Abstract
The diagnosis of acute coronary syndrome (ACS) in patients with cancer constitutes a therapeutic challenge. We aimed to assess the clinical presentation and management of ACS as well as 1-year survival in patients hospitalized for cancer.
This retrospective study included patients hospitalized between 2012 and 2018 in a nonacademic center. The inclusion criteria were diagnosis of active cancer and ACS recognized using standard criteria. Patients were assessed with respect to invasive or conservative ACS strategy. The primary endpoint was all-cause mortality, and the secondary endpoint was cardiovascular mortality during 1-year follow-up.
We screened 25,165 patients, of whom 36 (0.14%) had ACS (mean [SD] age, 71.9 [9.8] years). The most common presentation was non–ST-segment elevation myocardial infarction (61% of patients). Coronary angiography was performed in 47% of patients, while 53% were treated conservatively. Overall, the primary endpoint occurred in 67% of patients and secondary endpoint in 28% during follow-up. The predictors of better outcome in a univariate analysis were invasive strategy, lack of metastases, aspirin use, and no cardiogenic shock. Invasive treatment and aspirin use remained significant predictors of better survival when adjusted for the presence of metastases (hazard ratio [HR] 0.37, confidence interval [CI] 0.15–0.92 and HR 0.39, CI 0.16–0.94, respectively) and ineligibility for cancer treatment (HR 0.37, CI 0.15–0.93 and HR 0.30, CI 0.12–0.73, respectively).
The incidence of ACS in cancer patients is low but 1-year mortality rates are high. Guideline-recommended management was frequently underused. Our results suggest that invasive approach and aspirin use are associated with better survival regardless of cancer stage and eligibility for cancer treatment.
Keywords: acute coronary syndrome, aspirin, cancer, invasive strategy
1. Introduction
Cancer disease at various stages may cooccur in about 15% of patients with acute coronary syndrome (ACS), which constitutes a significant therapeutic challenge.[1,2] It is estimated that the incidence of ACS in patients with newly diagnosed cancer is particularly high in the first 6 months since diagnosis and in advanced cancer stages.[1–4] The pathophysiology of ACS in cancer is complex, and often involves not only classic type 1 myocardial infarction but also type 2 (caused by ischemia as a consequence of imbalance between oxygen demand and supply).[5] The risk of ACS in oncologic patients seems to depend on an interplay between multiple factors including classic cardiovascular risk factors, cancer type and stage, strategy of cancer treatment, and malignancy-related factors contributing to a prothrombotic state.[6,7]
The occurrence of ACS in cancer patients raises numerous concerns, such as the often challenging diagnosis of ACS itself, therapeutic decision making (including use of antiplatelet agents in case of thrombocytopenia), indications for invasive revascularization, bleeding complications, and continuation of cancer treatment. In addition, the current ACS guidelines[8–10] are based on trials that have excluded patients with active cancer, so they cannot be easily applied to oncologic patients. The available literature data on patients with ACS and cancer are scarce,[11–14] and no studies have been published so far in Poland. Therefore, the aim of our study was to assess the clinical presentation and management of ACS as well as 1-year survival in patients hospitalized due to cancer.
2. Methods
This retrospective analysis was conducted at Specialist Hospital in Brzozów, Subcarpathian Oncological Center, from January 2012 to December 2018. Annually, there are 3595 patients hospitalized for various types of cancer. The hospital statistical data were used to determine the number of cancer patients according to various types of cancer treatment and the number of patients with cancer complicated by ACS. The inclusion criteria were as follows:
-
(1)
diagnosis of active cancer (encompassing patients undergoing current cancer treatment and those who completed treatment in the past 6 months [including chemotherapy, immune therapy, hormonal therapy, radiotherapy, or oncologic surgery], as well as patients not receiving treatment because of significant disease progression); and
-
(2)
occurrence of ACS during hospitalization, including ST-segment elevation myocardial infarction (STEMI), non-STEMI (NSTEMI), and unstable angina (UA), recognized with standard criteria.[9,10]
The data were obtained using a standardized data collection form and included demographic characteristics, medical history, clinical symptoms, echocardiography and laboratory test findings, treatment modalities, significant bleeding events (requiring blood transfusion or being the cause of death), and patient survival at 1 year.
The primary endpoint was all-cause mortality during 1-year follow-up, whereas the secondary endpoint was cardiovascular mortality at 1-year.
The study was conducted in accordance with the principles of the Declaration of Helsinki, and the study protocol was approved by a local institutional ethics committee. All alive patients provided written informed consent to participate in the study.
All statistical analyses were performed using R 3.4 (R Foundation for Statistical Computing, Vienna, Austria). Categorical variables were described using numbers and percentages. Quantitative variables were expressed as medians and quartiles or means (SD), as appropriate. The null hypothesis was tested using the Mann-Whitney test or t test. For categorical variables, the between-group differences were determined using the χ2 or Fisher exact test. The Kaplan–Meier method was used to estimate the survival function with a log-rank comparison. The effect of individual variables on survival was evaluated using the univariate Cox proportional hazards model. Owing to a low number of cases, a full multivariable analysis was not feasible. Instead, we analyzed a relationship between selected variables and mortality risk, with metastases (a marker of disease progression) and cancer treatment as cofactors in the Cox proportional hazard analysis. The results of the Cox models were presented as hazard ratios and 95% confidence interval (CI). A P value of less than .05 was considered significant.
