Supplemental Digital Content is available in the text
Keywords: assessment tools, iatrogenic withdrawal syndrome, opiates, paediatric intensive care units, sedatives, treatment
Abstract
Background:
Sedoanalgesia secondary iatrogenic withdrawal syndrome (IWS) in paediatric intensive units is frequent and its assessment is complex. Therapies are heterogeneous, and there is currently no gold standard method for diagnosis. In addition, the assessment scales validated in children are scarce. This paper aims to identify and describe both the paediatric diagnostic and assessment tools for the IWS and the treatments for the IWS in critically ill paediatric patients.
Methods:
A systematic review was conducted according to the PRISMA guidelines. This review included descriptive and observational studies published since 2000 that analyzed paediatric scales for the evaluation of the iatrogenic withdrawal syndrome and its treatments. The eligibility criteria included neonates, newborns, infants, pre-schoolers, and adolescents, up to age 18, who were admitted to the paediatric intensive care units with continuous infusion of hypnotics and/or opioid analgesics, and who presented signs or symptoms of deprivation related to withdrawal and prolonged infusion of sedoanalgesia.
Results:
Three assessment scales were identified: Withdrawal Assessment Tool-1, Sophia Observation Withdrawal Symptoms, and Opioid and Benzodiazepine Withdrawal Score. Dexmedetomidine, methadone and clonidine were revealed as options for the treatment and prevention of the iatrogenic withdrawal syndrome. Finally, the use of phenobarbital suppressed symptoms of deprivation that are resistant to other drugs.
Conclusions:
The reviewed scales facilitate the assessment of the iatrogenic withdrawal syndrome and have a high diagnostic quality. However, its clinical use is very rare. The treatments identified in this review prevent and effectively treat this syndrome. The use of validated iatrogenic withdrawal syndrome assessment scales in paediatrics clinical practice facilitates assessment, have a high diagnostic quality, and should be encouraged, also ensuring nurses’ training in their usage.
1. Introduction
In paediatric intensive care units (PICU), sedoanalgesia is an essential part of the treatment of critical patients. Some of its main objectives consist in avoiding stress and other negative psychological effects, as well as the physical suffering related to invasive procedures, preventing accidental removal of vascular catheters and self-extubation, and encouraging the adaptation of the patient to mechanical ventilation (MV).[1,2] However, it is difficult to achieve optimal sedation and analgesia in children. In fact, it is only achieved in 60% of patients.[1,3] That is why mismatches frequently occur in PICU regarding over or under sedoanalgesia alike. These phenomena are related to increased hospital stays, prolongation of MV, increased morbidity and mortality, inadequate management of pain, increased risk of nosocomial infections, and occurrence of iatrogenic syndrome, among others.[1–3]
There are few recommendations on the drugs that must be used for sedation and analgesia in the paediatric critical patient. In the clinical practice of PICUs, the most used pharmacological agents are opioids and benzodiazepines (BZD), being fentanyl (FNT) the analgesic of choice, followed by paracetamol and metamizole and, as sedative, midazolam (MDZ), followed by lorazepam, ketamine, and propofol.[3,4] The benefits of both pharmacological groups on the critical patient are evident, but they also generate tolerance and physical dependence, making higher doses and prolonged infusions necessary in order to maintain the desired effects and avoid the WS.[1,5]
The iatrogenic withdrawal syndrome (IWS) consists in the set of signs and symptoms that appear after removing or decreasing psychoactive drugs that have been administered for a long time or in high doses.[6] Its incidence in the PICU is highly variable. In Spain, it affects 50% of the patients who have a continuous infusion of sedoanalgesia for 48 hours, increasing to more than 80% when infusion lasts more than 5 days.[7] In other countries, the incidence of the IWS is similar, reaching 50% in patients with infusions of more than 24 hours of duration, and an increase from 80% to 100% when exceeding 5 days of treatment.[7–10] Regarding the IWS signs and symptoms, these vary depending on the drug and patient characteristics, such as age, cognitive status, etc. The most common manifestations are at breathing level (tachypnoea), gastrointestinal (nausea, vomiting, diarrhea), nervous system (sweating, tachycardia, mydriasis), and motor level (tremors, abnormal movements, hyperreflexivity, hypertonia).[1,8] In addition, it is worth noting that opioid abstinence originates more superficial movement disorders and gastrointestinal disorders, as opposed to the withdrawal of BZD.[11]
