Supplemental Digital Content is available in the text
Keywords: bronchoscopic lung volume reduction, emphysema, network meta-analysis
Abstract
Bronchoscopic lung volume reduction (BLVR) offers alternative novel treatments for patients with emphysema. Comprehensive evidence for comparing different BLVR remains unclear. To estimate the effects of different BLVR on patients with emphysema. PubMed, EMBASE, Cochrane Library, and Web of Science databases from January 2001 to August 2017 were searched. Randomized clinical trials evaluated effects of BLVR on patients with emphysema. The relevant information was extracted from the published reports with a predefined data extraction sheet, and the risk of bias was assessed with the Cochrane risk of bias tools. Pair-wise metaanalyses were made using the random-effects model. A random-effects network meta-analysis was applied within a Bayesian framework. The quality of evidence contributing to primary outcomes was assessed using the GRADE framework. 13 trials were deemed eligible, including 1993 participants. The quality of evidence was rated as moderate in most comparisons. Medical care (MC)was associated with the lowest adverse events compared with intrabronchial valve (IBV)(-2.5,[-4.70 to -0.29]), endobronchial valve (EBV) (-1.73, [-2.37 to -1.09]), lung volume reduction coils (LVRC) (-0.76, [-1.24 to -0.28]), emphysematous lung sealant (ELS) (-1.53, [-2.66 to -0.39]), and airway bypass(-1.57, [-3.74 to 0.61]). Adverse events in LVRC were lower compared with ELS (-0.77,[-2.00 to 0.47]). Bronchoscopic thermal vapor ablation (BTVA) showed significant improvement in FEV1 compared with MC (0.99, [0.37 to 1.62]), IBV (1.25, [0.25 to 2.25]), and LVRC (0.72, [0.03 to 1.40] ). Six minute walking distance (6 MWD) in ELS was significantly improved compared with other four BLVR, sham control, and MC (-1.96 to 1.99). Interestingly, MC showed less improvement in FEV1 and 6MWDcompared with EBV (-0.45, [-0.69 to -0.20] and -0.39, [-0.71 to -0.07], respectively). The mortality in MC and EBV was lower compared with LVRC alone (-0.38, [-1.16 to 0.41] and -0.50, [-1.68 to 0.68], respectively). BTVA and EBV led to significant changes in St George's respiratory questionnaire (SGRQ) compared with MC alone (-0.74, [-1.43 to -0.05] and 0.44, [0.11 to 0.78], respectively). BLVR offered a clear advantage for patients with emphysema. EBV had noticeable beneficial effects on the improvement of forced expiratory volume 1, 6MWD and SGRQ, and was associated with lower mortality compared with MC in different strategies of BLVR.
1. Introduction
Chronic obstructive pulmonary disease (COPD) is the most common and a leading cause of mortality and morbidity worldwide. COPD was ranked among the top 20 conditions causing disability globally and the eighth cause of disease burden measured by disability-adjusted life years in 2015.[1] The morbidity and mortality leaded by COPD have been still increasing unceasingly, and COPD is expected to be the third most common cause of death by 2020 worldwide.[2–4] Emphysema is the main pathological features of COPD. Loss of lung elastic recoil leads to airflow obstruction, gas trapping and increased operating lung volumes. Despite optimal pharmacological therapy consisting of both short-acting and long-acting β2 agonist sand anticholinergic agents, COPD patients with a predominant emphysema phenotype remain significantly disabled. Medical treatment for emphysema does not prevent disease progression.[5–7]
Appendix 3
Air flows preferentially through collateral pathways, which is associated with the destruction of alveoli and small airway, the properties changes of elastic recoil and radial support in emphysema.[8] During exhalation, alveolar and small airway collapse lead to air gather in dysfunctional small airway without exchange and collateral ventilation in emphysema. Collateral ventilation is regarded as a kind of invalid ventilation, but a key issue for lung volume reduction when treat for emphysema.[9] Lung volume reduction surgery (LVRS) is able to improve pulmonary function parameters significantly. However, 6-minute walk distance (6MWD) cannot be significantly improved after the initial intervention. Meanwhile, LVRS is associated with risks of early mortality and adverse events.[7,10]
In the recent years, a variety of bronchoscopic lung volume reduction (BLVR) procedures offer alternative novel treatments for selected patients with emphysema.[11] However, some important issues, especially the effect on BLVR procedures, are insufficiently addressed, which provides an unbalanced view on the topic.[12] Here, our study provides the first network meta-analysis of all available randomized trials, which comprehensively compared and ranked different strategies of BLVR and medical treatment in COPD with emphysema phenotype. We did network metaanalysis to identify both direct and indirect evidence from relevant trials. Adverse events, treatment effects percent change in forced expiratory volume (FEV1), change in 6MWD and St George's respiratory questionnaire (SGRQ) and risks of mortality are under the evaluation to present a clear advantage for patients with emphysema.
