Abstract
This study analyzed independent risk factors that could improve the qSOFA scoring system among sepsis patients.
This retrospective study evaluated 821 patients (2015–2016) who fulfilled the 2001 International Sepsis Definitions Conference diagnostic criteria. Patients were classified based on their survival outcomes after 28 days, and the predictive values of various predictive scores at admission were compared.
The independent risk factors for 28-day mortality were fibrinogen, plasma lactic acid, albumin, oxygenation index, and procalcitonin level >0.5 ng/mL (all P < .05). The “PqSOFA” score combined the qSOFA score with procalcitonin, which provided an area under the curve value of 0.751 (95% CI: 0.712–0.790) for predicting 28-day mortality. A cut-off score of 2 points provided sensitivity of 83.2%, specificity of 54.9%, negative predictive value (NPV) of 33.03%, positive predictive value (PPV) of 92.47%, positive-likelihood ratio (PLR) of 1.85, and negative-likelihood ratio (NLR) of 0.31. The area under the curve for predicting 28-day mortality was significantly greater for the PqSOFA score than for the qSOFA score (Z = 7.019, P < .0001). The PqSOFA score was comparable to the SOFA and APACHE II scores.
The PqSOFA score independently predicted poor short-term outcomes among high-risk sepsis patients.
Keywords: acute sepsis in adults, PqSOFA score, qSOFA score
1. Introduction
Sepsis refers to a systemic inflammatory response syndrome (SIRS) that is caused by the confirmed presence of bacteria or highly suspected infectious lesions, clinically.[1] Epidemiological investigations have demonstrated that the incidence of sepsis is increasing, with an increase in the United States from 164,000 cases in 1979 to 660,000 cases in 2000, based on the results of one retrospective American study.[2] Moreover, a study by Dombrovskiy et al on hospitalized patients in American community hospitals with confirmed sepsis revealed an increase in the proportion of severe sepsis cases from 25.6% in 1993 to 43.8% in 2003.[3] Although the mortality rate for sepsis has decreased during recent years, the increasing incidence has led to an increasing number of deaths due to sepsis.[4] Various studies[5–7] and guidelines[8] regarding sepsis indicate that early assessment, prognostication, and treatment can decrease the mortality rate among sepsis patients.
In this context, non-specific scoring systems have been created for critical illnesses, such as the Acute Physiology and Chronic Health Evaluation (APACHE) II score and Modified Early Warning Score (MEWS) score, as well as sepsis-specific scoring systems, including the Mortality in Emergency Department Sepsis (MEDS) score and Sepsis-related Organ Failure Assessment (SOFA) score. The MEDS score was developed to rapidly assess patients with suspected infection,[9] although it may underestimate the mortality rate among patients with severe sepsis. The SOFA score objectively and dynamically assesses the development and progression of organ dysfunction based on four laboratory parameters,[10] although each laboratory parameter is based on the patient's worst condition on that day, which can interfere with a timely diagnosis and prognostication. The MEWS score only requires six physiological parameters and can be scored rapidly,[11] although it has inadequate ability to assess the criticality of sepsis. The APACHE II score has been considered the gold standard for assessing criticality and prognosis, although it requires 12 clinical and 7 laboratory parameters, which makes it a complicated and time-consuming tool with limited application in emergency medicine.[12] Therefore, there is a need for a simple, rapid, and accurate scoring system for assessing the condition and prognosis of patients with sepsis.
The qSOFA score was initially proposed by Seymour et al in 2015, based on their retrospective study of 148,907 patients with suspected infections.[13] This scoring system uses three parameters: a respiratory rate of ≥22 breaths/min, a systolic blood pressure of ≤100 mmHg, and an altered mental status. Because this system is simple and easy to implement, it was once widely regarded and applied. However, a retrospective study of adult patients with acute sepsis by Wang et al revealed that the qSOFA score only provided an area under the curve (AUC) value of 0.666 for predicting 28-day sepsis mortality that was weaker than the predictive value of the MEDS score.[14] Moreover, Williams et al evaluated 8,871 cases of organ dysfunction in patients with acute infections, and revealed that a qSOFA score of ≥2 provided limited sensitivity (29.7%) that was inadequate for identifying potentially critically ill patients.[15] Thus, it may be difficult to accurately use APACHE II, SOFA, MEDS, MEWS, and qSOFA scores for early and rapid assessment of sepsis severity and prognosis among adult patients.
