Abstract
Background:
Cancer patients with hepatitis B or C virus (HBV/HCV) infection are commonly seen in clinical practice, however, the data of safety and efficacy of immune checkpoint inhibitors (ICIs) among them are sparse, because active HBV/HCV infected patients were generally excluded by clinical trials and the correlation between previous infection and treatment-related adverse events was rarely reported. This review is the first to summarize the results on the safety and efficacy of immune checkpoint inhibitors (ICIs) in HBV/HCV infected cancer patients.
Method:
We searched literature and conference abstracts in PubMed and Embase followed the PRISMA guideline, using the keywords hepatitis B, hepatitis C, immune checkpoint inhibitor, ipilimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, avelumab, tremelimumab. Studies described patients with HBV/HCV infection treated with ICIs for advanced stage cancer were included.
Findings:
One hundred eighty six patients were identified from 14 articles (8 case reports, 4 case series, 2 trials). Eighty nine patients had HBV infection and 98 had HCV infection (1 both had HBV and HCV). The majority of patients were treated with PD-1 inhibitor monotherapy (140 of 186, 75.3%) and anti-CTLA-4 monotherapy (36 of 186, 19.4%). No treatment-related death was reported. The incidence of grade 3 or 4 hepatic transaminase elevating (HTE) in HBV and HCV infected patients were 3.4% (3/89) and 17.3% (17/98), respectively. 2.8% patients without antivirus therapy experienced virus load increasing, and 1.9% presented virus-related hepatitis. In terms of efficacy, 22 of 118 (18.6%) patients with liver cancer, 11 of 34 (32.4%) with melanoma, 1 of 6 (16.7%) with NSCLC showed objective responses (CR and PR) to ICIs in spite of lines of therapies.
Conclusion:
ICIs is considered to be safe and effective in advanced cancer patients with hepatitis B or C infection, but still has possibilities to reactive hepatitis virus due to uncertain mechanisms. We recommend that those with viral hepatitis be monitored closely and treated with antiviral therapy if indicated before or during ICIs treatment.
Keywords: cancer, efficacy, hepatitis B, hepatitis C, immune checkpoint inhibitors, safety
1. Introduction
Up to the latest report, there are approximately 380 millions of individuals that have hepatitis B virus (HBV) or hepatitis C virus (HCV) infection.[1,2] Based on the large number of carriers, many cancer patients also concurrently have hepatitis virus infection. Conventional chemotherapy has potential reactivation of HBV/HCV in these patients because of immunosuppression of cytotoxic drugs,[3] so that HBV/HCV should be controlled before receiving this kind of treatment. Some targeted therapies also have this side effect.[4] It is a common view of antiviral prophylaxis before initiation of rituximab even just in viral carriers.[5] Immune checkpoint inhibitors (ICIs), such as anti-cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) monoclonal antibody, anti-programmed death 1 (PD-1) antibody, anti-programmed death ligand 1 (PD-L1) antibody, are hot spots in immunotherapy that have shown high response rate and survival benefit in many kinds of advanced stage cancers,[6] such as melanoma[7] and non-small cell lung cancer (NSCLC),[8,9] but limited evidence exists on the safety and efficacy of ICIs in cancer patients with HBV or HCV infection. Prior studies represented that chronic HBV or HCV infection lead to specific T cell exhausting[10] which may theoretically affect the efficacy of ICIs and even improve the risk of triggering exacerbations of autoimmune disease and viral infections. Reactivation of HBV/HCV virus during anticancer therapy would badly damage liver function and led to therapy interruption. Thus appropriate strategy of ICIs in HBV/HCV infected cancer patients needs in-depth assessment and there is not any consensus yet. Here, we conducted a systematic review of the literatures to evaluate the safety and efficacy of ICIs in advanced-stage cancer patients with HBV or HCV infection to explore an appropriate treatment strategy for them.
