Abstract
Background:
Heart failure with reduced ejection fraction (HFrEF) has contributed to an increasing number of deaths and readmissions over the past few decades. Despite the appearance of standard treatments, including diuretics, β-receptor blockers and angiotensin-converting enzyme inhibitor (ACEI), there are still a large number of patients who have progressive deterioration of heart function and, inevitably, end-stage heart failure. In recent years, new medications for treating chronic heart failure have been clinically applied, but there is controversy surrounding drug selection and whether patients with HFrEF benefit from these medications. Therefore, we aimed to compare and rank different new pharmacological treatments in patients with HFrEF.
Methods:
We performed a network meta-analysis to identify both direct and indirect evidence from relevant studies. We searched MEDLINE, EMBASE, and PsycINFO through the OVID database and CENTRAL through the Cochrane Library for clinical randomized controlled trials investigating new pharmacological treatments in patients with HFrEF published up to September 30, 2018. We included trials of ivabradine, levosimendan, omega-3, tolvaptan, recombinant human B-type natriuretic peptide (rhBNP), isosorbide dinitrate and hydralazine (ISDN/HYD) and angiotensin-neprilysin inhibition (LCZ696). We extracted the relevant information from these trials with a predefined data extraction sheet and assessed the risk of bias with the Cochrane risk of bias tool. Based on these items, more than half of the entries were judged as having an overall low to moderate risk of bias; the remaining studies had a high or unclear risk of bias. The outcomes investigated were left ventricle ejection fraction (LVEF %), heart rate (HR) and serum level of B-type natriuretic peptide (BNP). We performed a random-effects network meta-analysis within a Bayesian framework.
Results:
We deemed 32 trials to be eligible that included 3810 patients and 32 treatments. Overall, 32 (94.1%) trials had a low to moderate risk of bias, while 2 (5.9%) trials had a high risk of bias. The quality of the included studies was rated as low in regard to allocation concealment and blinding and high in regard to other domains according to the Cochrane tools. As for increasing LVEF%, levosimendan was better than placebo (–3.77 (–4.96, –2.43)) and was the best intervention for improving ventricle contraction. As for controlling HR, n3-PUFA was better than placebo (4.01 (–0.44, 8.48)) and was the best choice for regulating HR. As for decreasing BNP, omega-3 was better than placebo (941.99 (–47.48, 1952.89) and was the best therapy for improving ventricle wall tension.
Conclusions:
Our study confirmed the effectiveness of the included new pharmacological treatments for optimizing the structural performance and improving the cardiac function in the management of patients with HFrEF and recommended several interventions for clinical practice.
Keywords: heart failure with reduced ejection fraction, network meta-analysis, pharmacological treatments
1. Introduction
For patients with chronic heart failure with reduced ejection fraction (HFrEF), multiple medication therapy that includes angiotensin converting enzyme inhibitors or angiotensin-receptor antagonists (ACEI/ARB), β-receptor blocker and spironolactone has been proven to decrease mortality and hospitalization rates in large randomized controlled trials (RCTs).[1,2] The clinical benefits of these medical therapies have generally been applied in routine clinical practice.[3] Therefore, these drugs form the cornerstone of contemporary evidence-based HFrEF care and are supported by class I indications in clinical treatment guidelines.[1,2]
Despite their proven benefits and strong guideline recommendations, these traditional medications are restricted in application because of the complicated condition of patients and their many contraindications. With the high prevalence and mortality of patients with HFrEF each year, starting from the pathogenesis of the neural fluid mechanism of heart failure, a series of new clinical drugs that break through the limitations of traditional medicine have emerged.[4,5] On this basis, several RCTs have been designed to evaluate the advantages and disadvantages of the new pharmacological therapy and traditional drugs using the cardiac function and structural optimization as the clinical outcomes.[6–9] However, there is still a lack of direct comparisons between the efficacies of the new medications. To obtain high-quality evidence for making clinical decisions, we performed a Bayesian network meta-analysis to compare and rank different new pharmacological therapies for the management of patients with HFrEF.
2. Methods
This study was conducted in accordance with the Cochrane Handbook for the Systematic Review of Interventions (for details, see at http://training.cochrane.org/handbook) and the Preferred Reporting Items for Systematic Review and Meta-Analyses.[10] The included studies were classified according to the types of pharmacological treatments.
2.1. Search strategy
For the network meta-analysis, we searched MEDLINE, EMBASE, and PsycINFO through the OVID database and searched CENTRAL through the Cochrane Library. We searched studies published from their inception to September 30, 2018, and compared different pharmacological treatments for clinical outcomes in patients with HFrEF (Appendix 1).
2.2. Study selection
2.2.1. Types of studies
All RCTs with a sample size >10 per arm.
2.2.2. Types of participants
The inclusion criteria were as follows: diagnosis of HFrEF according to the report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Heart failure patients with preserved ejection fraction, acute or chronic infectious or inflammatory diseases and recent myocardial infarction (<8 weeks) or active ischemia were excluded. The details of eligibility criteria PICOS are shown in Table 1.
Table 1.
Eligibility criteria PICOS.

2.2.3. Types of interventions
Ivabradine, levosimendan, omega-3, tolvaptan, recombinant human B-type natriuretic peptide (rhBNP), isosorbide dinitrate and hydralazine (ISDN/HYD) and angiotensin-neprilysin inhibitor (LCZ696) were included. However, the data form the LCZ696 clinical trials did not satisfy the requirements of the network meta-analysis. In the control group, any of the above seven pharmacological treatments (positive control), placebo and usual care (blank control) were included.
2.2.4. Types of outcome measures
The primary outcomes were LVEF, heart rate (HR) and the serum level of the B-type natriuretic peptide (BNP), which were also analyzed by network meta-analysis.
2.3. Data extraction and quality assessment
Two investigators (HL, YTD) independently selected the studies. The review of the main reports and supplementary materials, the extraction of the relevant information from the included trials with a predetermined data extraction sheet, and the assessment of the risk of bias with the Cochrane risk of bias tool were independently performed by 3 investigators (BFC, YZ, JMW). Any disagreements were resolved through discussion. When the investigators did not reach a consensus, the final decision regarding each question was made by other investigators within the review team (SW, WSH, and LML).