Although the sensitivity of qSOFA score is low, the scoring method is simple and practical. Therefore, our study aimed to identify independent predictors of sepsis severity and prognosis by analyzing the clinical characteristics of adult sepsis patients who were treated at our emergency department. These factors were then combined with the qSOFA parameters in an attempt to improve it. Finally, the predictive efficacy of the improved qSOFA score was evaluated to examine whether it was appropriate for the rapid assessment of sepsis status, criticality, and prognosis in emergency departments.
2. Materials and methods
2.1. Study design
This single-center retrospective observational study evaluated the clinical characteristics of adult patients with sepsis at the Emergency Department of West China Hospital, Sichuan University. Multiple factor analysis was used to identify factors that independently predicted sepsis criticality and prognosis that were combined with the qSOFA score parameters to create a new assessment system. A substitution model was used to compare the new scoring system with existing scoring systems for predicting the incidences of 7-day mortality, 28-day mortality, 28-day septic shock, 28-day mechanical ventilation, and 28-day intensive care unit (ICU) admission. The patients were grouped according to their survival status at the 28-day follow-up. The study complied with the Declaration of Helsinki, and the study protocol was approved by the Human Ethical Committee of West China Hospital of Sichuan University.
2.2. Data collection and analysis
Electronic and paper medical records were searched for patients who were treated at the Emergency Department of West China Hospital, were >14 years old, and fulfilled the sepsis diagnostic criteria (Sepsis 2.0)[16] between July 1, 2015 and June 30, 2016. Patients were excluded if they were pregnant, in cardiac arrest at hospital arrival, receiving mechanical ventilation support or vasoactive drugs (which may affect the qSOFA score), or could not be scored within 24 hours after their admission. The patients’ general characteristics data (age and sex) were collected, as well as their vital signs data from the triage pre-examination. Blood gas analysis and plasma lactic acid results were obtained within 1 hour after hospital arrival, and laboratory results regarding blood panels, biochemistry, coagulation panels, and procalcitonin (PCT) (>0.5ng/mL suggests sepsis)[13] were measured within 24 hours after hospital arrival. Based on these data, each patient's MEDS, SOFA, MEWS, APACHE II, qSOFA, and GCS scores were calculated. The incidences of 28-day mortality, 7-day mortality, 28-day septic shock, 28-day mechanical ventilation (non-invasive or invasive), and 28-day ICU admission were recorded.
2.3. Statistical analysis
All analyses were performed using IBM SPSS software (version 21.0) and Microsoft Office Excel 2007 software. Normally distributed continuous data were reported as mean ± standard deviation and non-normally distributed continuous data were reported as median and interquartile range. The t test for independent samples was used to compare normally distributed data between the 2 groups, while the rank sum test was used to compare non-normally distributed data between the 2 groups. Categorical data were reported as number and percentage. Multifactor two-category logistic regression analysis was used to identify factors that independently predicted 28-day sepsis mortality. The area under the curve (AUC) was calculated from the receiver operating characteristics (ROC) and the values for sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (+LR), and negative likelihood ratio (-LR) were also calculated. The ROC analysis was also used to compare the abilities of the improved and existing scoring systems for predicting sepsis severity and prognosis. Differences with a two-tailed P value of <.05 were considered statistically significant.
3. Results
3.1. General characteristics
The present study evaluated 841 cases of sepsis, although 20 patients were lost to follow-up (2.38%). Thus, 821 cases, which included 528 men (64.31%) and 293 women (35.69%), were included in the study. After 28 days, 648 patients (78.93%) were alive and 173 patients (21.07%) had died. There were 462 cases (56.27%) of respiratory system infections, 204 cases (24.85%) of abdominal infections, and 155 cases (18.88%) of infection involving other systems (Fig. 1).
Figure 1.
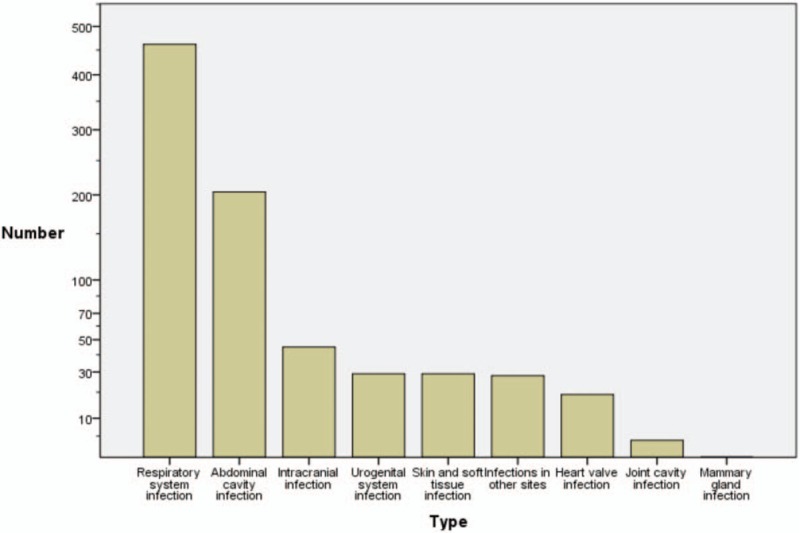
The sites of infection.