2. Methods
2.1. Search strategy and selection criteria
For this network metaanalysis, we searched PubMed, Cochrane Library, Embase, and Web of Science from January 2001 to August 2017 to collect randomized clinical trials (RCTs) about BLVR for emphysema. The search terms included “lung volume reduction procedures,” “Lung volume Reduction coils,” “emphysema,” “Intrabronchial valve,” “Endobronchial valve,” “Emphysematous lung sealant,” “Airway bypass,” “Bronchoscopic thermal vapor ablation,” “ endobronchial valve ,” “ intra-bronchial valve,” “ lung volume reduction coils,” “ABS,” and “ emphysematous lung sealant.” No language restrictions and time limits were applied to the initial search.
We included the following interventions: lung volume reduction coils (LVRC), intra-bronchial valve, endobronchial valve (EBV), emphysematous lung sealant (ELS), airway bypass, medical care (MC), bronchoscopic thermal vapor ablation (BTVA), and sham control. Patients involved in trials were with moderate to severe emphysema. We excluded studies that recruited participants with giant or bullous emphysema; however, RCTs recruiting participants with treatment duration of less than 4 weeks, or with an overall sample size of fewer than ten patients were excluded.
2.2. Data extraction and quality assessment
Two authors (Wu Xu and Guoping Li.) extracted the data with a predefined data extraction sheet and assessed the risk of bias with the Cochrane risk of bias tool. Some articles were excluded on account of the inclusion criteria that they do not meet by reading the full text independently. Any discrepancies were resolved by consensus and arbitration by a panel of other investigators within the review team. Two authors (Wu Xu and Guoping Li.) reviewed the full text of all the RCTs on the basis of their own study design and assigned a value of ‘high risk’, ‘low risk’ or ‘unclear risk’ to the following: random sequence generation (selection bias); allocation concealment (selection bias); blinding of participants and personnel (performance bias); blinding of outcome assessment (detection bias); incomplete outcome data (attrition bias); selective reporting (reporting bias); and other biases. Some articles were excluded on account of the inclusion criteria that they do not meet by reading the full text independently. Any discrepancies were resolved by consensus and arbitration by a panel of other investigators within the review team. The study is not involved in ethical approval.
3. Outcomes
We considered the lung capacity, survival, and the health-related quality of life as the primary outcomes. The percent change in forced expiratory volume in one second was used to evaluate the lung capacity. The perioperative and postoperative mortality was used to evaluate the survival. The Georges respiratory questionnaire was used to evaluate the health-related quality of life. Secondary outcomes included exercise capacity and adverse events. The 6MWD was used to evaluate the exercise capacity. We defined acute treatment duration between 12 and 48 weeks, and we gave preference to the time-point used in the original study as the study endpoint.
3.1. Statistical analysis
The network meta-analysis was performed with the random-effects model using STATA 14.0 (STATA Corp, College Station, TX) software. The standardized mean difference (SMD) was calculated as the effect size for continuous outcomes, and the odds ratio (OR) was calculated for dichotomous outcomes, both with 95% confidence intervals (CIs). We assessed statistical heterogeneity in each pair-wise comparison with the I2 statistic and P value.
A network plot was produced to represent the overall information of the trials included in the analysis. We used the funnel plot to detect publication bias. The funnel plot should be symmetrical near the zero line if there is no publication bias.[13] The contribution of each direct comparison to each network estimate was calculated according to the variance of the direct treatment effect and the network structure, later summarized in a contribution plot. The node splitting method was used to calculate the inconsistency of the model, which separated evidence on a particular comparison into direct and indirect evidence.[14] We estimated the ranking probabilities for all treatments of being at each possible rank for each intervention. The probability of a treatment being ranked was summarized and reported as surface under the cumulative ranking curve (SUCRA).[15] Additionally, we assessed the quality of evidence contributing to each network estimate using the GRADE framework, which characterizes the quality of a body of evidence on the basis of the study limitations, imprecision, inconsistency, indirectness, and publication bias for the primary outcomes.[16] To assess the influence of single study on the overall results, sensitivity analyses were conducted by discarding individual studies sequentially. We calculated statistical heterogeneity in each pair wise comparison with the I2 statistic and P value by R (version 3.4.1) software.[17]
3.2. Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report, or in the decision to submit for publication.
4. Results
Overall, a total of 4942 studies were identified by our literature search. After scanned titles and abstracts, we identified 1871 duplicate articles retrieved through multiple search engines. Another 2946 irrelevant articles were excluded based on the titles and abstracts. Then the full texts of the 125 remaining articles were reviewed, thirteen RCTs (1993 patients) were published from 2010 to 2016, and 6 BLVR procedures, sham control, or MC compared (Fig. 1 and Table 1). The search strategy and results were given in the appendix page 5. The full references for all trials were given in the appendix page 3. The mean study sample size was 145 participants, ranging between 46 and 321 patients. Overall, 1157 participants were randomly assigned to BLVR, 533 participants to MC, and 303 to sham control. The median duration of the acute treatment was 27 weeks, ranging between 12 and 48weeks. The basic characteristics of the included studies were presented in Table 1.
