Abstract
Background:
To systematically evaluate the clinical efficacy of salbutamol treatment in infants with bronchiolitis.
Methods:
A systematic review and meta-analysis of randomized controlled trials (RCTs) investigating the use of salbutamol in infants with bronchiolitis was performed. The Cochrane Risk of Bias Assessment Tool was used to evaluate the quality of RCTs. Data were extracted and meta-analyzed using STATA version 12.0 (StataCorp, College Station, TX).
Results:
Thirteen RCTs, including a total of 977 participants, were assessed in the present meta-analysis. Results indicated that salbutamol therapy for bronchiolitis in infants led to an increase in respiratory rate (weighted mean difference [WMD] 2.26 [95% confidence interval {CI} 0.36–4.16]) and higher heart rate (WMD 12.15 [95% CI 9.24–15.07]). However, as a selective β2-agonist, salbutamol did not improve the clinical severity score of infants with bronchiolitis (WMD –0.11 [95% CI –0.26 to 0.03]), length of hospital stay (WMD 0.12 [95% CI –0.32 to 0.56]), or oxygen saturation (WMD 0.20 [95% CI –0.35 to 0.75]).
Conclusion:
Based on the results of this systematic review, the use of salbutamol had no effect on bronchiolitis in children <24 months of age. Moreover, the treatment can also lead to side effects, such as high heart rate. As such, salbutamol should not be recommended for treatment of bronchiolitis in infants.
Keywords: bronchiolitis, infants, salbutamol therapy
1. Introduction
Bronchiolitis is a disease of the lower respiratory tract, generally caused by viral infection, and most commonly occurs in infants in the first 2 years of life.[1] In the United States, the diagnosis of bronchiolitis in children <12 months of age results in 57,000 to 172,000 hospitalizations annually.[2] Human respiratory syncytial virus (RSV) is responsible for most cases of infant bronchiolitis, while other viral pathogens, including parainfluenza, adenovirus, human metapneumovirus, influenza, and rhinovirus are less commonly reported in cases of bronchiolitis involving young children.[3] Infection with RSV does not offer long-term immunity, and commonly leads to recurrence of bronchiolitis throughout life. In 2015, it was estimated that RSV accounted for 28% episodes of all acute lower respiratory tract infection globally, with an overall mortality rate of 13% to 22% in young children.[4] The cause of bronchiolitis also varies according to geographical region and income. For example, in the United States, fewer than 100 young children with bronchiolitis die each year due to RSV infection.[5] Young children with bronchiolitis typically present with fewer symptoms of respiratory tract illness (cough, wheezing, tachypnea, and increased respiratory effort) after 2 to 4 days of low-grade fever, rhinorrhea, and nasal congestion.[2,6,7] The primary findings in bronchiolitis pathophysiology include acute inflammation, mucosal and submucosal edema, necrosis of epithelial cells of the small airways, increased mucus secretion, and impaired mucus clearance. These changes eventually obstruct the airways, leading to impaired gas exchange in patients.[8,9]
Although bronchiolitis can threaten the health of young children, it is difficult to define the best treatment for infants with this illness because no available therapies can shorten the course of bronchiolitis or the length of hospital stay.[2] The mainstay management for young children with bronchiolitis is supportive care when necessary, including supplementary oxygen, and adequate nutrition and hydration. Corticosteroids and antibacterial agents are generally not recommended.[1] Although a previous systematic review suggested that use of nebulized epinephrine for bronchiolitis may have the effect of reducing hospital admissions, there is little to no evidence supporting a shortening of the length of hospital stay.[10] The effect of nebulized hypertonic saline (3%) for bronchiolitis is actively being discussed. A recent Cochrane review reported that hypertonic saline (3%) appears to be safe and reduces the length of hospitalization in infants, with an average reduction of 10 hours.[3] Currently, evidence supporting the routine use of epinephrine or nebulized hypertonic saline remains insufficient. Beta-2 (β2) agonists, such as salbutamol (albuterol), are not recommended because they do not shorten the length of hospital stay; however, they may slightly improve clinical symptom scores in the short term.[11,12] Several studies have suggested that salbutamol can increase heart rate and oxygen saturation levels in infants with bronchiolitis.[13,14] An updated meta-analysis, including the most recent trials of salbutamol, may be helpful for better understanding of the role of salbutamol in the treatment of bronchiolitis in infants. In the present study, we searched the literature for published studies in attempt to explore the efficacy of salbutamol treatment in young children with acute bronchiolitis.