2. Method
The systematic review was performed by the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline. Literatures, with the following terms: “hepatitis B” or “hepatitis C” and ipilimumab or nivolumab or pembrolizumab or atezolizumab or durvalumab or avelumab or tremelimumab or “immune checkpoint inhibitor” published on PubMed and Embase till June 2019 were collected. We also searched related review articles to ensure comprehensive retrieval of articles. The inclusion criterions were:
-
1.
patients diagnosed as advanced stage cancer;
-
2.
with HBV/HCV infection;
-
3.
treated with ICIs.
The exclusion criterions were:
-
1.
insufficient data of safety and efficacy;
-
2.
studies on animals or in vitro;
-
3.
review.
Articles were searched and selected independently by 2 investigators. This is a literature analysis and does not involve ethical review and informed consent.
3. Statistical analysis
Descriptive statistics was performed to summarize the literature findings by using Excel 15.19.1 (Microsoft). Chi-Squared was performed to analyze the difference between 2 groups by SPSS 24.
4. Results
One hundred forty four papers were identified, among which 54 were excluded as duplications, then 67 were excluded as they definitely met the exclusion criterions. Nine conference meeting abstracts met our inclusion criterions, but excluded by duplicated with full-text papers and insufficient data.
Finally, we identified 14 articles published between 2013 and 2018 that describe patients with hepatitis B or C and advanced stage cancers treated with ICIs. Among them, 8 were case reports, 4 were retrospective case series, 2 were prospective clinical trials. A total of 186 patients were included. The characteristics of these reports were listed in Table 1. The mean age was 55.6 years. One hundred twenty two (65.6%) were male, 36 (19.4%) were female, 28 (15.1%) were not mentioned. One hundred twenty four (66.7%) patients suffered from hepatocellular cancer (HCC), 46 (24.7%) patients got melanoma and 7 (3.8%) patients got non-small cell lung cancer (NSCLC). The majority of patients (137 of 182, 75.3%) were treated with PD-1 inhibitor monotherapy (nivolumab or pembrolizumab), 35 (19.2%) received anti-CTLA-4 monotherapy (Ipilimumab or tremelimumab). Only 1 patients received PD-L1 inhibitor (Atezolizumab), 5 (2.7%) received anti-PD-1, and anti-CTLA-4 combination therapy and the rest 4 (2.2%) received sequential anti-PD-1 and anti-CTLA-4 therapy. In terms of viral infection, 160 (86.0%) patients had viral load supervision before and during ICIs therapy. Eighty nine (47.8%) patients had HBV infection, among which 67 (67/89, 75.3%) received anti-virus therapy before and during ICIs therapy. Ninety eight (52.7%) patients had HCV infection and 13 (13/98, 13.3%) received antivirus therapy (Table 3).
Table 1.
Characteristics of 186 advanced-stage cancer patients with HBV/HCV infection included in this review.

Table 3.
The virus load and liver function tests in all the patients (n = 186).

5. Safety
In general, ICIs therapy was well tolerated in advanced stage cancer patients with hepatitis virus B or C infection (Table 2 showed the details of the 14 studies). No treatment-related death was reported. The most prevalence side-effects were dermatic (64/186, 34.4%), liver function (22.0%, 41/186), gastric (31/186, 16.7%), and incretory (6/186, 3.2%) abnormality. Grade 3 or 4 adverse event (AE) happened in 30 patients (30/186, 16.1%), including 20 hepatic transaminase elevating (HTE)(10.8%), 4 diarrhea or colitis (2.2%), 3 rashes (1.6%), 2 adrenal insufficiency (1.1%), 1 grade 3 arthritis (0.5%), among them, 11 received nivolumab, 11 tremelimumab, 2 pembrolizumab, 2 ipilimumab, 2 combined nivolumab and ipilimumab, 1 combined pembrolizumab and ipilimumab, 1 sequential ipilimumab and nivolumab.
Table 2.
Selected studies of ICIs therapy in patients with advanced cancer and hepatitis virus[11,12,15,23,37–46].