On the other hand, factors that are related to the appearance of the IWS include the use of hypnotics and analgesics, duration, and abrupt interruption of the sedoanalgesia, the patients’ characteristics, and health care system factors.[1,12,13] Regarding the characteristics of the medications used in the treatment, it has been observed that the risk of IWS is higher when given synthetic and/or short half-life opioids. Similarly, other factors have been associated with an increased incidence of IWS, such as cumulative doses of hypnotics and opioids (MDZ ≥ 40–60 mg/kg FNT ≥ 0.48–1.5 mg/kg, sufentanil ≥95.61 μg/kg/hour and propofol ≥4 mg/kg/hour); continuous infusions of sedoanalgesia for a period longer than 3–5 days; simultaneous use of muscle relaxants; and sudden interruption of sedoanalgesia.[1,7–9] As for the characteristics of the patient, a relationship has been observed between the IWS, age, and clinical status of the patient. In children, the younger they are, the greater the vulnerability to IWS. However, the symptoms are more pronounced in term infants that in preterm infants.[14] Likewise, neurological conditions such as brain injury and ischemia and the presence of cognitive and functional decline are linked to an increased risk of IWS.[15] With respect to the health system factors, the absence of an interdisciplinary group, the lack of training and professional experience, the small number of sedation protocols, and the withdrawal of sedoanalgesia increase the incidence of IWS.[12]
The IWS is usually under diagnosed and undertreated, as its signs and symptoms often change and may be confused with other more frequent conditions in the paediatric critical patient. In fact, there is no gold standard for the diagnosis or assessment of the IWS by opioids and/or BZD.[1] The majority of studies on IWS carried out until now have used unvalidated scales for its evaluation in the paediatric population. Furthermore, most of the scales used in research and clinical practice employ tools specifically designed for newborns but that are also applied to the rest of paediatric patients, such as the Finnegan scale, the neonatal withdrawal index to narcotics, etc.[12] However, in recent years, new IWS diagnostic and monitoring instruments have been specifically designed and validated for infants and children of older ages. Therefore, the lack of knowledge on a validated IWS diagnostic and assessment scale in paediatric patients makes the evaluation of the effectiveness of treatments for this syndrome complex, and may even lead to wrong conclusions.[16] Recently, there have been studies that propose prevention strategies and treatments for the IWS, including the daily interruption of sedation, the gradual reduction of sedoanalgesia, a decrease in analgesics doses, the administration of alpha-2 agonists, or the transition to long half-life barbiturates, among others. However, the best treatment for the IWS in critical paediatric patients and the necessary conditions for its safe usage have not been established.[17]
The present work proposes a twofold objective: to identify and describe both the paediatric diagnostic and assessment tools for the IWS, and the treatments for the IWS in paediatric critical patients.
2. Materials and methods
A systematic review was carried out following the recommendations for systematic reviews collected in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).[18] The checklist and flow diagram are included as supplementary files (Fig. 1 and Supplementary File 1).
Figure 1.

PRISMA flow chart of the study selection process.
2.1. Search strategy
An electronic search of articles was carried out during the months of February to April, 2018 in the following databases: Medline-PubMed, Embase-Elsevier, Cochrane Central Register of Controlled Trials (CENTRAL), Latin American and Caribbean Literature in Health Sciences (LILACS, for its acronym in Spanish), Web of Science, CINAHL, Scopus, and Google Scholar.
The terms were found in the Medical Subject Headings (MeSH), in addition to free terms: Opioid; Benzodiazepines (MeSH); Hypnotics and Sedatives (MeSH); Iatrogenic Withdrawal Syndrome; Substance Withdrawal Syndrome (MeSH); Child∗(MeSH); Pediatric intensive care units (MeSH); Treatment; Therapy (MeSH); Substance Withdrawal Syndrome (MeSH); Assessment tool; Scale. With these terms, along with the truncation characters (∗) and Boolean AND and OR operators, the search strategies for each of the objectives of the review were built.
