Abstract
Background:
Although memory loss and other symptoms of dementia pose tremendous burdens on patients and societies, there is currently no cure for dementia.
Methods:
We conducted a systematic review and meta-analysis of the anti-dementia effects of Danggui-Shaoyao-San (DSS), which is derived from natural resources. We searched for randomized controlled trials (RCTs) from inception to June 2019. We searched PubMed, Embase, Korean databases (Research Information Service System and Oriental Medicine Advanced Searching Integrated System), Chinese databases (China Knowledge Resource Integrated Database and Wanfang Database), and Japanese databases (CiNii and J-STAGE). Studies were included if they were a RCT, investigated the efficacy of DSS or its modified form, and included participants with dementia. Use of DSS with other treatment (eg, acupuncture, anti-dementia drugs, etc) was included. Items of each trial were evaluated by 2 independent reviewers. Data were pooled by using random-effect models.
Results:
A total of 482 studies were identified, and 5 eligible studies for Alzheimer disease (AD) and 4 studies for vascular dementia (VD) were included in the final analysis, representing a total of 567 participants. As for AD, pooled results of the Mini-Mental State Examination (MMSE) (mean differences [MD] 4.60; 95% confidence interval [CI] 4.29, 4.91) and activities of daily living (MD 11.40; 95% CI 10.94, 11.86) favored DSS. DSS had synergistic effect with acupuncture over acupuncture alone in MMSE (MD 1.69; 95% CI 1.05, 2.34), Hasegawa Dementia Scale (MD.62; 95% CI –0.20, 1.44), and activities of daily living (MD 2.38; 95% CI 1.92, 2.85). In VD, pooled results showed a significant difference in the score of dementia scales such as MMSE and Hasegawa Dementia Scale compared with nootropic drugs. DSS significantly reduced symptoms (odds ratio 5.02, 95%, CI 2.76–9.11) in patients with VD. The respective size of each RCTs was small and some included studies were of low quality due to their limited description on methodological issues.
Conclusion:
These estimates suggest that DSS provides clinically important reductions in symptoms of AD and VD and can be a promising anti-dementia drug candidate.
Keywords: Alzheimer disease, Dangguijakyaksan, Danggui-Shaoyao-San, dementia, systematic review, Toki-shakuyaku-san, vascular dementia
1. Introduction
Dementia, also classified as neurocognitive disorder in Diagnostic and Statistical Manual of Mental Disorders Fifth Edition, is a group of degenerative neurological disorders that are disturbance of multiple higher cortical functions, and is characterized by progressive decline of cognitive function and interference of daily living activities.[1,2] Approximately, 35.6 million people currently suffer from dementia worldwide and this number is estimated to rise to 65.7 million in 2030.[3] Alzheimer disease (AD), which is the most frequent type of dementia, requires the biggest medical expenses among the 10 major geriatric diseases. Its pathological hallmarks are amyloid plaque, neurofibrillary tangles, and neuronal and synaptic loss.[4] Vascular dementia (VD), which is induced by cerebrovascular pathology, is the second most common form of dementia. Current studies found that relation among neuron, glia, and vascular cells attributes to cognitive impairment in VD.[5]
Acetylcholine esterase inhibitors (eg, donepezil, rivastigmine, and galantamine) or N-methyl-D-aspartate receptor antagonist (eg, memantine) is currently used to stall or delay the progression of both AD and VD, but these medications cannot fully treat any form of the disease.[6,7] Many pharmaceutical companies rushing to develop next-generation anti-dementia medications have to deal with challenges such as a variety of mechanism such as neuronal damage caused by acetylcholine synthesis, amyloid beta protein deposition, and hyperphosphorylation of tau protein. Many drug candidates were successful in animal experiments, but did not prove to be effective in clinical trials. For this reason, researchers turned their attention to natural resources or herbal formulae that can simultaneously act on multiple targets, rather than a single compound focusing on a certain mechanism. EGb 761 (Tanakan), the extract of Ginkgo biloba, has been used in traditional medicine to treat cognitive disorders such as AD.[8] Huperzine A, alkaloid compound in Huperzia serrata, was proven to be acetylcholine esterase inhibitor and N-methyl-D-aspartate receptor antagonist.[9] In phase II trial for mild-to-moderate AD patients, the dose of 400 μg/d Huperzine A did not show significant effect but the higher dose of 800 μg/d showed cognitive improvement.[10] Regarding VD, China has made it a national priority to proceed with a phase IV clinical trial of Tian zhi granule, derived from a traditional Chinese herbal formula.
Given the current trend of mining natural resources for anti-dementia drug candidates, Danggui-Shaoyao-San (DSS) could be viewed as another promising medication to treat dementia. DSS, one of the most widely used formula, is called Toki-shakuyaku-san in Japan and Dangguijakyakan in Korea. DSS was first introduced in “Synopsis of Prescriptions of the Golden Chamber,”  and it consists of Angelica gigas, Paeonia lactiflora, Ligusticum chuanxiong, Poria cocos, Atractylodes macrocephala, and Alisma orientalis. DSS is sold on the market as TJ-23 (Tsumura) and K-15 (Kracie Pharma, Ltd.) in Japan, Tang-Kuei & Peony Formula (Sun Ten Pharmaceutical Co.) in Taiwan, and as Dangguijakyaksan (Hankook Shinyak Pharmaceutical Co., Ltd., Kyungjin Pharm, Co., Ltd., etc) in Korea.
and it consists of Angelica gigas, Paeonia lactiflora, Ligusticum chuanxiong, Poria cocos, Atractylodes macrocephala, and Alisma orientalis. DSS is sold on the market as TJ-23 (Tsumura) and K-15 (Kracie Pharma, Ltd.) in Japan, Tang-Kuei & Peony Formula (Sun Ten Pharmaceutical Co.) in Taiwan, and as Dangguijakyaksan (Hankook Shinyak Pharmaceutical Co., Ltd., Kyungjin Pharm, Co., Ltd., etc) in Korea.