We evaluated the quality of the included studies with the Cochrane Collaboration Recommendations assessment tool. The tool for assessing 7 domains, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding (or masking) of outcome assessors, incomplete outcome data, selective reporting and other biases, is described in the Cochrane Handbook for Systematic Reviews of Interventions (see details at http://training.cochrane.org/handbook). Based on these items, more than half of the entries had an overall low to moderate risk of bias, and the remaining entries had a high or unclear risk of bias.
2.4. Statistical analysis
A network meta-analysis with a Bayesian framework with Aggregate Data Drug Information System (ADDIS, version 1.16.8) was conducted to assess the clinical outcomes of pharmacological interventions. This software is based on the Bayesian framework and the Markov chain Monte Carlo method, which can evaluate a priori and process research data. We used a random-effects model to analyze the effect sizes in this study. The effect sizes for continuous outcomes were the mean difference (MD). Consistency and inconsistency were the 2 models used to estimate the effect size in ADDIS. A consistency assessment drew conclusions on the effect sizes of the included interventions and estimated the ranking probabilities for all the interventions. The consistency test results were judged by node-splitting analysis and an inconsistence model. When the P value of the node-splitting analysis was greater than .05, a consistency mode was selected.[11] Otherwise, an inconsistency model was used. The potential scale reduction factor (PSRF) was used to evaluate the convergence of the model. The closer the PSRF value was to 1, the better the convergence. The convergence of the model was still acceptable if the PSRF value was less than 1.2. For each intervention, we estimated the ranking probabilities for each treatment at each possible rank.
3. Results
3.1. Study identification and selection
In total, 28,051 citations published between 1981 and September 30, 2018, were identified by the search. After removing duplicates and unrelated articles, 32 articles describing 32 RCTs including 3495 patients were eligible for further quantitative analyses. The flow chart of the specific screening procedures is shown in Figure 1.
Figure 1.
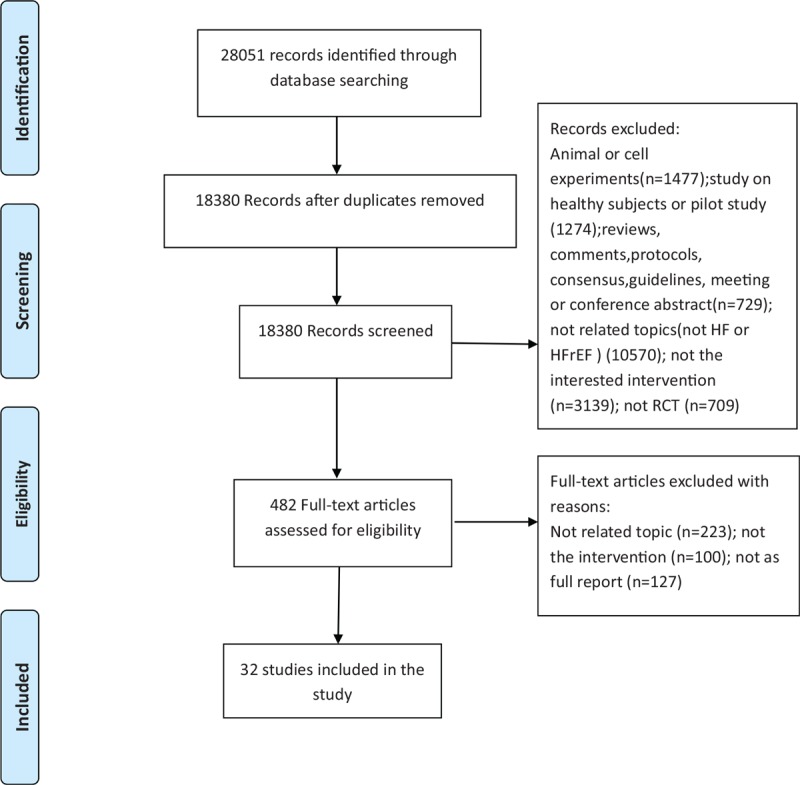
Study selection.
A total of 3495 participants were included, with sample sizes that ranged from 25 to 341. Participants’ mean age in the included studies ranged from 53 to 74, and the intervention duration was in the range of 24 hours to 12 months. All of the studies were parallel, randomized, and controlled, among which 2 studies (6.3%) were single-blinded, 9 studies (28.1%) were double-blinded, 13 studies (40.6%) were open-label and the remaining studies had 2 designs. Among the included studies, levosimendan (65.6%) was the main therapy in the treatment group, 6 studies (18.8%) employed ivabradine as the treatment group, while the other 4 drugs (omega-3, tolvaptan, rhBNP, ISDN/HYD) were used as treatments in the remaining studies. Outcome measures such as LVEF%, HR, and the serum level of BNP were used to evaluate the cardiac function. Eleven studies (34.4%) also treated New York Heart Association (NYHA) heart function and mortality as observation outcomes. All the characteristics of the included studies are shown in Table 2 .
Table 2.
The characteristics of the included studies.
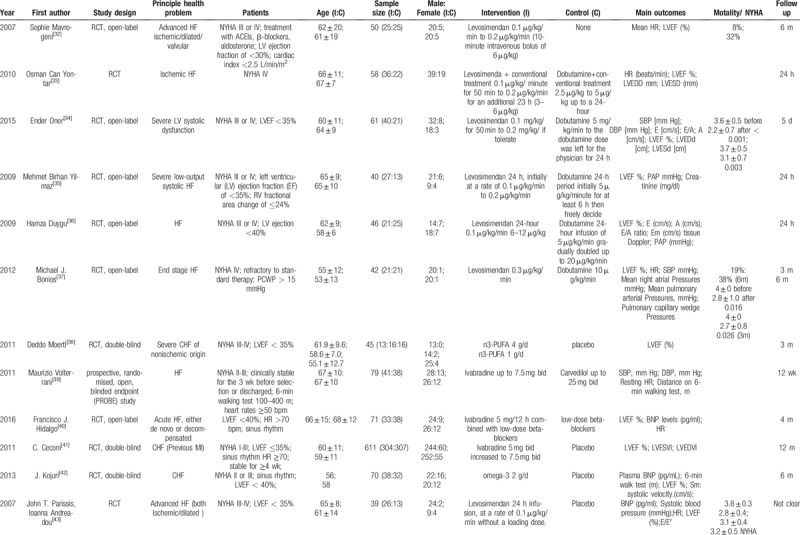
Table 2 (Continued).
The characteristics of the included studies.