3.2. Differences according to survival outcomes
When we compared the groups that were alive or deceased after 28 days, we detected significant differences in age, heart rate, respiratory rate, peripheral oxygen saturation, lactic acid, oxygenation index, platelet count, hemoglobin, albumin, urea nitrogen, creatinine, fibrinogen, D-dimer, PCT, and the qSOFA, SOFA, APACHE II, MEDS, and MEWS scores (Table 1).
Table 1.
Comparison of the groups’ general characteristics.
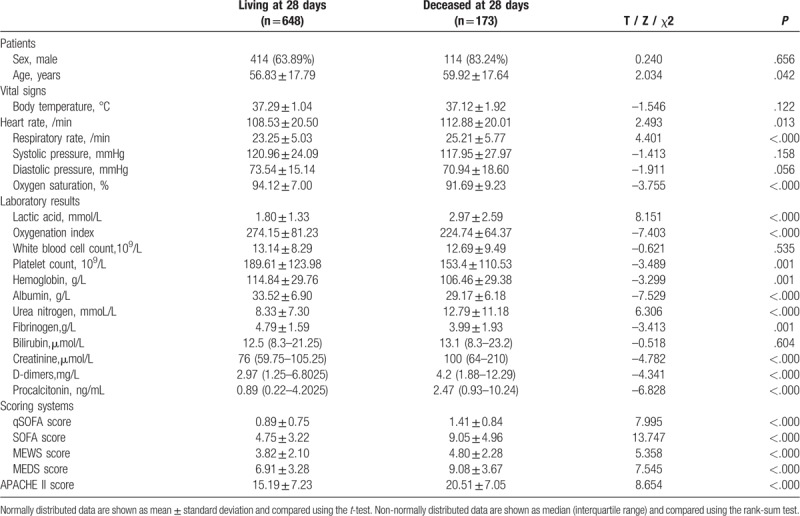
3.3. Independent predictors of 28-day sepsis mortality
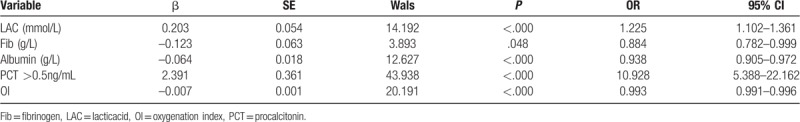
The multifactorial two-category logistic regression analysis revealed that 28-day sepsis mortality was independently predicted by fibrinogen, lactic acid, albumin, oxygenation index, and a PCT level of >0.5 ng/mL (Table 2).
Table 2.
Multifactor two-category logistic regression analysis of factors predicting 28-day sepsis mortality.
3.4. Combined ROC analysis
The ROC curve analyses revealed that the AUC values were 0.578 for fibrinogen, 0.68 for albumin, 0.681 for oxygenation index, 0.655 for lactic acid, and 0.7 for PCT. The PCT parameter provided the largest AUC value (0.7) and good sensitivity (94.2%) (Table 3, Fig. 2).
Table 3.
ROC analysis of factors independently predicting 28-day sepsis mortality.
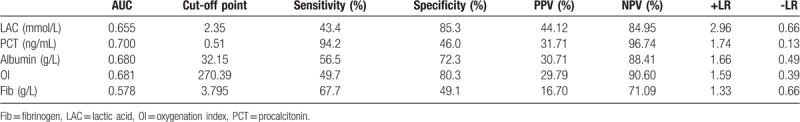
Figure 2.