Figure 1.
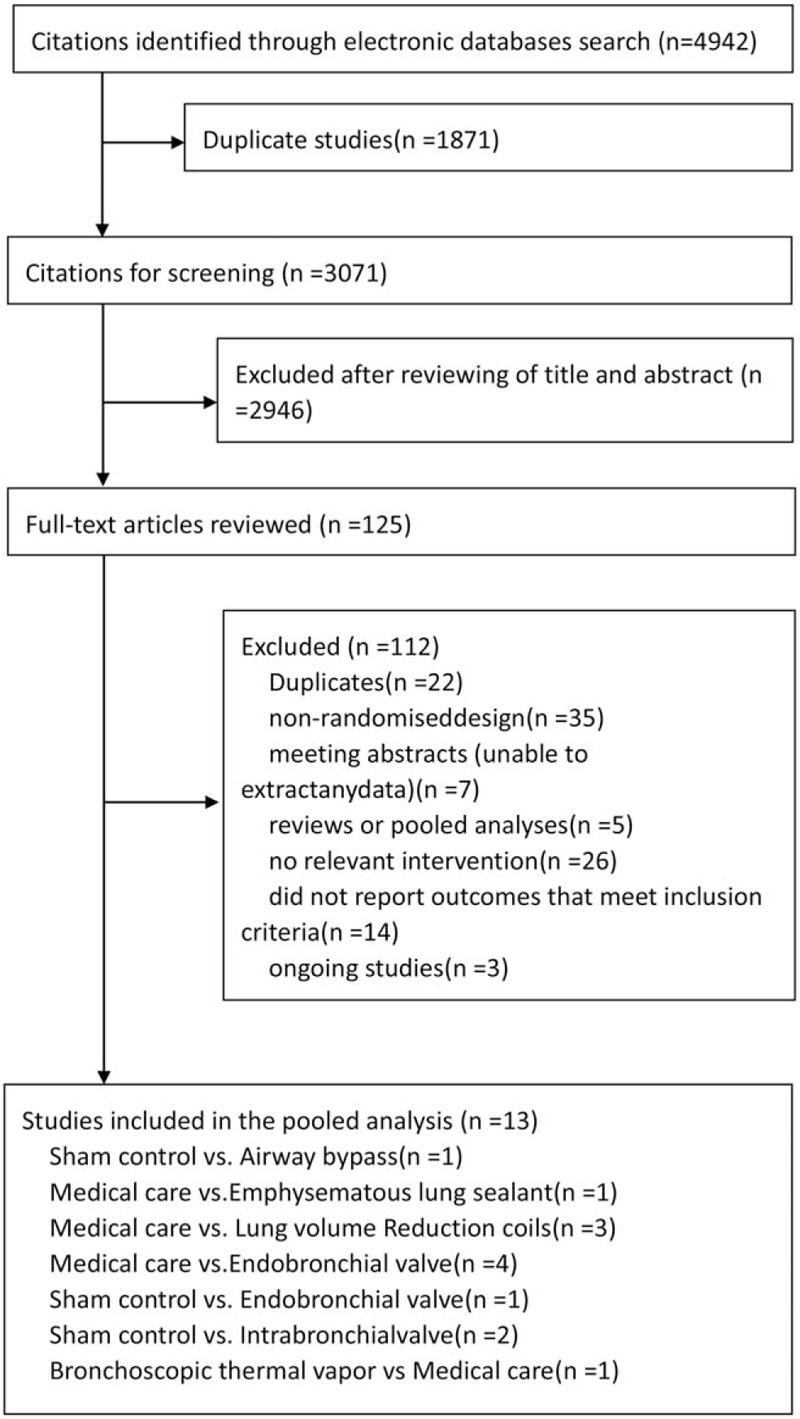
Study selection. RCT=randomised controlled trial.
Table 1.
Full references for all trials are given in the appendix (p 14).