As one of the basic formula in traditional medicine, DSS can be a useful preventive or therapeutic agent for cognitive decline. This widely used formula has many reports of a cognitive function from Japan.[11,12] Korean researchers presented long-term case series on mild cognitive impairment (MCI).[13] In Korea, 1 pharmaceutical company has developed an extract of A gigas, one of the main ingredients of DSS, as a therapeutic agent for AD and has completed phase III trials.[14,15] With the aim to validating the efficacy of DSS on dementia, we have conducted a systematic review on DSS and meta-analyses of its efficacy regarding cognition and other functions.
2. Methods
2.1. Search strategy and eligibility criteria
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and recommendations of the Cochrane Collaboration were followed for the reporting of this review (www.prisma-statement.org) (Fig. 1). We searched online databases such as PubMed, Embase, Korean databases (Research Information Service System and Oriental Medicine Advanced Searching Integrated System), Chinese databases (China Knowledge Resource Integrated Database and Wanfang Database), and Japanese databases (CiNii and J-STAGE) from inception to June 2019. The search terms were adapted to suit each database. For PubMed, we used the following search strategy: {(“Dangguijakyaksan”[tiab] OR “toki-shakuyaku-san”[tiab] OR “Danggui Shaoyao Powder”[tiab] OR “TJ-23”[tiab] OR “Dangguijakyak-san”[tiab]) OR (“Angelica gigas”[tw] AND “Paeonia lactiflora”[tw]) OR (Angelica[tw] AND Peony[tw]) OR “Danggui-Shaoyao-San”[mh] OR “toki-shakuyaku-san”[mh]} AND (dementia[tiab] OR “mild cognitive impairment”[tiab] OR MCI[tiab] OR Alzheimer[tiab] OR “Pick disease”[tiab] OR “Pick's disease”[tiab] OR “lewy body”[tiab] OR memory[tiab] OR cognitive[tiab] OR dementia[mh] OR “memory disorders”[mh] OR “cognitive dysfunction”[mh]).
Figure 1.
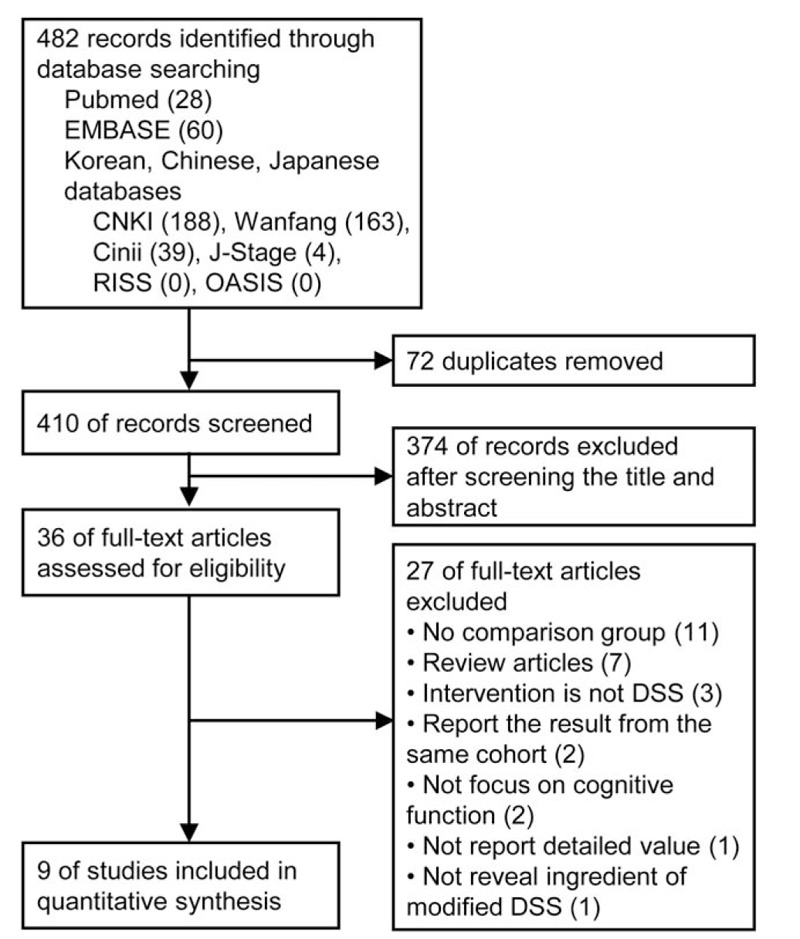
. Study flow diagram. DSS = Danggui-Shaoyao-San, OASIS = Oriental Medicine Advanced Searching Integrated System, RISS = Research Information Service System.
Studies were included if they
-
1.
were a randomized controlled trial (RCT);
-
2.
investigated the efficacy of DSS or its modified form that the author mentioned originates from DSS and comprises A gigas, P lactiflora, L chuanxiong, P cocos, A macrocephala, and A orientalis; and
-
3.
included participants with dementia (AD, VD, Lewy body dementia, Pick disease). Use of DSS with other treatment (eg, acupuncture, anti-dementia drugs, etc) was included.
Studies were excluded if they met any of the following criteria:
-
1.
the difference of intervention group and control group is the component other than DSS;
-
2.
studies did not use outcome measures of interest;
-
3.
studies were not published either in English, Korean, Chinese, or Japanese; or
-
4.
studies reported duplicate data.
Ethical approval was not necessary in this study as we used aggregated data from the included studies.
2.2. Data extraction and items
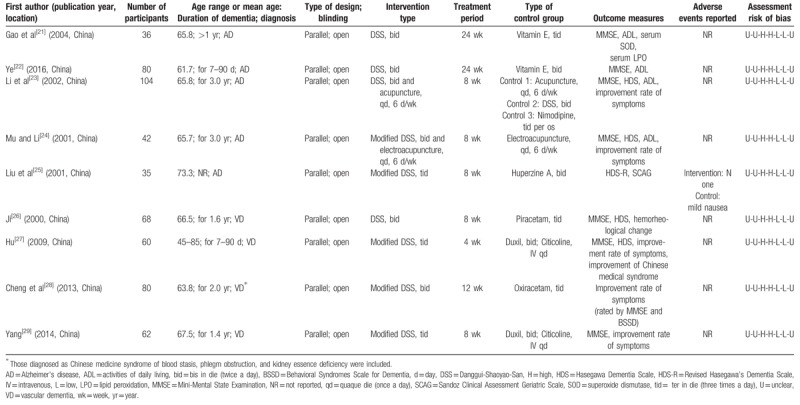
Two reviewers independently reviewed the titles and abstracts. Irrelevant or duplicate articles were removed in this step. The abstracts of the remaining articles were reviewed using the eligibility criteria. If the abstract did not provide sufficient information, the full-text article was reviewed. One reviewer conducted a full abstraction of all data, and 2 reviewers verified accuracy. The identification of relevant studies is presented in Figure 1. Data from each eligible study were extracted, and details of the author, publication year, location, participant characteristics, setting, intervention, duration of intervention, control, outcome measures, adverse events, and assessment of risk of bias were placed in summary tables (Table 1). The risk of bias was independently assessed using the Cochrane Risk of Bias Assessment Tool[16] by 2 authors. An “unclear” judgment was made if the insufficient detail was provided.