2. Methods
2.1. Data sources and search strategy
The PubMed, Embase, Web of Science, CBM (Chinese Biomedical Literature Database), and Chinese National Knowledge Infrastructure (CNKI) databases were searched for all relevant articles published before January 1, 2019, without any language restrictions. The following search terms were adopted in the meta-analysis: (salbutamol or albuterol) and (infants with bronchiolitis). In addition, 2 investigators (ZC and YL) performed a manual search of the reference lists of all potentially eligible studies.
2.2. Inclusion criteria
Only randomized controlled trials (RCTs) fulfilling the following criteria were included in the present systematic review: patients <24 months of age with acute bronchiolitis; sufficient data to calculate weighted mean differences (WMDs) and corresponding 95% confidence intervals (CIs); and the correlation between salbutamol treatment and children with acute bronchiolitis was estimated. Case reports, systematic reviews, and letters to the editor were excluded. All participants were included without limitations on sex, ethnicity, disease course, and severity of disease. All disagreements between/among the investigators were resolved though discussion and consensus.
2.3. Data extraction
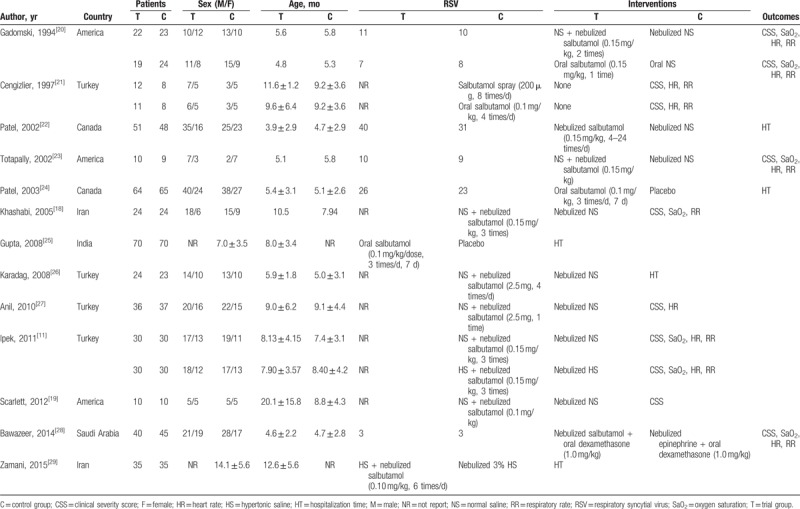
All relevant data were extracted from the 13 included studies, and independently entered into a predesigned and standardized spreadsheet file (Excel, Microsoft Corporation, Redmond, WA) by 2 investigators (ZC and YL). For controlled trials, the full text article was read before deciding whether it should be included. The 2 investigators organized their quality evaluations independently, and consensus was reached through discussion to resolve any disagreements. Extracted information from the identified RCTs included the first author's name in the original article, year of publication, number of patients, sex, age, inclusion period, method of evaluating heart rate, respiratory rate, length of hospital stay, oxygen saturation, and clinical store at different years in infants with bronchiolitis. Only the most recent or complete study was enrolled if a particular article was published in duplicate. All disagreements were thoroughly discussed until consensus was reached.
2.4. Quality assessment
The Cochrane Risk of Bias Assessment tool was used to perform quality evaluation of the included RCTs, which included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias.[15] Every RCT was rated accordingly, as follows: “high risk of bias” referred to incorrect random methods, no allocation concealment, or blinding; “unclear risk of bias” referred to no description in the text with which to assess bias; and “low risk of bias” referred to correct randomization methods, appropriate blinding without being violated through implementation, and detailed description in the RCTs.
2.5. Statistical analysis
The correlation between salbutamol treatment and bronchiolitis in infants was evaluated by calculating relative ratios and corresponding 95% CIs. Between-study heterogeneity in this meta-analysis was assessed using the I2 statistic, a quantitative measure describing inconsistency across studies measured from 0% to 100%.[16] When heterogeneity was significant (I2 > 50%), the potential sources of heterogeneity were identified by analyzing the methodological variability of the included studies or by omitting studies one by one to evaluate the impact of a single trial on the overall pooled estimate. If heterogeneity across studies persisted, a random-effect model was used to calculate the pooled relative ratio and corresponding 95% CI. When the heterogeneity was low (I2 < 50%), a fixed-effect model was applied. The Egger test was used to evaluate the possibility of publication bias. STATA version 12.0 software (StataCorp, College Station, TX) was used for statistical analysis, and differences with P < .05 were considered to be statistically significant.
2.6. Ethics statement
The present meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[17] Ethics approval and patient consent were not required for this meta-analysis because the data were merely gathered from RCTs retrieved in the literature search; as such, all personal patient data were completely anonymous.