3. Results
3.1. Incidence of ACS
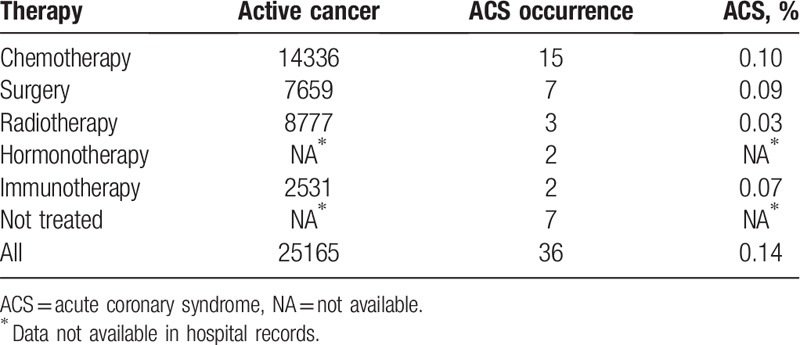
We reviewed 25,165 medical records of patients hospitalized due to cancer. Overall, the incidence of ACS in all patients hospitalized in the years 2012 to 2018 was 0.14%. The prevalence of ACS with respect to the type of cancer treatment is presented in Table 1. There were no significant differences between the frequency of ACS for various cancer treatments and no treatment.
Table 1.
Prevalence of acute coronary syndrome in all patients hospitalized due to cancer in the years 2012 to 2018 by the type of therapy.
3.2. Baseline characteristics of patients with ACS
We identified 36 patients with active cancer who experienced ACS during hospitalization between 2012 and 2018, which constituted 4.7% of all in-hospital ACS cases (760 patients, including patients with and without cancer). Data are presented in Table 1.
The mean (SD) age of patients with ACS was 71.9 (9.8) years. Men constituted 58% of the study group. The baseline characteristics of patients are shown in Tables 2 and 3. The most common ACS symptom was chest pain. Nontypical symptoms such as dyspnea and weakness were reported less frequently, in 25% of cases. The mean time from cancer diagnosis to ACS was 15 months (median, 4.5 months [range, 1–12 months]). ACS was observed most often in patients with gastrointestinal cancer (45% of all cancers), followed by those with hematologic malignancies (16%) and lung cancer (14%). Hypertension was the most common cardiovascular risk factor, observed in 58% of cases, while 40% of patients had a history of coronary artery disease. Of note, lipid profile analysis was not performed in two-thirds of patients during the acute phase of ACS.
Table 2.
Characteristics of patients with acute coronary syndrome according to type of cancer (n = 36).
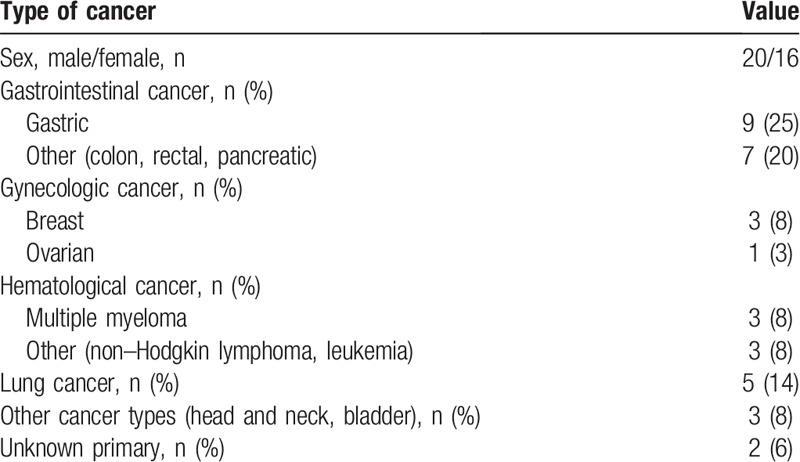
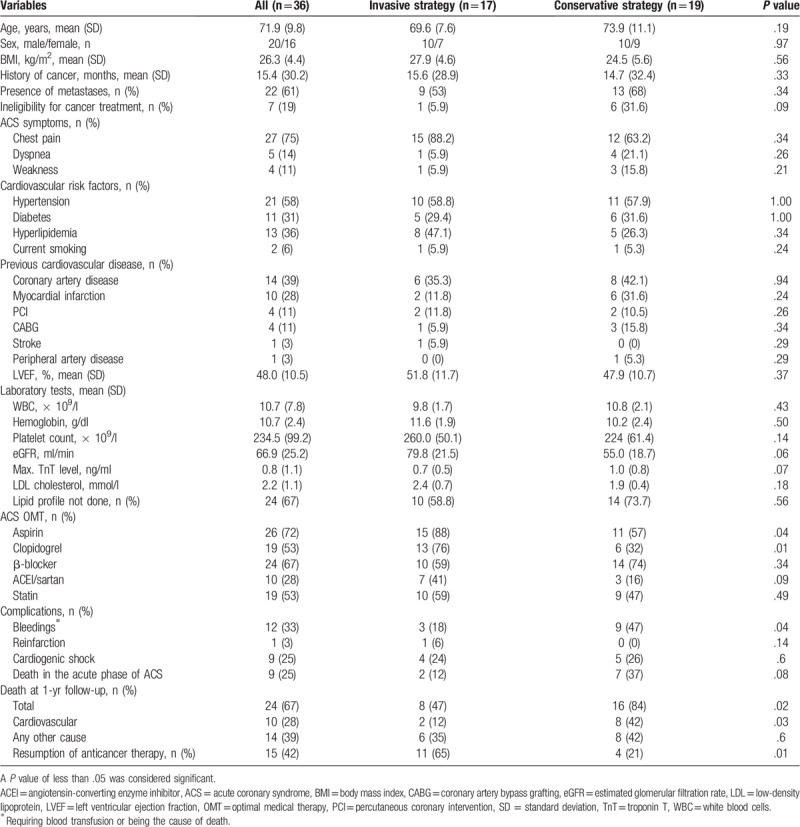
Table 3.
Comparison of clinical status, in-hospital optimal medical therapy, complications, and outcome according to the type of acute coronary syndrome treatment strategy.
3.3. Clinical presentation and management of ACS
The clinical presentation of ACS according to treatment strategy (invasive vs conservative) is shown in Figure 1A, and the distribution of treatment methods for ACS, in Figure 1B. We recorded NSTEMI in 22 patients (61%), STEMI in 10 (28%), and UA in 4 (11%).
Figure 1.