3.2. Quality assessment of the included studies
We evaluated the quality of included studies with the Cochrane Collaboration Recommendations assessment tools.[12] Among 32 trials, 32 studies (100%) described a random component in the sequence generation process, such as a computer-generated random number or a random number table. Allocation concealment was performed using an appropriately sealed method in 25% (8) of the studies, while 46.9% (15) either did not describe concrete methods or used an inappropriate allocation concealment method. In regard to performance bias, 34.4% (11) of the included trials reported the methods of blinding for both participants and personnel. In regard to detection bias, 53.1% (17) of the outcome assessors in the studies either could not be blinded or were unclear. In regard to attrition bias, 30 studies were deemed to have low-risk outcome data (ie, the reported dropout rates were within the range of the statistical estimations, provided detailed explanations of dropout rates or performed intention-to-treat analysis). Other risks were unclear due to insufficient information in 1 study. A detailed quality assessment is presented in Figures 2 and 3.
Figure 2.
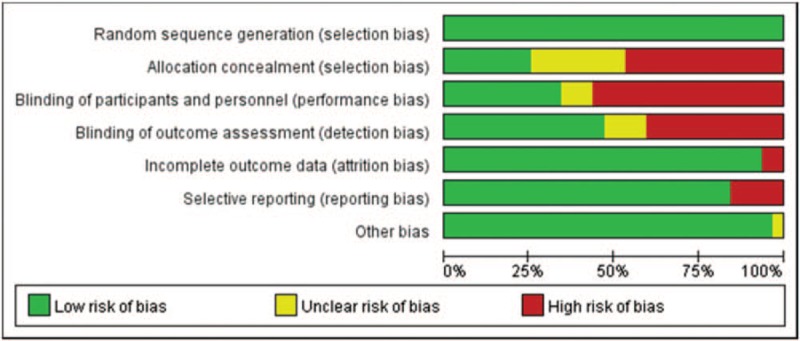
Risk of bias of included studies.
Figure 3.
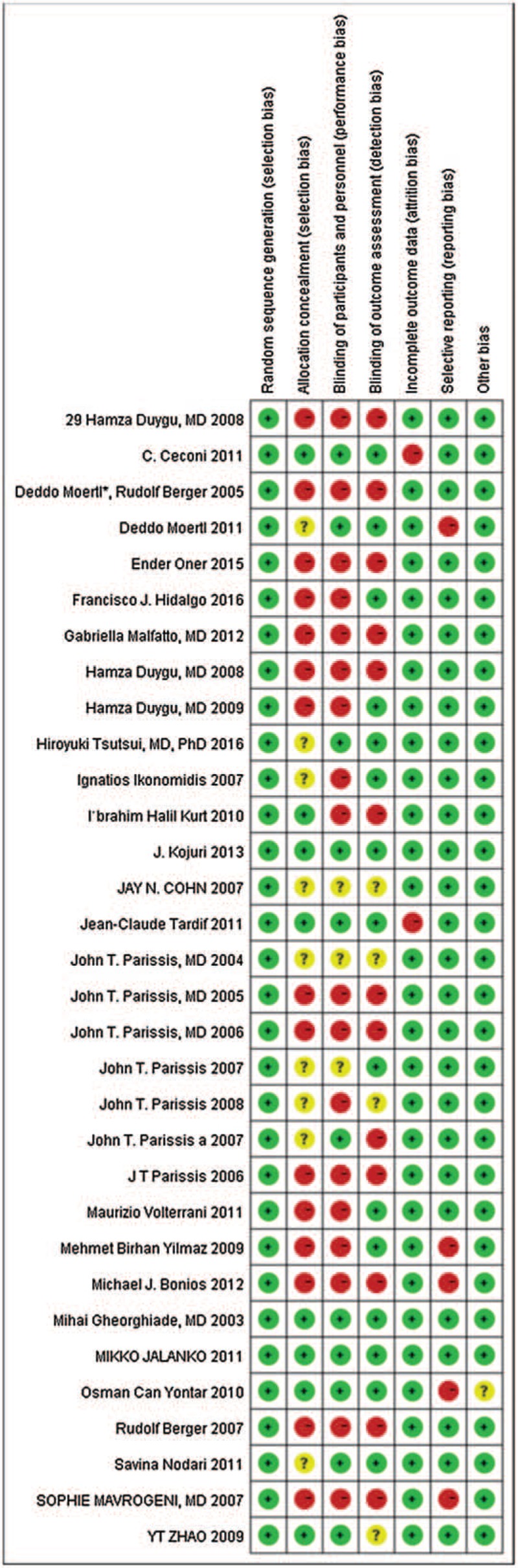
Risk of bias summary of included studies.
3.3. Bayesian network meta-analyses
3.3.1. Outcome 1: LVEF%
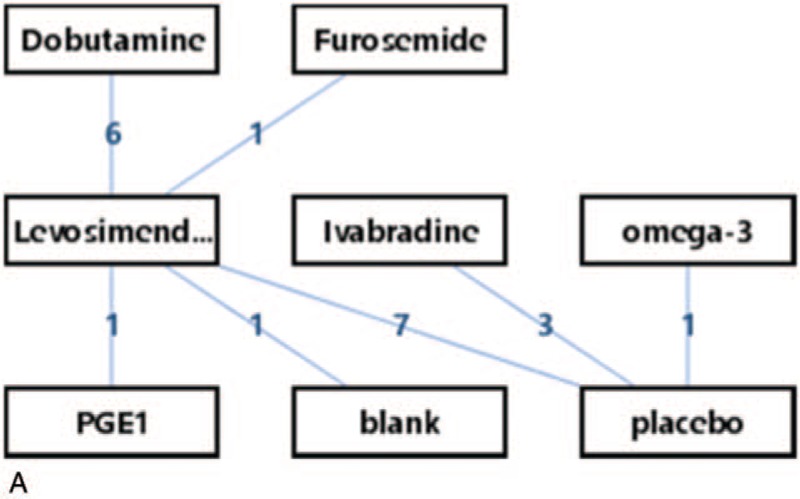
The network of eligible comparisons for efficacy consisted of 20 studies and 8 treatments (16 arms of levosimendan; 6 arms of dobutamine; 3 arms of ivabradine; 1 arm of PGE1, omega-3 and furosemide; 1 arm of blank; 11 arms of placebo). The specific network is presented in Figure 4 A.
Figure 4.