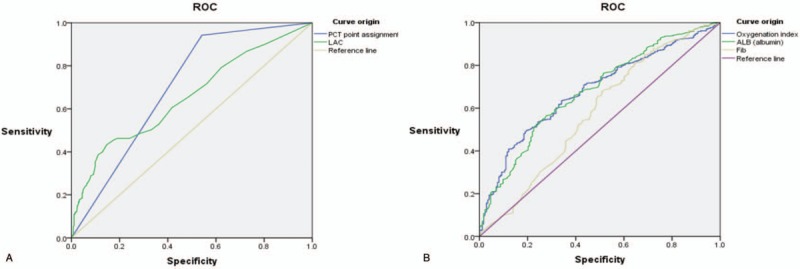
Comparisons of the ROC curves for predicting 28-day sepsis mortality based on plasma lactic acid, PCT (Panel A) or albumin, fibrinogen, and the oxygenation index (Panel B).
3.5. Improvements based on the qSOFA scoring system
The AUC for predicting 28-day sepsis mortality using the qSOFA score was only 0.669, with 2 points identified as the best clinical cut-off point (sensitivity: 45.1%, specificity: 80.7%). The AUC for predicting 28-day sepsis mortality using a PCT level of >0.5 ng/mL was 0.7, with sensitivity of 94.2%, a best cut-off point of 0.51, and an OR value of 10.928. Based on the qSOFA score assignment method, a PCT of >0.5 ng/mL was assigned 1 point, which created the improved “PqSOFA score” with a total score of 4 points (Table 4).
Table 4.
The PqSOFA scoring system.

3.6. The predictive ability of the improved PqSOFA score
The AUC for predicting 28-day sepsis mortality using the PqSOFA score was 0.751 (95% confidence interval [CI]: 0.712–0.790). The best clinical cut-off was 2 points, with sensitivity of 83.2%, specificity of 54.9%, PPV of 33.03%, NPV of 92.47%, +LR of 1.85, and -LR of 0.31 (Fig. 3A andTable 5). The AUC for predicting 28-day sepsis mortality was significantly greater for the PqSOFA score than for the qSOFA assessment (Z = 7.019, P < .0001) (Table 6).
Figure 3.
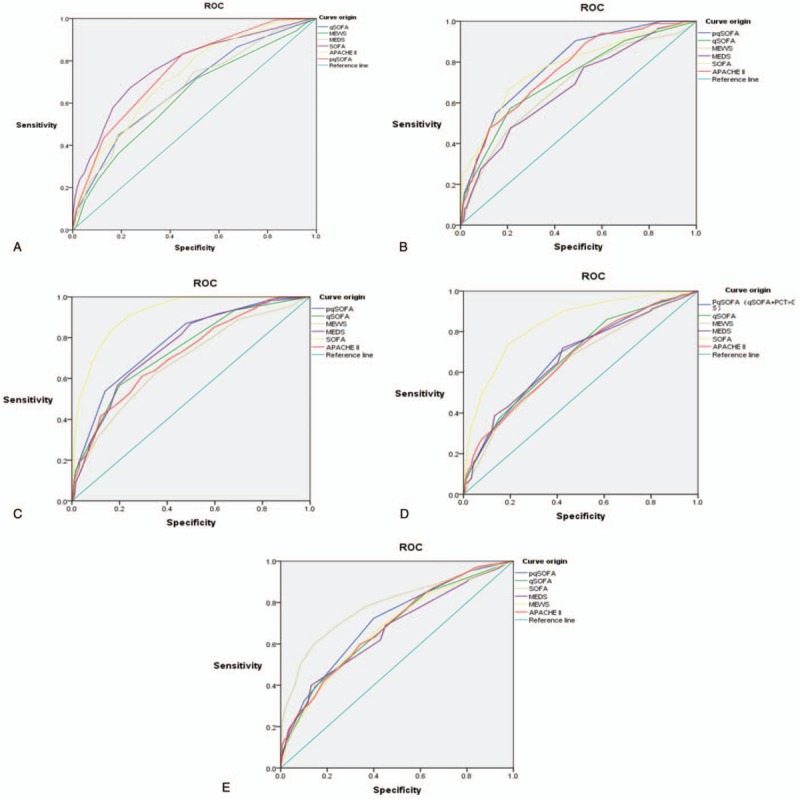
The ROC analysis of each scoring system for predicting the development of 28-day sepsis mortality (Panel A), 7-day sepsis mortality(Panel B), 28-day septic shock (Panel C), 28-day mechanical ventilation(Panel D), and 28-day ICU admission (Panel E).
Table 5.
ROC analysis of each scoring system for predicting sepsis severity and prognosis.