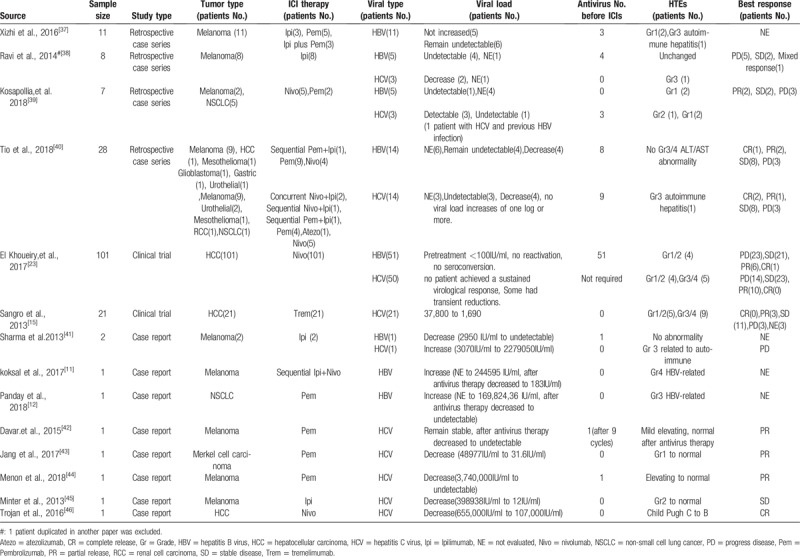
Liver function abnormalities were specifically analyzed. 13.5% (12/89) patients with HBV and 29.6% (29/98) patients with HCV experienced HTE after ICIs therapy. The incidence of grade 3 or 4 HTE in HBV and HCV infected patients were 3.4% (3/89) and 17.3% (17/98), respectively. Notably, 2.2% (2/89) and 7.1% (7/98) patients with HBV and HCV that had HTE before treatment showed hepatic transaminase decreasing after treatment (Table 3). Two of the 3 patients with HBV infection that got grade 3 or 4 HTE had normal range of liver functions before ICIs and did not receive antivirus therapy. Both of them presented HBV load increasing, leading to virus-related HTEs. Notably, their liver function abnormalities and HBV loads gradually returned to normal range after tenofovir treatment without cease of PD-1 inhibitors.[11,12] The other patient that also experienced grade 3 or 4 HTE received antivirus therapy before and during ICI treatment and got HBV inactive. His HTE was normalized by using steroid and related to autoimmune hepatitis. Among the 17 HCV infected patients that had grade 3 or 4 HTEs, only 1 patient was confirmed HCV load significantly increasing, but his HTE was due to autoimmune and recovered by using steroid. One patient's HCV load was not evaluated before treatment, so the role of HCV in this adverse event was uncertain. The mean HCV load of the other 15 patients decreased.
Considering the relationship between hepatitis virus and HCC, 14 (14/124, 11.3%) patients of HCC and 6 (6/62, 9.7%) patients with other kinds of cancers presented grade 3 or 4 HTE. There was no significant difference between HCC and other kinds of cancers (11.3% vs. 9.7%, P = .738).
6. Efficacy
There were 106 patients that did not receive extra antivirus therapy, among which 2.8% (3/106) experienced virus load increasing and 1.9% (2/106) presented virus-related hepatitis.
Specifically, among the 89 patients with HBV infection, HBV load were increased in 2 patients, unchanged in 65 patients and decreased in 7 patients. Twenty two patients with HBV infection did not receive antivirus therapy. two of them experienced HBV reactivation with virus load increasing, leading to virus-related hepatitis. The HBV load in the rest 20 patients remained inactive. While all the 7 patients with HBV load decreasing received extra antivirus therapy. In 98 patients had HCV infection, HCV load were increased in 1 patient, unchanged in 54 patients and decreased in 32 patients. Eighty five patients with HCV did not receive antivirus therapy, including the one whose HCV load increased dramatically. While there were also 81.3% (26/32) patients in the decreasing 32 patients that did not get extra antivirus drugs (Table 3).
One hundred sixty six of 186 patients had tumor response reported. ICIs showed remarkable antitumor activity in patients with HBV or HCV infection. Overall, 22 of 118 (18.6%) patients with liver cancer, 11 of 34 (32.4%) with melanoma, 1 of 6 (16.7%) with NSCLC showed objective responses (CR and PR) to ICIs in spite of lines of therapies (Table 4).
