Abstract
Background:
We conducted a meta-analysis to assess the efficacy and safety of mirabegron on overactive bladder (OAB) induced by benign prostatic hyperplasia (BPH) in men receiving tamsulosin therapy.
Methods:
We performed the analysis by using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. The databases including MEDLINE, EMBASE, and the Cochrane Controlled Trials Register were retrieved to get information regarding randomized controlled trials of mirabegron on OAB induced by BPH in men receiving tamsulosin therapy. We also searched the references of included literatures.
Results:
Three randomized controlled trials containing a total of 1317 BPH patients were included in the analysis. Co-primary efficacy end points: the mean number of micturitions per day [the mean difference (MD) = –0.27, 95% confidence interval (CI): –0.46 to –0.09, P = .004], the urgency episodes per day (the MD = –0.50, 95% CI: –0.77 to –0.22, P = .0004), the total OAB symptom score (the MD = –0.69, 95% CI: –1.00 to –0.38, P < .0001), and mean volume voided (the MD = 10.76, 95% CI: 4.87–16.64, P = .0003) indicated that mirabegron was effective in treating OAB induced by BPH in men receiving tamsulosin therapy. Safety assessments that included treatment-emergent adverse events (odds ratio = 0.88, 95% CI: 0.68–1.13, P = .31) indicated that mirabegron was well tolerated with the exception of post-void residual urine volume (MD = 12.02, 95% CI: 6.01–18.04, P < .0001).
Conclusions:
This analysis demonstrates that mirabegron is an effective and safe treatment for OAB symptoms induced by BPH in men receiving tamsulosin therapy with a low occurrence of side effects. Besides, we should be aware that the administration of mirabegron might have the risk of increasing post-void residual urine volume.
Keywords: meta-analysis, mirabegron, overactive bladder, randomized controlled trial, tamsulosin
1. Introduction
Overactive bladder (OAB) is a common condition in aging population, associated with detrimental effects on quality of life and huge economic burden.[1] In men, OAB is caused by bladder dysfunction or bladder outlet obstruction (BOO).[2] According to the 2019 European Association of Urology Guideline for non-neurogenic male lower urinary tract symptoms (LUTS) including benign BOO, patients having BOO with OAB symptoms and patients with BOO still having OAB symptoms after α-blocker administration are recommended to use anticholinergic agents simultaneously or in the add-on setting. In addition, the effectiveness of anticholinergic agents has been shown by meta-analysis and large-scale, well-designed randomized controlled trials (RCT).[3–5] At the same time, patients often discontinue antimuscarinics because of side effects such as blurred vision, dry mouth, and constipation.[6]
Mirabegron is a β3-adrenoceptor agonist that can be used for the treatment of OAB symptoms.[7] Besides, the rate of adverse effects such as blurred vision, dry mouth, and constipation with mirabegron is significantly lower than that with traditional anticholinergic drugs.[8] So far, there are a few clinical study on its efficacy and safety for OAB induced by benign prostatic hyperplasia (BPH) in men receiving tamsulosin therapy. The evidence for these studies is insufficient because the sample size is small.
Due to the lack of available literature, we conducted a meta-analysis to evaluate the efficacy and safety of mirabegron on OAB induced by BPH in men receiving tamsulosin therapy.
2. Methods
2.1. Inclusion and exclusion criteria
The included studies must meet the following requirements:
-
1.
The studies should be associated with the topic: mirabegron on OAB induced by BPH in men receiving tamsulosin therapy;
-
2.
RCT; and
-
3.
There should be similar characteristic between mirabegron+ tamsulosin group and tamsulosin group in addition to the content of the study.
The following studies were excluded:
-
1.
Studies having incomplete data;
-
2.
The type of study was abstract, comment, or review; and
-
3.
The patient with other disease, such as history of urinary retention, performance of clean intermittent catheterization or prior diagnosis of neurogenic bladder, with severe bladder diverticulum or urethral stricture.
2.2. Literature search and data sources
We performed a systematic search of PubMed, EMBASE, and Cochrane Library databases until September 2019 for studies of mirabegron on OAB induced by BPH in men receiving tamsulosin therapy. The keywords and medical subject headings used for searching were “mirabegron,” “tamsulosin,” “OAB,’ “BPH,” and “RCT.” There was not any restriction on the sample size. We also did a manual search of all retrieved references to get original text and reviewed them.