The following strategies were used for the search of IWS assessment and diagnostics scales: Substance Withdrawal Syndrome AND Assessment tool; Substance Withdrawal Syndrome AND scale AND child∗; Substance Withdrawal Syndrome AND Assessment tool AND child∗; Opioid AND Benzodiazepine AND Substance Withdrawal Syndrome AND Assessment tool; Hypnotics AND Sedatives AND Substance Withdrawal Syndrome AND Assessment tool; Iatrogenic Withdrawal Syndrome AND Assessment tool AND pediatric intensive care.
The following strategies were used for the IWS treatments search: Hypnotics AND Sedatives AND Iatrogenic Withdrawal Syndrome; Opioid AND Benzodiazepine AND (Iatrogenic Withdrawal Syndrome OR Substance Withdrawal Syndrome); Opioid AND Benzodiazepine AND Substance Withdrawal Syndrome AND Child∗; Opioid OR benzodiazepine AND Substance Withdrawal Syndrome AND pediatric intensive care; Iatrogenic Withdrawal Syndrome AND Therapy; Iatrogenic Withdrawal Syndrome AND Treatment AND Child∗; Iatrogenic Withdrawal Syndrome OR Substance Withdrawal Syndrome) AND Therapy AND Child∗.
Additionally, the references of relevant articles were reviewed to identify studies that met the objectives of the review and to include them in the screening process.
2.2. Selection criteria
The selected studies for the development of this systematic review met the following inclusion criteria:
Types of studies: randomized clinical trials, observational and descriptive studies published in Spanish or English, available in full text. Due to the limited number of records on this subject, documents from 2000 to 2018 were included.
Participants: Studies involving neonates, newborns, infants, pre-schoolers, and adolescents up to age 18 who were admitted to the PICU with continuous infusion of hypnotics and/or opioid analgesics and who presented signs or symptoms of deprivation related to withdrawal and prolonged infusion of sedoanalgesia.
Intervention: Studies in which drugs were administered for the treatment or prophylaxis of the IWS, produced by continuous infusions of hypnotics and/or opioids such as α2-adrenergic agonists, buprenorphine, methadone, naloxone, and dexmedetomidine. In addition, studies that compared the efficacy of different drugs, dosages, and posology for the treatment or prevention of the IWS were selected. On the other hand, articles that estimated the validity, specificity and sensitivity of different diagnostic and evaluation instruments of the paediatric IWS were included. In addition, records that compared the effectiveness of these scales were selected.
Studies that met the following criteria were excluded:
Researches involving participants with neonatal withdrawal syndrome (newborns to mothers with substance dependence or exposed to opiates and illicit drugs in utero), cognitive alterations, IWS due to drug abuse, IWS related to drugs other than hypnotics and/or opioids, and who were also admitted to non-critical care units.
Publications that were oriented to the treatment or prophylaxis of tolerance to hypnotics and opiates.
Studies that did not specify the administered treatment of IWS, nor the assessment methods or the diagnostic criteria for the IWS (cut-off score of a scale, number of manifestations of the IWS, severity of the symptoms, etc.).
Articles analyzing assessment and/or diagnostic tools of neonatal IWS and delirium.
2.3. Selection of studies and data extraction
The studies selection was carried out in 3 phases. In the first phase, the titles of all the results were screened by a reviewer. Then, 2 reviewers read the summary of the articles selected in the previous phase, discarding irrelevant documents. The third phase consisted of the final selection of studies for the review, after the full text was read and the verification of compliance with the inclusion and exclusion criteria was carried out. In situations of disagreement, a consensus was reached between the 2 reviewers.
Once the final selection of articles for the review was made, the analytical reading, data extraction and classification of the information was carried out in evidence tables that were specifically designed for this purpose. In these tables, the following items were extracted: first author, year of publication, design of the study, objectives, population, intervention, assessment method, main results, and conclusions.