Table 4.
Objective response rates per disease Type.

7. Discussion
In this paper, we review, to our knowledge, all the published cases of HBV/HCV infected patients with advanced stage cancer treated with ICIs. Pembrolizumab and nivolumab were anti-PD-1 antibody, atezolizumab was anti-PD-L1 antibody, ipilimumab and tremelimumab were anti-CTLA-4 antibody. Under normal circumstances, T cells in the human body can monitor and clear tumor cells. However, the expression of PD-L1/PD-L2 on tumor cells binds to PD-1 on the surface of T cells, affecting T cell function and allowing cancer cells to evade attacks by the immune system. CTLA-4 plays a similar role as PD-1 and both of them are members of negative costimulatory factors. Blocking even one of the negative costimulatory pathways often leads to enhanced T cell function. By now, anti-PD-1 antibody have been approved in more than 10 kinds of cancers, including melanoma, renal cell cancer, NSCLC, urinary tract cancer, and colorectal cancer. The main indications for usage of anti-CTLA-4 antibody are melanoma, renal cell cancer and colorectal cancer. Anti-PD-L1 antibody is relatively new and it has been approved in urinary tract cancer, small cell lung cancer and triple-negative breast cancer. The indications of these drugs are supposed to keep expanding as numerous potential clinical trials are on the way. As these agents function through activating the immune system, they can lead to immune-related adverse events, such as dermatological events, endocrine disorders, liver function abnormalities, which are distinct inflammatory side effects characteristics.[13]
There is a theoretical risk of hepatitis B or C virus reactivation and potential for immune-mediated liver damage in them as their liver function may be compromised at baseline. The situation of chronic viral infection, similar to tumor microenvironment, was a strong immunosuppression environment. Specific T cell exhaustion partially through negative co-stimulatory molecules. On the one hand, T regulatory cells (Treg), a sub-population of T cells that suppresses the activation of CD4+ and CD8+ cells, express up-regulated CTLA-4, thus blocking CTLA-4 might impair the ability of T cells to keep hepatic virus suppressed by activating Treg.[14] A small scale clinical research had pointed that CTLA-4 inhibitor-tremelimumab increased Treg numbers in the peripheral blood.[15] On the other hand, specific CD8+ T cell plays the main role in controlling HBV and HCV by recognizing and destroying infected cells.[16–18] The persistent existing of hepatitis B or C led to CD8+ T cell exhaustion by negative co-stimulatory molecules, such as CTLA-4 and PD-1. Blockade these molecules could reverse this kind of T cell exhaustion and thus enhance the antiviral response. However, these effects could damage the balance between host immune system and viral control, which means a risk of liver function damage. In addition, the exhaustion of T cells might lead to poor response to ICIs, but the mechanisms and clinical outcomes behind these events require further elucidation.
From all the 14 case reports, case series and clinical trials that had published till March, 2019, we found 186 patients with HBV/HCV infection and advanced stage cancers that had ICI therapies. No treatment-related death was reported. 22.0% patients with HBV/HCV experienced HTE after ICIs therapy and the incidence of grade 3 or 4 HTE were 10.8%. According to other reports, the incidence of hepatic toxicity in anti PD-1/PD-L1 antibody ranged in different studies from 3% to 5%[19,20] and in anti CTLA-4 antibody is up to 10%,[21,22] which were lower than this review. An open-label clinical trial (NCT01658878) showed that the AST and ALT increases were more prevalence in HCC patients (∼20%)[23] or in anti PD-1 antibody and anti CTLA-4 antibody combination (NCT01024231).[24] In this review, 14 (14/124, 11.3%) patients of HCC and 6 (6/62, 9.7%) patients with other kinds of cancers presented grade 3 or 4 HTE and showed no significant difference (p = 0.738). This was reasonable, because in this review, most non-HCC patients with HBV/HCV were reported as case reports, leading to selected bias. Despite of the high incidence of hepatic toxicities, all the grade 3 or 4 HTEs were reversible by antivirus or using steroid without cease of ICIs, so the side-effect was considered to be acceptable. Other common adverse events were dermatic (64/186, 34.4%), gastric (31/186, 16.7%) and incretory (6/186, 3.2%) abnormality, including 4 grade 3/4 diarrhea or colitis (2.2%), 3 grade 3/4 rashes (1.6%), 2 grade 3 adrenal insufficiencies (1.1%) and 1 grade 3 arthritis (0.5%). In former studies, dermatological toxicities were observed up to 29.1%, gastric toxicities were 16.9% in patients received anti-PD-1 antibody.[7] The incidence of grade 3 or 4 diarrhea or colitis was 1% to 5% in late phase studies with anti-CTLA-4 antibody or anti-PD-1/PD-L1 antibody.[7,25] Grade 3 or 4 rashes, incretory abnormalities and arthritis were approximately 1%, which were similar with these HBV/HCV infected patients. To summarize, the incidence of these adverse events in patients with HBV/HCV were not significantly increased.