Rank probability of LVEF% in pharmacological treatments. LVEF = left ventricular ejection fraction, PGE1 = prostaglandin E1.
Node-splitting analysis was used to assess consistency. All of the P values between the direct and indirect effects in node-splitting analysis were >.05 (Table 3). A PSRF value closer to 1 indicated convergence and stable results for the model. Therefore, the consistency model was selected for the subsequent network analysis.
Table 2 (Continued).
The characteristics of the included studies.
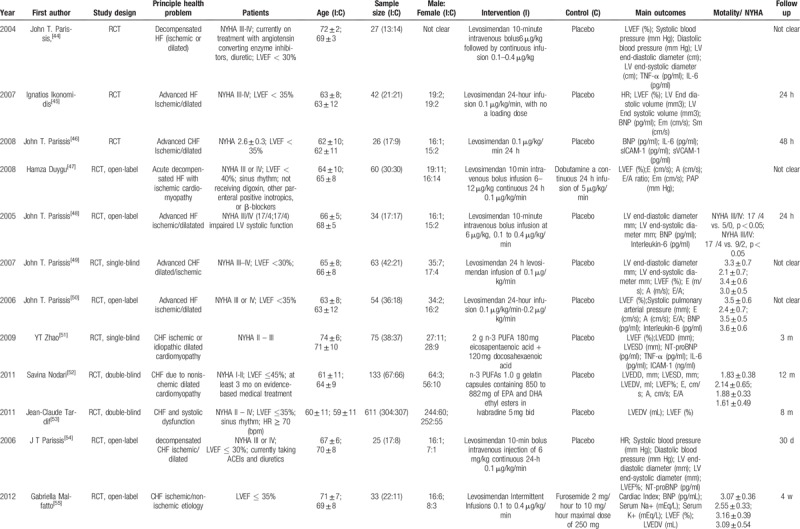
Table 3.
Direct and indirect effects between drugs.

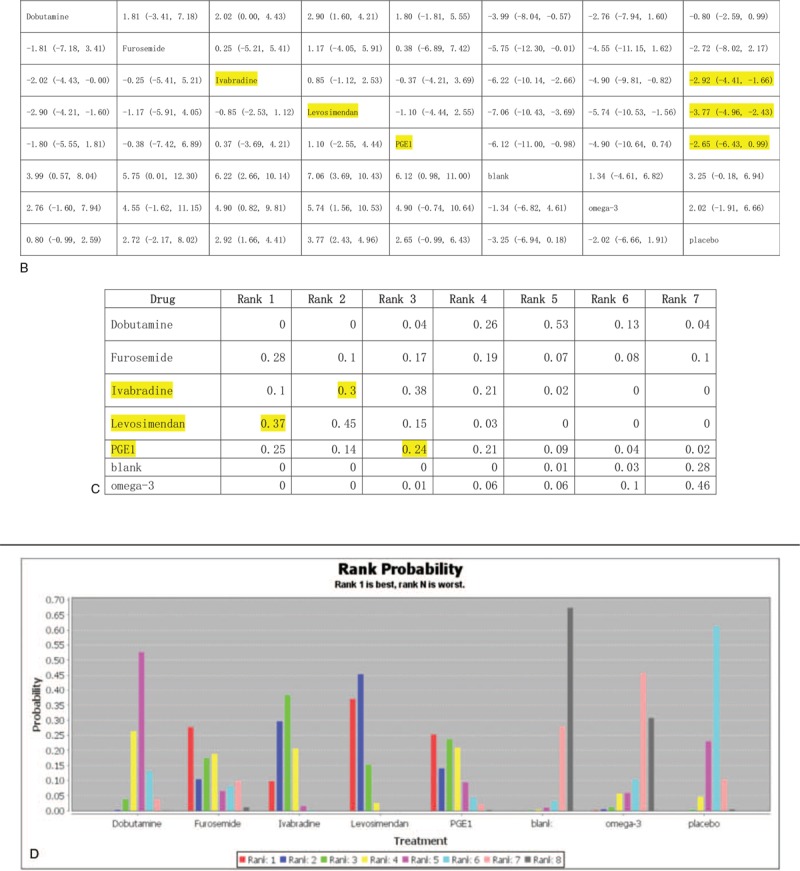
The results of the network meta-analyses for LVEF% are presented as a league table in Figure 4 B. In terms of efficacy, levosimendan was better than placebo (–3.77 (–4.96, –2.43)) and was the best intervention for improving ventricle contraction. The efficacies of ivabradine and PGE1 were also better than that of placebo (–2.92 (–4.41, –1.66)), –2.65 (–6.43, 0.99), respectively).
The ranking probability of treatments is presented in Figure 4 C and D. The results indicated that levosimendan was significantly more effective than the other treatments. The second and third most effective interventions were ivabradine and PGE1, respectively.
3.3.2. Outcome 2: HR
The network of eligible comparisons for efficacy consisted of 11 studies and 6 treatments (10 arms of levosimendan; 2 arms of dobutamine and PGE1; 1 arm of n3-PUFA; 1 arm of blank; 6 arms of placebo). The specific network is presented in Figure 5 A.
Figure 4 (Continued).
Rank probability of LVEF% in pharmacological treatments. LVEF = left ventricular ejection fraction, PGE1 = prostaglandin E1.
Figure 5 (Continued).
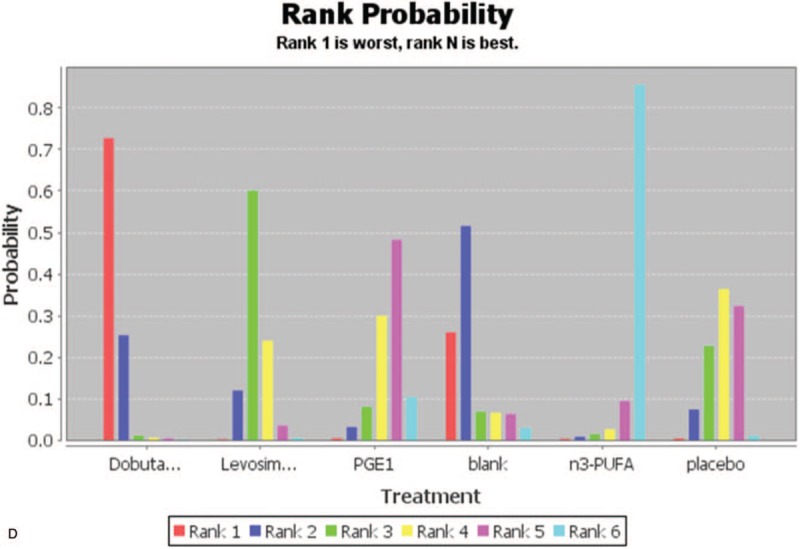
Rank probability of HR in pharmacological treatments. HR = heart rates, n3-PUFA = n-3 polyunsaturated fatty acids, PGE1 = prostaglandin E1.