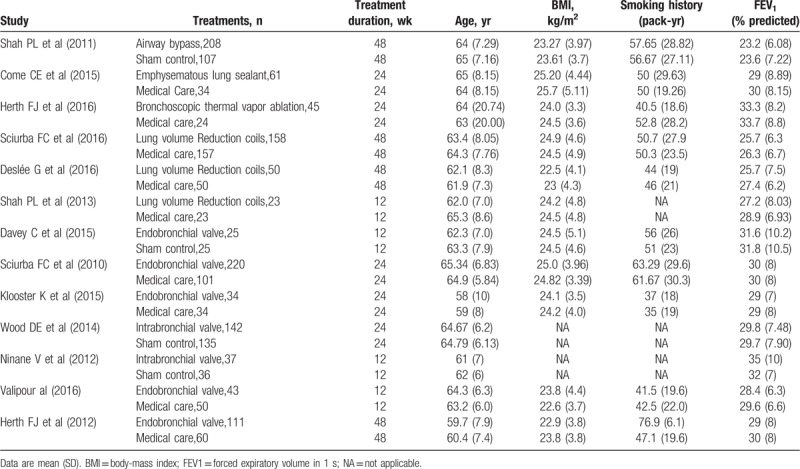
Figure 2 showed the network of eligible comparisons for mortality at end of follow-up. For graphical representation of the other networks see appendix 4. The width of the lines is proportional to the number of trials comparing every pair of treatments, and the size of every circle is proportional to the number of randomly assigned participants (sample size). The results from pair wise metaanalysis for each outcome about numbers, estimates, and heterogeneity see appendix 10. The comparison-adjusted funnel plot for each outcome from the network meta-analysis was in appendix 5. The contribution plot for each outcome from the network meta-analysis was in appendix 6. The contribution plot for mortality, adverse events, percent change in FEV1, and units SGRQ change from baseline in all comparisons of the network suggested that the comparison of EBV versus MC had the largest contribution in the entire network (24.2%). The contribution plot for meters change from baseline in 6MWD in all comparisons of the network suggested that the comparison of EBV versus MC or sham control had the largest contribution in the entire network (25.0%).
Figure 2.
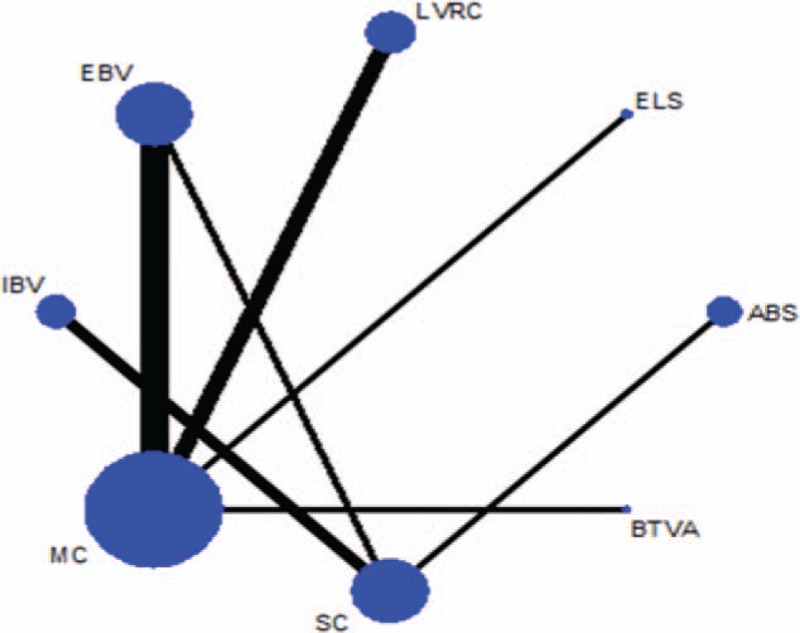
Network of eligible comparisons for mortality at end of follow-up. The width of the lines is proportional to the number of trials comparing every pair of treatments, and the size of every circle is proportional to the number of randomly assigned participants (sample size). ABS = airway bypass; BTVA = bronchoscopic thermal vapor ablation; EBV = endobronchial valve; ELS = emphysematous lung sealant; IBV = intrabronchial valve; LVRC = lung volume reduction coils; MC = medical care; SC = sham control.
The results of the network meta-analyses for the primary outcomes were presented as a league table in Figures 3 and 4. MC and EBV were also associated with a decreased proportion of mortality compared with MC LVRC alone (OR -0.38, 95% CI -1.16 to 0.41 and OR -0.50, 95% CI -1.68 to 0.68, respectively). The actual number of patients with mortality was in Table 2 and adverse events were in Table 3. BTVA and EBV were associated with a significantly change in SGRQ compared with MC alone (SMD -0.74, 95% CI -1.43 to -0.05 and SMD 0.44, 95% CI 0.11 to 0.78, respectively). BTVA significantly improved percent change in FEV1 compared with MC (SMD 0.99, 95% CrI 0.37 to 1.62), intra-bronchial valve (IBV) (SMD 1.25, 95% CrI 0.25 to 2.25), and LVRC (SMD 0.72, 95% CrI 0.03 to 1.40). MC was associated with a significantly lower improving percent change in FEV1 than EBV (SMD -0.45, 95% CrI -0.69 to -0.20). Results for secondary outcomes of exercise capacity and adverse events see appendix (page 28–31). ELS was associated with a greater improvement in 6MWD in COPD patients with predominant emphysema compared with other four BLVR procedures, sham control, and MC (SMD ranging between -1.96 and 1.99).In addition, MC was associated with less improvement in 6MWD in patients with emphysema than LVRC (SMD -0.38, 95% CrI -0.75 to -0.01) and EBV (SMD -0.39, 95% CrI -0.71 to -0.07). MC was associated with lower adverse events compared with IBV (OR -2.5, 95% CrI -4.70 to -0.29), EBV (OR-1.73, 95% CrI -2.37 to -1.09),LVRC (OR -0.76, 95% CrI -1.24 to -0.28), ELS (OR -1.53, 95% CrI -2.66 to -0.39), and airway bypass (OR -1.57, 95% CrI -3.74 to 0.61). LVRC showed lower adverse events than ELS (OR -0.77, 95% CrI -2.00 to 0.47).