2.4. Assessment of the methodological quality of the studies
The methodological quality of cohort studies was assessed through the Critical Appraisal Skills Programme España (CASPe) templates[19,20] in order to identify possible biases. Articles that obtained more than 6 points were included, considering as good quality scores those > 8, 6–8 as average quality, and ≤5 as low quality. Reports and case series were assessed with the CARE Guide.[21] Articles that fulfilled at least 80% of the items and contained all the sections of these types of studies were selected.
In situations of discordance, a critical analysis of the study was repeated, and discordance was resolved by mutual agreement.
2.5. Ethical considerations
Ethical considerations were identified in the reviewed articles, and no ethical issues were raised.
3. Results
A total of 4740 bibliographical references were initially identified by following the search strategy, and 17 additional records through the assessment of the references of the screened studies. 3586 duplicated items were removed.
In the first screening (title reading), 1053 studies were excluded. Subsequently, the analysis of summaries of 118 records was developed, eliminating 93 of them. Therefore, 25 articles were examined in full text, of which 11 met the inclusion criteria and underwent methodological assessment.[22–32] Finally, 10 studies were included in the review.[22–30,32] The degree of concordance between the 2 reviewers was determined with the Cohen Kappa index, which was 0.73 (confidence interval, CI 95%: 0.47–0.88). The studies selection process for the review developed according to PRISMA is reflected in Figure 1.
Regarding the type and design of the studies, 6 were observational and 4 were descriptive. The first 6 consisted of 4 prospective cohort studies and 2 case-control analyses. As for the descriptive records, there were 3 case reports and 1 case series.
3.1. Methodological quality
Of the observational studies, 1 did not report follow-up losses, originating a selection bias and affecting its validity.[30]
As for the descriptive articles, only 1 case report specified the study design in the title.[27] Although no study indicated whether the patient's informed consent was requested, all were approved by an ethics committee. On the other hand, 1 study represented the most relevant events in a table.[26] Finally, a case series was the only study that did not comply with at least 80% of the items included in the CARE Guide, and it was decided to exclude it from the review by mutual agreement between the reviewers.[31] The results of the methodological assessment are collected in Tables 1–3.
Table 1.
Methodological assessment of observational cohort studies, according to the Critical Appraisal Skills Programme (CASP).

Table 3.
Methodological assessment of case report studies, according to the CARE Guide.

Table 2.
Methodological assessment of observational studies, including cases and controls, according to the Critical Appraisal Skills Programme (CASP).

3.2. IWS assessment instruments
Four observational articles focused on the analysis of IWS assessment scales related to opioids and sedatives in critically ill children.[22,23,25,32] All of them were prospective cohort studies, involving 378 patients and performing 6323 IWS tests. Two of them examined the Withdrawal Assessment Tool-1 (WAT-1) scale[22,23]; another one analyzed the Opioid and Benzodiazepine Withdrawal Score (OBWS)[32]; and finally, 1 study assessed the Sophia Observation Withdrawal Symptoms (SOS).[25]Table 4 describes the studies that analyze the IWS assessment tools included in this review.
Table 4.
Description of the studies on IWS assessment tools included in the review.

Three validated IWS assessment scales, specific for the paediatric population, were identified: WAT-1, SOS, and OBWS.[22,23,25,32] As for the structure, the OBWS complies with 21 items that estimate the frequency and severity of withdrawal symptoms.[32] The WAT-1 is constituted by 11 items and it values the presence and severity of the manifestations of deprivation.[22,23] The SOS has 15 items, each one corresponding to a withdrawal symptom, obtaining a positive score if the symptom has been observed at some point in 4 hours.[25]Table 5 summarizes the characteristics of these tools.
Table 5.
Main features of the IWS diagnostic tools.

3.3. IWS treatment
Among the reviewed papers, 2 studies analyzed clonidine as a pharmacological treatment for IWS associated with continuous and prolonged infusion of benzodiazepines and opioids.[27,30] Two other studies examined methadone to reduce and prevent IWS caused by prolonged administration of opiates. Likewise, 1 study focused on phenobarbital and clonidine,[26] and another one on dexmedetomidine, as IWS treatments, when it was developed for the same previous causes.[24]Table 6 details the selected records that studied IWS treatments in critical paediatric patients for this review.
