study in daily clinical practice. J Thromb Haemost 2017;15:74–9. [DOI] [PubMed] [Google Scholar]
- [29].Prins MH, Lensing AWA, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PE): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol 2014;1:e37–46. [DOI] [PubMed] [Google Scholar]