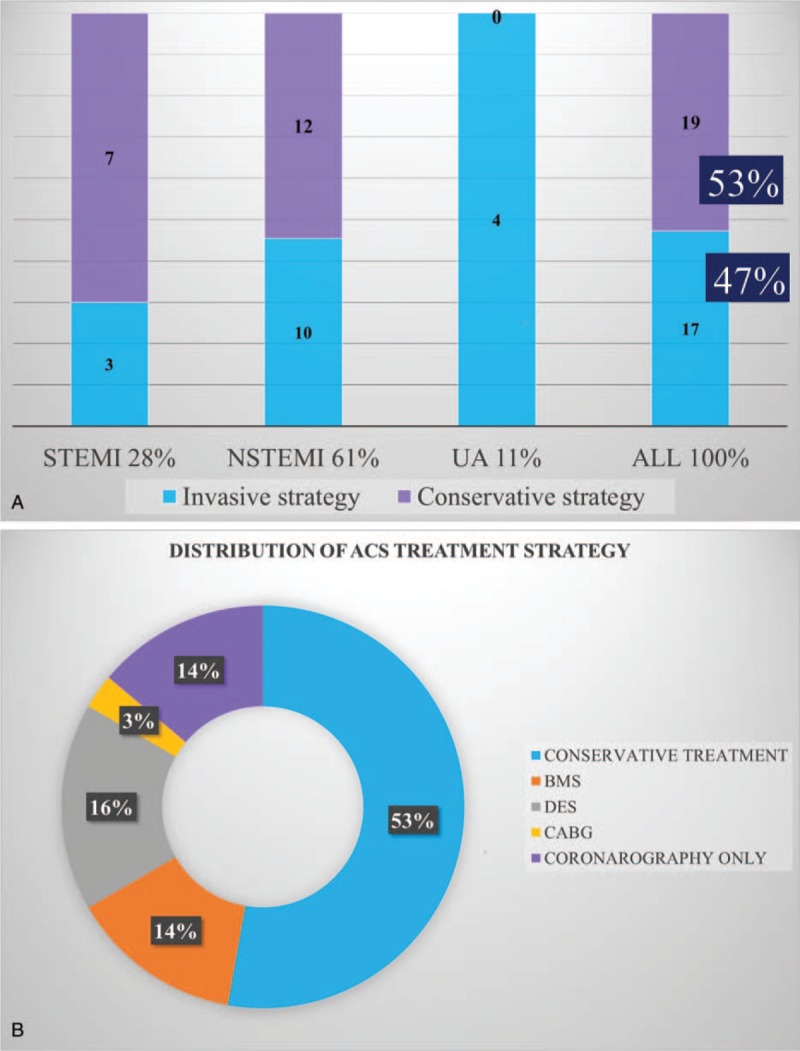
A – clinical presentation of acute coronary syndrome with regard to treatment strategy; B – distribution of treatment strategy for acute coronary syndrome. BMS = bare metal stent, CABG = coronary artery bypass grafting, DES = drug-eluting stent, NSTEMI = non–ST-segment elevation myocardial infarction, STEMI = ST-segment elevation myocardial infarction, UA = unstable angina; others, see Table 1.
Conservative strategy was the most common, reported in 19 patients (53%). Coronary angiography was performed in 17 cases (47%) (Fig. 1A). Invasive strategy was applied in 3 patients (30%) with STEMI, 10 patients (45%) with NSTEMI, and in all patients with UA. Fibrinolytic therapy was not performed in any patients. Coronary angiography revealed no significant stenosis or coronary artery lesions in 5 patients (14% of all ACS cases); in 5 patients (14%), bare metal stents were implanted, and in 6 (16%), drug-eluting stents. Successful coronary artery bypass surgery was reported in 1 patient (3%). No balloon angioplasties were performed. In 2 cases, the diagnosis of ACS was questioned on the basis of negative coronary angiography and these patients were finally diagnosed as type 2 of myocardial infarction and did not receive double antiplatelet therapy.
3.4. Comparison of invasive and conservative treatment
We compared patients on invasive (n = 17) and conservative treatment (n = 19) with respect to in-hospital optimal medical therapy (OMT), complications, cancer status, as well as acute and 1-year outcomes (Table 3). Aspirin and clopidogrel treatment of ACS was more common in the invasive-treatment group than in the conservative-treatment group. There were no differences for other OMT drugs. Significant bleeding at the acute phase of ACS was about 3-fold more common in patients on conservative treatment than in those on invasive treatment. Death during the acute phase of ACS occurred in 2 patients (12%) treated invasively and in 7 patients (37%) treated conservatively (P = .08). Death at 1 year was noted in 8 patients (47%) on invasive treatment and in 16 patients (84%) on conservative treatment (P = .02). Anticancer therapy was more often reintroduced in patients referred for invasive treatment after the acute phase of ACS.
3.5. One-year survival
During 1-year follow-up, 24 patients (67%) died, including 10 patients (28%) due to cardiovascular reasons (Table 3). All-cause and cardiovascular mortality rates were significantly higher in the conservative-treatment group in comparison with patients treated invasively in the acute phase of ACS.
3.6. Predictors of outcome
In the Kaplan–Meier analysis of overall survival stratified by the ACS treatment method, patients undergoing invasive treatment had significantly better outcome than those on conservative treatment (Fig. 2 A). Overall survival was worse in patients with the presence of metastases (Fig. 2 B), in patients considered ineligible for cancer treatment (Fig. 2 C), and in those with ACS complicated by cardiogenic shock (Fig. 2 D). Use of aspirin (Fig. 2 E) and angiotensin-converting enzyme inhibitor or sartans (small statistical significance- P = .048, Fig. 2 F), but not β-blockers or statins was associated with better survival.
Figure 2.