Table 6.
Description of the studies on pharmacological treatment of the IWS included in the review.
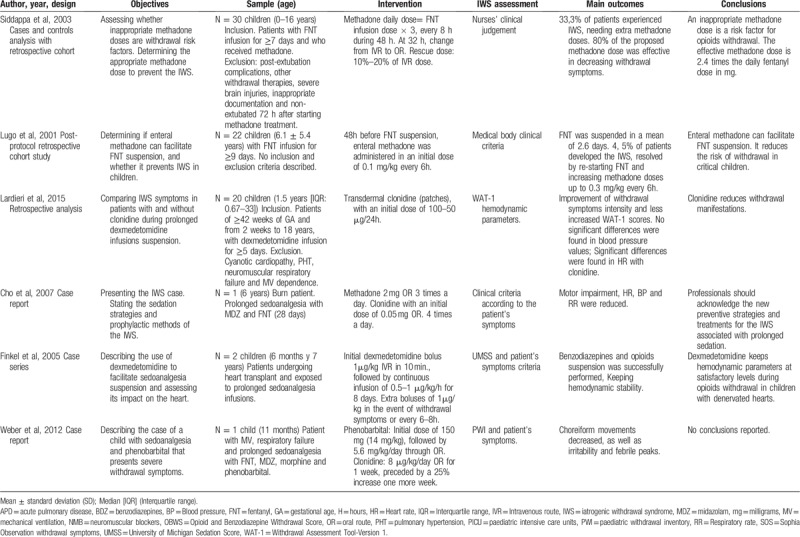
Three of them were descriptive studies and the other 3 were observational. These involved a total of 75 very heterogeneous patients. The demographic characteristics of these patients are reflected in Table 6. All subjects received multiple sedatives and analgesics during their hospital stay, being benzodiazepines and opiates the most usually employed ones. These were administered continuously for 37.92 ± 28.50 days in average, simultaneously with extra doses (boluses) of sedoanalgesia in case of loss of adaptation to the mechanical ventilation or given withdrawal indications in the process of withdrawal of these drugs. The sedoanalgesia treatment is described in Table 6.
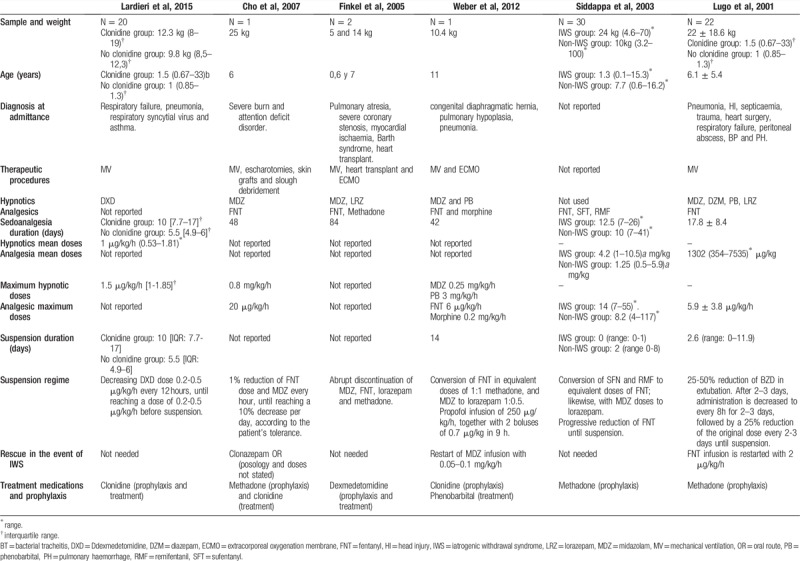
Three different methods were used in the process of withdrawing the sedoanalgesia. On the one hand, in 2 of the reviewed studies, analgesia and sedation withdrawal were carried out progressively, according to the child's tolerance, until they were completely withdrawn.[27,30] On the other hand, 2 articles used sequential sedation prior to extubation of the patient, which consisted in converting doses of analgesics and opioids into equipotent doses of morphine, methadone or FNT, and MDZ, respectively. Then, they were withdrawn.[24,28] Finally, 2 studies applied the usual hospital units’ protocol of sedoanalgesia withdrawal.[26,29]Table 7 details the sedoanalgesia withdrawal strategies.