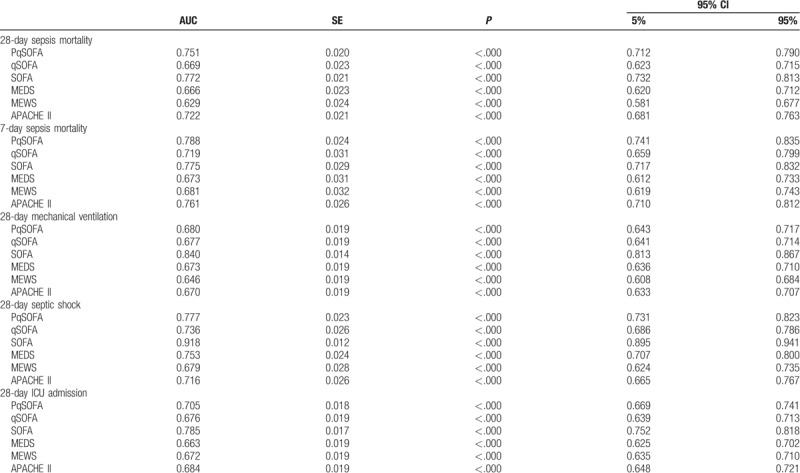
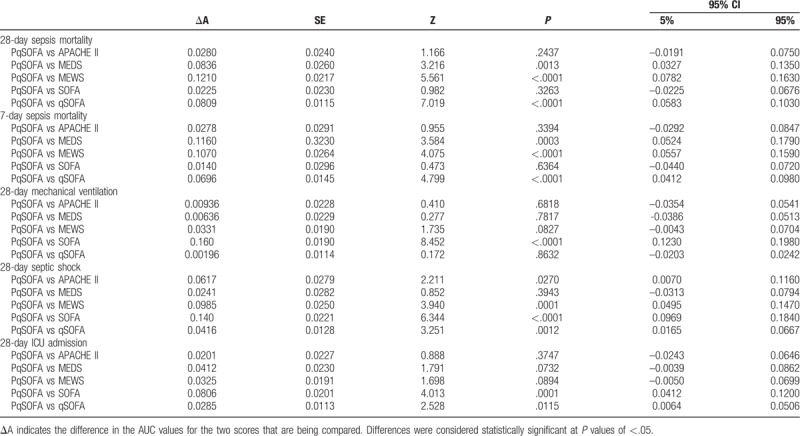
Table 6.
Comparing thePqSOFA to other scores for predicting sepsis severity and prognosis.
3.7. Comparing each scoring system's ability to predict sepsis severity and prognosis
The comparison of the ROCs for the PqSOFA score and the SOFA or APACHE II scores revealed no significant differences in their ability to predict 28-day and 7-day sepsis mortality (P > .05). However, the PqSOFA score provided significantly better predictive ability than the MEDS, MEWS, and qSOFA scores (P < .05) (Fig. 3, Tables 5 and 6).
4. Discussion
Many scoring systems exist for determining the severity and prognosis of sepsis. The APACHE II and SOFA scores have important applications in ICUs globally, although they require a time-consuming and complicated calculation, which limits their application in emergency departments. The present study revealed that the MEWS scores is simple but lacks accuracy, and there is no clear standard for “end-stage disease” in the MEDS score. Moreover, the rating item in “nursing homes” is not suitable for China's national conditions; thus, they are unsuitable for use in China. The qSOFA score was proposed in 2016 based on the newest definition of sepsis and was subsequently found to be significantly better for predicting non-ICU mortality than the SOFA and SIRS scores. For example, Freundet et al prospectively evaluated patients with suspected infection in emergency departments, and revealed that the qSOFA was better than SIRS for predicting in-hospital mortality.[17] Furthermore, Donnellyet et al retrospectively evaluated 2593 cases of infection and reported higher rates of in-hospital and 1-year mortality among patients who fulfilled the qSOFA criteria (score of ≥2 + infection) than among patients who fulfilled the SIRS criteria (score of ≥2 + infection) or SOFA criteria (score of ≥2 + infection).[18] Thus, the qSOFA and SOFA scores are recommended for identifying sepsis patients with poor prognosis. However, many subsequent studies have shown that the qSOFA score has relatively poor ability to predict sepsis severity and prognosis, and that its poor sensitivity limited its clinical application, despite its high specificity.[19,20] Churpek et al compared the NEWS, MEWS, SIRS, and qSOFA scores for predicting non-ICU in-hospital mortality among patients with infection, and reported that the predictive ability of the qSOFA score was not superior to that of the other early warning scoring systems.[21] Other studies also showed that qSOFA had a suboptimal level of predictive value in sepsis.[22,23]
To further improve the predictive efficacy of the qSOFA score, the present study evaluated factors that independently predicted 28-day sepsis mortality. This study showed that lactic acid, albumin, oxygenation index, PCT, and fibrinogen are independent risk factors affecting 28-day mortality in emergency adults with sepsis. The results of this study are consistent with those of other studies.[24–29] We used ROC curves to analyze the predictive efficacy of each of the above independent risk factors for 28-day mortality in sepsis. The results revealed that PCT had a large AUC and relatively high sensitivity, based on a cut-off point of 0.51 ng/mL, which is similar to the optimal cut-off point of a previous study (0.47 ng/mL).[30] In this context, PCT is a widely accepted biomarker for bacterial infection, during which PCT release is induced via two pathways.[31] The first pathway occurs through the organizational structure of bacteria, which induces intracellular signal transduction and hence, PCT release, while the second involves the stimulated production of pro-inflammatory factors and other intermediates by pathogenic microorganisms, which act on target cells to produce PCT. During the progression of infectious diseases, PCT can amplify inflammatory reactions and lead to the worsening of disease status, which explains its significant association with infection severity and prognosis.