It is a big challenge to achieve complete HBV clearance[26] and prevent HCV relapse after direct-acting antiviral agent failure.[27] Preclinical data suggest that immune checkpoint inhibitor may be a new therapeutic approach based on its function of immune reconstruction. Studies showed that CD8+ cell function could be partially restored in vitro by blocking PD-1 pathway.[28,29] Former study established proof-of-concept that nivolumab could suppress HCV replication in some patients with chronic infection, including those who do not respond to interferon-alpha therapy.[30] But its clinical application of this approach in HCV infection is limited by the recent. In this review, among the 85 patients with HCV infection that did not have extra antivirus therapy, 1 had HCV load increasing and 32 had reductions, but no one achieved sustained virological response. In 22 patients with HBV activation that did not receive antiviral therapy (entecavir or tenofovir), 2 had HBV load elevating and HBV related HTE, the others remained stable. These indicated that ICIs applied as antiviral drugs needs further consideration, because other surface ligand-receptors were also implicated in suppression of immune responses against chronic viral infections, such as T cell immunoglobulin-3 (Tim-3) and lymphocyte activation gene-3 (Lag-3),[10,31,32] which might limit the antiviral efficacy of monotherapy of PD-1 or CTLA-4 inhibitors.
The treatment of advanced cancers, especially progressed after first-line therapy, is tough problem. Patients suffered from HCC or NSCLC usually had even poorer survival. Keynote-224 (NCT02702414) was a non-randomized, open-label phase 2 trial that enrolled 104 pretreated HCC patients, most of which without HBV (78%) or HCV (75%). The ORR of pembrolizumab was 17%.[33] In this review, the ORR in HBV/HCV infected HCC patients was 18.6%, comparable with former study, which means that virus infection did not significantly impact anticancer effect in HCC. In terms of NSCLC, keynote-010 (NCT01905657) compared pembrolizumab and docetaxel in pretreated NSCLC showed ORR of 18% and 9%, respectively.[34] In addition, the ORR in NCT01642004 and NCT01673867 that evaluated effect of nivolumab in NSCLC as second-line was 20% and 19%.[34,35] Although the number of patients are small in this review, 16.7% NSCLC with HBV/HCV infection show objective response in spite of lines of therapies. In melanoma, former studies showed 33% ORR in first-line and 22% in second-line therapy in patients without HBV/HCV infection (NCT01866319, NCT01704287),[7,36] which was similar with our result (32.4%). It seems that HBV/HCV infection would not significantly impact the effect of ICIs, so that HBV/HCV infection should not be an obstacle for these patients to receive ICIs.
8. Limitations
There were several limitations of this review. Data from retrospective cases and case reports had selection bias and heterogeneity in the evaluation of tumor response, virus response, and adverse events. Data from 2 clinical trials was lack of detailed individual information, so that viral load trends were not all available. The types of cancer in these published articles were not comprehensive, HCC and melanoma consisted most of them, so the conclusion may not apply to everyone. The correlation between hepatitis B or C virus infection and PD-L1 expression, tumor mutation burden should be taken into account. Our data should therefore be interpreted with caution. Further prospective studies are still needed.