Figure 3.
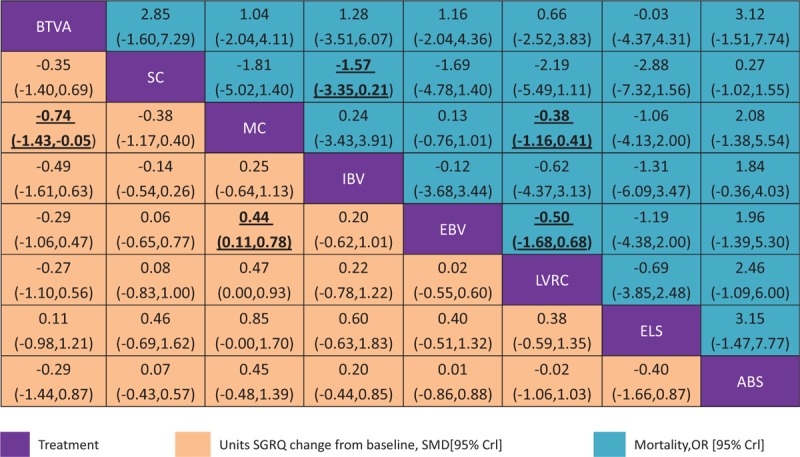
Network meta-analysis of units SGRQ change from baseline and mortality at end of follow-up Comparisons should be read from left to right. The SGRQ and mortality estimate is located at the intersection often column-defining treatment and the row-defining treatment. For mean overall change in SGRQ, an SMD below 0 favours the column-defining treatment. For mortality, an OR below 1 favours the row-defining treatment. To obtain SMDs for comparisons in the opposing direction, negative values should be converted into positive values and vice versa. To obtain ORs for comparisons in the opposing direction, reciprocals should be taken. Significant results are in bold and underlined. BTVA=bronchoscopic thermal vapor ablation. ABS = airway bypass; EBV = endobronchial valve; ELS = emphysematous lung sealant; IBV = intrabronchial valve; LVRC = lung volume reduction coils; MC = medical care; OR = odds ratio; SC = Sham control; SGRQ = St George's respiratory questionnaire; SMD = standardized mean difference.
Figure 4.
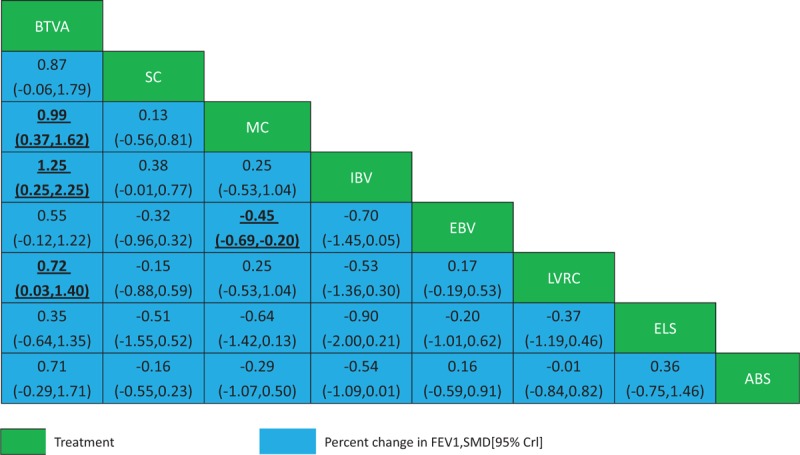
Network meta-analysis of percent change in FEV1. Comparisons should be read from left to right. The percent change in FEV1 estimate is located at the intersection of the column-defining treatment and the row-defining treatment. For percent change in FEV1, an SMD below 0 favours the row-defining treatment. To obtain SMDs for comparisons in the opposing direction, negative values should be converted into positive values and vice versa. Significant results are in bold and underlined. ABS = airway bypass; BTVA = bronchoscopic thermal vapor ablation; EBV = endobronchial valve; ELS = emphysematous lung sealant; FEV1 = forced expiratory volume in 1 s; IBV = intra-bronchial valve; LVRC = lung volume reduction coils; MC = medical care; SC = Sham control; SMD = standardized mean di-erence.
Table 2.
Number of patients with mortality according to study treatment.