Table 1.
Characteristics of RCTs of Danggui-Shaoyao-San for dementia.
2.3. Outcome measures and analysis
The primary outcome measures were scales for dementia such as Mini-Mental State Examination (MMSE) and Hasegawa Dementia Scale (HDS). Secondary outcome measures were activities of daily living (ADL) and the improvement rate of symptoms that assessed the change of dementia symptom determined by criteria from “Guiding principle of clinical research on new drugs of traditional Chinese medicine.”[17]
For the meta-analysis, in terms of pooled results, continuous data were expressed as mean differences (MD) and dichotomous data were presented as odds ratios with associated 95% confidence intervals (CI). MD for changes of each outcome measure was used. If the trial reported only the value at pretreatment and that at posttreatment, the mean change was subtracting pre-measurement from post-measurement. If the standard deviation (s.d) for change was missing, it was calculated using the following formula.
The correlation coefficients between pre- and post-measurements (rpre,post) were calculated from a study with detailed value[18] in synthesis of AD studies with comparison with vitamin E or was assumed to be 0.5[19,20] in synthesis of VD studies. Random-effects model was used because the number of participants of the included studies was small. I2 statistic was used to present statistical heterogeneity. Due to its small number of included studies, the existence of publication bias was not assessed. Meta-analysis was performed using RevMan 5.3.5 software (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
3. Results
3.1. Characteristics of included studies
Among 482 relevant articles identified in the initial search, 9 studies[21–29] met our inclusion criteria and were subjected to our systematic review. A flow chart detailing the search and screening process can be found in Figure 1. We excluded 446 studies by removing duplicates and screening. We collected remaining 36 studies as full-text articles to evaluate eligibility and we excluded 27 studies with reasons (no comparison group,[30–40] review articles,[41–47] used different or multiple herbal formula as intervention,[48–50] report on the result from the same cohort,[51,52] no focus on cognitive function,[53,54] no report on detailed value of outcomes,[55] ingredient of modified DSS was not revealed[56]). Table 1 summarizes the characteristics of the included studies in terms of patients’ age, duration of disease, and elements of study quality. These studies enrolled 567 patients with a mean study size of 63 patients, with ages ranging from 40 to 85 years. The duration that the studies lasted ranged from 4 to 24 weeks. Five studies[21–25] were subjected to AD, and the rest of them[26–29] were to VD. Outcome measures used by the researchers of enrolled studies were mostly scales of dementia (eg, MMSE,[21–24,26–29] HDS[23–27]), ADL,[21–24] and improvement rate of symptoms.[23,24,27–29]
DSS was orally administered in the form of aqueous extract in every included study. Modified prescription respectively added Codonopsis pilosula Radix,[25]Polygonatum sibiricum,[28]Cistanche deserticola, Cuscutae Semen, Polygala tenuifolia, Alpiniae Fructus, Acorus gramineus Solander,[27,29] and other additional herbs according to traditional medical diagnosis (pattern differentiation).[24] DSS was administered 3 times a day in 3 studies,[25,27,29] and twice a day in the rest of the studies.[21–24,26,28] Two studies assessed the efficacy of treatment by both DSS and acupuncture.[23,24] No studies reported the case of cointervention of DSS with anti-dementia drugs. Two[21,22] out of 5 AD studies that assessed single effect of DSS were compared with vitamin E and 1 study[25] was compared with Huperzine A. Two AD studies that assessed the effect of both DSS and acupuncture[23,24] were compared with acupuncture alone,[23,24] DSS alone,[23] or calcium channel blocker, nimodipine.[23] All the included VD studies were compared with nootropic drugs that were of racetam groups[26,28] or dual use of Duxil and Citicoline.[27,29]
All eligible studies were parallel, open RCTs, and their risk of bias is reported in Table 1. All the included studies did not mention the detail of randomization and were at high risk of bias in regard to blinding.
3.2. Synthesis of studies of DSS for AD
All RCTs reported positive effects of DSS on AD.[21–25] Meta-analyses of 2 studies comparing DSS with vitamin E resulted in significant difference in both MMSE (MD 4.60; 95% CI 4.29, 4.91, Z = 28.72, P < .001) and ADL (MD 11.40; 95% CI 10.94, 11.86, Z = 48.49, P < .001) with low heterogeneity (I2 = 0%) (Fig. 2).
Figure 2.
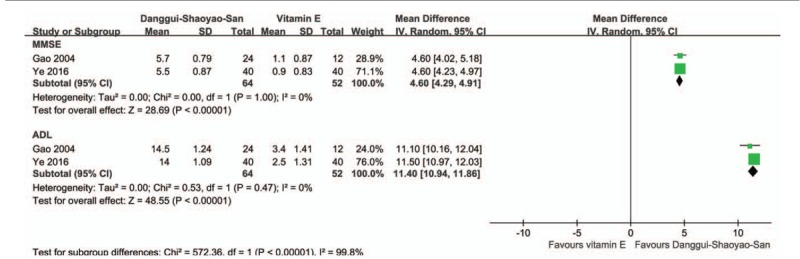
. Forest plot of comparison of clinical efficacy in Danggui-Shaoyao-San versus vitamin E on Alzheimer disease. ADL = activities of daily living, MMSE = Mini-Mental State Examination.