3. Results
3.1. Analysis of the literature and assessment of quality
In total, 2425 relevant articles were retrieved through searching the titles and abstracts, while duplications, apparently non-relevant articles, nonclinical studies, reviews, and commentaries were excluded. A total of 45 studies investigating salbutamol treatment efficacy in infants with bronchiolitis was identified. After reading the full texts of potentially relevant studies, 32 articles were removed because they did not provide sufficient data to calculate WMDs and corresponding 95% CIs. Ultimately, 13 articles, published between 1994 and 2015, were included in the present meta-analysis.[11,18–29] The detailed selection process for the included studies is presented as a flow diagram in Fig. 1. The age of the patients in the trials ranged from 3 to 20 months. The most often used dose of salbutamol was 0.1 mg/kg. Additional details regarding the individual trials are summarized in Table 1.
Figure 1.
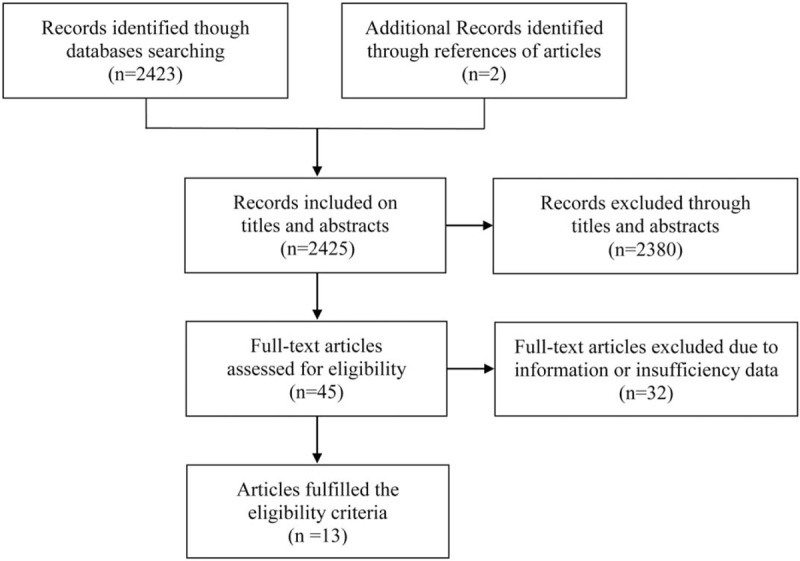
Selection process for eligible studies in the present meta-analysis.
Table 1.
Main characteristics of included studies.
The Cochrane Risk of Bias Assessment Tool was used to perform a quality evaluation on the included trials. The results revealed that none of these included studies were of low quality in terms of selection and blinding data; however, 2 RCTs were evaluated to have a high risk of reporting incomplete outcome data. Overall, however, the quality of the included trials was generally high. Detailed information regarding risk of bias is shown in Fig. 2.
Figure 2.
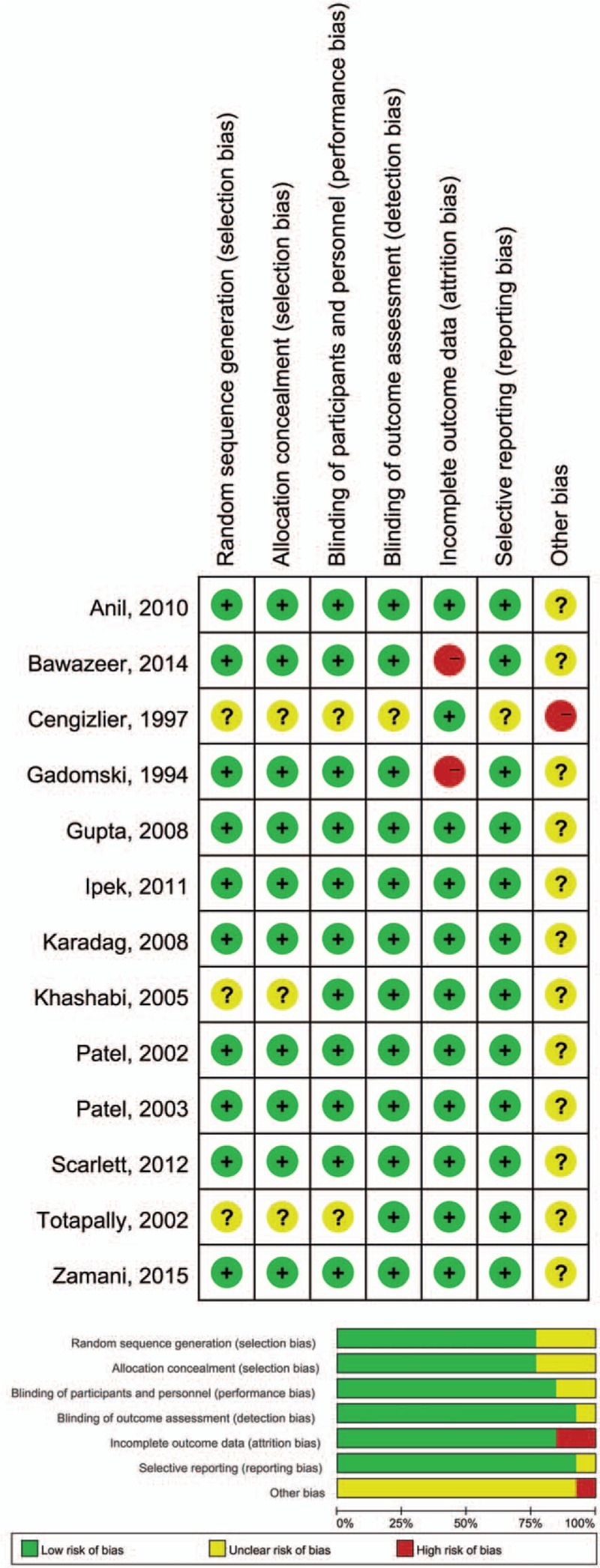
Risk of bias summary.