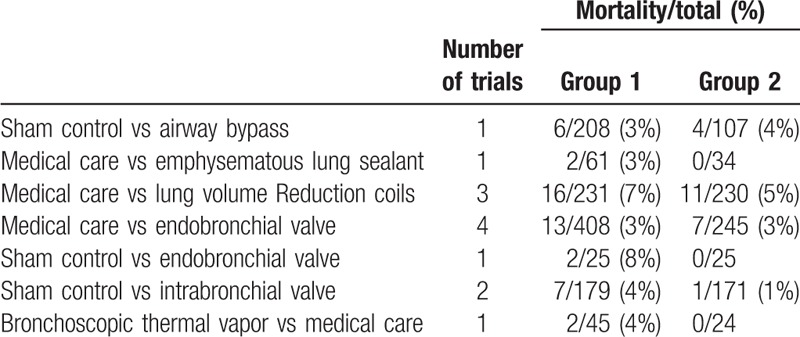
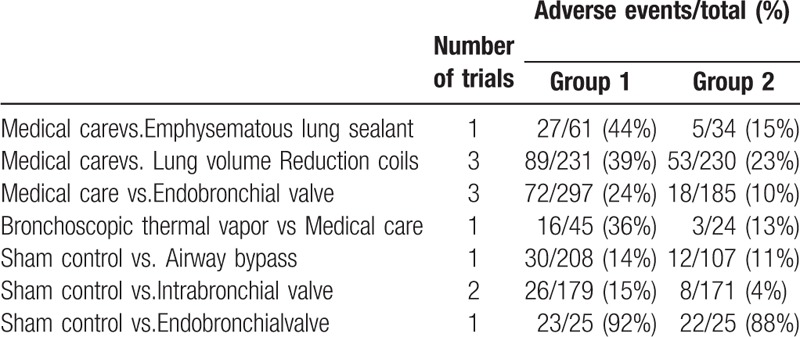
Table 3.
Number of patients with adverse events according to study treatment.
The test of inconsistency from the node-splitting model showed no significant differences between some comparisons in mortality, adverse events, the percent change in FEVI, meters change from baseline in 6MWD, and units SGRQ change from baseline see appendix 7. The comparison-adjusted funnel plot for each outcomes were not suggestive of any publication bias see appendix see appendix 5, . The comparison-adjusted funnel plot for mortality, adverse events, percent change in FEV1, meters change from baseline in 6MWD, and units SGRQ change from baseline in all comparisons appeared symmetric, implying the absence of small study effects in the network.
The ranking of treatments based on estimated probability plots and SUCRAs see appendix 9. EBV (47.3%), airway bypass (13.0%) and IBV (54.3%) showed lower SUCRA value for mortality compared with LVRC, ELS and BTVA.LVRC (28.2%), airway bypass (57.7%) and BTVA (52.5%) showed lower SUCRA value for adverse events compared with EBV, ELS and IBV. ELS (73.3%), EBV (66.6%) and BTVA (93.7%) showed higher SUCRA value for FEV1 improvement compared with LVRC, airway bypass, and IBV. ELS (99.4%), EBV (73.5%) and LVRS (71.0%) have higher SUCRA value for change in 6MWD than IBV and airway bypass. For change in SGRQ scores, IBV (68.1%), EBV (47.7%), and LVRS (47.0%) were better than other three BLVR. According to GRADE, the quality of evidence for primary outcomes (mortality at end of follow-up, percent change in FEV1, and units SGRQ change from baseline) were moderate inmost comparisons see appendix 11.
5. Discussion
Different strategies of BLVR have been studied. LVRC is designed to enhance lung recoil, compress emphysematous tissue, and improve ventilatory mechanical function.[18] EBV is designed to mimic the physiological effects of LVRS by unilaterally excluding target diseased lobe of the lung. IBV is small umbrella-shaped devices designed to limit airflow to distal diseased lobe of the lung.[19] ELS are designed to facilitate deflation through irreversibly blocking small airways and collateral channels.[20] Small bronchial holes reinforced by stents were created next to severely emphysematous regions to reduce lung hyperinflation through improved collateral ventilation (airway bypass).[21] BTVA induces a localized inflammatory response by delivering heated water vapour to targeted diseased lobe of the lungs, bringing about volume reduction.[22] Previous trials of BLVR procedures were very unlikely to directly compare different strategies of BLVR. A network metaanalysis can be very informative to different strategies of BLVR. In our present studies, we deemed 13 RCT trials eligible. The duration of the acute treatment was ranged between 12 and 48weeks. We found that the quality of evidence was rated as moderate in most comparisons. Our results provide a comprehensive and systematic analyze evidence for BLVR treatments in patients with emphysema by comparing with MC and sham control.