Table 7.
Description of demographic characteristics, sedoanalgesia strategies and withdrawal regimes employed in studies on the pharmacological treatment of the IWS included in the review.
3.3.1. Clonidine
Three studies analyzed the effects of clonidine on the IWS manifestations and as a prophylactic therapy for this phenomenon.
On the one hand, Ladrieri et al[30] compared withdrawal symptoms in subjects receiving transdermal clonidine and those who did not receive it. In his research, it was found that, 24 hours after the withdrawal of dexmedetomidine, patients without clonidine presented greater intensity of deprivation symptoms with a WAT-1: 3.2 score (range 0-8) vs 0.8 (range 0–6), (P = .49), as compared to patients who did have clonidine administered. The most pronounced symptoms in patients without clonidine were trembling, repetitive movements, startles, time to calm, increased muscle tone, and higher score in the Behavioural State Scale (BSS). No significant differences were observed in systolic (SBP) and diastolic (DBP) blood pressure figures, neither prior to the sedoanalgesia withdrawal (P = .624 and P = .910) and after this (P = .851 and P = .678), nor between both groups. However, the average heart rate (HR) in the clonidine group 24 hours after the withdrawal of sedoanalgesia was significantly lower than in the group without clonidine: 112 bpm (88.5–151.5) vs 138.4 bpm (range 117.8–168.3); P = .03. Also, the HR mean change of pre-and post-discontinuation was significantly higher in the group without clonidine versus the clonidine group [29.9 bpm (range: 5.5–74.7) vs 3.6 bpm (range 39.6–47.5); P = 0.042]. Finally, rebound arterial hypertension was the only adverse effect related to clonidine.
On the other hand, Cho et al[27] employed clonidine to decrease the sympathetic activation of the patient. In this study, it was found that this was effective for lowering HR, RR, and blood pressure, and although they failed to eliminate the trembling, they did reduce its intensity. Finally, Weber et al[26] found that clonidine along with dexmedetomidine as prophylactic treatments for the IWS did not obtain results, presenting the subject fever, insomnia, extreme agitation, nervousness, and inconsolable crying 72 hours after the discontinuation of the sedoanalgesia.
3.3.2. Methadone
Two studies assessed methadone as IWS treatment in critically ill paediatric patients. In the study by Siddappa et al,[28] 66.6% (n = 20) of patients presented less than 3 withdrawal symptoms and, therefore, did not require additional doses of methadone. On the other hand, 33.3% (n = 10) of patients showed more than 3 signs of high intensity withdrawal, coming to need additional doses of this drug. Additionally, in this analysis, the optimal dose of methadone was determined to prevent the IWS and it was found that, with doses ≤80% of the used formula (methadone = 3× daily dose of fentanyl), withdrawal symptoms would occur in 23.3% (n = 7) of the subjects (OR: 21), and with a dose of >80% in 10% (n = 3). There were no secondary effects derived from methadone administration.
In the research by Lugo et al,[29] 95.5% (n = 21) of children did not present IWS during the decrease in fentanyl infusion. The only patient who developed IWS manifested diaphoresis, agitation, arterial hypertension (mean blood pressure: 90–100 mm Hg), diarrhoea, tachypnoea, disorientation, abdominal pain, and trembling. However, with an increase of 0.30 mg/kg in the dose of methadone every 6 hours, simultaneously with diazepam 0.1 mg/kg every 6 hours, the symptoms were resolved.