3.2. Effects on respiratory rate
A total of 5 studies provided data regarding respiratory rate in the trial and control groups.[11,18,20,23,28] Pooling of data revealed that infants treated with salbutamol experienced an overall statistically significant increase in respiratory rate (WMD 2.26 [95% CI 0.36–4.16]) (Fig. 3). A fixed-effect model was used for statistical analysis, and no significant heterogeneity was found (I2 = 0.0%; P for heterogeneity = .492).
Figure 3.
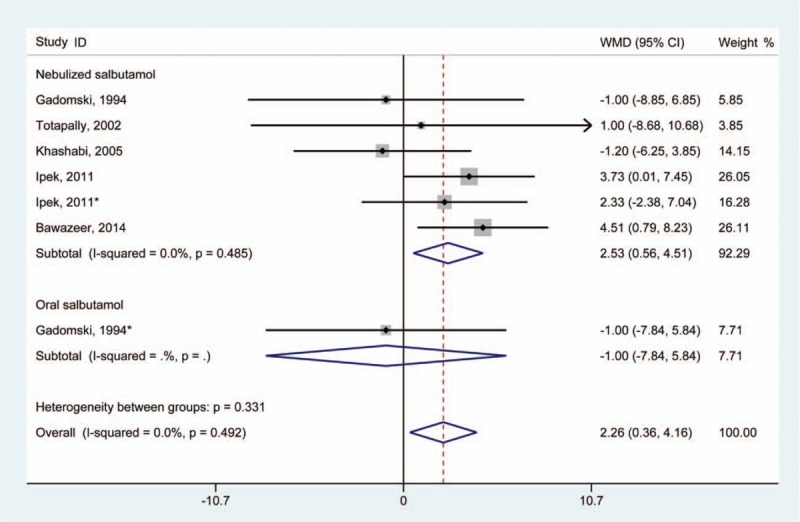
Results of meta-analysis regarding respiratory rate associated with salbutamol in the treatment of infants with bronchiolitis. ∗Same article but different dataset.
3.3. Effects on heart rate
Five studies provided data regarding heart rate in the treatment and control groups.[11,20,23,27,28] The aggregated results suggested that salbutamol treatment in infants was significantly associated with a higher heart rate compared with placebo (WMD 12.15 [95% CI 9.24–15.07]) (Fig. 4). A fixed-effect model was used for statistical analysis and statistically significant heterogeneity was found among these studies (I2 = 90.3%; P for heterogeneity = .000). Sensitivity analysis demonstrated that the results were stable and unchanged.
Figure 4.
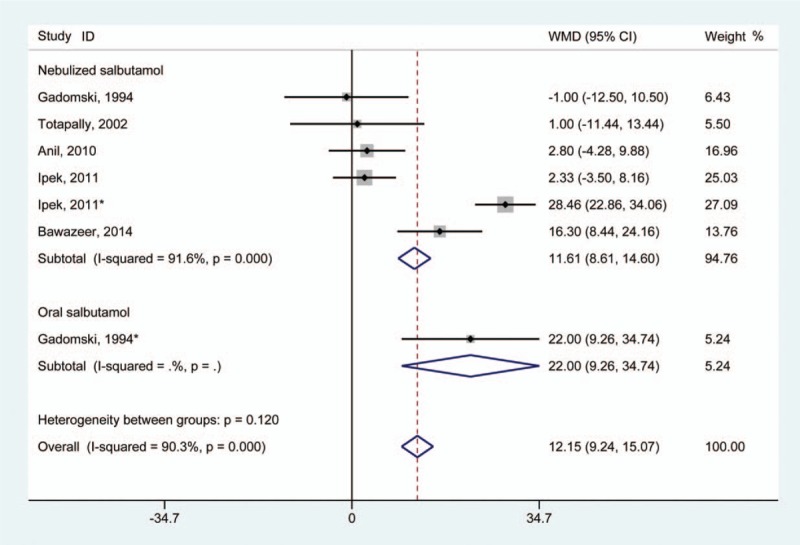
Results of meta-analysis regarding heart rate associated with salbutamol in the treatment of infants with bronchiolitis. ∗Same article but different dataset.