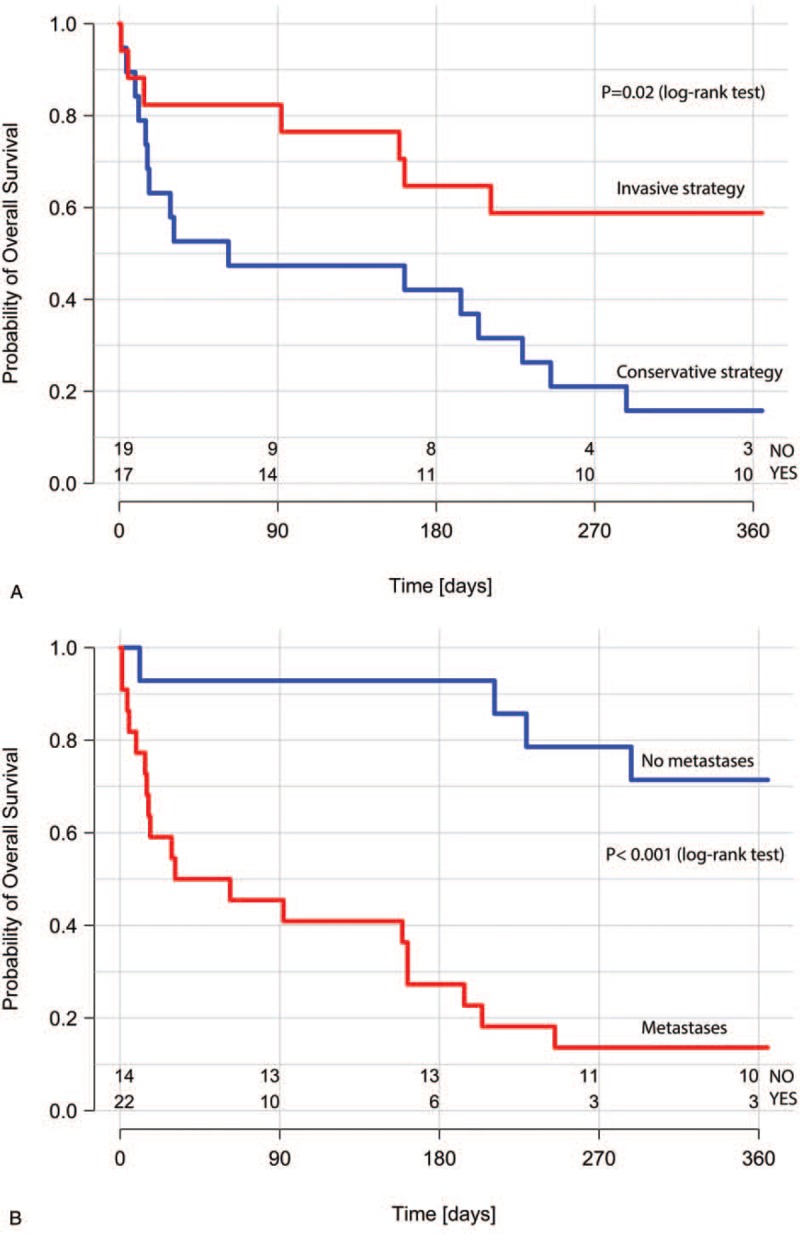
Kaplan–Meier survival curves for ACS treatment strategy (A); the presence of metastases (B); cancer treatment (C); ACS complicated by acute heart failure or cardiogenic shock (D); use of aspirin in the acute phase of ACS (E); use of ACEI or sartans in the acute phase of ACS (F). ACEI = angiotensin-converting enzyme inhibitors, ACS = acute coronary syndrome.
Figure 2 (Continued).
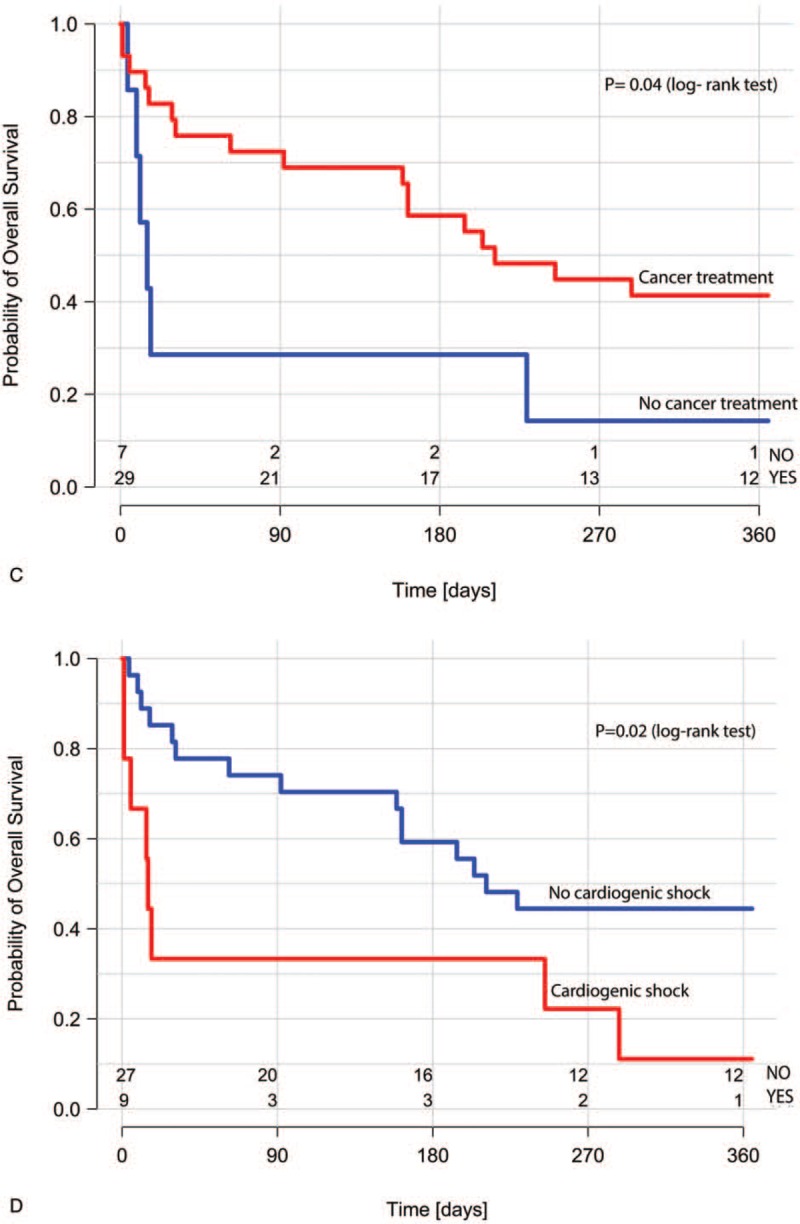
Kaplan–Meier survival curves for ACS treatment strategy (A); the presence of metastases (B); cancer treatment (C); ACS complicated by acute heart failure or cardiogenic shock (D); use of aspirin in the acute phase of ACS (E); use of ACEI or sartans in the acute phase of ACS (F). ACEI = angiotensin-converting enzyme inhibitors, ACS = acute coronary syndrome.