3.3.3. Phenobarbital
Weber et al[26] developed the only study that assessed phenobarbital associated with clonidine as IWS treatment. The subject experienced cessation of choreiform movements, and decreased stress and fever after 2 days of the onset of phenobarbital. In addition, it reduced the need for additional doses of sedatives and analgesics, allowing to be withdrawn in less than a week. No adverse effects associated with this drug were developed.
3.3.4. Dexmedetomidine
One study analyzed the use of dexmedetomidine to facilitate withdrawal of opioid analgesics.[24] In this research, this drug showed to be useful by decreasing sympathetic activation, reducing the heart rate from 135–176 bpm to 110–147 bpm, SBP mean from 90–150 mm Hg to 88–123 mm Hg, and slightly decreasing the DBP mean from 55–98 mm Hg to 50–70 mm Hg. Similarly, it eliminated disorganized movements and maintained the level of sedation, according to the ≤ 2 sedation score of the University of Michigan (UMSS). However, nausea was present on the fifth day of infusion of this medicine. Finally, rebound arterial hypertension was not reported when lowering the dose or after removing the dexmedetomidine.
4. Discussion
This review of the literature identifies 3 IWS assessment scales validated for the paediatric population and describes a clinical variability in relation to the diagnosis and treatment of the IWS. These results are of great relevance since the absence of an early and correct diagnosis of the IWS physically and psychologically affects patients’ well-being and increases health expenditure.[6,33]
Harris et al[34] recommend the use of validated IWS assessment tools and whose reliability, validity and clinical utility have been tested and demonstrated in children. The WAT-1, SOS, and OBWS are validated scales. However, their items do not conform to the different paediatric ages. In the case of OBWS, the Moro reflex is included which, despite being a significant manifestation of withdrawal in newborns, disappears by 3 months of age.[35] WAT-1, on the contrary, does not include any specific symptoms at any stage of the child's development.[22,23] However, Frank et al[22] indicate that estimating each of these characteristics would seriously compromise the utility of the tool. Consequently, future research to define a new IWS assessment tool for collecting the specific manifestations at each age or for carrying out adaptations of the existing scales for each stage of development are necessary.
In relation to the statistical characteristics, it was found that the WAT-1 and the SOS possess high sensitivities and specificities, and have similar psychometric characteristics.[22,23,25] The OBWS, on the contrary, presents an excellent specificity but a reduced sensitivity, which translates into a low capacity for the scale to detect the IWS in really sick children and high competence in ruling out the disease in healthy subjects.[32] On the other hand, the SOS and WAT-1 obtained a greater diagnose accuracy against the OBWS, reflected with higher areas below the ROC curve.[22,23,25,32] Finally, the SOS is a scale with a high possibility of making strong changes in the pre and post-test probability.[25] However, the OBWS showed good predictive validity and, according to its likelihood ratio, it is an acceptable diagnostic test which slightly increases the probability of a positive diagnosis of the IWS.[32]
In addition, none of the 3 instruments, despite contemplating different signs and symptoms of withdrawal, can discern between the IWS caused by opioids and the IWS caused by BZD.[11,23,25] However, Franck et al[23] suggest that WAT-1 is more effective in detecting symptoms of opioid withdrawal than in detecting symptoms of BZD withdrawal. This may be because, unlike the SOS and OBWS, this scale does not contemplate the specific withdrawal manifestations to these sedatives: hallucinations, grimaces, and considerable disorganized movements.[11,25,32,36] It could even be said that the SOS is more sensitive than the OBWS in detecting BZD withdrawal manifestations because, in addition to assessing the hallucinations and alterations of the movement, it also comprises facial expressions.[25,32] Finally, the WAT-1 faces this drawback by incorporating in its items the SBS, which requires a painful stimulus for its assessment.[22,23]
In this review, clonidine was revealed to be effective in subsiding withdrawal symptoms from sedoanalgesia. On the one hand, Ladrieri et al[30] employed transdermal clonidine and, on the other hand, Cho et al[27] and Weber et al[26] used it in an enteral way, achieving similar effects and even facilitating the withdrawal of opioid analgesics, although it was not possible to eliminate the trembling.