Pharmacologic therapy does not lead to a repair of emphysema and long-term decline of lung function as well as survival time of COPD patients.[23] Alternative minimally invasive approaches using BLVR have been at the forefront of these developments. BLVR might have benefits that are comparable with pharmacologic therapy. In our present analysis, BTVA significantly improved percent change in FEV1 compared with MC, IBV, and LVRC. EBV was associated with a significantly improving percent change in FEV1compared with MC. BTVA and EBV were associated with a significantly change in SGRQ compared with MC. LVRC, ELS and EBV were also associated with a greater improvement compared with MC in 6MWD in COPD patients with predominant emphysema, but not in IBV and Airway bypass (Supplementary appendix 8 to the manuscript, Fig. 4). SUCRA value for providing the hierarchy of BLVR on emphysema, BTVA has the best SUCRA value for FEV1 improvement. ELS and EBV also have higher SUCRA value for FEV1 improvement. IBV, EBV and LVRC have high SUCRA value for the change in 6MWD in patients with predominant emphysema. Notably, our data suggested that EBV had noticeable beneficial effects on the improvement of FEV1, 6MWDand SGRQ in different strategies of BLVR.
Although medical treatment regiment like inhaled corticosteroid increased the risk of osteoporosis and pneumonia,[24–26] pharmacologic therapy has low severe adverse events. Moreover, robust evidence suggests a safety profile of Long-acting beta2-agonist which has been considered as potentially proarrhythmic agent.[27,28] It has been showed that the procedures of lung volume reduction were associated with risks of early mortality and adverse events. However, long-term mortality after lung volume reduction may be lower than standard MC.[10,12] In our studies, MC was associated with a significantly lower proportion of adverse events compared with IBV, EBV, LVRC, ELS, and airway bypass. In different strategies of BLVR, LVRC showed low adverse events compared with ELS. Adverse events observed in network meta-analysis had no significant difference among LVRC, IBV, EBV, and BTVA (Supplementary appendix 8 to the manuscript, Fig. 3). EBV showed a decreased proportion of mortality compared with LVRC. However, IBV was associated with a significantly greater proportion of mortality compared with sham control. LVRC was also associated with a significantly greater proportion of mortality compared with MC (Fig. 3). In SUCRA rankings for different strategies of BLVR and MC, EBV and airway bypass have the lower SUCRA value for mortality compared with MC. But ELS, LVRC, IBV, and BTVA have higher SUCRA value for mortality compared with MC. Indeed, MC exhibited the lowest SUCRA value for adverse events. However, EBV exhibited decreased mortality compared with MC. Our results provided a strong evidence of EBV, which was also associated with lower mortality compared with MC.
This study has some limitations. In the GRADE framework, the comparisons for mortality at end of follow-up, percent change in FEV1, and units SGRQ change from baseline were assessed as moderate. Those may restrict the interpretation of the improvement inFEV1, mortality, and SGRQ. The evaluation of both heterogeneity and inconsistency is also important to ensure valid results from a network meta-analysis.[29] In our present studies, a node-splitting analysis was performed to assess evidence consistency. We did not find inconsistency for mortality, adverse events, FEV1 improvement, 6MWD, and SGRQ in direct and indirect comparisons. Even though we identified both direct and indirect evidence from these relevant trials, we still faced whether our analytical results were credible enough for clinical directive significance. Unfortunately, there still exist limitations for clinical directive significance in this network meta-analysis. Under our rigorous screenings, only thirteen relevant published trials were included in our estimate for addressing this important but inconclusive issue of treatment option to participants suffering from emphysema. The efficiency and reliability of these data contained in these randomized trials are uncertain. Network meta-analysis is a statistical method integrating massive features and data so that we lack of definite individual patient-level data, the accuracy and reliability of published information is questionable.[30] The potential bias cannot be ruled out because of the situation of selective reporting, overestimate of treatment efficacy or small sample size.[7,31]
In summary, this comprehensive network-meta analysis provided some evidences that BLVR had noticeable beneficial effects on the improvement of FEV1, 6MWDand SGRQ. BTVA, IBV, LVRC, and EBV significantly improved FEV1 compared with MC. BTVA and EBV were associated with a significantly change in SGRQ compared with MC. LVRC, ELS and EBV were associated with a greater improvement compared with MC in 6MWD in patients with emphysema as well. Our results also showed that MC was associated with a significantly lower proportion of adverse events compared with IBV, EBV, LVRC, ELS, and airway bypass. Importantly, EBV had noticeable beneficial effects on the improvement of FEV1, 6MWD and SGRQ, and was associated with lower mortality compared with MC in different strategies of BLVR. We further found that the evidence of comparisons for primary outcomes was assessed as moderate in the GRADE framework. Those may restrict the interpretation of network-meta analysis.
Author contributions
Conceptualization: Li Guoping.
Data curation: Wu Xu, Junyi Wang.
Formal analysis: Wu Xu, Xiang He.
Methodology: Dehong Wu.
Writing – original draft: Li Guoping, Wu Xu, Junlan Wang.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: 6 MWD = 6-minute walking distance, BLVR = bronchoscopic lung volume reduction, BTVA = bronchoscopic thermal vapor ablation, COPD = chronic obstructive pulmonary disease, EBV = endobronchial valve, ELS = emphysematous lung sealant, IBV = intra-bronchial valve, LVRC = lung volume reduction coils, MC = medical care, RCT = randomized clinical trials, SGRQ = St George's respiratory questionnaire.