Figure 2 (Continued).
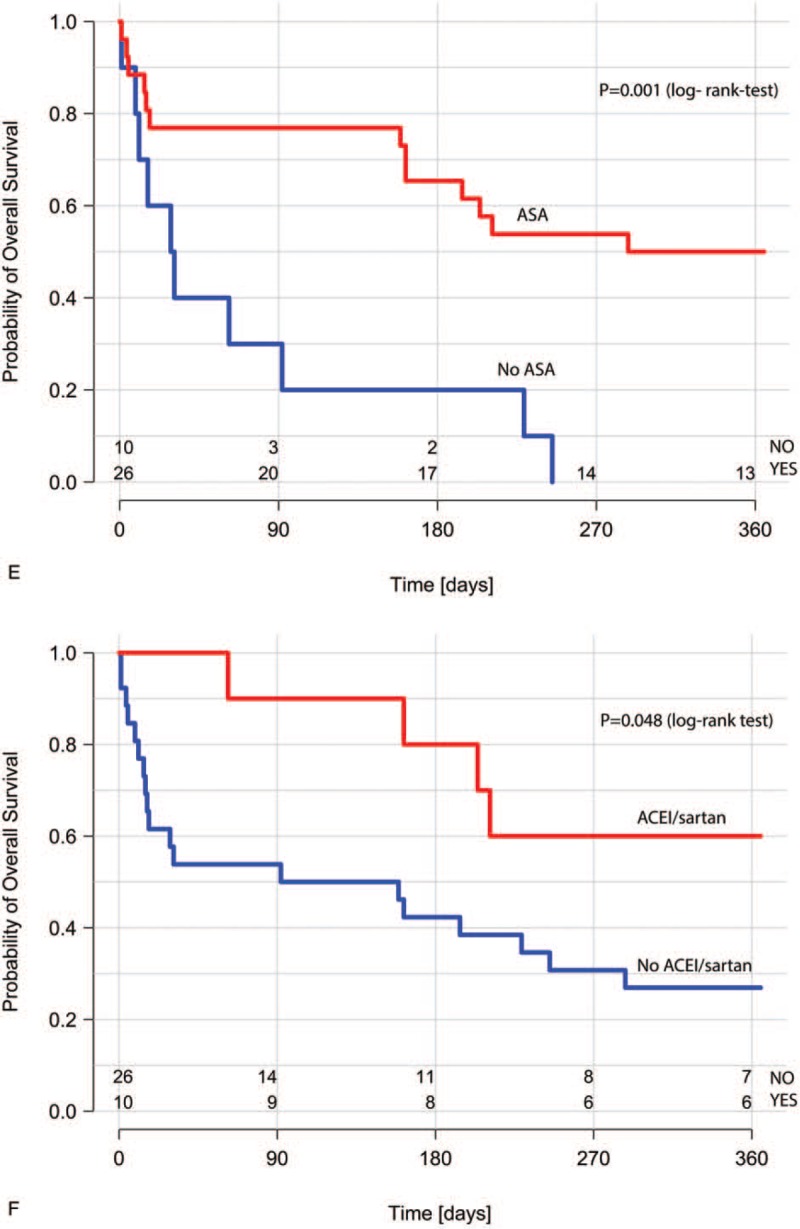
Kaplan–Meier survival curves for ACS treatment strategy (A); the presence of metastases (B); cancer treatment (C); ACS complicated by acute heart failure or cardiogenic shock (D); use of aspirin in the acute phase of ACS (E); use of ACEI or sartans in the acute phase of ACS (F). ACEI = angiotensin-converting enzyme inhibitors, ACS = acute coronary syndrome.
In the univariate Cox proportional hazards model assessing predictors of 1-year mortality, lack of metastases, invasive ACS strategy, and ACS not complicated by cardiogenic shock were associated with better prognosis (Table 4). Older age, STEMI, and significant bleedings at the acute phase of ACS had no impact on the risk of death; cancer treatment lost significance (P = .05). The use of aspirin was associated with a reduced risk of death. No association with the mortality risk was observed for other OMT drugs for ACS treatment, including angiotensin-converting enzyme inhibitor and sartans.
Table 4.
Univariate Cox proportional hazard model of 1-year mortality predictors.
