Abstract
Background:
Acute pancreatitis (AP) is one of the common diseases with increasing incidence in clinical surgery and other gastrointestinal-digestive departments. Despite the rapid development of modern medicine, the overall mortality rate of AP is still high. Xuebijing (XBJ) injection (a traditional Chinese patent medicine) is a potentially effective drug for AP. This study is designed to assess the efficacy and safety of XBJ injection for AP.
Methods:
We will extract data and assess methodological quality of included studies from 7 electronic databases from their inception to December 31, 2019. The primary outcomes include the mortality, surgical intervention, systemic inflammatory response syndrome (SIRS), local complications, systemic infections, gastrointestinal symptoms, and normal blood amylase recovery time. The statistical analysis will be performed using RevMan 5.3 software.
Results:
This study will provide high-quality evidence for the efficacy of XBJ injection as an adjuvant therapy for AP.
Conclusion:
The study will provide the key evidence for clinical doctors and the development of clinical guidelines.
Keywords: acute pancreatitis, protocol, randomized controlled trials, systematic review, Xuebijing injection
1. Introduction
Acute pancreatitis (AP) is a potentially fatal disease that can cause patients to enter the emergency room or intensive care unit.[1–3] Despite the rapid development of modern medicine and the discovery of a large number of prognostic markers and predictors of inflammation, the total mortality of AP is still very high.[4,5] In addition, anti-inflammatory treatment of acute pancreatitis, including prophylactic antibiotics, remains controversial.[6–8]
With the rapid and extensive development of the treatment of AP with the combination of traditional Chinese and Western medicine, the incidence and mortality of sepsis, abdominal compartment syndrome, and other complications of AP have been effectively reduced.[9–12] At present, Xuebijing (XBJ) injection, a mixture of 5 Chinese herbs, has been proved to protect vascular endothelial cells, improve microcirculation and tissue perfusion, regulate immunity, attenuate acute organ injury and dysfunction, and relieve epigastric pain.[13–15] XBJ injection has treatment effects on sepsis and multiple organ dysfunction syndrome (MODS).[13,16] It has the anti-inflammatory effect by reducing the expression of inflammatory factors such as Toll-like receptor-4 and NF-KB.[16] So it has been paid more attention to the treatment of other critical diseases. Some researchers have used it to treat AP and have achieved convincing evidence.[17,18] With new published high-quality researches, it is important to evaluate the efficacy of XBJ injection for AP. Therefore, our aim is to systematically evaluate the efficacy of XBJ injection for AP.
2. Methods
This protocol will follow the Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P).
2.1. Criteria for inclusion in the study
2.1.1. Types of studies
Randomized controlled trials (RCTs) will be included. We will exclude quasi-randomized controlled trials (quasi-RCTs) with non-random methods, such as date of admission, date of birth, or clinic record number. The language will be unlimited.
2.1.2. Types of participants
Patients with AP (age ≥18 years) will be included. There is no restriction on gender. AP is diagnosed based on internationally recognized diagnostic standards.[19–21] Patients with AP should be hospitalized within 48 hours.
2.1.3. Types of interventions
The intervention is XBJ plus routine treatment in the treatment group and only routine treatment in the control group. The routine treatment includes fluid resuscitation, antibiotic therapy, nutritional support, or mechanical ventilation.
2.1.4. Types of outcomes
The primary outcomes include mortality, surgical intervention, systemic inflammatory response syndrome, local complications, systemic infection (septicemia, urinary tract infection, and pneumonia), gastrointestinal symptoms (the relief time of abdominal pain, bloating relief time, anal exhaust recovery time, defecation recovery time, bowel sound recovery time), and normal blood amylase recovery time.
Secondary outcomes include Acute Physiology and Chronic Health Evaluation II score, hospitalization time, inflammatory markers (such as C-reactive protein), and adverse events.