[32,33] PCT as a marker for distinguishing between bacterial and non-bacterial infections, is highly correlated with SOFA and APACHE II scores in non-surviving septic patients.[34] A study by Zhenyu et al on 102 sepsis cases also revealed that PCT independently predicted 28-day sepsis mortality, with an AUC value of 0.792.[35] Moreover, Castelliet et al reported that PCT can predict sepsis severity and prognosis,[36] while other studies also indicated that PCT concentrations are directly correlated with sepsis criticality and positively correlated with SOFA score.[19,35,36] Hua et al conducted a retrospective multicenter cohort study with independent validation in a prospectively collected cohort in three tertiary medical centers. The study showed that qSOFA score, by adding the ordinal scale of PCT value, could greatly improve the sensitivity to 86.5%.[37] It can be consistently concluded from our study that the inclusion of PCT clearly improves the predictive ability of the qSOFA score. Moreover, the PqSOFA provided significantly higher AUC for predicting 7-day sepsis mortality, relative to the SOFA score, which may indicate that it is useful in predicting the short-term prognosis among adult patients with acute sepsis.
The present study is limited by its retrospective cross-sectional design and inability to prospectively validate the predictive ability of the PqSOFA score. In addition, approximately 20% of the patients were referred to our hospital from other centers, where interventional measures could have been taken that could affect the values of plasma lactic acid, PCT, and other indices, which reflect sepsis severity and prognosis. Finally, a small proportion of the study population was excluded because they were lost to follow-up.
5. Conclusions
Combining PCT and the qSOFA score can facilitate an early assessment of acute sepsis severity and prognosis among adult patients, although its predictive ability is less than ideal. Nevertheless, the PqSOFA score can independently identify critically ill patients with sepsis, predict their short-term adverse events, and their 28-day prognosis. Furthermore, the PqSOFA score had superior predictive value than the qSOFA score, although its performance was comparable to the SOFA or APACHE II scores.
Author contributions
Conceptualization: Yiqin Xia.
Data curation: Yiqin Xia, LiQun Zou.
Formal analysis: Yiqin Xia.
Funding acquisition: LiQun Zou.
Investigation: LiQun Zou.
Methodology: Yiqin Xia.
Project administration: Hai Hu.
Resources: LiQun Zou.
Software: Qin Qin, Yiwu Zhou.
Supervision: Dongze Li.
Validation: Hai Hu, Yiwu Zhou.
Visualization: Dongze Li.
Writing – original draft: Qin Qin.
Writing – review & editing: Yu Cao.
Footnotes
Abbreviations: +LR = positive likelihood ratio, ACCP = American College Of Chest Physicians, APACHE = acute physiology and chronic health evaluation, AUC = area under the curve, COPD = chronic obstructive pulmonary disease, CURB-65 = confusion, urea, respiratory rate and age 65, ESICM = European Society of Intensive Care Medicine, Fib = Fibrinogen, FiO2 = fraction of inspiration O2, GCS = Glasgow coma scale, ICU = intensive care unit, LAC = lactic acid, -LR = negative likelihood ratio, MEDS = mortality in emergency department sepsis score, MEWS = modified early warning score, NPV = negative predictive value, OI = oxygenation index, PaO2 = arterial oxygen pressure, PCT = procalcitonin, PPV = positive predictive value, PqSOFA = Procalcitonin and Quick Sequential Organ Failure Assessment, qSOFA = quick sequential organ failure assessment, ROC curve = receiver operating characteristic curve, SCCM = society of critical care medicine, SIRS = systemic inflammatory response syndrome, SOFA = sequential organ failure Assessment, SPO2 = blood oxygen saturation.