9. Conclusion
Based on the present studies, ICIs is considered to be safe and effective in advanced cancer patients with hepatitis B or C infection. Although the incidence of virus reactivation and virus-related liver dysfunction was very low, we recommend that those with active viral hepatitis be monitored closely in consultation with a hepatologist and treated with antiviral therapy if indicated.
Author contributions
Data extraction: Yuwen Zhou, Wen Li.
Data sorting: Lin Huang.
Resources: Dan Pu, Liyuan Yin.
Supervision: Qinghua Zhou.
Visualization: Liang Cai.
Writing: Dan pu
Review & editing: Qinghua Zhou
Writing – original draft: Dan Pu.
Footnotes
Abbreviations: Atezo = atezolizumab, CR = complete release, Gr = grade, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, HTE = hepatic transaminase elevating, ICI = immune checkpoint inhibitor, Ipi = ipilimumab, NE = not evaluated, Nivo = nivolumab, NSCLC = non-small cell lung cancer, ORR = objective response rate, PD = progress disease, Pem = pembrolizumab, PR = partial release, RCC = renal cell carcinoma, SD = stable disease, Trem = tremelimumab.
How to cite this article: Pu D, Yin L, Zhou Y, Li W, Huang L, Cai L, Zhou Q. Safety and efficacy of immune checkpoint inhibitors in patients with HBV/HCV infection and advanced-stage cancer: a systematic review. Medicine. 2020;99:5(e19013).
The authors have no funding and conflicts of interests to disclose.
References
- [1].Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018;3:383–403. [DOI] [PubMed] [Google Scholar]
- [2].Gower E, Estes C, Blach S, et al. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 2014;61: 1 Suppl: S45–57. [DOI] [PubMed] [Google Scholar]
- [3].Paul S, Saxena A, Terrin N, et al. Hepatitis B virus reactivation and prophylaxis during solid tumor chemotherapy: a systematic review and meta-analysis. Ann Intern Med 2016;164:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yazici O, Sendur MA, Aksoy S. Hepatitis C virus reactivation in cancer patients in the era of targeted therapies. World J Gastroenterol 2014;20:6716–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gutierrez Garcia ML, Alonso Lopez S, Martin Rios MD, et al. [Hepatitis B virus reactivation in rituximab-treated patients: incidence and risk factors]. Gastroenterol Hepatol 2015;38:1–6. [DOI] [PubMed] [Google Scholar]
- [6].Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–32. [DOI] [PubMed] [Google Scholar]
- [8].Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018–28. [DOI] [PubMed] [Google Scholar]
- [10].Nakamoto N, Cho H, Shaked A, et al. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS pathogens 2009;5:e1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Koksal AS, Toka B, Eminler AT, et al. HBV-related acute hepatitis due to immune checkpoint inhibitors in a patient with malignant melanoma. Ann Oncol 2017;28:3103–4. [DOI] [PubMed] [Google Scholar]
- [12].Pandey A, Ezemenari S, Liaukovich M, et al. A rare case of pembrolizumab-induced reactivation of hepatitis B. Case Rep Oncol Med 2018;2018:5985131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kleiner DE, Berman D. Pathologic changes in ipilimumab-related hepatitis in patients with metastatic melanoma. Dig Dis Sci 2012;57:2233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stoop JN, van der Molen RG, Baan CC, et al. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology 2005;41:771–8. [DOI] [PubMed] [Google Scholar]
- [15].Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013;59:81–8. [DOI] [PubMed] [Google Scholar]
- [16].Webster GJ, Reignat S, Maini MK, et al. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology 2000;32:1117–24. [DOI] [PubMed] [Google Scholar]