2.3. Data extraction
One author extracted the following data by reading the articles: the general data of the test (eg, the name of the first author, publication time, country, and the study design), the characteristics of the patients (eg, age), the interventions of the different groups (eg, mirabegron+tamsulosin or tamsulosin, dosage, usage, and duration time), and the data on effectiveness of OAB [eg, the mean number of micturitions per day, the urgency episodes per day, the total OAB symptom score (total OABSS), mean volume voided (MVV)]. All the extracted data were checked by another author.
2.4. Quality assessment
Jadad scale was used to classify the quality of each study.[9] We also used all of the measurable methods of assessment to evaluate the quality of the individual studies, including blindness of process, concealing distributive process, distributive method, results of loss to follow, and whether there is intention-to-treat analysis or calculation of sample size. Studies were graded in line according to the principles derived from the Cochrane Handbook for Systematic Reviews of Interventions version 5.10.[10] All of the RCTs were allotted according to the following quality classification standards:
-
1.
A: Satisfying almost all of the quality criteria, study would be considered to have a low probability of bias;
-
2.
B: Satisfying the partial quality criteria or unclear, the study was thought of having a secondary probability of bias; or
-
3.
C: Satisfying bare quality criteria, the study was considered to have a high probability of bias.
Each author participated in the assessment of all RCTs, and all agreed with the results. All reviewers independently assessed whether the study was suitable for the criteria. All discrepancies were discussed, recorded, and settled among authors.
2.5. Statistical analysis and meta-analysis
The RevMan 5 (Cochrane Collaboration, Oxford, UK) (version 5.10[10]) was used to complete meta-analysis of the continuous and dichotomous data. The mean difference (MD) with 95% confidence interval (CI) was employed to compare the continuous data and the odds ratio (OR) with 95%CI was used to compare the dichotomous data among the different groups. The I2 test and Mantel–Haenszel chi-square test were employed to evaluate the statistical heterogeneity, and then we chose a fixed-effects model if the P > .05, otherwise the random-effects model would be chosen. This meta-analysis does not need the ethical approval and the patient consent because all the data are available from previously published articles.
3. Results
3.1. Characteristics and quality of the studies
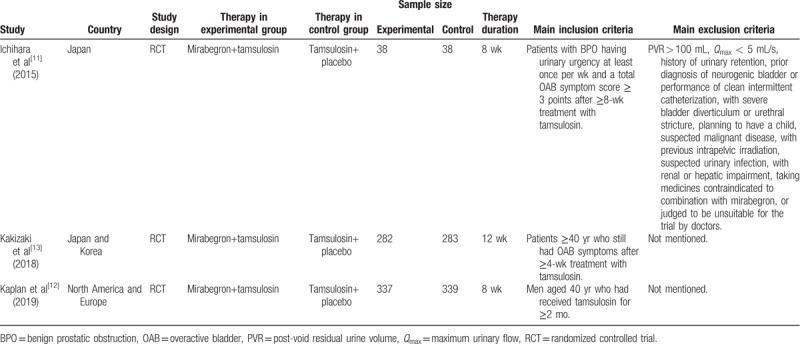
The study selection process is shown in PRISMA flow diagram. We found 50 original articles retrieved from the commonly used database. Based on the abstract, the inclusion and exclusion criteria of our meta-analysis, 26 articles were excluded. Fourteen studies without useful data were excluded. In total, 3[11–13] RCTs were included in our analysis. The condition of the studies and characteristics of the patients are shown in Table 1.
Table 1.
The details of individual study.
All of the 3 studies included in our analysis followed the randomization process, and no study showed an intention-to-treat analysis. The quality level of individual identified trials is shown in Table 2. The plot was symmetrical and 3 squares were contained in the large triangle, and no obvious evidence of bias was found (Fig. 1).
Table 2.
Quality assessment of individual study.
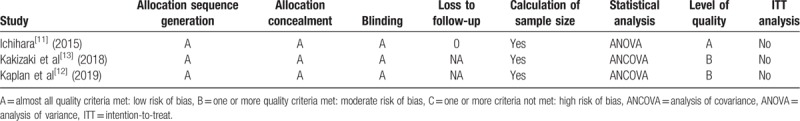
Figure 1.