3.4. Effects on oxygen saturation
Five studies reported data regarding oxygen saturation in infants.[11,18,20,23,28] Compared with infants without salbutamol treatment, the use of salbutamol failed to demonstrate statistical improvement in oxygen saturation level in infants (WMD 0.20 [95% CI –0.35 to 0.75]) (Fig. 5). A fixed-effect model was adopted and revealed no clear heterogeneity among the studies (I2 = 10.0%; P for heterogeneity = .353).
Figure 5.
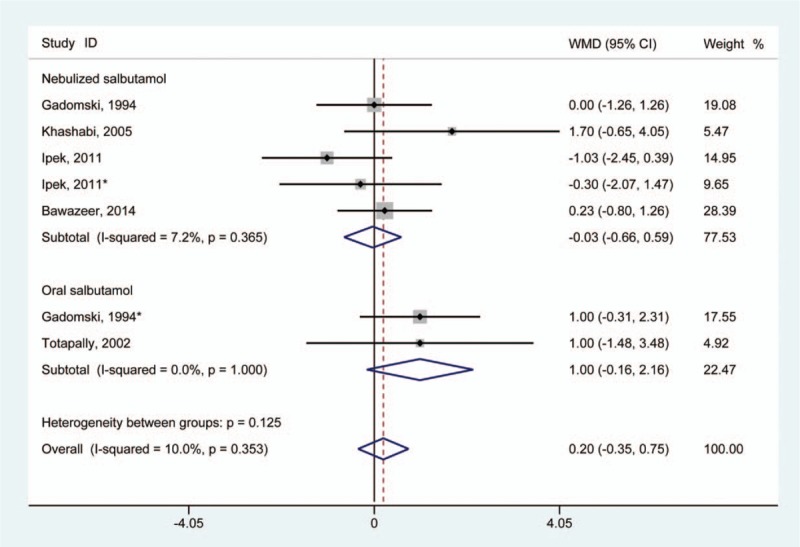
Results of meta-analysis regarding oxygen saturation associated with salbutamol in the treatment of infants with bronchiolitis. ∗Same article but different dataset.
3.5. Effects on clinical severity score
In total, 8 studies provided data regarding the evaluation of clinical severity score.[11,18–21,23,27,28] Meta-analysis of 6 of these studies revealed no evidence of statistically significant improvement in clinical severity score in infants treated with salbutamol compared with those without treatment (WMD –0.11 [95% CI –0.26 to 0.03]) (Fig. 6). A fixed-effect model was adopted for statistical analysis, and no significant heterogeneity was found (I2 = 40.5%; P for heterogeneity = .079).
Figure 6.
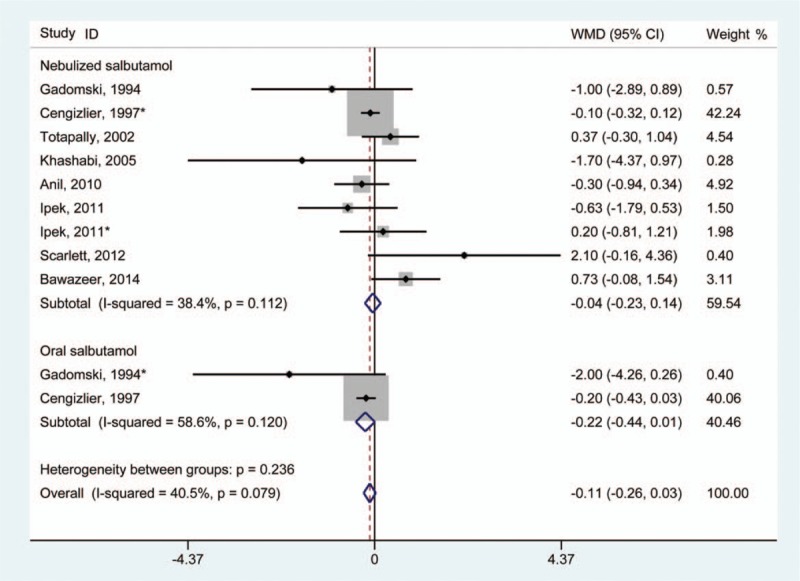
Results of meta-analysis regarding clinical severity score associated with salbutamol in the treatment of infants with bronchiolitis. ∗Same article but different dataset.