- [17].Larrubia JR, Moreno-Cubero E, Lokhande MU, et al. Adaptive immune response during hepatitis C virus infection. World J Gastroenterol 2014;20:3418–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Balmasova IP, Yushchuk ND, Mynbaev OA, et al. Immunopathogenesis of chronic hepatitis B. World J Gastroenterol 2014;20:14156–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nishijima TF, Shachar SS, Nyrop KA, et al. Safety and Tolerability of PD-1/PD-L1 inhibitors compared with chemotherapy in patients with advanced cancer: a meta-analysis. Oncologist 2017;22:470–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bernardo SG, Moskalenko M, Pan M, et al. Elevated rates of transaminitis during ipilimumab therapy for metastatic melanoma. Melanoma Res 2013;23:47–54. [DOI] [PubMed] [Google Scholar]
- [22].Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol 2013;31:616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 2010;11:155–64. [DOI] [PubMed] [Google Scholar]
- [26].Bitton Alaluf M, Shlomai A. New therapies for chronic hepatitis B. Liver Int 2016;36:775–82. [DOI] [PubMed] [Google Scholar]
- [27].European Association for Study of L. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol 2014;60:392–420. [DOI] [PubMed] [Google Scholar]
- [28].Nakamoto N, Kaplan DE, Coleclough J, et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology 2008;134:1927–37. 1937.e1921–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Peng G, Li S, Wu W, et al. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol 2008;45:963–70. [DOI] [PubMed] [Google Scholar]
- [30].Gardiner D, Lalezari J, Lawitz E, et al. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PloS One 2013;8:e63818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Burke KP, Cox AL. Hepatitis C virus evasion of adaptive immune responses: a model for viral persistence. Immunol Res 2010;47:216–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang Y, Ma CJ, Wang JM, et al. Tim-3 negatively regulates IL-12 expression by monocytes in HCV infection. PloS One 2011;6:e19664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940–52. [DOI] [PubMed] [Google Scholar]
- [34].Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wen X, Wang Y, Ding Y, et al. Safety of immune checkpoint inhibitors in Chinese patients with melanoma. Melanoma Res 2016;26:284–9. [DOI] [PubMed] [Google Scholar]
- [38].Ravi S, Spencer K, Ruisi M, et al. Ipilimumab administration for advanced melanoma in patients with pre-existing Hepatitis B or C infection: a multicenter, retrospective case series. J Immunother Cancer 2014;2:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kothapalli A, Khattak MA. Safety and efficacy of anti-PD-1 therapy for metastatic melanoma and non-small-cell lung cancer in patients with viral hepatitis: a case series. Melanoma Res 2018;28:155–8. [DOI] [PubMed] [Google Scholar]
- [40].Tio M, Rai R, Ezeoke OM, et al. Anti-PD-1/PD-L1 immunotherapy in patients with solid organ transplant, HIV or hepatitis B/C infection. Eur J Cancer 2018;104:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sharma A, Thompson JA, Repaka A, et al. Ipilimumab Administration in Patients With Advanced Melanoma and Hepatitis B and C. J Clin Oncol 2013;31:E370–2. [DOI] [PubMed] [Google Scholar]
- [42].Davar D, Wilson M, Pruckner C, et al. PD-1 blockade in advanced melanoma in patients with hepatitis C and/or HIV. Case Rep Oncol Med 2015;2015:737389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jang S, Venna S. Antitumor and anti-hepatitis C viral response after administration of the anti-programmed death 1 antibody pembrolizumab. J Oncol Pract 2017;13:462–4. [DOI] [PubMed] [Google Scholar]
- [44].Menon LL, Chin B, Khattak MA. Safety of pembrolizumab while commencing treatment for hepatitis C with Harvoni (ledipasvir and sofosbuvir). Intern Med J 2018;48:1542–3. [DOI] [PubMed] [Google Scholar]
- [45].Minter S, Willner I, Shirai K. Ipilimumab-induced hepatitis C viral suppression. J Clin Oncol 2013;31:e307–8. [DOI] [PubMed] [Google Scholar]
- [46].Trojan J, Sarrazin C. Complete response of hepatocellular carcinoma in a patient with end-stage liver disease treated with nivolumab: whishful thinking or possible? Am J Gastroenterol 2016;111:1208–9. [DOI] [PubMed] [Google Scholar]