Two studies focused on cointervention of DSS and acupuncture versus acupuncture.[23,24] Pooled results showed that scores of MMSE (MD 1.69; 95% CI 1.05, 2.23, Z = 5.15, P < .001, I2 = 7%) and ADL (MD 2.38; 95% CI 1.92, 2.85, Z = 10.08, P < .001, I2 = 0%) differed significantly between DSS combined with acupuncture and acupuncture only. HDS tended to be in favor of cointervention, but it was not statistically significant (MD.62; 95% CI –0.20, 1.44, Z = 1.48, P = .14) with relatively higher heterogeneity (I2 = 31%) than other analyses (Fig. 3). The odds of enhancing symptom with DSS and acupuncture were 2.14 times higher (95% CI 0.99, 4.64, Z = 1.93, P = .05, I2 = 0%) than those with acupuncture alone in elderly with AD.
Figure 3.
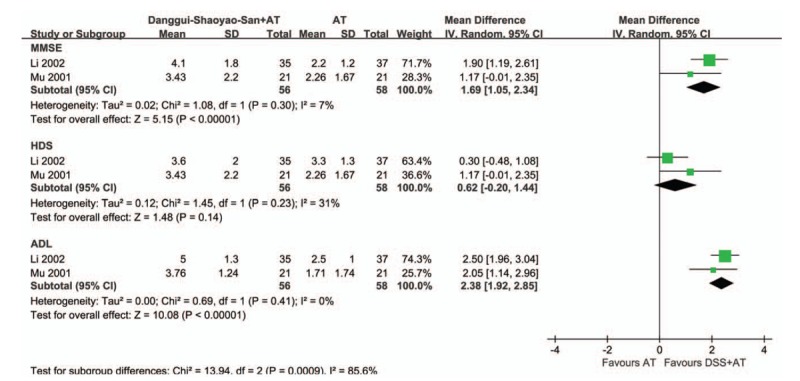
. Forest plot of comparison of clinical efficacy in Danggui-Shaoyao-San and acupuncture versus acupuncture alone on Alzheimer disease. ADL = activities of daily living, AT = acupuncture treatment, CI = confidence intervals, DSS = Danggui-Shaoyao-San, HDS = Hasegawa Dementia Scale, IV = intravenous, MMSE = Mini-Mental State Examination, SD = standard deviation.
3.3. Synthesis of studies of DSS for vascular dementia
Four VD studies[26–29] were selected, and they all included comparison with nootropics as a control. Nootropics used in the trials were Piracetam,[26] Oxiracetam,[28] and Duxil and Citicoline injection.[27,29] In the analysis of MMSE from 3 studies,[26,27,29] the width of CI decreased (MD 3.07; 95% CI 1.71, 4.43, Z = 4.43, P < .001). Two studies[26,27] reported modest positive change of HDS (MD 2.57; 95% CI 0.53, 4.61, Z = 2.47, P = .01) (Fig. 4A). All 4 RCTs[26–29] (n = 270) were included in the improvement rate of symptoms analysis. Oral administration of DSS significantly increased the odds of improvement rate of symptoms by 4 times (odds ratio 5.02; 95% CI 2.76, 9.11, Z = 5.29, P < .001) comparing with nootropic drugs (Fig. 4B). The I2 statistic in every meta-analysis did not identify the presence of heterogeneity among the studies (I2 = 0%).
Figure 4.
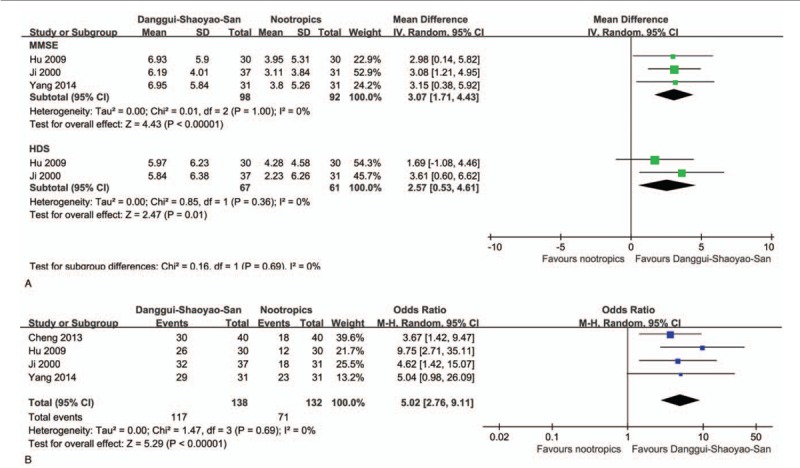
. Forest plot of comparison of clinical efficacy in Danggui-Shaoyao-San versus nootropics on vascular dementia. (A) Comparison of MMSE and HDS and (B) comparison of improvement rate of symptoms are presented. CI = confidence intervals, HDS = Hasegawa Dementia Scale, IV = intravenous, MMSE = Mini-Mental State Examination, SD = standard deviation.
3.4. Adverse events
Adverse events were not specifically reported in most studies. One study mentioned adverse events that the Huperzine A, which was used as control, had mild nausea, whereas DSS had no adverse event.[25]
4. Discussion
The current systematic review and meta-analysis compares the efficacy of DSS on dementia in elderly. There were 2 main findings. First, in cognition and ADL of AD, DSS was better than vitamin E supplement and has a synergistic effect when used with acupuncture. Second, DSS had a more beneficial effect than nootropic drugs on cognitive impairment of VD. No adverse event was reported. To the best of our knowledge, this is the first systematic review and meta-analysis of a certain type of herbal formula for AD while there barely exists a systematic review on a single type of herb or herbal prescription for cognitive function of dementia patients. Systematic reviews previously conducted on herbal medicine for dementia analyzed multiple types of herbal medicines and they were not free from heterogeneity.[57–59] They also mostly focused on herbal prescriptions that are only purchasable in China or studied on behavioral and psychological symptoms. DSS, the widely available formula, has been investigated for cognitive impairment since the late 1980s in east Asian countries either by experiments or by clinical studies. The current evidence from this systematic review demonstrated that DSS could improve symptoms of dementia patients in clinical practice.