How to cite this article: Xia Y, Zou L, Li D, Qin Q, Hu H, Zhou Y, Cao Y. The ability of an improved qSOFA score to predict acute sepsis severity and prognosis among adult patients. Medicine. 2020;99:5(e18942).
YX and LZ contributed equally to this work.
This work was supported by Scientific Research of the Science and Technology Project of the Health Planning Committee of Sichuan (150169).
The authors have no conflicts of interest to disclose.
References
- [1].Bone RC, Balk RA, Cerra FB, et al. American college of chest physicians/society of critical care medicine consensus conference: definition for sepsis and guideline for the use of innovative therapies in sepsis. Crit Care Med 1992;20:864–74. [PubMed] [Google Scholar]
- [2].Martin GS, Mannino DM, Eaton S, et al. The epidemiology of severe sepsis in the United States from 1979 through 2000. N Engl J Med 2003;348:1546–54. [DOI] [PubMed] [Google Scholar]
- [3].Dombrovskiy VY, Martin AA, Sunderram J, et al. Rapid increase inhospitalization and mortality rates for severe sepsis in the United States: a tread analysis from 1993 to 2003. Crit Care Med 2007;35:1244–50. [DOI] [PubMed] [Google Scholar]
- [4].Lagu T, Rothberg MB, Shieh MS, et al. Hospitalizations, costsand outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med 2012;40:754–61. [DOI] [PubMed] [Google Scholar]
- [5].Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–10. [DOI] [PubMed] [Google Scholar]
- [6].Kumar G, Kumar N, Taneja A, et al. Nationwide trends of severe sepsis in the 21st century (2000-2007). Chest 2011;140:1223–31. [DOI] [PubMed] [Google Scholar]
- [7].Seymour CW, Gesten F, Prescott H, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med 2017;376:2235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med 2018;44:925–8. [DOI] [PubMed] [Google Scholar]
- [9].Shapiro NI, Wolfe RE, Moore RB. Mortality in Emergency Department Sepsis (MEDS) score: a prospectively derived and validated clinical predictionrule. Crit Care Med 2003;31:670–5. [DOI] [PubMed] [Google Scholar]
- [10].Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis related Organ Failure Assessment) score to describe organ dysfuntion/failure. Intensive Care Med 1996;22:707–10. [DOI] [PubMed] [Google Scholar]
- [11].Subbe CP, Kruger M, Rutherford P, et al. Validation of a Modified earlywarning score inmedical admissions. QJM 2001;94:521–6. [DOI] [PubMed] [Google Scholar]
- [12].Knaus WA, Draper EA, Waagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818–29. [PubMed] [Google Scholar]
- [13].Singer M, Deutschman CS, Seymour CW, et al. The Third International consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang JY, Chen YX, Guo SB, et al. Predictive performance of quick Sepsis-related Organ Failure Assessment for mortality and intensive care unit admission in patients with infection at the ED. Am J Emerg Med 2016;34:1788–93. [DOI] [PubMed] [Google Scholar]
- [15].Williams JM, Greenslade JH, McKenzie JV, et al. Systemic inflammatory response syndrome, quick sequential organ function assessment, and organ dysfunction: insights from a prospective database of ED patients with infection. Chest 2017;151:586–96. [DOI] [PubMed] [Google Scholar]
- [16].Levy MM, Fink MP, Marshall JC, et al. International Sepsis Definitions Conference. 200l SCCM/ESICM, ACCP/ATS/SIS International sepsis definitions conference. Intensive Care Med 2003;29:530–8. [DOI] [PubMed] [Google Scholar]
- [17].Freund Y, Lemachatti N, Krastinova E, et al. Prognostic accuracy of sepsis-3 criteria for in-hospital mortality among patients with suspected infection presenting to the emergency department. JAMA 2017;317:301–8. [DOI] [PubMed] [Google Scholar]
- [18].Donnelly JP, Safford MM, Shapiro NI, et al. Application of the Third International Consensus Definitions for Sepsis (Sepsis-3) Classification: a retrospective population-based cohort study. Lancet Infect Dis 2017;S1473-3099:30117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang JY, Chen YX, Guo SB, et al. Predictive performance of quick Sepsis-related Organ Failure Assessment for mortality and ICU admission in patients with infection at the ED. Am J Emerg Med 2016;34:1788–93. [DOI] [PubMed] [Google Scholar]