2.2. Search strategy
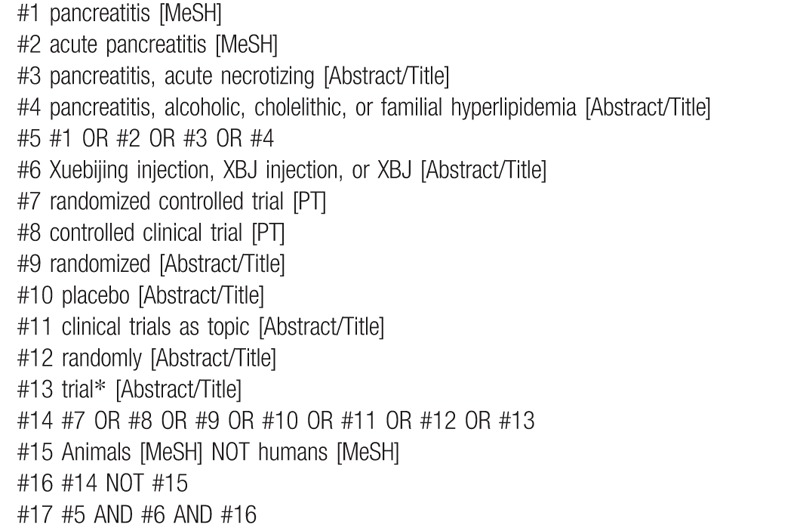
Two reviewers (CG and KZ) will independently search PubMed, Web of Science, EMBASE, Cochrane Central Register of Controlled Trials, Wan Fang Data, Chinese Scientific Journal Database, and China National Knowledge Infrastructure (CNKI) from their inception to December 31, 2019. Table 1 shows the search strategy for PubMed. ClinicalTrials.gov will also be searched to identify potentially eligible studies.
Table 1.
Search strategy for PubMed.
2.3. Selection of studies
The titles and abstracts will be checked to exclude irrelevant papers. Then, filter the remaining studies by reading the full texts. Two authors will cross-check results, discuss, and resolve the disagreement.
2.4. Data extraction and management
Two reviewers (BHL and NS) will extract the relevant data independently. Any difference will be evaluated by a third reviewer (KZ). The information such as study design, characteristics of patients, interventions, and outcomes will be extracted. If the data is incomplete, we will try to contact the first author or corresponding author for obtaining the missed information.
2.5. Risk of bias
Two authors (CG and KZ) will independently investigate the risk of bias for each included study with a tool in the Cochrane handbook. It will be graded as low, unclear, or high. Disagreements will be resolved by discussion or consultation with another author (QLT).
2.6. Measures of treatment effect
We will conduct statistical analysis by RevMan 5.3 software. Risk ratio (RR) will be used to estimate the effect for the dichotomous variables. Mean difference (MD) will be used to represent the estimate of the effect for continuous variables. We will also report the 95% confidence intervals (CIs). When the same result is measured in a variety of ways, standard mean difference (SMD) will be used to describe the intervention effect.
2.7. Assessment of heterogeneity
The I2 value will be computed to quantify the statistical heterogeneity in the meta-analysis. The I2 value will be divided into 4 categories:
-
(1)
0% to 40%;
-
(2)
30% to 60%;
-
(3)
50% to 90%;
-
(4)
75% to 100%
2.8. Data synthesis
Heterogeneity test will be conducted before conducting the meta-analysis. If I2 < 50% and P > .10, the heterogeneity will be low. The fixed-effects model will be used to estimate the effect. On the contrary, the random-effects model will be adopted. Descriptive analysis will be performed when clinical heterogeneity is not neglected. Publication bias will be graphically examined by the funnel plot when the meta-analysis contains 10 or more studies.[22] We will conduct subgroup analysis according to mild and severe AP, age, race, and drug dosage.[19,23–25] The robustness of the meta-analysis will be tested based on risk of bias and sample size by sensitivity analysis.
2.9. Assessment of evidence quality
We will investigate the quality of evidence for all outcomes by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool. The level of evidence is divided into 4 types (very low, low, medium, or high).
2.10. Ethics and dissemination
There is no need for ethical approval because individual information cannot be identified. The findings and important protocol amendments will be reported in a peer-reviewed journal.
3. Discussion
This study will provide the high-quality evidence on XBJ injection for acute pancreatitis, and a reference for clinical doctors and the development of clinical guidelines. Some potential limitations may affect the conclusions drawn from the study. First, different drug doses, the severity of acute pancreatitis may increase the risk of heterogeneity. Second, it is difficult to adopt the blinding in RCTs about traditional Chinese medicine injections for AP.[26–30] We need to explain results with caution.