The results of the network meta-analyses for HR are presented as a league table in Figure 5 B. In terms of efficacy, n3-PUFA was better than placebo (4.01 (–0.44, 8.48)) and was the best intervention for regulating HR. The efficacies of PGE1 was also better than placebo (0.85 (–4.48, 5.64)).
The ranking probability of treatments is presented in Figure 5 C and D. The results indicated that ivabradine was significantly more effective than the other treatments. The next most effective interventions were PGE1 respectively.
3.3.3. Outcome 3: BNP
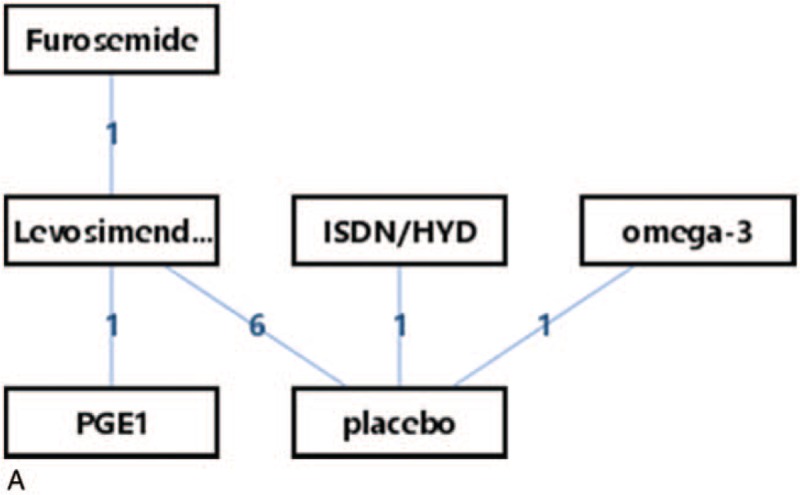
The network of eligible comparisons for efficacy consisted of 10 studies and 6 treatments (8 arms of levosimendan; 1 arm of omega-3, ISDN/HYD, PGE1 and furosemide; 8 arms of placebo). The specific network is presented in Figure 6 A.
Figure 5.

Rank probability of HR in pharmacological treatments. HR = heart rates, n3-PUFA = n-3 polyunsaturated fatty acids, PGE1 = prostaglandin E1.
Figure 6.
Rank probability of BNP in pharmacological treatments. BNP = brain natriuretic peptide, PGE1 = Prostaglandin E1, ISDN/HYD = isosorbide dinitrate and hydralazine.
Figure 6 (Continued).

Rank probability of BNP in pharmacological treatments. BNP = brain natriuretic peptide, PGE1 = Prostaglandin E1, ISDN/HYD = isosorbide dinitrate and hydralazine.
The results of the network meta-analyses for BNP are presented as a league table in Figure 6 B. In terms of efficacy, omega-3 was better than placebo (941.99 (–47.48, 1952.89)) and was the best therapy for improving ventricle wall tension. The efficacies of levosimendan and PGE1 were also better than that of placebo (365.88 (199.34, 550.01)), 306.39 (–159.12, 753.17)), respectively).
The ranking probability of treatments is presented in Figure 6 C and D. The results indicated that omega-3 was significantly more effective than the other treatments. The second and third most effective interventions were levosimendan and PGE1, respectively.
4. Discussion
4.1. Summary of results
This comprehensive network meta-analysis found that levosimendan was superior to the other therapeutic drugs in improving the ventricular systolic function and reducing ventricular wall tension. In the reduction of HR, n3-PUFA plays a critical role that is compatible with its pharmacological effect. The effects of omega-3 in reducing rhBNP were better than that of the control group, suggesting that they were only used in specific circumstances.
4.2. Clinical implications
As a new medication designed for improving cardiac contractility, levosimendan can obtain improved myocardial contraction and blood oxygen supply without increasing the intracellular Ca2+ concentration and avoid adverse events, such as myocardial stunning and malignant arrhythmia.[13,14] A series of clinical studies, including LIDO, RUSSLAN, CASINO, SURVICE, and REVIVE, have confirmed that levosimendan can improve the clinical outcome in patients with congestive heart failure caused by systolic dysfunction.[15–19] In this study, it was found that levosimendan was superior to other drugs in regard to improving myocardial contraction (higher LVEF%, SMD –3.77 (–4.96, –2.43)) and reducing ventricular wall tension (lower serum BNP level, SMD: 365.88 (199.34, 550.01)) mainly because of its unique biological effects in vivo. Levosimendan increases myocardial contraction and improves ventricular diastolic function during the cardiac cycle by pulsed binding to troponin C at low Ca2+ concentrations, which has been demonstrated in laboratory and clinical studies.
Given that the latest guidelines consider HR (frequency) control to be an important component of heart failure management, the use of ivabradine has increased. Unlike the negative muscle force and conduction induced by a β receptor blocker, ivabradine reduces both atrial rhythm and ventricular nonconduction by specifically inhibiting the cationic current If (funny current), which is activated by the hyperpolarization of the sinoatrial node. Studies such as SHIFT and BEAUTIFUL have shown that ivabradine can translate HR reduction into beneficial effects for improving the prognosis of heart failure.[20,21] As a third generation β receptor blocker, carvedilol regulates the adverse effects of catecholamines on the heart and kidneys via non-selective inhibition of the β receptor and selective inhibition of the α1 receptor, thereby improving the long-term prognosis of patients with HFrEF. Further clinical studies have also confirmed that patients with HFrEF taking carvedilol have improved survival compared to those taking a metoprolol succinate or tartrate formulation.[22,23]
As a supplement to traditional diuretics, tolvaptan is mainly used by patients with heart failure with high volume of hyponatremia. EVEREST and other trials have shown that tolvaptan can only alleviate short-term symptoms and signs (sodium retention and dyspnea), but does not help decrease mortality.[24,25] Similar to atorvastatin, exogenous rhBNP (nesiritide) supplementation may improve short-term hemodynamics and acute symptoms in patients with HFrEF, but is not helpful for improving the long-term prognosis.[26,27]
Considered the antiarrhythmic, anti-inflammatory and antioxidant effects of omega-3 polyunsaturated fatty acids, GISSI-HF study from Tavazzi et al. further revealed that omega-3 supplementation may reduce heart failure-related hospitalizations and death in patients with HFrEF (56 patients needed to be treated for a median duration of 3.9 years to avoid one death or 44 to avoid one event like death or admission to hospital for cardiovascular reasons)[28,29]. It was also found in this study that omega-3 polyunsaturated fatty acids supplements improved myocardial performance for patients with HFrEF. Therefore, we suggest that HFrEF patients may benefit from omega-3 supplementation to lower their risk of congestive heart failure-related hospitalizations and death.