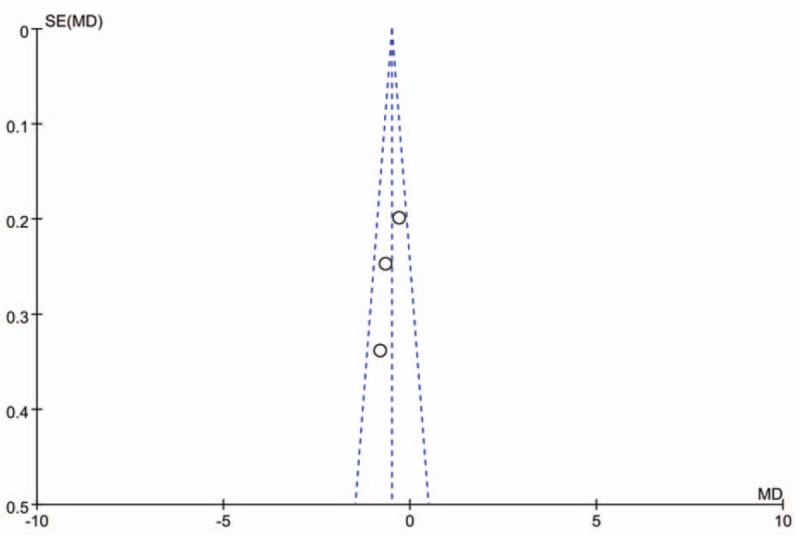
. Funnel plot of the studies represented in our analysis. MD = mean difference, SE = standard error.
3.2. Efficacy
3.2.1. The mean number of micturitions per day
Three RCTs, representing 1317 participants (657 in the mirabegron+tamsulosin group and 660 in the tamsulosin group), contributed to access mean number of micturitions per day data (Fig. 2a). No heterogeneity was found among the trials, and the fixed-effects model was thus chosen for the analysis. The estimate of MD was –0.27, the 95% CI was –0.46 to –0.09, P = .004. This result suggests that mirabegron+tamsulosin group showed statistically significant reductions in the mean number of micturitions per day compared with tamsulosin group.
Figure 2.
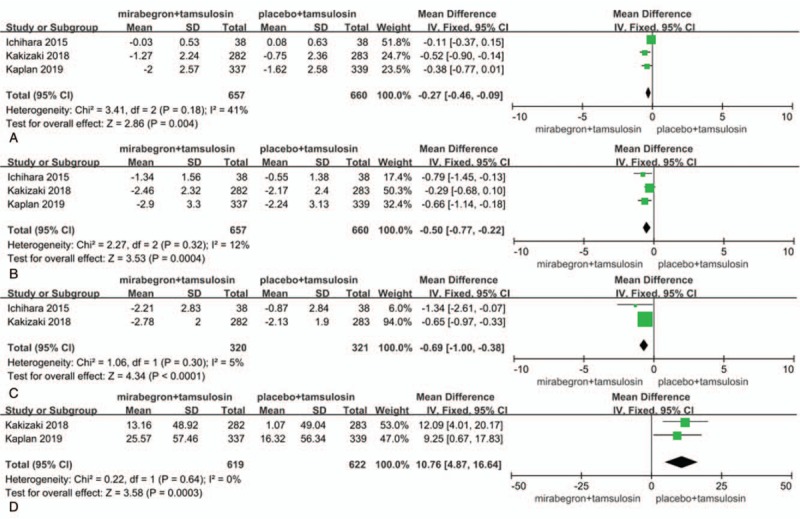
. Forest plots showing changes in (a) the mean number of micturitions per day, (b) the urgency episodes per day, (c) the total OAB symptom score (total OABSS), and (d) mean volume voided. CI = confidence interval, SD = standard deviation, IV = inverse variance.
3.2.2. The urgency episodes per day
Three RCTs, containing 1317 participants (657 in the mirabegron+tamsulosin group and 660 in the tamsulosin group), included data on the urgency episodes per day (Fig. 2b). The fixed-effects estimate of the MD was –0.50, 95% CI was –0.77 to –0.22, P = .0004. The forest plot demonstrates that mirabegron+tamsulosin group showed significantly greater reductions in the urgency episodes per day compared with tamsulosin group.