- [20].April MD, Aguirre J, Tannenbaum LI, et al. Sepsis Clinical Criteria in Emergency Department patients admitted to an intensive care unit: an external validation study of quick seguential organ failure assessment. J Emerg Med 2016;52:622–31. [DOI] [PubMed] [Google Scholar]
- [21].Churpek MM, Snyder A, Han X, et al. Quick sepsis-related organ failure assessment, systemic inflam matory response syndrome, and early warning scores for detecting clinical deterioration in infected patients outside the intensive care unit. Am J Respir Crit Care Med 2017;195:906–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Umemura Y, Ogura H, Gando S, et al. Assessment of mortality by qSOFA in patients with sepsis outside ICU: a post hoc subgroup analysis by the japanese association for acute medicine sepsis registry study group. J Infect Chemother 2017;23:757–62. [DOI] [PubMed] [Google Scholar]
- [23].Askim A, Moser F, Gustad LT, et al. Poor performance of quick-SOFA (qSOFA) score in predicting severe sepsis and mortality - a prospective study of patients admitted with infection to the emergency department. Scand J Trauma Resusc Emerg Med 2017;25:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Song JE, Kim MH, Jeong WY, et al. Mortality risk factors for patients with septic shock after implementation of the surviving sepsis campaign bundles. Infect Chemother 2016;48:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mikkelsen ME, Miltiades AN, Gaieski DF, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med 2009;37:1670–7. [DOI] [PubMed] [Google Scholar]
- [26].Lee YK, Hwang SY, Shin TG, et al. Prognostic value of lactate and central vennous oxygen saturation after early resuscitation in sepsis patients. PLOS One 2016;11:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Houwink AP, Rijkenberg S, Bosman RJ, et al. The association between lactate, mean arterial pressure, central venous oxygen saturation and peripheral temperature and mortality in severe sepsis: a retrospective cohort analysis. Crit Care 2016;20:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vorwerk C, Loryman B, Coats TJ, et al. Prediction of mortality in adult emergency department patients with sepsis. Emerg Med J 2009;26:254–8. [DOI] [PubMed] [Google Scholar]
- [29].Kao HC, Lai TY, Hung HL, et al. Sequential oxygenation index and organ dysfunction assessment within the first 3 days of mechanical ventilation predict the outcome of adult patients with severe acute respiratory failure. ScientificWorldJournal 2013;413216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hu L, Zhu Y, Chen M, et al. Development and validation of a disease severity scroring model for pediatric sepsis. Iran J Public Health 2016;45:875–84. [PMC free article] [PubMed] [Google Scholar]
- [31].Matwiyoff GN, Prahl JD, Miller RJ, et al. Immune regulation of procalcitonin: a biomarker and mediator of infection. Inflamm Res 2012;61:401–9. [DOI] [PubMed] [Google Scholar]
- [32].Kenzaka T, Okayama M, Kuroki S, et al. Use of a semiquantitative procalcitonin kit for evaluating severity and predicting mortality in patients with sepsis. Int J Gen Med 2012;5:483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Arkader R, Troster EJ, Lopes MR, et al. Procalcitonin does discriminate between sepsis and systemic inflammatory response syndrome. Arch Dis Child 2006;91:117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mustafić S, Brkić S, Prnjavorac B, et al. Diagnostic and prognostic value of procalcitonin in patients with sepsis. Med Glas (Zenica) 2018;15:93–100. [DOI] [PubMed] [Google Scholar]
- [35].Zhenyu Li, Hongxia Wang, Jian Liu, et al. Serum soluble triggering receptor expressed on myeloid cells-1 and procalcitonin can reflect sepsis severity and predict prognosis: a prospective cohort study. Mediators Inflamm 2014;641039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Castelli GP, Pognani C, Cita M, et al. Procalcitonin, C-reactive protein, white blood cells and SOFA score in ICU: diagnosis and monitoring of sepsis. Minerva Anestesiol 2006;72:69–80. [PubMed] [Google Scholar]
- [37].Yu H, Nie L, Liu A, et al. Combining procalcitonin with the qSOFA and sepsis mortality prediction. Medicine 2019;98:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