4.3. Limitations
There were several limitations in the current study. First, the quality of several of the included studies was not optimal. When evaluating these studies, we found that many lacked details on allocation concealment or blinding. Additionally, several studies had high dropout rates, inevitably due to the lengths of the trials. Second, although we evaluated the studies according to the tool, any evaluation of bias is subjective. There is no quantitative index that can evaluate only an artificial risk of bias. Third, because we used strict inclusion and exclusion criteria, the number of included studies was low, which may have influenced the strength of the evidence. For example, 2 RCTs on LCZ696 were not included in this study due to the lack of the main outcomes required for meta-analysis. Nonetheless, as a revolutionary drug that is most likely able to change the status of heart failure, LCZ696 has been shown to significantly reduce the risk of cardiovascular death and readmission due to heart failure by 20%, while the total mortality is reduced by approximately 20%.[30,31]
5. Conclusion
Our study confirmed the effectiveness of the included new pharmacological treatments for optimizing the structural performance and improving the cardiac function in the management of patients with HFrEF and recommended several interventions for clinical practice. No single clinical trial can answer all pertinent questions, nor can all trial results be perfectly replicated in clinical practice. Additional high-quality RCTs should be performed to provide more powerful evidence in a wider population of heart failure patients.
Author contributions
Conceptualization: Benfa Chen.
Data curation: Yuting Duan, Benfa Chen.
Formal analysis: Yuting Duan.
Funding acquisition: Liming Lu.
Investigation: Shanhua Wang.
Methodology: Jiaming Wu, Liming Lu.
Resources: Yu Zhao.
Software: Yuting Duan.
Supervision: Liming Lu.
Validation: Weiping Su.
Writing – original draft: Heng Li, Yuting Duan.
Footnotes
Abbreviations: ACEI = angiotensin-converting enzyme inhibitor, ADDIS = aggregate data drug information system, ARB = angiotensin-receptor antagonists, BNP = B-type natriuretic peptide, HFrEF = heart failure with reduced ejection fraction, HR = heart rate, HYD = hydralazine, ISDN = isosorbide dinitrate, LVEF = left ventricular ejection fraction, MD = mean difference, NYHA = New York Heart Association, PSRF = potential scale reduction factor, RCTs = randomized controlled trials, rhBNP = recombinant human B-type natriuretic peptide.
How to cite this article: Li H, Duan Y, Chen B, Zhao Y, Su W, Wang S, Wu J, Lu L. New pharmacological treatments for heart failure with reduced ejection fraction (HFrEF): a Bayesian network meta-analysis. Medicine. 2020;99:5(e18341).
HL and YD contributed equally to this study.
This project was funded by the First-Class Discipline Construction Foundation of Guangzhou University of Chinese Medicine (Chinese medicine discipline), the Young Top Talent Project of Scientific and Technological Innovation in Special Support Plan for Training High-Level Talents in Guangdong (No. 2017TQ04R627), and the Guangdong Natural Science Foundation (Project No. 2016A030310290).
The authors have no conflicts of interests to disclose.
References
- [1].Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:147–239. [DOI] [PubMed] [Google Scholar]
- [2].Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- [3].Fonarow GC, Albert NM, Curtis AB, et al. Incremental reduction in risk of death associated with use of guideline-recommended therapies in patients with heart failure: a nested case-control analysis of IMPROVE HF. J Am Heart Assoc 2012;1:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fonarow GC, Hernandez AF, Solomon SD, et al. Potential mortality reduction with optimal implementation of angiotensin receptor neprilysin inhibitor therapy in heart failure. JAMA Cardiol 2016;1:714–7. [DOI] [PubMed] [Google Scholar]
- [5].Clyde W. Yancy, Mariell Jessup, Biykem Bozkurt, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2016;134:e282–93. [DOI] [PubMed] [Google Scholar]
- [6].Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010;376:875–85. [DOI] [PubMed] [Google Scholar]
- [7].Ruilope LM, Dukat A, Böhm M, et al. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet 2010;375:1255–66. [DOI] [PubMed] [Google Scholar]
- [8].McMurray JJV, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- [9].Rognoni A, Lupi A, Lazzero M, et al. Levosimendan: From Basic Science to Clinical Trials. Recent Patents on Cardiovascular Drug Discovery 2011;6:9–15. [DOI] [PubMed] [Google Scholar]
- [10].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932–44. [DOI] [PubMed] [Google Scholar]
- [12].Savovic J, Weeks L, Sterne JA, et al. Evaluation of the Cochrane Collaboration's tool for assessing the risk of bias in randomized trials: focus groups, online survey, proposed recommendations and their implementation. Syst Rev 2014;3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rognoni A, Lupi A, Lazzero M, et al. Levosimendan: from basic science to clinical trials. Recent Pat Cardiovasc Drug Discov 2011;6:9–15. [DOI] [PubMed] [Google Scholar]
- [14].Burger AJ, Horton DP, LeJemtel T, et al. Effect of nesiritide (B-type natriuretic peptide) and dobutamine on ventricular arrhythmias in the treatment of patients with acutely decompensated congestive heart failure: the PRECEDENT study. Am Heart J 2002;144:1102–8. [DOI] [PubMed] [Google Scholar]
- [15].Moiseyev VS, Poder P, Andrejevs N, et al. Safety and efficacy of a novel calcium sensitizer, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction. A randomized placebo controlled, double-blind study (RUSSLAN). Eur Heart J 2002;23:1422–32. [DOI] [PubMed] [Google Scholar]