In this study, DSS worked both on cognitive function of AD and on VD as presented in MMSE and HDS scores. Although the RCT for MCI was not included in this research, several previous studies regarding MCI also imply that DSS might enhance cognitive function. Ninety-five MCI patients who administered DSS twice a day showed significantly higher MMSE and Montreal Cognitive Assessment after the intervention, and its effect persisted in 1-year follow-up.[13] Its efficacy on cognitive impairment was also assessed using brain imaging tools in observational studies. DSS improved decreased alpha activity of AD and VD patients in electroencephalography.[37] Some MCI cases were reported that patients who were treated by DSS for 8 weeks had improved regional cerebral blood flow on single photon emission computed tomography imaging.[11] DSS made regional cerebral blood flow in the posterior cingulate higher in MCI and AD patients’ brain, and orientation score in MMSE also improved.[12]
Furthermore, according to the results of this review, DSS not only affected cognitive function but also improved ADL. Aside from this, even though only 2 RCTs systemically assessed,[25,28] most observational studies reported that DSS effectively diminished behavioral and psychological symptoms such as wandering, agitation, hyperactivity, insomnia, delusion, and depressive mood.[30,34,36–40] DSS diminishes the possibility of geriatric depression that is raised as an intermediary of dementia.[60] In the 1-year follow-up study on MCI, DSS improved Geriatric Quality of Life-Dementia and Geriatric Depression Scale-Korean.[13] This fact implies that DSS not only simply improves memory but also works on global function. DSS can be suggested as a potential agent for multiple symptoms of dementia including cognitive impairment, BPDS, and other accessory symptoms.
According to previous pharmacological researches, DSS was reported to have a neuroprotective effect on neuronal damage and regain reduced long-term potentiation induced by neurotoxicity of amyloid beta protein.[61–63] DSS had a positive effect on free radical-mediated neurological disorders, showed anti-inflammatory and antioxidant activities, and reduced cell apoptosis in the hippocampus.[64,65] DSS have an influence on synthesizing and releasing neurotransmitter including acetylcholine, dopamine, and norepinephrine.[64,66,67] It also shown neuroprotective effect in microglia cell inflammation[68] or in neuronal damage caused by hypoglycemia/hypoxia[69] and glutamate.[70,71] Individual herbal components were previously studied regarding cognition. INM-176, ethanolic extract of A gigas, improved cognitive impairment in scopolamine and amyloid beta protein models of mice by acetylcholine esterase inhibition and neuroprotection.[14] This agent also completed phase III trial for Alzheimer type dementia.[72]P lactiflora was superior to Angelica root in terms of memory decline, both of which significantly improved performance in radial maze in AD mouse model.[73] Paeonol, which is the ingredient of root of P lactiflora, improved cognitive decline and neurodegeneration in hippocampus and cortex, and it was supported by its antioxidant effect.[74] Paeoniflorin, also isolated from the root of P lactiflora, ameliorated ischemic pathology and neurological symptoms including cognitive impairment in middle cerebral artery occlusion model of rat.[75] The series of results potentially implies that DSS has a neuroprotective effect and can be another candidate for agents of AD and VD.
This wide range that DSS works on reminds us of 2 possibilities of DSS. One is that DSS operates on multitarget as revealed in a previous system pharmacology study.[76] Second is that DSS may act on a fundamental mechanism that contributes to brain function related to both AD and VD rather than specific pathology of each disease such as amyloid beta protein and tau.
DSS was more effective than vitamin E that can be considered as placebo,[77] and its efficacy is, thus, promising in AD. Even though DSS was superior to Huperzine A regarding cognitive function and dementia-related condition,[25] comparison with commonly used anti-dementia drugs or combining use with them should be investigated further. The difference between DSS combined with acupuncture and acupuncture alone was not as remarkable as that between DSS and vitamin E in AD, and difference of HDS did not differ significantly. As acupuncture is reported to have a therapeutic effect on dementia,[78,79] this may attribute to ceiling effect of treating dementia. The relatively higher value of I2 in MMSE and HDS comparing with other analyses might be owing to different types of acupuncture that were body acupuncture[23] and electroacupuncture.[24]
Regarding its noticeable effect on VD, DSS basically has a positive effect on cerebrovascular diseases. DSS improved both hemorheological abnormalities and cognitive function in VD patients.[26] On a 1-year RCT, DSS helped in the recovery of stroke patients and reduced its worsening.[53] In case series of asymptomatic cerebral infarction, microcirculation of bulbar conjunctiva improved and hemorheological factors also significantly changed after administration of DSS.[80]
The RCTs included in this study did not report adverse effect of DSS. Most observational studies also did not report adverse effect so it was hard to confirm whether DSS had no risk. Assessment of adverse effect found in some observational studies, though, helped us assume its possible side effect. Two studies that tested 8-week administration of DSS neither showed abnormality in laboratory examination nor subjective/objective symptom.[12,36] Eighty cases of AD or VD who were administered DSS for 12 weeks report nothing other than decrease of average blood pressure and decrease of average serum sodium level within normal range.[38] Two cases of indigestion occurred among 95 MCI patients who took DSS for 12 weeks.[13] Safety of DSS could be suggested considering these cases of the elderly, even though elaborately designed RCT should be performed in the future.
We also acknowledge the limitations of this review. The number of RCTs was small, and the respective size of the trial was also small. In the case that the included studies were of the small number, we were unable to assess risk of bias across studies. As they were all written by Chinese authors, potential publication bias may be considered. Since all the studies did not fully describe methodological issues, they were of low quality, or they have barely undergone an adequate assessment of the risk of bias. Every included study was randomized in parallel, but the exact method of randomization was not designated. Blinding was hardly conducted at least in terms of participants. Most studies had short duration varying from 4 to 12 weeks, but its long-term efficacy and side effect need to be investigated as dementia agents are prescribed for a longer period.
Limitations aside, this review provides important evidence that DSS is effective for major types of dementia. Based on the available evidence, DSS showed a considerable change on cognitive function and accompanied symptoms of dementia with low risk of side effect. Accordingly, practitioners can consider DSS as another option for AD and VD.
Author contributions
Conceptualization: Yunna Kim, Seung-Hun Cho.
Formal analysis: Yunna Kim.
Investigation: Yunna Kim, Seung-Hun Cho.
Methodology: Yunna Kim, Seung-Hun Cho.
Validation: Seung-Hun Cho.