3.6. Effects on length of hospitalization
Only 4 studies reported data regarding the length of hospitalization.[22,24–26] Result of analysis suggested that neither nebulized or oral salbutamol treatment demonstrated effects on infants with bronchiolitis in terms of reducing length of hospitalization compared with the control group (WMD 0.12 [95% CI –0.32 to 0.56]) (Fig. 7). A fixed-effect model was used for statistical analysis, and no significant heterogeneity was found (I2 = 0.0%; P for heterogeneity = .902).
Figure 7.
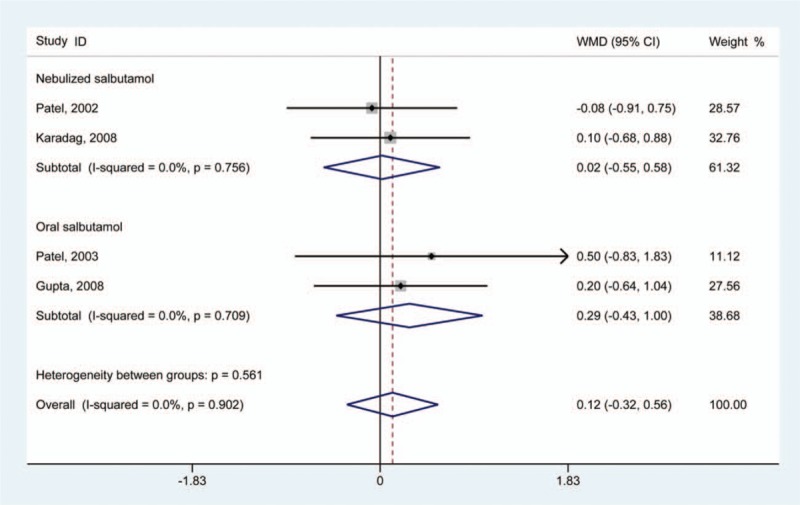
Results of meta-analysis regarding hospitalization time of salbutamol in the treatment of infants with bronchiolitis.
4. Discussion
Multiple studies have attempted to determine the positive treatment efficacy of bronchitis medications. Because airway edema and mucus plugging are the predominant pathological features of bronchiolitis, any therapy that can reduce these changes and improve the clearance of airway secretions may be beneficial. Therefore, salbutamol, as a selective β2 agonist, is a common medication studied in the treatment of asthma attacks and bronchiolitis, and many studies have attempted to explore the therapeutic relationship between salbutamol and bronchiolitis.[30]
Salbutamol has a theoretical effect on acute bronchiolitis. The tertiary butyl group in salbutamol makes it more selective for β2 receptors, which are the predominant receptors in bronchial smooth muscles. As a β2 adrenoreceptor stimulant, salbutamol also exerts some β-1 inhibition function at a high dosage level. However, our meta-analysis revealed that salbutamol can significantly increase the respiratory rate of infants with acute bronchiolitis after treatment compared with the control group. This treatment also elevates heart rate among infants with bronchiolitis, which was considered to be a side effect.[12] Moreover, treatment with salbutamol demonstrated no improvement in clinical severity score and oxygen saturation in our meta-analysis. We also found that salbutamol could not reduce the need for hospitalization nor shorten the length of hospital stay compared with the control group in infants with bronchiolitis.
A previous reappraisal and meta-analysis indicated that outpatient studies do not support the use of β2-agonist therapy for bronchiolitis in children. That study indicated that, compared with the placebo group, β2-agonist therapy had no impact on hospitalization rate and none of the event rate differences for individual trials were statistically significant. However, the present meta-analysis also demonstrated that β2-agonist therapy could result in statistically significant increases in oxygen saturation and heart rate, which resulted in different conclusions than the previous study.[31] Our review suggests that nebulized salbutamol results in no improvement in the level of oxygen saturation, and only contributes to increases in patient respiratory rate. By adding recent trials and increasing the sample size, we believe that our updated meta-analysis has better statistical power and provides convincing results that show that the efficacy of treatment with salbutamol is limited to increased respiratory rate. Administration of salbutamol does not improve oxygen saturation and does not shorten the length of hospital stay in infants with bronchiolitis.