- [16].Zairis MN, Apostolatos C, Anastasiadis P, et al. The effects of a calcium sensitizer or an inotrope or none in chronic low output decompesated heart failure: Results from the Calcum Sensitizer or Inotrope or None in Low Output Heart Failure Study (CASINO). J Am Coll Cardiol 2004;43: Suppl A: 206A–7A. [Google Scholar]
- [17].Follath F, Cleland JG, Just H, et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low - output heart failure (the LIDO stud): A randomized double- blind trial. Lancet 2002;360:196–202. [DOI] [PubMed] [Google Scholar]
- [18].Packer M, Colucci W, Fisher L, et al. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail 2013;1:103–11. [DOI] [PubMed] [Google Scholar]
- [19].Cleland JG, Freemantle N, Coletta AP, et al. Clinical trials update from the American Heart Association: REPAIR-AMI, ASTAMI, JELIS, MEGA, REVIVE-II, SURVIVE, and PROACTIVE. Eur J Heart Fail 2006;8:105–10. [DOI] [PubMed] [Google Scholar]
- [20].Danchin N. Impact of a pure reduction in heart rate for the treatment of left ventricular dysfunction: clinical benefits of ivabradine in the BEAUTIFUL trial. Therapie 2009;64:111–4. [DOI] [PubMed] [Google Scholar]
- [21].Voors AA, van Veldhuisen DJ, Robertson M, et al. The effect of heart rate reduction with ivabradine on renal function in patients with chronic heart failure: an analysis from SHIFT. Eur J Heart Fail 2014;16:426–34. [DOI] [PubMed] [Google Scholar]
- [22].Ajam T, Ajam S, Devaraj S, et al. Effect on Mortality of Higher Versus Lower β-Blocker (Metoprolol Succinate or Carvedilol) Dose in Patients With Heart Failure. Am J Cardiol 2018;122:994–8. [DOI] [PubMed] [Google Scholar]
- [23].Ajam T, Ajam S, Devaraj S, et al. Effect of carvedilol vs metoprolol succinate on mortality in heart failure with reduced ejection fraction. Am Heart J 2018;199:1–6. [DOI] [PubMed] [Google Scholar]
- [24].Gheorghiade M, Konstam MA, Burnett JC, Jr, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA 2007;297:1332–43. [DOI] [PubMed] [Google Scholar]
- [25].Ambrosy AP, Khan H, Udelson JE, et al. Changes in Dyspnea Status During Hospitalization and Postdischarge Health-Related Quality of Life in Patients Hospitalized for Heart Failure: Findings From the EVEREST Trial. Circ Heart Fail 2016;9:e002458. [DOI] [PubMed] [Google Scholar]
- [26].Silver MA, Horton DP, Ghali JK, et al. Effect of nesiritide versus dobutamine on short-term outcomes in the treatment of patients with acutely decompensated heart failure. J Am Coll Cardiol 2002;39:798–803. [DOI] [PubMed] [Google Scholar]
- [27].Colucci WS, Elkayam U, Horton DP, et al. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N Engl J Med 2000;343:246–53. [DOI] [PubMed] [Google Scholar]
- [28].Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008;372:1223–30. [DOI] [PubMed] [Google Scholar]
- [29].Rimm EB, Appel LJ, Chiuve SE, et al. Seafood Long-Chain n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation 2018;138:e35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- [31].Packer M, McMurray JJ, Desai AS, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015;131:54–61. [DOI] [PubMed] [Google Scholar]
- [32].Mavrogeni S, Giamouzis G, Papadopoulou E, et al. A 6-Month Follow-up of Intermittent Levosimendan Administration Effect on Systolic Function, Specific Activity Questionnaire, and Arrhythmia in Advanced Heart Failure. J Card Fail 2007;13:556–9. [DOI] [PubMed] [Google Scholar]
- [33].Osman Can Yontar, Mehmet Birhan Yilmaz, Kenan Yalta, et al. Acute Effects of Levosimendan and Dobutamine on QRS Duration in Patients with Heart Failure. Arq Bras Cardiol 2010;95:738–42. [DOI] [PubMed] [Google Scholar]
- [34].Oner E, Erturk M, Birant A, et al. Assessment of sustained effects of levosimendan and dobutamine on left ventricular systolic functions by using novel tissue Doppler derived indices in patients with advanced heart failure. Cardiol J 2015;22:87–93. [DOI] [PubMed] [Google Scholar]
- [35].Mehmet Birhan Yilmaz, Can Yontar, Alim Erdem, et al. Comparative effects of levosimendan and dobutamine on right ventricular function in patients with biventricular heart failure. Heart Vessels 2009;24:16–21. [DOI] [PubMed] [Google Scholar]
- [36].Hamza Duygu, Sanem Nalbantgil, Mehdi Zoghi, et al. Comparison of Ischemic Side Effects of Levosimendan and Dobutamine with Integrated Backscatter Analysis, Clin. Cardiol 2009;32:187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bonios MJ, Terrovitis JV, Drakos SG, et al. Comparison of three different regimens of intermittent inotrope infusions for end stage heart failure. Int J Cardiol 2012;159:225–9. [DOI] [PubMed] [Google Scholar]
- [38].Moertl D, Hammer A, Steiner S, et al. Dose-dependent effects of omega-3-polyunsaturated fatty acids on systolic left ventricular function, endothelial function, and markers of inflammation in chronic heart failure of nonischemic origin: a double-blind, placebo-controlled, 3-arm study. Am Heart J 2011;161:915.e1-9. [DOI] [PubMed] [Google Scholar]
- [39].Volterrani M, Cice M, Caminiti G, et al. Effect of Carvedilol, Ivabradine or their combination on exercise capacity in patients with Heart Failure (the CARVIVA HF trial). Int J Cardiol 2011;151:218–24. [DOI] [PubMed] [Google Scholar]
- [40].Hidalgo FJ, Anguita M, Castillo JC, et al. Effect of early treatment with ivabradine combined with beta-blockers versus beta-blockers alone in patients hospitalised with heart failure and reduced left ventricular ejection fraction (ETHIC-AHF): A randomised study. Int J Cardiol 2016;217:7–11. [DOI] [PubMed] [Google Scholar]