Visualization: Yunna Kim.
Writing – original draft: Yunna Kim.
Writing – review & editing: Seung-Hun Cho.
Footnotes
Abbreviations: AD = Alzheimer disease, ADL = activities of daily living, CI = confidence intervals, DSS = Danggui-Shaoyao-San, HDS = Hasegawa Dementia Scale, MCI = mild cognitive impairment, MD = mean differences, MMSE = Mini-Mental State Examination, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RCT = randomized controlled trial, VD = vascular dementia.
How to cite this article: Kim Y, Cho SH. Danggui-Shaoyao-San for dementia: a PRISMA-compliant systematic review and meta-analysis. Medicine. 2020;99:4(e18507).
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2018R1D1A1B07050445).
The authors report no conflicts of interest.
References
- [1].World Health Organization. International Statistical Classification of Diseases and Related Health Problems. Vol 1. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- [2].McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Alzheimer's Disease International. World Alzheimer Report 2010: The Global Economic Impact of Dementia. London, UK: Alzheimer's Disease International; 2010. [Google Scholar]
- [4].Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol 2010;9:1118–27. [DOI] [PubMed] [Google Scholar]
- [5].Ladecola C. The pathobiology of vascular dementia. Neuron 2013;80:844–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bachurin SO, Bovina EV, Ustyugov AA. Drugs in clinical trials for Alzheimer's disease: the major trends. Med Res Rev 2017;37:1186–225. [DOI] [PubMed] [Google Scholar]
- [7].O’Brien JT, Thomas A. Vascular dementia. Lancet 2015;386:1698–706. [DOI] [PubMed] [Google Scholar]
- [8].Lang F, Hoerr R, Noeldner M. Wagner H, Ulrich-Merzenich G, et al. Ginkgo biloba extract EGb 761®: from an ancient Asian plant to a modern European herbal medicinal product. Evidence and Rational Based Research on Chinese Drugs. Basel, Switzerland: Springer Nature; 2013. 431–70. [Google Scholar]
- [9].Ferreira A, Rodrigues M, Fortuna A, et al. Huperzine A from Huperzia serrata: a review of its sources, chemistry, pharmacology and toxicology. Phytochem Rev 2016;15:51–85. [Google Scholar]
- [10].Rafii MS, Walsh S, Little JT, et al. A phase II trial of huperzine A in mild to moderate Alzheimer disease. Neurology 2011;76:1389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kitabayashi Y, Shibata K, Nakamae T, et al. Effect of traditional Japanese herbal medicine toki-shakuyaku-san for mild cognitive impairment: SPECT study. Psychiatry Clin Neurosci 2007;61:447–8. [DOI] [PubMed] [Google Scholar]
- [12].Matsuoka T, Narumoto J, Shibata K, et al. Effect of toki-shakuyaku-san on regional cerebral blood flow in patients with mild cognitive impairment and Alzheimer's disease. Evid Based Complement Alternat Med 2012;2012:245091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kim KH, Go HY, Lee JA, et al. The effect of dangguijagyag-san on mild cognitive impairment. J Altern Complement Med 2016;22:509–14. [DOI] [PubMed] [Google Scholar]
- [14].Park SJ, Jung JM, Lee HE, et al. The memory ameliorating effects of INM-176, an ethanolic extract of Angelica gigas, against scopolamine-or Aβ 1-42-induced cognitive dysfunction in mice. J Ethnopharmacol 2012;143:611–20. [DOI] [PubMed] [Google Scholar]
- [15].Berk C, Sabbagh M. Successes and failures for drugs in late-stage development for Alzheimer's disease. Drugs Aging 2013;30:783–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zheng X. Guiding Principle of Clinical Research on New Drugs of Traditional Chinese Medicine. Vol 143. Beijing, China: China Medic-Pharmaceutical Sciences and Technology Publishing House; 2002. [Google Scholar]
- [18].Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Vol 4. Chichester, UK: John Wiley & Sons; 2011. [Google Scholar]
- [19].Follmann D, Elliott P, Suh I, et al. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 1992;45:769–73. [DOI] [PubMed] [Google Scholar]
- [20].Abrams KR, Gillies CL, Lambert PC. Meta-analysis of heterogeneously reported trials assessing change from baseline. Stat Med 2005;24:3823–44. [DOI] [PubMed] [Google Scholar]
- [21].Gao D, Huang J, He H. A clinical study on 36 cases of Alzheimer disease treated with Danggui Shaoyao San [in Chinese]. Chin Gen Pract 2004;7:782–3. [Google Scholar]
- [22].Ye Y. Clinical analysis of Danggui Shaoyao San on senile dementia [in Chinese]. J North Pharm 2016;13:100–1. [Google Scholar]
- [23].Li ZR, Mu YY, Ouyang Q. Clinic control research of Alzheimer's disease by the combination of acupuncture and Danggui Shaoyao San (DGSYS) of TCM. Chin J Clin Rehabil 2002;6:2848–9. [Google Scholar]
- [24].Mu Y-Y, Li Z-R. The treatment of Alzheimer's disease by the combination of acupuncture and medicine [in Chinese]. Shanghai J Acupunct Moxibustion 2001;20:3–5. [Google Scholar]
- [25].Liu M, Wang D, Liu Y, et al. Effects of modified Danggui Shaoyao powder for Alzheimer's disease [in Chinese]. J Guangzhou Univ Tradit Chin Med 2001;18:30–3. [Google Scholar]
- [26].Ji H. Clinical observation on the treatment of vascular dementia by Danggui Shaoyao powder [in Chinese]. Shanxi J Tradit Chin Med 2000;16:10–1. [Google Scholar]
- [27].Hu J. Clinical observation on treatment of vascular dementia with modified Danggui Shaoyao San [in Chinese]. Chin J Clin Ration Drug Use 2009;2:62–3. [Google Scholar]