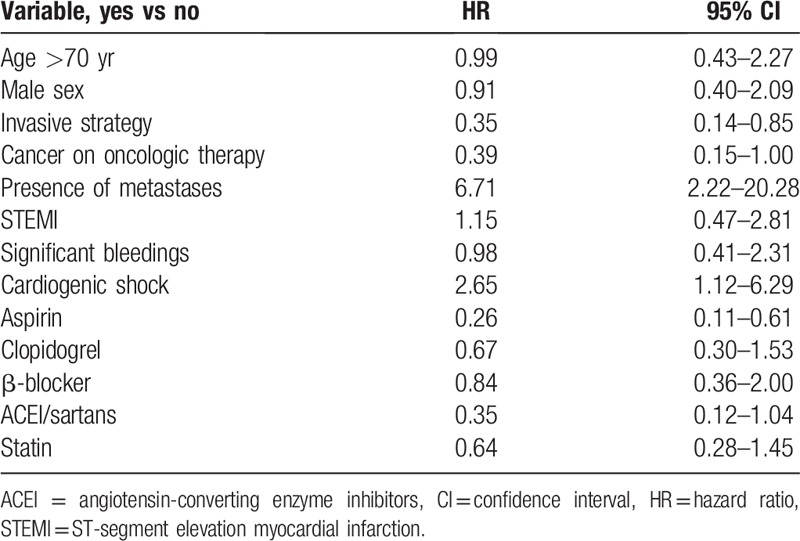
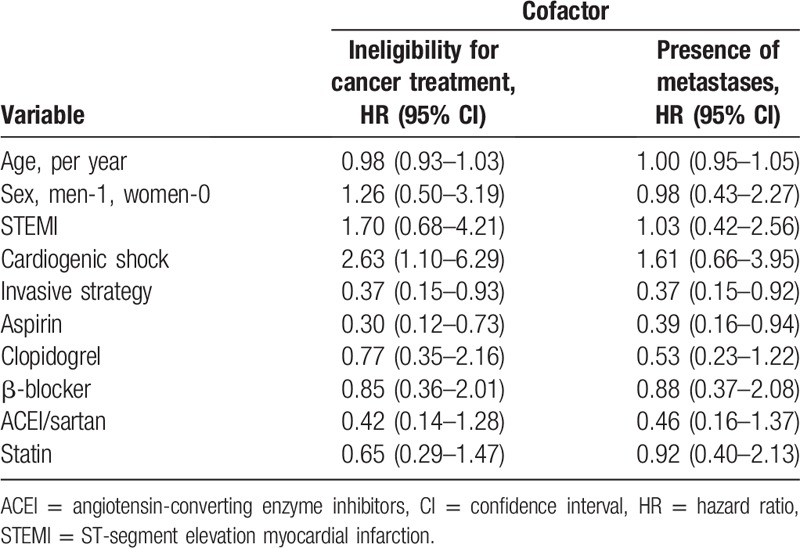
The relationship of age, sex, type of myocardial infarction, presence of cardiogenic shock, and treatment of ACS with the risk of death in the Cox proportional model with the presence of metastases and cancer treatment as cofactors is presented in Table 5. The invasive treatment and aspirin use at the acute phase of ACS remained significant predictors of death even when adjusted for the presence of metastases and ineligibility for cancer treatment. Cardiogenic shock complicating ACS was significantly related to the risk of all-cause death when adjusted for the presence of metastases, but not when ineligibility for cancer treatment was considered.
Table 5.
Relationship between age, sex, type of myocardial infarction, presence of cardiogenic shock, and treatment of acute coronary syndrome and the risk of death in the Cox proportional hazard analysis with oncologic variables as cofactors.
4. Discussion
To our best knowledge, this is the first Polish study addressing the management of ACS as well as 1-year survival in hospitalized patients with active cancer. Our results indicate that although the incidence of ACS in patients with active cancer is low, this group is characterized by a high mortality rate that could be reduced if patients were encouraged to undergo appropriate treatment for ACS, whenever possible. Based on our results, it may be speculated that the invasive treatment and aspirin use in the acute phase of ACS are related to better survival, even when cancer progression and ineligibility for cancer treatment are considered.
In this retrospective analysis, over 25,000 patients hospitalized for active cancer in the years 2012 to 2018 were screened, and 36 cases of ACS were identified. Our population is much larger than that in a recent retrospective analysis by Park et al,[15] who screened 5300 patients with active hematologic malignancies and identified 73 cases of ACS. Another retrospective study by Yusuf et al[14] included 456 patients with ACS and cancer, but the authors did not specify if the cancer disease was active.
Our study provides an estimated incidence of ACS depending on various cancer therapies as well as describes the clinical presentation and outcome of patients with active cancer. The incidence of ACS was found to be low (about 0.14% of all patients with active cancer). As a comparison, the incidence of in-hospital cancer-related pulmonary embolism in our center was estimated at 2% for the same population of cancer patients and the same time period (unpublished hospital statistics data).
Surprisingly, cancer patients with ACS in our center were significantly more often treated invasively than in other studies,[14,15] although similarly to the study by Yusuf et al,[14] conducted for the period between December 2000 and October 2006, we also do not have hemodynamic facilities at our institution. In our study, invasive ACS treatment was applied in 47% of patients, of whom 71% underwent revascularization. Moreover, in our patients, no fibrinolytic therapies were applied and no balloon angioplasties procedures were performed, recently indicated to be harmful in cancer population.[11] In the study by Yusuf et al,[14] only 15 patients (3.3%) with ACS underwent catheter-based revascularization, while 7 patients (1.5%) were administered fibrinolytic therapy. In a study by Park et al,[15] conducted between 2004 and 2014,[15] invasive strategy was applied in 18 cases (24.7%), although percutaneous coronary intervention (PCI) was performed only in 55.6% of patients. The low number of cancer patients considered for interventional treatment in those studies is notable, as this method is the treatment of choice in STEMI and, in most cases, in NSTEMI and UA.[8–10] The lower rate of PCI procedures in those studies compared with our analysis may be related to advancement of invasive techniques over time as well as to better availability of hemodynamic facilities in Poland. According to data from the Malopolska Registry of ACS (covering the region close to our center) from the years 2005 and 2006, invasive treatment was applied in 42% of general STEMI population, as compared with 24% of patients with NSTEMI.[16] Currently, the rates reach about 90% in the general population according to the Polish National Health Fund (unpublished data).
Based on the 2015 European Society of Cardiology guidelines,[9] the decision on an invasive strategy should carefully weigh the risks of invasive diagnostics against the benefits of revascularization. Although cancer patients have been excluded from randomized clinical trials, the guidelines indicate that in cancer patients, similarly to other mentioned groups (ie, very elderly and frail patients), the decision on the invasive treatment is at the discretion of the treating physician. This indicates that such patients undergo subjective and individual assessment by the physician, which is probably the major reason for substantial undertreatment in this group. In our study, the use of invasive strategy was closer to the recommendations on ACS management. Only in 2 cases (6%), the diagnosis of ACS was rejected, which in the context of the frequently atypical presentation of ACS[14] in patients with advanced cancer (25% of patients in our group did not report chest pain), is an acceptable rate for the initial cardiac assessment and assessing eligibility for invasive procedures.