Author contributions
YL conceived the study and provided general guidance for the drafting of the protocol. CG and KZ drafted the protocol. QLT, LXT, and KZ designed the search strategy. QLT, LXT, CG, KZ, NS, BHL, JBZ, and SL drafted the manuscript. QLT, LXT, CG, KZ, NS, BHL, JBZ, SL, and YL reviewed and revised the manuscript. All authors have read and approved the final version of the manuscript.
Footnotes
Abbreviations: AP = acute pancreatitis, CIs = confidence intervals, CNKI = China National Knowledge Infrastructure, GRADE = Grading of Recommendations Assessment, Development and Evaluation, MD = mean difference, MODS = multiple organ dysfunction syndrome, PRISMA-P = Preferred Reporting Items for Systematic Review and Meta-analysis Protocols, RCTs = randomized controlled trials, RR = risk ratio, SMD = standard mean difference, XBJ = Xuebijing.
How to cite this article: Tang Q, Tian L, Gao C, Zhang K, Su N, Liu B, Zhai J, Liu S, Li Y. The efficacy and safety of Xuebijing Injection as an adjunctive treatment for acute pancreatitis: Protocol for a systematic review and meta-analysis of randomized controlled trials. Medicine. 2020;99:4(e18743).
QT, LT, CG, KZ, NS, BL, and JZ contributed equally to this work and are considered co-first authors.
There is no need for ethical approval because individual information cannot be identified. The findings will be reported in a peer-reviewed journal.
Prospero registration number: CRD42018115113.
This study is supported by National Natural Science Foundation of China (grant number: 81373839).
The authors have no conflicts of interest to disclose.
References
- [1].Forsmark CE, Swaroop Vege S, Wilcox CM. Acute pancreatitis. N Engl J Med 2016;375:1972–81. [DOI] [PubMed] [Google Scholar]
- [2].Munigala S, Subramaniam D, Subramaniam DP, et al. Predictors for early readmission in acute pancreatitis (AP) in the United States (US) – a nationwide population based study. Pancreatology 2017;17:534–42. [DOI] [PubMed] [Google Scholar]
- [3].Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol 2019;16:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].van Dijk SM, Hallensleben NDL, van Santvoort HC, et al. Acute pancreatitis: recent advances through randomised trials. Gut 2017;66:2024–32. [DOI] [PubMed] [Google Scholar]
- [5].Waller A, Long B, Koyfman A, et al. Acute pancreatitis: updates for emergency clinicians. J Emerg Med 2018;55:769–79. [DOI] [PubMed] [Google Scholar]
- [6].Mourad MM, Evans R, Kalidindi V, et al. Prophylactic antibiotics in acute pancreatitis: endless debate. Ann R Coll Surg Engl 2016;99:107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bai Y, Gao J, Zou D, et al. Prophylactic antibiotics in acute pancreatitis: further high-quality trials are still warranted. Am J Gastroenterol 2008;103:1838–9. [Google Scholar]
- [8].Lata J, Stibůrek O. Prophylactic antibiotics and probiotics in acute pancreatitis. Vnitr Lek 2010;56:582–4. [PubMed] [Google Scholar]
- [9].Zhang XP, Shi Y, Zhang L. Progress in the study of therapeutic effects of traditional Chinese medicine and extracts in treating severe acute pancreatitis. JOP 2007;8:704–14. [PubMed] [Google Scholar]
- [10].Zhang M, Zhang G, Yuan W, et al. Treatment of abdominal compartment syndrome in severe acute pancreatitis patients with traditional Chinese medicine. World J Gastroenterol 2008;14:3574–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chen H, Li F, Jia J, et al. Effects of traditional Chinese medicine on intestinal mucosal permeability in early phase of severe acute pancreatitis. Chin Med J (Engl) 2010;123:1537–42. [PubMed] [Google Scholar]