- [28].Cheng S, Wei Y, Wang J. Clinical observation of “Guishaoliaochitang” to improve vascular dementia cognitive abilities of 40 patients [in Chinese]. Jiangsu J Tradit Chin Med 2013;45:36–7. [Google Scholar]
- [29].Yang J. Effect of modified Danggui Shaoyao San on vascular dementia [in Chinese]. Contemp Med Forum 2014;12:34–5. [Google Scholar]
- [30].Zhang S, Wu F. Treatment of 21 cases of senile dementia with Danggui-Shaoyao-San [in Chinese]. J Anhui Tradit Chin Med Coll 1996;15:20–1. [Google Scholar]
- [31].Zhang S, He X. Experience of Danggui-Shaoyao-San in treating Alzheimer's disease [in Chinese]. Guangming J Tradit Chin Med 1996;1996:23. [Google Scholar]
- [32].Zhao Q, Hu Y, Lu Y, et al. Treatment of 42 cases of senile dementia with Danggui-Shaoyao-San [in Chinese]. Tradit Chin Med Res 2000;13:56–7. [Google Scholar]
- [33].Han Z, Shi F, Wang L. Treatment of 36 cases of vascular dementia with Danggui-Shaoyao-San [in Chinese]. Shanxi J Tradit Chin Med 1998;19:16. [Google Scholar]
- [34].Nobushi M, Teruhiko I. Efficacy of Toki-shakuyaku-san for senile dementia [in Japanese]. The general meeting of Medical and Pharmaceutical Society for Wakan-yaku abstracts 1989;6:127. [Google Scholar]
- [35].Sakakura M, Tamai T, Yagyu K, et al. Clinical data; effects of Tsumura Toki-Shakuyaku-San on patients with dementia [in Japanese]. Sci Kampo Med 1992;16:77–81. [Google Scholar]
- [36].Fukushima T, Tomonaga M, Tanaka A, et al. Clinical effect of Tsumura Toki-shakuyaku-san on cerebrovascular disorder sequelae [in Japanese]. Pract Res 1994;71:1065–70. [Google Scholar]
- [37].Yagyu T, Su G, Nobuhara K, et al. Effect of Tsumura Toki-shakuyaku-san (TJ-23) in elderly with dementia–quantitative EEG study [in Japanese]. Ther Res 1994;15:969–76. [Google Scholar]
- [38].Inanaga K, Dainoson K, Ninomiya Y, et al. Effects of Toki-Shakuyaku-San in patients with senile cognitive disorders [in Chinese]. Prog Med 1996;16:293–300. [Google Scholar]
- [39].Harada H. The effects of Toki-Shakuyaku-San on Alzheimer's disease. Japn J Orient Med 1997;47:861–7. [Google Scholar]
- [40].Egami H, Fukutomi T, Kamura T, et al. Therapeutic experience of Toki-Shakuyaku-San and Yokukansan on dementia and mental disorders in elderly. Japn J Orient Med 2007;58:201. [Google Scholar]
- [41].Tang Q. Study on treatment of senile vascular dementia with traditional Chinese medicine [in Chinese]. J Beijing Univ Tradit Chin Med 1996;19:32–3. [Google Scholar]
- [42].Liu M, Liu H. Study on treatment of senile dementia with Danggui-Shaoyao-San [in Chinese]. Chin Tradit Patent Med 1999;21:428–30. [Google Scholar]
- [43].Hagino N. Effect of toki-shakuyaku-san on central nervous system and its effectiveness as anti-dementia drug [in Japanese]. The general meeting of Medical and Pharmaceutical Society for Wakan-yaku abstracts 1989;6:12. [Google Scholar]
- [44].Kudo C, Sugiura K. Clinical effect of Tsumura Toki-Shakuyaku-San on senile dementia [in Japanese]. Sci Kampo Med 1992;16:387–91. [Google Scholar]
- [45].Hiramatsu M. Preventive effect of Toki-Shakuyaku-San the kampo medication on dementia [in Japanese]. J Tohoku Univ Community Serv Sci 2002;3:a13–27. [Google Scholar]
- [46].Inaka K. Aging of brain and mind: drug treatment of behavioral and psychological symptoms (BPSD) of dementia [in Japanese]. Psychiatria et neurologia Japonica 2007;109:703–8. [PubMed] [Google Scholar]
- [47].Mizukami K, Kitada S, Goto H, et al. Efficacy and evaluation of Kampo treatment in geriatrics. Kampo Med 2009;60:253–81. [Google Scholar]
- [48].Mou Y, Ge C, Li H, et al. Clinical study on treatment of senile dementia with traditional Chinese medicine [in Chinese]. Acta Acad Med Nantong 2000;20:354–5. [Google Scholar]
- [49].Sun X. Clinical observation on 42 cases of dementia after multiple infarction treated by integrative Chinese and Western medicine [in Chinese]. Yunan J Tradit Chin Med Mater Med 2001;22:22–3. [Google Scholar]
- [50].Yang J, Lu J. Clinical study of combination traditional Chinese medicine with Western medicine therapy on senile dementia [in Chinese]. Hebei J Tradit Chin Med 2005;27:687–8. [Google Scholar]
- [51].Liu M, Wang D, Liu Y, et al. Therapeutic effect of laozhifu on the treatment of senile dementia [in Chinese]. J Chin Med Mater 2001;24:73–5. [Google Scholar]
- [52].Liu M, Wang D, Liu Y, et al. Observation on therapeutic effect of modified Danggui-Shaoyao-San on Alzheimer's disease [in Chinese]. Jiangxi J Tradit Chin Med 2001;32:14. [Google Scholar]
- [53].Goto H, Satoh N, Hayashi Y, et al. A Chinese herbal medicine, tokishakuyakusan, reduces the worsening of impairments and independence after stroke: a 1-year randomized, controlled trial. Evid Based Complement Alternat Med 2011;2011:194046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Goto H. Effects of Toki-Shakuyaku-San on malfunction and decreased independence of patients with sequela of cerebrovascular disease. Japn J Orient Med 2008;59:90. [Google Scholar]
- [55].Yamamoto T, Kono K. Kampo therapy on Alzheimer type dementia (DAT). The general meeting of Medical and Pharmaceutical Society for Wakan-yaku abstracts 1989;6:126. [Google Scholar]
- [56].Li Z, Zhang H, Cao Z, et al. Clinical study on Guiqi Yinao mixture formula in treating vascular dementia [in Chinese]. J Weifang Med Coll 1998;20:39–41. [Google Scholar]