The choice of an invasive strategy in our study was not related to the presence of metastatic cancer disease, and there were no significant differences in the rate of metastases between invasive- and conservative-treatment groups. Nevertheless, similarly to an invasive treatment strategy, the lack of metastases significantly influenced patients’ prognosis, and these patients had significantly better survival rates at 1 year compared with patients with metastatic disease and undergoing conservative treatment.
Generally, the use of OMT for ACS in the whole study group was rather low, except for aspirin in the invasively treated group and β-blockers in both groups. Of note, in our group, almost two-thirds of patients did not undergo lipid profile analysis. This confirms previous observations that cancer patients receive worse prevention care and do not receive optimal pharmacological treatment although available data indicate that it improves their outcome.[1,11–15,17–20] In this context, various coordinated care programs have been proposed and studied.[21]
The pathomechanisms of ACS in cancer patients differ significantly from those in the general population. They include not only coronary artery plaque rupture but also increased prothrombotic status, impaired endothelial function, vasospasms, supply-demand mismatch, and accelerated premature arteriosclerosis as a consequence of various anticancer therapies and of cancer itself.[6,7,22,23] As in previous studies, we observed that ACS occurred mostly in patients with advanced cancer (61% of patients had metastases)[1,14,23] and with NSTEMI as a predominant ACS type, which is in line with observations for the general population.[24] In our study, ACS was most common in patients with gastric cancer. This might be explained by high arterial thromboembolic potential of this cancer,[3] increased further by cancer treatment and additionally by sharing some common risk factors with coronary disease, including poor dietary and lifestyle habits.[25] Of note, the previous studies reported that ACS was most common in patients with lung cancer, followed by those with gastric cancer.[3,23]
The BleeMACS substudy[11] demonstrated that the presence of cancer in patients undergoing PCI negatively affects the prognosis and is the strongest predictor of death or reinfarction and bleedings after ACS in 1-year follow-up.[11] On the other hand, as shown also by our analysis, patients with cancer who develop ACS and undergo interventional treatment benefit from such therapy and have better cardiovascular prognosis than those who are not considered for invasive treatment.[1,11,12] Indeed, in our study, after invasive treatment of ACS, 11 patients (65%) could continue anticancer therapy, while it was possible in only 4 patients (21%) treated conservatively. However, the worse prognosis of patients treated conservatively might be related not to the lack of invasive reperfusion but to a lower rate of prescribed antiplatelet therapy and higher rate of bleedings. The importance of OMT and interventional treatment in cancer patients with ACS was highlighted in a large retrospective analysis by Guddati et al,[12] who showed that even patients with metastatic disease and limited life expectancy benefit from this therapy. The higher significant bleeding rate in the conservatively treated patients possibly could be the consequence of already existing less severe bleedings in this group, which discouraged cardiologists from qualification to invasive treatment and later were aggravated after implementation of antiplatelet therapy.
Our results indicate that factors negatively affecting the prognosis, apart from conservative strategy and the presence of metastases, were lack of aspirin use (with no impact shown for other OMT drugs) during the acute phase of ACS and cardiogenic shock complicating ACS. After adjustment for markers of cancer progression, invasive treatment and aspirin use in the acute phase were associated with better survival at 1 year. In our opinion, each patient with ACS and cancer should be considered for guideline-recommended therapy including not only interventional treatment but also OMT, especially aspirin use if not contraindicated.
4.1. Limitations
One of the study limitations is its retrospective design and potential bias associated with this type of studies. The major limitation is the low number of cases despite the fact that we screened over 25,000 patients. The small sample size made it impossible to perform a full multivariable analysis. However, the study period was 7 years, and if it was further extended into the past, we might have risked another bias as there were different guidelines and indications for invasive ACS treatment, including also the availability of hemodynamic units. Finally, our study included only patients who had experienced ACS during hospitalization at our center and not patients referred to our hospital with an ACS diagnosis as our hospital does not have a hemodynamic unit. All these limitations could be overcome in a large multicenter prospective registry of cancer patients with ACS diagnosis.
5. Conclusions
The incidence of ACS in patients hospitalized due to cancer in a nonacademic center is low; however, the 1-year mortality rate in this population is high. The majority of patients experienced NSTEMI. The management recommended by current guidelines was frequently underused, probably due to unfavorable assessment of the risk-benefit ratio. Our results suggest that an invasive approach and aspirin use are associated with better survival regardless of cancer stage and eligibility for cancer treatment. Large prospective registries are needed to validate the effectiveness of cardiovascular interventions in cancer patients with ACS.
Author contributions
Conceptualization: Katarzyna Styczkiewicz, Marek Styczkiewicz, Sabina Mędrek, Tomasz Kondraciuk, Sebastian Szmit, Piotr Jankowski.
Formal analysis: Katarzyna Styczkiewicz, Sabina Mędrek, Anna Czerkies-Bieleń.
Investigation: Katarzyna Styczkiewicz, Marek Styczkiewicz, Monika Myćka, Sabina Mędrek, Anna Czerkies-Bieleń, Andrzej Wiśniewski.
Methodology: Katarzyna Styczkiewicz, Tomasz Kondraciuk, Anna Czerkies-Bieleń, Andrzej Wiśniewski.
Project administration: Katarzyna Styczkiewicz, Monika Myćka, Tomasz Kondraciuk.
Resources: Monika Myćka, Andrzej Wiśniewski.
Software: Piotr Jankowski.
Supervision: Katarzyna Styczkiewicz, Tomasz Kondraciuk, Sebastian Szmit, Piotr Jankowski.
Writing – original draft: Katarzyna Styczkiewicz, Marek Styczkiewicz, Piotr Jankowski.
Writing – review & editing: Katarzyna Styczkiewicz, Sebastian Szmit, Piotr Jankowski.