- [41].Ceconi C, Freedman SB, Tardif JC, et al. Effect of heart rate reduction by ivabradine on left ventricular remodeling in the echocardiographic substudy of BEAUTIFUL. Int J Cardiol 2011;146:408–14. [DOI] [PubMed] [Google Scholar]
- [42].Kojuri J, Ostovan MA, Rezaian GR, et al. Effect of omega-3 on brain natriuretic peptide and echocardiographic findings in heart failure: Double-blind placebo-controlled randomized trial. Journal of Cardiovascular Disease Research 2013;4:20–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Parissis JT, Andreadou I, Markantonis SL, et al. Effects of Levosimendan on circulating markers of oxidative and nitrosative stress in patients with advanced heart failure. Atherosclerosis 2007;195:e210–5. [DOI] [PubMed] [Google Scholar]
- [44].Parissis JT, Adamopoulos S, Antoniades C, et al. Effects of Levosimendan on Circulating Proinflammatory Cytokines and Soluble Apoptosis Mediators in Patients With Decompensated Advanced Heart Failure. Am J Cardiol 2004;15:1309–12. [DOI] [PubMed] [Google Scholar]
- [45].Ignatios Ikonomidis, John T. Parissis, Ioannis Paraskevaidis, et al. Effects of levosimendan on coronary artery flow and cardiac performance in patients with advanced heart failure. European Journal of Heart Failure 2007;9:1172–7. [DOI] [PubMed] [Google Scholar]
- [46].Parissis JT, Karavidas A, Bistola V, et al. Effects of levosimendan on flow-mediated vasodilation and soluble adhesion molecules in patients with advanced chronic heart failure. Atherosclerosis 2008;197:278–82. [DOI] [PubMed] [Google Scholar]
- [47].Duygu H, Nalbantgil S, Ozerkan F, et al. Effects of Levosimendan on Left Atrial Functions in Patients with Ischemic Heart Failure. Clin Cardiol 2008;31:607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Parissis JT, Panou F, Farmakis D, et al. Effects of Levosimendan on Markers of Left Ventricular Diastolic Function and Neurohormonal Activation in Patients With Advanced Heart Failure. Am J Cardiol 2005;96:423–6. [DOI] [PubMed] [Google Scholar]
- [49].Parissis JT, Papadopoulos C, Nikolaou M, et al. Effects of Levosimendan on Quality of Life and Emotional Stress in Advanced Heart Failure Patients. Cardiovasc Drugs Ther 2007;21:263–8. [DOI] [PubMed] [Google Scholar]
- [50].Parissis JT, Paraskevaidis I, Bistola V, et al. Effects of Levosimendan on Right Ventricular Function in Patients with Advanced Heart Failure. Am J Cardiol 2006;98:1489–92. [DOI] [PubMed] [Google Scholar]
- [51].Zhao YT, Shao L, Teng LL, et al. Effects of n-3Polyunsaturated Fatty Acid Therapy on Plasma Inflammatory Markers and N-Terminal Pro-brain Natriuretic Peptide in Elderly Patients with Chronic Heart Failure. J Int Med Res 2009;37:1831–41. [DOI] [PubMed] [Google Scholar]
- [52].Savina Nodari, Marco Triggiani, Umberto Campia, et al. Effects of n-3 Polyunsaturated Fatty Acids on Left Ventricular Function and Functional Capacity in Patients With Dilated Cardiomyopathy. J Am Coll Cardiol 2011;7:870–9. [DOI] [PubMed] [Google Scholar]
- [53].Tardif JC, O‘Meara E, Komajda E, et al. Effects of selective heart rate reduction with ivabradine on left ventricular remodeling and function: results from the SHIFT echocardiography substudy. Eur Heart J 2011;32:2507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Parissis JT, Adamopoulos S, Farmakis D, et al. Effects of serial levosimendan infusions on left ventricular performance and plasma biomarkers of myocardial injury and neurohormonal and immune activation in patients with advanced heart failure. Heart 2006;92:1768–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Malfatto G, Della Rosa F, Villani A, et al. Intermittent Levosimendan Infusions in Advanced Heart Failure: Favourable Effects on Left Ventricular Function, Neurohormonal Balance, and One-Year Survival. J Cardiovasc Pharmacol 2012;5:450–5. [DOI] [PubMed] [Google Scholar]
- [56].Cohn JN, Tam SW, Anand IS, et al. Isosorbide Dinitrate and Hydralazine in a Fixed-Dose Combination Produces Further Regression of Left Ventricular Remodeling in a Well-Treated Black Population With Heart Failure: Results From A-HeFT. J Card Fail 2007;5:331–9. [DOI] [PubMed] [Google Scholar]
- [57].Berger R, Moertl D, Huelsmann M, et al. Levosimendan and prostaglandin E1 for uptitration of beta-blockade in patients with refractory, advanced chronic heart failure. Eur J Heart Fail 2007;9:202–8. [DOI] [PubMed] [Google Scholar]
- [58].Duygu H, Turk U, Ozdogan O, et al. Levosimendan versus Dobutamine in Heart Failure Patients Treated Chronically with Carvedilol. Cardiovasc Ther 2008;26:182–8. [DOI] [PubMed] [Google Scholar]
- [59].Jalanko M, Kivikko M, Harjola VP, et al. Oral levosimendan improves filling pressure and systolic function during long-term treatment. Scandinavian Cardiovascular Journal 2011;45(2):91–7. [DOI] [PubMed] [Google Scholar]
- [60].Kurt IH, Yavuzer K, Batur MK, et al. Short-term effect of levosimendan on free light chain kappa and lambda levels in patients with decompensated chronic heart failure. Heart Vessels 2010;25:392–9. [DOI] [PubMed] [Google Scholar]
- [61].Moertl D, Berger R, Huelsmann M, et al. Short-term effects of levosimendan and prostaglandin E1 on hemodynamic parameters and B-type natriuretic peptide levels in patients with decompensated chronic heart failure. Eur J Heart Fail 2005;7:1156–63. [DOI] [PubMed] [Google Scholar]
- [62].Gheorghiade M, Niazi I, Ouyang J, et al. Vasopressin V2-receptor blockade with tolvaptan in patients with chronic heart failure: results from a double-blind, randomized trial. Circulation 2003;107:2690–6. [DOI] [PubMed] [Google Scholar]
- [63].Tsutsui H, Momomura S, Yamashina A, et al. Heart Rate Control with If Inhibitor, Ivabradine, in Japanese Patients with Chronic Heart Failure. Circ J 2016;80:668–76. [DOI] [PubMed] [Google Scholar]