- [57].Hyde AJ, May BH, Dong L, et al. Herbal medicine for management of the behavioural and psychological symptoms of dementia (BPSD): a systematic review and meta-analysis. J Psychopharmacol 2017;31:169–83. [DOI] [PubMed] [Google Scholar]
- [58].Xu Q-Q, Shan C-S, Wang Y, et al. Chinese herbal medicine for vascular dementia: a systematic review and meta-analysis of high-quality randomized controlled trials. J Alzheimers Dis 2018;62:429–56. [DOI] [PubMed] [Google Scholar]
- [59].Zeng L, Zou Y, Kong L, et al. Can Chinese herbal medicine adjunctive therapy improve outcomes of senile vascular dementia? Systematic review with meta-analysis of clinical trials. Phytother Res 2015;29:1843–57. [DOI] [PubMed] [Google Scholar]
- [60].Dillon C, Tartaglini MF, Stefani D, et al. Geriatric depression and its relation with cognitive impairment and dementia. Arch Gerontol Geriatr 2014;59:450–6. [DOI] [PubMed] [Google Scholar]
- [61].Egashira N, Iwasaki K, Akiyoshi Y, et al. Protective effect of Toki-shakuyaku-san on amyloid β25–35-induced neuronal damage in cultured rat cortical neurons. Phytother Res 2005;19:450–3. [DOI] [PubMed] [Google Scholar]
- [62].Hu ZY, Yang S, Zhou WX, et al. JD-30, an active constituent extracted from Danggui Shaoyao San, ameliorates amyloid beta-protein fragment 25-35 induced inhibition of long-term potentiation of CA1 area in rat hippocampal slices. Chin J Pharmacol Toxicol 2007;21:185–9. [Google Scholar]
- [63].Hu ZY, Liu G, Cheng XR, et al. JD-30, an active fraction extracted from Danggui-Shaoyao-San, decreases β-amyloid content and deposition, improves LTP reduction and prevents spatial cognition impairment in SAMP8 mice. Exp Gerontol 2012;47:14–22. [DOI] [PubMed] [Google Scholar]
- [64].Fu X, Wang Q, Wang Z, et al. Danggui-Shaoyao-San: new hope for Alzheimer's disease. Aging Dis 2016;7:502–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ueda Y, Komatsu M, Hiramatsu M. Free radical scavenging activity of the Japanese herbal medicine toki-shakuyaku-san (TJ-23) and its effect on superoxide dismutase activity, lipid peroxides, glutamate, and monoamine metabolites in aged rat brain. Neurochem Res 1996;21:909–14. [DOI] [PubMed] [Google Scholar]
- [66].Hagino N. An overview of Kampo medicine: Toki-Shakuyaku-San (TJ-p23). Phytother Res 1993;7:391–4. [Google Scholar]
- [67].Sakamoto S, Hagino N, Toriizuka K. Effect of Toki-Shakuyaku-San (TJ-23) on the activity of choline acetyltransferase in the brain of menopausal rats. Phytother Res 1994;8:208–11. [Google Scholar]
- [68].Ding R-R, Chen W, Guo C-Y, et al. Dangguishaoyao-San attenuates LPS-induced neuroinflammation via the TLRs/NF-κB signaling pathway. Biomed Pharmacother 2018;105:187–94. [DOI] [PubMed] [Google Scholar]
- [69].Kataoka Y, Kouzuma M, Koizumi S, et al. Neuroprotective effect of Toki-Shakuyaku-San on hypoglycemia/hypoxia-induced neural damage in rat striatal slices. Phytother Res 1991;7:S67–9. [Google Scholar]
- [70].Watanabe Y, Zhang XQ, Liu J-S, et al. Protection of glutamate induced neuronal damages in cultured cerebellar granule cells by Chinese herbal. J Tradit Med 1995;12:93–101. [Google Scholar]
- [71].Zhang Xq, Hagino N, Nozaki T. Neuroprotective effects of Toki-Shakuyaku-San (TJ-23) on glutamate induced neuronal death in cultured cerebellar granule cells. Phytother Res 1997;11:107–12. [Google Scholar]
- [72].Whanin Pharmaceutical Company. 2010. An efficacy and safety study of INM-176 for the treatment of patients with Alzheimer type dementia (NCT01245530). Available at: https://clinicaltrials.gov/ct2/show/NCT01245530 [Accessed June 30, 2019]. [Google Scholar]
- [73].Ohta H, Ni JW, Matsumoto K, et al. Peony and its major constituent paeoniflorin, improve radial maze performance impaired by scopolamine in rats. Pharmacol Biochem Behav 1993;45:719–23. [DOI] [PubMed] [Google Scholar]
- [74].Zhong S-Z, Ge Q-H, Qu R, et al. Paeonol attenuates neurotoxicity and ameliorates cognitive impairment induced by d-galactose in ICR mice. J Neurol Sci 2009;277:58–64. [DOI] [PubMed] [Google Scholar]
- [75].Xiao L, Wang Y-Z, Liu J, et al. Effects of paeoniflorin on the cerebral infarction, behavioral and cognitive impairments at the chronic stage of transient middle cerebral artery occlusion in rats. Life Sci 2005;78:413–20. [DOI] [PubMed] [Google Scholar]
- [76].Luo Y, Wang Q, Zhang Y. A systems pharmacology approach to decipher the mechanism of danggui-shaoyao-san decoction for the treatment of neurodegenerative diseases. J Ethnopharmacol 2016;178:66–81. [DOI] [PubMed] [Google Scholar]
- [77].Farina N, Llewellyn D, Isaac MG, et al. Vitamin E for Alzheimer's dementia and mild cognitive impairment. Cochrane Database Syst Rev 2017;1:CD002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wang Z, Nie B, Li D, et al. Effect of acupuncture in mild cognitive impairment and Alzheimer disease: a functional MRI study. PLoS One 2012;7:e42730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zhou J, Peng W, Xu M, et al. The effectiveness and safety of acupuncture for patients with Alzheimer disease: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2015;94:e933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Yang Q, Goto H, Hikiami H, et al. Effects of Toki-shakuyaku-san on microcirculation of bulbar conjunctiva and hemorheological factors in patients with asymptomatic cerebral infarction. J Tradit Med 2004;21:170–3. [Google Scholar]