Footnotes
Abbreviations: ACS = acute coronary syndrome, CI = confidence interval, HR = hazard ratio, NSTEMI = non-ST-segment elevation myocardial infarction, OMT = optimal medical treatment, PCI = percutaneous coronary intervention, SD = standard deviation, STEMI = ST-segment elevation myocardial infarction, UA = instable angina.
How to cite this article: Styczkiewicz K, Styczkiewicz M, Myćka M, Mędrek S, Kondraciuk T, Czerkies-Bieleń A, Wiśniewski A, Szmit S, Jankowski P. Clinical presentation and treatment of acute coronary syndrome as well as 1-year survival of patients hospitalized due to cancer: a 7-year experience of a nonacademic center. Medicine. 2020;99:5(e18972).
The authors have no funding and conflicts of interests to disclose.
References
- [1].Banasiak W, Zymliński R, Undas A. Optimal management of cancer patients with acute coronary syndrome. Pol Arch Intern Med 2018;128:244–53. [DOI] [PubMed] [Google Scholar]
- [2].Chen HY, Saczynski JS, McManus DD, et al. The impact of cardiac and noncardiac comorbidities on the short-term outcomes of patients hospitalized with acute myocardial infarction: a population-based perspective. Clin Epidemiol 2013;5:439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Navi BB, Reiner AS, Kamel H, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol 2017;70:926–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Navi BB, Reiner AS, Kamel H, et al. Arterial thromboembolic events preceding the diagnosis of cancer in older persons. Blood 2019;133:781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018) [in Polish]. Kardiol Pol 2018;76:1383–415. [DOI] [PubMed] [Google Scholar]
- [6].Zamorano JL, Lancellotti P, Muñoz DR, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines [in Polish]. Kardiol Pol 2016;74:1193–233. [DOI] [PubMed] [Google Scholar]
- [7].Aronson D, Brenner B. Arterial thrombosis and cancer. Thromb Res 2018;164: Suppl 1: S23–8. [DOI] [PubMed] [Google Scholar]
- [8].Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization [in Polish]. Kardiol Pol 2018;76:1585–664. [DOI] [PubMed] [Google Scholar]
- [9].Roffi M, Patrono C, Collet JP, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation [in Polish]. Kardiol Pol 2015;73:1207–94. [DOI] [PubMed] [Google Scholar]
- [10].Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation [in Polish]. Kardiol Pol 2018;76:229–313. [DOI] [PubMed] [Google Scholar]
- [11].Iannaccone M, D’Ascenzo F, Vadalà P, et al. Prevalence and outcome of patients with cancer and acute coronary syndrome undergoing percutaneous coronary intervention: a BleeMACS substudy. Eur Heart J Acute Cardiovasc Care 2018;7:631–8. [DOI] [PubMed] [Google Scholar]
- [12].Guddati AK, Joy PS, Kumar G. Analysis of outcomes of percutaneous coronary intervention in metastatic cancer patients with acute coronary syndrome over a 10-year period. J Cancer Res Clin Oncol 2016;142:471–9. [DOI] [PubMed] [Google Scholar]
- [13].D Iannaccone M, Ascenzo F, De Filippo O, et al. Optimal medical therapy in patients with malignancy undergoing percutaneous coronary intervention for acute coronary syndrome: a BleeMACS Sub-Study. Am J Cardiovasc Drugs 2017;17:61–71. [DOI] [PubMed] [Google Scholar]
- [14].Yusuf SW, Daraban N, Abbasi N, et al. Treatment and outcomes of acute coronary syndrome in the cancer population. Clin Cardiol 2012;35:443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Park JY, Guo W, Al-Hijji M, et al. Acute coronary syndromes in patients with active hematologic malignancies: incidence, management, and outcomes. Int J Cardiol 2019;275:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dziewierz A, Siudak Z, Dykla D, et al. Management and mortality in patients with non-ST-segment elevation vs. ST-segment elevation myocardial infarction. Data from the malopolska registry of acute coronary syndromes. Kardiol Pol 2009;67:115–20. [PubMed] [Google Scholar]
- [17].Wilk M, Walaszkowska-Czyż A, Rak A, et al. Acute coronary syndromes in oncology: conservative or invasive strategies? Case study and literature review. OncoReview 2018;1:5–10. [Google Scholar]
- [18].Sarkiss MG, Yusuf SW, Warneke CL, et al. Impact of aspirin therapy in cancer patients with thrombocytopenia and acute coronary syndromes. Cancer 2007;109:621–7. [DOI] [PubMed] [Google Scholar]
- [19].Podolec P, Filipiak KJ, Undas A, et al. Polish forum for prevention guidelines on prophylactic pharmacotherapy: update 2017. Kardiol Pol 2017;75:508–11. [DOI] [PubMed] [Google Scholar]
- [20].Cybulska B, Szostak WB, Filipiak KJ, et al. Polish forum for prevention guidelines on dyslipidaemia: update 2016. Kardiol Pol 2017;75:187–90. [DOI] [PubMed] [Google Scholar]
- [21].Styczkiewicz K, Styczkiewicz M, Mędrek S, et al. Tele-cardio-onco AID: a new concept for a coordinated care program in breast cancer (BREAST-AID): rationale and study protocol. Pol Arch Intern Med 2019;129:295–8. [DOI] [PubMed] [Google Scholar]
- [22].Rohrmann S, Witassek F, Erne P, et al. Treatment of patients with myocardial infarction depends on history of cancer. Eur Heart J Acute Cardiovasc Care 2018;7:639–45. [DOI] [PubMed] [Google Scholar]
- [23].Ogawa A, Kanda T, Sugihara S, et al. Risk factors for myocardial infarction in cancer patients. J Med 1995;26:221–33. [PubMed] [Google Scholar]
- [24].McManus DD, Gore J, Yarzebski J, et al. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am J Med 2011;124:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yusefi AR, Bagheri Lankarani K, Bastani P, et al. Risk factors for gastric cancer: a systematic review. Asian Pac J Cancer Prev 2018;19:591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]