How to cite this article: Xu W, Wang J, He X, Wang J, Wu D, Li G. Bronchoscopic lung volume reduction procedures for emphysema: A network meta-analysis. Medicine. 2020;99:5(e18936).
This project was supported by National major research program on Natural Science of China (91542164).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Collaborators GBDCRD. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med 2017;5:691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fragoso CA. Epidemiology of chronic obstructive pulmonary disease (COPD) in aging populations. COPD 2016;13:125–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Niewiadomska E, Kowalska M. Chronic obstructive pulmonary disease (COPD) - epidemiology in Silesian voivodeship. Przegl Epidemiol 2017;71:237–50. [PubMed] [Google Scholar]
- [4].Glaab T, Hohlfeld JM, Jorres RA, et al. [Pathomechanisms in chronic obstructive pulmonary disease (COPD)]. Med Klin (Munich) 2006;101:951–6. [DOI] [PubMed] [Google Scholar]
- [5].Davey C, Zoumot Z, Jordan S, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi trial): study design and rationale. Thorax 2015;70:288–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Aggelou K, Siafakas N. Medical lung volume reduction for severe emphysema: a review. Respir Med 2017;131:141–7. [DOI] [PubMed] [Google Scholar]
- [7].Copetti M, Fontana A, Graziano G, et al. Advances in meta-analysis: examples from internal medicine to neurology. Neuroepidemiology 2014;42:59–67. [DOI] [PubMed] [Google Scholar]
- [8].Chahla M, Larson CD, Parekh KR, et al. Transpleural ventilation via spiracles in severe emphysema increases alveolar ventilation. Chest 2016;149:e161–7. [DOI] [PubMed] [Google Scholar]
- [9].Cetti EJ, Moore AJ, Geddes DM. Collateral ventilation. Thorax 2006;61:371–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].van Agteren JE, Carson KV, Tiong LU, et al. Lung volume reduction surgery for diffuse emphysema. Cochrane Database Syst Rev 2016;10:CD001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].van Agteren JE, Hnin K, Grosser D, et al. Bronchoscopic lung volume reduction procedures for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017;2:CD012158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Franzen D, Weder W. Lung volume reduction for emphysema. Lancet Respir Med 2017;5:e23. [DOI] [PubMed] [Google Scholar]
- [13].Peters JL, Sutton AJ, Jones DR, et al. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med 2007;26:4544–62. [DOI] [PubMed] [Google Scholar]
- [14].Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932–44. [DOI] [PubMed] [Google Scholar]
- [15].Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–71. [DOI] [PubMed] [Google Scholar]
- [16].Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS One 2014;9:e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Higgins J, Green SE. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The cochrane collaboration (Eds) Naunyn-Schmiedebergs archiv für experimentelle pathologie und pharmakologie 2011;5:S38. [Google Scholar]
- [18].Sciurba FC, Criner GJ, Strange C, et al. Effect of endobronchial coils vs usual care on exercise tolerance in patients with severe emphysema: the RENEW randomized clinical trial. JAMA 2016;315:2178–89. [DOI] [PubMed] [Google Scholar]
- [19].Mahajan AK, Doeing DC, Hogarth DK. Isolation of persistent air leaks and placement of intrabronchial valves. J Thorac Cardiovasc Surg 2013;145:626–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Come CE, Kramer MR, Dransfield MT, et al. A randomised trial of lung sealant versus medical therapy for advanced emphysema. Eur Respir J 2015;46:651–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Weder W, Russi EW. Lung-volume reduction by airway bypass. Lancet 2011;378:966–7. [DOI] [PubMed] [Google Scholar]
- [22].Herth FJ, Valipour A, Shah PL, et al. Segmental volume reduction using thermal vapour ablation in patients with severe emphysema: 6-month results of the multicentre, parallel-group, open-label, randomised controlled STEP-UP trial. Lancet Respir Med 2016;4:185–93. [DOI] [PubMed] [Google Scholar]
- [23].Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775–89. [DOI] [PubMed] [Google Scholar]
- [24].Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med 2014;371:1285–94. [DOI] [PubMed] [Google Scholar]
- [25].McAllister DA, Maclay JD, Mills NL, et al. Arterial stiffness is independently associated with emphysema severity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;176:1208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yang IA, Clarke MS, Sim EH, et al. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012;CD002991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest 2004;125:2309–21. [DOI] [PubMed] [Google Scholar]
- [28].Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J 2017;49. [DOI] [PubMed] [Google Scholar]
- [29].van Valkenhoef G, Dias S, Ades AE, et al. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods 2016;7:80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Le Noury J, Nardo JM, Healy D, et al. Restoring study 329: efficacy and harms of paroxetine and imipramine in treatment of major depression in adolescence. BMJ 2015;351:h4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Whittington CJ, Kendall T, Fonagy P, et al. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet 2004;363:1341–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