An updated guideline from the Canadian Paediatric Society does not recommend salbutamol therapy.[8] It has been established that the clinical manifestations of wheeze in children with bronchiolitis share the same features of clinical asthma. Moreover, the pathophysiology of bronchiolitis is such that the airways are obstructed rather than constricted.[27] It also appears that infants and younger children have a deficiency in β-agonist lung receptor sites and immature bronchiolar smooth muscles. Although some studies have shown that treatment using salbutamol can improve clinical scores and oxygen saturation in infants, none have demonstrated evidence of reduction in admission rates or the length of hospital stay, which are 2 main evaluation standards.[8,32] In addition, an updated review suggested that albuterol or salbutamol are ineffective in the treatment of bronchiolitis in infants.[8] From our meta-analysis, we reached a similar conclusion in that salbutamol has no significant efficacy in the treatment of bronchiolitis in young children. Hence, we would not recommend salbutamol treatment in infants with acute bronchiolitis.
Several limitations to our meta-analysis merit consideration. First, although all included RCTs included investigated bronchiolitis in infants, overestimation, and underestimation cannot be excluded due to differences in the severity of bronchiolitis. Second, many articles were eliminated due to insufficient data to calculate the WMD and corresponding 95% CI, which may have biased our conclusions. Third, characteristic information from the studies was possibly incomplete. Although we attempted to retrieve all information from original articles, some was still unavailable.
In summary, results of the present systematic review and meta-analysis indicate that salbutamol, as an agonist, should not be recommended for therapy for bronchiolitis in children. Primary treatments for acute bronchiolitis should remain supportive care including adequate oxygen exchange, nutrition, and hydration.[3]
5. Conclusion
Current evidence indicates that salbutamol significantly increases heart rate and respiratory rate, and cannot improve clinical severity scores in infants with bronchiolitis, the length of hospital stay, or oxygen saturation. Due to the limited quality of the included studies, more high-quality studies are needed to verify this conclusion.
Acknowledgments
The authors thank department members for assistance with this study.
Author contributions
Conceptualization: Zhibo Cai.
Data curation: Zhibo Cai, Yan Lin.
Formal analysis: Jianfeng Liang.
Investigation: Zhibo Cai, Yan Lin, Jianfeng Liang.
Methodology: Yan Lin, Jianfeng Liang, Zhibo Cai.
Project administration: Yan Lin.
Resources: Yan Lin.
Software: Zhibo Cai, Yan Lin, Jianfeng Liang.
Supervision: Zhibo Cai.
Validation: Zhibo Cai, Yan Lin.
Writing – original draft: Zhibo Cai.
Writing – review & editing: Jianfeng Liang.
Footnotes
Abbreviations: CI = confidence interval, RCT = randomized controlled trial, RSV = respiratory syncytial virus, WMD = weighted mean difference.
How to cite this article: Cai Z, Lin Y, Liang J. Efficacy of salbutamol in the treatment of infants with bronchiolitis: A meta-analysis of 13 studies. Medicine. 2020;99:4(e18657).
The authors have no conflicts of interest to disclose.
References
- [1].Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014;134:e1474–502. [DOI] [PubMed] [Google Scholar]
- [2].Meissner HC. Viral bronchiolitis in children. N Engl J Med 2016;374:1793–4. [DOI] [PubMed] [Google Scholar]
- [3].Zhang L, Mendoza-Sassi RA, Wainwright C, et al. Nebulised hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database Syst Rev 2017;12:1–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017;390:946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Byington CL, Wilkes J, Korgenski K, et al. Respiratory syncytial virus-associated mortality in hospitalized infants and young children. Pediatrics 2015;135:e24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].National Collaborating Centre for Ws, Children's H. National Institute for Health and Care Excellence: Clinical Guidelines. Bronchiolitis: Diagnosis and Management of Bronchiolitis in Children. London: National Institute for Health and Care Excellence (UK) National Collaborating Centre for Women's and Children's Health; 2015. [Google Scholar]
- [7].Smith DK, Seales S, Budzik C. Respiratory syncytial virus bronchiolitis in children. Am Fam Physician 2017;95:94–9. [PubMed] [Google Scholar]