3.2.3. Total OABSS
Two RCTs included the total OABSS data representing a cohort of 641 participants (320 in the mirabegron+tamsulosin group and 321 in the control group) (Fig. 2c). The fixed-effects estimate of the MD was –0.69, 95% CI was –1.00 to –0.38, P < .0001. This result suggests that mirabegron+tamsulosin group had significantly greater decreases in the total OABSS.
3.2.4. Mean volume voided
Two of the RCTs included the MVV data representing a cohort of 1239 participants (619 in the mirabegron+tamsulosin group and 622 in the control group) (Fig. 2d). The fixed-effects estimate of the MD was 10.76, 95% CI was 4.87 to 16.64, P = .0003. This result suggests that mirabegron+tamsulosin group had significantly greater increases in the MVV compared with tamsulosin group.
3.3. Safety
3.3.1. Treatment-emergent adverse events
Three RCTs, representing 1317 participants (657 in the mirabegron+ tamsulosin group and 660 in the tamsulosin group), included the treatment-emergent adverse events (TEAEs) data (Fig. 3a). The effect size for meta-analysis was denoted as the OR. The estimate of OR was 0.88, and the 95% CI was 0.68 to 1.13, P = .31. The result suggests that both groups are similar in terms of the incidence of TEAEs.
Figure 3.
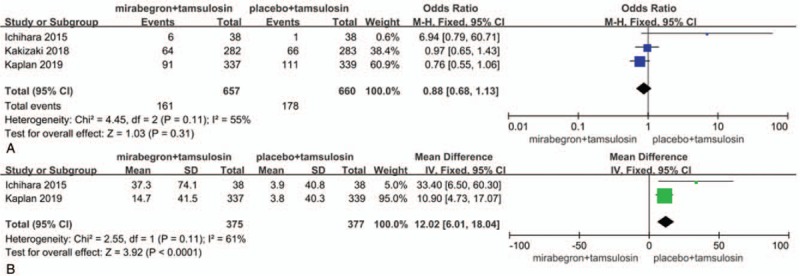
. Forest plots showing changes in (a) treatment-emergent adverse events and (b) post-void residual urine volume. CI = confidence interval, IV = inverse variance, M-H = Mantel–Haenszel, SD = standard deviation.
3.3.2. Post-void residual urine volume
Two RCTs included the post-void residual urine volume (PVR) data representing a cohort of 752 participants (375 in the mirabegron+ tamsulosin group and 377 in the control group) (Fig. 3b). The estimate of MD was 12.02, 95% CI was 6.01 to 18.04, P < .0001. The result suggests that the administration of mirabegron+ tamsulosin might have the risk of increasing PVR.
4. Discussion
There is an increasing evidence of a link between inflammation and development and progression of benign and malignant diseases of prostate and bladder. LUTS often represent the starting point for identifying such conditions.[14] So, there is a growing interest in combination treatment of LUTS, whether with tamsulosin in combination with finasteride,[15] solifenacin,[16] or tadalafil[17] or with solifenacin in combination with mirabegron.[18] The combination treatment causes a significant improvement in patients with LUTS in the mean number of micturitions per day, total OABSS, and MVV. Generally, combination therapy appeared more effective than monotherapy in many aspects. Although adding a second drug to monotherapy may indeed be helpful in patients experiencing insufficient LUTS, parallel group articles such as all of the above may be not adequate to demonstrate such benefit. Novel nomograms based on age, Prostate Specific Antigen, digital rectal examination, prostate volume, and PVR also can help patients to understand their risk of harboring LUTS and prostate cancer.[19]
Our analysis proves that in men with OAB taking tamsulosin for BPH, addition of mirabegron 50 mg once daily for 8 to 12 weeks showed superior efficacy versus placebo in improving the mean number of micturitions per day, the urgency episodes per day, total OABSS, and MVV. One of the RCTs that evaluated the quality of life also showed that mirabegron adding to tamsulosin was superior to the control group in improving the symptoms of OAB. Besides, Wada et al[2] evaluated urodynamic parameters before and after mirabegron add-on treatment for men with persistent OAB symptoms after receiving tamsulosin and demonstrated mirabegron add-on treatment with tamsulosin has efficacy and safety because it improves storage symptom without impairment of bladder contractility during voiding in male patients with OAB.