- [12].Wang J, Chen G, Gong H, et al. Amelioration of experimental acute pancreatitis with Dachengqi Decoction via regulation of necrosis-apoptosis switch in the pancreatic acinar cell. PLoS One 2012;7:e40160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li C, Wang P, Zhang L, et al. Efficacy and safety of Xuebijing injection (a Chinese patent) for sepsis: a meta-analysis of randomized controlled trials. J Ethnopharmacol 2018;224:512–21. [DOI] [PubMed] [Google Scholar]
- [14].Chen G, Gao Y, Jiang Y, et al. Efficacy and safety of Xuebijing injection combined with ulinastatin as adjunctive therapy on sepsis: a systematic review and meta-analysis. Front Pharmacol 2018;9:743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen X, Feng Y, Shen X, et al. Anti-sepsis protection of Xuebijing injection is mediated by differential regulation of pro- and anti-inflammatory Th17 and T regulatory cells in a murine model of polymicrobial sepsis. J Ethnopharmacol 2018;211:358–65. [DOI] [PubMed] [Google Scholar]
- [16].He F, Wang J, Liu Y, et al. Xuebijing injection induces anti-inflammatory-like effects and downregulates the expression of TLR4 and NF-κB in lung injury caused by dichlorvos poisoning. Biomed Pharmacother 2018;106:1404–11. [DOI] [PubMed] [Google Scholar]
- [17].Huang P, Huang Z, Qin WBX, et al. Clinical observation of Xuebijing injection in adjunctive therapy of severe acute pancreatitis. China Pharm 2016;27:4580–1. [Google Scholar]
- [18].Liu XD. Xuebijing injection for treatment of severe acute pancreatitis: curative effect and influence on inflammatory factors. World Chin J Digestol 2017;25:929–33. [Google Scholar]
- [19].Sarr MG. 2012 revision of the Atlanta classification of acute pancreatitis. Polskie Archiwum Medycyny Wewnetrznej 2013;123:118–24. [DOI] [PubMed] [Google Scholar]
- [20].Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis – 2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102–11. [DOI] [PubMed] [Google Scholar]
- [21].Pancreatic Disease Group CSoG, Association CM. Consensus on the diagnosis and treatment of acute pancreatitis. Chin J Dig Dis 2010;6:47–51. [DOI] [PubMed] [Google Scholar]
- [22].Tarsilla M. Cochrane Handbook for Systematic Reviews of Interventions. 2008;142. [Google Scholar]
- [23].Crockett SD, Wani S, Gardner TB, et al. American Gastroenterological Association Institute guideline on initial management of acute pancreatitis. Gastroenterology 2018;154:1096–101. [DOI] [PubMed] [Google Scholar]
- [24].Zhang K, Li C, Gao C, et al. Efficacy and safety of acupuncture as an adjuvant treatment for acute pancreatitis: a protocol of systematic review and meta-analysis. BMJ Open 2019;9:e029327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang K, Gao C, Li C, et al. Acupuncture for acute pancreatitis: a systematic review and meta-analysis. Pancreas 2019;48:1136–47. [DOI] [PubMed] [Google Scholar]
- [26].Tang Q, Zhang K. Is acupuncture effective for knee osteoarthritis? Comment on a recent trial. Clin Rehabil 2019;33:1697–8. [DOI] [PubMed] [Google Scholar]
- [27].Zhang K, Tang Q, Zhao C. Traditional manual acupuncture combined with rehabilitation therapy for shoulder hand syndrome after stroke within the Chinese healthcare system. Clin Rehabil 2019;33:1699–700. [DOI] [PubMed] [Google Scholar]
- [28].Zhang K, Tang Q, Gao C. Non-pharmacologic treatments for symptoms of diabetic peripheral neuropathy: a systematic review – methodological issues are a matter for concern. Curr Med Res Opin 2019;35:1319–20. [DOI] [PubMed] [Google Scholar]
- [29].Zhang K, Tang Q. Acupuncture on aromatase inhibitor-induced arthralgia in patients with breast cancer. Breast 2019;119. [DOI] [PubMed] [Google Scholar]
- [30].Zhang K, Gao C, Tang Q. Acupuncture for reduction of symptom burden in multiple myeloma patients undergoing autologous hematopoietic stem cell transplantation: a randomized sham-controlled trial. Respond to author. Support Care Cancer 2019;27:3171–2. [DOI] [PubMed] [Google Scholar]