- [8].Friedman JN, Rieder MJ, Walton JM. Bronchiolitis: recommendations for diagnosis, monitoring and management of children one to 24 months of age. Paediatr Child Health 2014;19:485–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Flores P, Mendes AL, Neto AS. A randomized trial of nebulized 3% hypertonic saline with salbutamol in the treatment of acute bronchiolitis in hospitalized infants. Pediatr Pulmonol 2016;51:418–25. [DOI] [PubMed] [Google Scholar]
- [10].Hartling L, Bialy LM, Vandermeer B, et al. Epinephrine for bronchiolitis. Cochrane Database Syst Rev 2011;6:1–89. [DOI] [PubMed] [Google Scholar]
- [11].Ipek IO, Yalcin EU, Sezer RG, et al. The efficacy of nebulized salbutamol, hypertonic saline and salbutamol/hypertonic saline combination in moderate bronchiolitis. Pulm Pharmacol Ther 2011;24:633–7. [DOI] [PubMed] [Google Scholar]
- [12].Gadomski AM, Scribani MB. Bronchodilators for bronchiolitis. Cochrane Database Syst Rev 2014;6:1–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schuh S, Canny G, Reisman JJ, et al. Nebulized albuterol in acute bronchiolitis. J Pediatr 1990;117:633–7. [DOI] [PubMed] [Google Scholar]
- [14].Klassen TP, Rowe PC, Sutcliffe T, et al. Randomized trial of salbutamol in acute bronchiolitis. J Pediatr 1991;118:807–11. [DOI] [PubMed] [Google Scholar]
- [15].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Khashabi J, Salari Lak S, Karamiyar M, Mussavi H. Comparision of the efficacy of nebulized L-epinephrine, salbutamol and normal saline in acute bronchiolitis: a randomized clinical trial. Med J Islam Repub Iran 2005;19:119–25. [Google Scholar]
- [19].Elizabeth E, Scarlett SW, Amy R, et al. Tidal breathing responses to albuterol and normal saline in infants with viral bronchiolitis. Pediatr Allergy Immunol Pulmonl 2012;25:220–5. [Google Scholar]
- [20].Gadomski AM, Lichenstein R, Horton L, et al. Efficacy of albuterol in the management of bronchiolitis. Pediatrics 1994;93(6 pt 1):907–12. [PubMed] [Google Scholar]
- [21].Cengizlier R, Saraclar Y, Adalioglu G, et al. Effect of oral and inhaled salbutamol in infants with bronchiolitis. Acta Paediatr Jpn 1997;39:61–3. [DOI] [PubMed] [Google Scholar]
- [22].Patel H, Platt RW, Pekeles GS, et al. A randomized, controlled trial of the effectiveness of nebulized therapy with epinephrine compared with albuterol and saline in infants hospitalized for acute viral bronchiolitis. J Pediatr 2002;141:818–24. [DOI] [PubMed] [Google Scholar]
- [23].Totapally BR, Demerci C, Zureikat G, et al. Tidal breathing flow-volume loops in bronchiolitis in infancy: the effect of albuterol [ISRCTN47364493]. Crit Care 2002;6:160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Patel H, Gouin S, Platt RW. Randomized, double-blind, placebo-controlled trial of oral albuterol in infants with mild-to-moderate acute viral bronchiolitis. J Pediatr 2003;142:509–14. [DOI] [PubMed] [Google Scholar]
- [25].Gupta P, Aggarwal A, Gupta P, et al. Oral salbutamol for symptomatic relief in mild bronchiolitis a double blind randomized placebo controlled trial. Indian Pediatr 2008;45:547–53. [PubMed] [Google Scholar]
- [26].Karadag B, Ceran O, Guven G, et al. Efficacy of salbutamol and ipratropium bromide in the management of acute bronchiolitis--a clinical trial. Respiration 2008;76:283–7. [DOI] [PubMed] [Google Scholar]
- [27].Anil AB, Anil M, Saglam AB, et al. High volume normal saline alone is as effective as nebulized salbutamol-normal saline, epinephrine-normal saline, and 3% saline in mild bronchiolitis. Pediatr Pulmonol 2010;45:41–7. [DOI] [PubMed] [Google Scholar]
- [28].Bawazeer M, Aljeraisy M, Albanyan E, et al. Effect of combined dexamethasone therapy with nebulized r-epinephrine or salbutamol in infants with bronchiolitis: a randomized, double-blind, controlled trial. Avicenna J Med 2014;4:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zamani MA, Movahhedi M, Nourbakhsh SM, et al. Therapeutic effects of Ventolin versus hypertonic saline 3% for acute bronchiolitis in children. Med J Islam Repub Iran 2015;29:1–5. [PMC free article] [PubMed] [Google Scholar]
- [30].Chen YJ, Lee WL, Wang CM, et al. Nebulized hypertonic saline treatment reduces both rate and duration of hospitalization for acute bronchiolitis in infants: an updated meta-analysis. Pediatr Neonatol 2014;55:431–8. [DOI] [PubMed] [Google Scholar]
- [31].Flores G, Horwitz RI. Efficacy of beta2-agonists in bronchiolitis: a reappraisal and meta-analysis. Pediatrics 1997;100(2 pt 1):233–9. [DOI] [PubMed] [Google Scholar]
- [32].Bertrand P, Aranibar H, Castro E, et al. Efficacy of nebulized epinephrine versus salbutamol in hospitalized infants with bronchiolitis. Pediatr Pulmonol 2001;31:284–8. [DOI] [PubMed] [Google Scholar]