The adverse reaction such as TEAEs induced by mirabegron+ tamsulosin group and tamsulosin group were similar. The combination therapy was well tolerated, with no major safety concerns. Only 1 case of mild urinary retention was reported. And no severe adverse event was found between the 2 groups. This demonstrates the safety of mirabegron add-on treatment with tamsulosin in treating OAB symptoms. Besides, van Gelderen et al[20] conducted an open-label, randomized, 2-sequence, 2-arm study and demonstrated that the observed pharmacokinetic interactions upon add-on of tamsulosin or mirabegron to existing mirabegron or tamsulosin therapy did not lead to clinically relevant changes in cardiovascular safety or safety profiles.
Besides, one thing should be noticed that our result indicates that the administration of mirabegron+tamsulosin might have the risk of increasing PVR. Thus, based on the results, we should be aware that the administration of mirabegron might have the risk of increasing PVR or deteriorating the voiding condition like anticholinergic agents. Meanwhile, our result suggests that mirabegron+tamsulosin group and control group are similar in terms of the maximum urinary flow (MD = –1.01, 95% CI: –2.62 to 0.59, P = .22). These data demonstrated that mirabegron add-on treatment with tamsulosin for males having BOO was safe.
Based on our knowledge, this article is the first analysis forcing on the efficacy and safety of mirabegron on OAB induced by BPH in men receiving tamsulosin therapy, and the conclusions we draw have important clinical significance. However, some limitations should be emphasized. First, the number of included studies was not many. Second, data from the unpublished studies were not included in the analysis. The longer-term efficacy, safety, and persistence of mirabegron could not be extrapolated from this article. Besides, other end points (retention rate, episodes of nocturia, change in Qmax, and change of quality of life) are lacking because the data were too scarce to be officially analyzed. These factors may have resulted in a bias. However, all the included studies are in line with our inclusion criteria, and we hold the opinion that these limitations have less impact on the results. Furthermore, there is still a need for additional high-quality trials to provide more evidence.
5. Conclusions
This analysis demonstrates that mirabegron is an effective and safe treatment for OAB symptoms induced by BPH in men receiving tamsulosin therapy with a low occurrence of side effects. Besides, we should be aware that the administration of mirabegron might have the risk of increasing PVR.
Author contributions
Conceptualization: Jinlei Lin, Liqin Liang
Data curation: Shunye Su
Methodology: Ludong Liu
Software: Zhipeng Chen,
Supervision: Yuan Gao
Validation: Zhipeng Chen
Writing – original draft: Shunye Su
Writing – review & editing: Yuan Gao
Footnotes
Abbreviations: BOO = bladder outlet obstruction, BPH = benign prostatic hyperplasia, CI = confidence interval, LUTS = lower urinary tract symptoms, MD = mean difference, MVV = mean volume voided, OAB = overactive bladder, OR = odds ratio, PVR = post-void residual urine volume, RCT = randomized controlled trials, total OABSS = the total OAB symptom score.
How to cite this article: Su S, Lin J, Liang L, Liu L, Chen Z, Gao Y. The efficacy and safety of mirabegron on overactive bladder induced by benign prostatic hyperplasia in men receiving tamsulosin therapy: a systematic review and meta-analysis. Medicine. 2020;99:4(e18802).
Compliance with Ethical Standards: This article does not contain any studies with human participants or animals performed by any of the authors.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Sacco E, Tienforti D, D’Addessi A, et al. Social, economic, and health utility considerations in the treatment of overactive bladder. Open Access J Urol 2010;2:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wada N, Iuchi H, Kita M, et al. Urodynamic efficacy and safety of mirabegron add-on treatment with tamsulosin for Japanese male patients with overactive bladder. Low Urin Tract Symptoms 2016;8:171–6. [DOI] [PubMed] [Google Scholar]
- [3].Nishizawa O, Yamaguchi O, Takeda M, et al. Randomized controlled trial to treat benign prostatic hyperplasia with overactive bladder using an alpha-blocker combined with anticholinergics. Low Urin Tract Symptoms 2011;3:29–35. [DOI] [PubMed] [Google Scholar]
- [4].Takeda M, Nishizawa O, Gotoh M, et al. Clinical efficacy and safety of imidafenacin as add-on treatment for persistent overactive bladder symptoms despite alpha-blocker treatment in patients with BPH: the ADDITION study. Urology 2013;82:887–93. [DOI] [PubMed] [Google Scholar]
- [5].Gong M, Dong W, Huang G, et al. Tamsulosin combined with solifenacin versus tamsulosin monotherapy for male lower urinary tract symptoms: a meta-analysis. Curr Med Res Opin 2015;31:1781–92. [DOI] [PubMed] [Google Scholar]
- [6].Abrams P, Andersson KE, Buccafusco JJ, et al. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol 2006;148:565–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cui Y, Zong H, Yang C, et al. The efficacy and safety of mirabegron in treating OAB: a systematic review and meta-analysis of phase III trials. Int Urol Nephrol 2014;46:275–84. [DOI] [PubMed] [Google Scholar]
- [8].Chapple CR, Siddiqui E. Mirabegron for the treatment of overactive bladder: a review of efficacy, safety and tolerability with a focus on male, elderly and antimuscarinic poor-responder populations, and patients with OAB in Asia. Expert Rev Clin Pharmacol 2017;10:131–51. [DOI] [PubMed] [Google Scholar]
- [9].Jadad A. Randomised Controlled Trials. London: BMJ Publishing Group; 1998. [Google Scholar]
- [10].Higgins J, Green S. 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. Cochrane Collaboration. Available at: http://www.cochrane-handbook.org/. [Google Scholar]
- [11].Ichihara K, Masumori N, Fukuta F, et al. A randomized controlled study of the efficacy of tamsulosin monotherapy and its combination with mirabegron for overactive bladder induced by benign prostatic obstruction. J Urol 2015;193:921–6. [DOI] [PubMed] [Google Scholar]
- [12].Kaplan S, Herschorn S, McVary K. Efficacy and safety of mirabegron vs. placebo add-on therapy in men with overactive bladder symptoms receiving tamsulosin for underlying benign prostatic hyperplasia (PLUS). J Urol 2019;201: Suppl 4: e992–3. [DOI] [PubMed] [Google Scholar]
- [13].Kakizaki H, Lee KS, Yamamoto O, et al. Efficacy and safety of add-on mirabegron vs placebo to tamsulosin in men with overactive bladder symptoms (MATCH study). J Urol 2018;199:e988. [Google Scholar]
- [14].Mancin V, Balzarro M, Illiano E, et al. Lower urinary tract symptoms in elderly men: a simple yet comprehensive approach. J Clin Gerontol Geriatr 2018;66:245–52. [Google Scholar]
- [15].McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003;349:2387–98. [DOI] [PubMed] [Google Scholar]
- [16].Michel MC. Words of wisdom: re: a randomized controlled study of the efficacy of tamsulosin monotherapy and its combination with mirabegron for overactive bladder induced by benign prostatic obstruction. Eur Urol 2016;69:174. [DOI] [PubMed] [Google Scholar]
- [17].Singh DV, Mete UK, Mandal AK, et al. A comparative randomized prospective study to evaluate efficacy and safety of combination of tamsulosin and tadalafil vs. tamsulosin or tadalafil alone in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. J Sex Med 2014;11:187–96. [DOI] [PubMed] [Google Scholar]
- [18].Abrams P, Kelleher C, Staskin D, et al. Combination treatment with mirabegron and solifenacin in patients with overactive bladder: efficacy and safety results from a randomised, double-blind, dose-ranging, phase 2 study (Symphony). Eur Urol 2015;67:577–88. [DOI] [PubMed] [Google Scholar]
- [19].Cormio L, Cindolo L, Troiano F, et al. Development and internal validation of novel nomograms based on benign prostatic obstruction-related parameters to predict the risk of prostate cancer at first prostate biopsy. Front Oncol 2018;8:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].van Gelderen M, Tretter R, Meijer J, et al. Absence of clinically relevant cardiovascular interaction upon add-on of mirabegron or tamsulosin to an established tamsulosin or mirabegron treatment in healthy middle-aged to elderly men. Int J Clin Pharmacol Ther 2014;52:693–701. [DOI] [PubMed] [Google Scholar]