Additionally, in the Weber et al study,[26] clonidine did not have the expected prophylactic effect, and the IWS was triggered. Therefore, it might be suggested that transdermal clonidine is slightly more effective than enteral. This fact can be attributed, first, to the lower tissue infusion of the organs existing in critical patients which in turn causes atrophy and reduction of gastrointestinal motility. This translates into the decrease of the gastrointestinal absorption of the medicine.[17] Secondly, it has been observed that clonidine has a greater prophylactic utility for the IWS in the first 24 hours after the withdrawal of sedation, especially in children with respiratory failure.[30] Third, in several studies, it has been shown that transdermal clonidine is safer since it provides constant doses and, in turn, reduces peak doses and adverse reactions.[27,30,31]
The results of the reviewed articles are consistent with the review conducted by Duffet et al[37] on the effect of clonidine on sedation and withdrawal symptoms. In this review, it was described that with the use of clonidine, a reduction in withdrawal manifestations was obtained, along with an increase in the level of sedation and the need for sedatives. However, these results may be limited by the small sample size, the lack of precision to estimate the effect of clonidine, and the heterogeneity of the included studies.
According to the reviewed studies, methadone managed to reduce and prevent the IWS, with scarce side effects. Siddappa et al[28] converted the dose of intravenous fentanyl into equipotent doses of methadone that were subsequently enterally administered. This practice has been recommended and used by several researchers.[6,38,39] However, most of these formulas were made from the pharmacokinetic characteristics of healthy subjects.[40] In addition, regarding changes in the route of administration, it is necessary to consider the capacity of fentanyl (100 times greater than methadone) and the average life and the bio-viability of methadone (75%–100% and 75%–80%).[6] On the contrary, Lugo et al,[29] despite the previous recommendations, used a minimum initial dose of methadone, increased if necessary. It is argued that, even considering the capacity and average life of both drugs, the resulting equivalent dose is higher than the dose needed to prevent the IWS, thus lengthening dependence and opiates withdrawal time.[29]
This review identified a lack of agreement in relation to the most suitable initial dose to prevent the IWS. On the one hand, Siddappa et al[28] recommended 2.4 times the dose of fentanyl every 6 hours, in contrast to Lugo et al,[29] who proposed a dose of 0.1 mg/kg every 6 hours. However, Johnson et al,[40] in their review, advised more frequent intervals of administration, reaching ranges of 3.8 to 62 hours. According to the above-mentioned, using smaller intervals between doses seems to be more effective to achieve therapeutic concentrations in a more precocious way.
Dexmedetomidine was used in a descriptive study included in this review for the purpose of facilitating withdrawal of opiates in 2 hospitalized children in a PICU with denervated hearts by cardiac transplantation.[24] The initially used dexmedetomidine doses were recommended for adults and, in the event of withdrawal symptoms, additional boluses of dexmedetomidine were administered.[24] The use of non-paediatric doses responds to the lack of studies on the use of dexmedetomidine in children with transplanted hearts. So far, it had only been proven to be used in animals with similar characteristics. However, the results partly coincide with another descriptive study in which the dexmedetomidine effectively controlled the manifestations of the IWS without producing hemodynamic disturbances.[31]
Phenobarbital, like other IWS treatments, managed to eliminate withdrawal signs and symptoms, being this a case of resistant IWS to other treatments such as dexmedetomidine, clonidine and methadone. In the case of dexmedetomidine, a loading dose was not administered, being this, a priori, the reason for its ineffectiveness.[24] There are studies that prove that the efficacy of dexmedetomidine depends both on the loading dose and on the procedure of its withdrawal.[31]
Finally, in this review, the relevance of the appropriate dosage administration in all the analyzed IWS treatments was proven. This is because, regardless of the drug used, an excessive or insufficient dose increases the risk of IWS. Likewise, the withdrawal regime must be closely controlled and set by objectives, with the purpose of not developing the IWS through the same drug that was administered to prevent it.
4.1. Limitations
This review has several limitations: due to the limited number of studies, subjects, and the diversity of variables (sedoanalgesia regimen, IWS assessment, concomitant use of other drugs, polypathological patients, and interventions), it is difficult to quantify the effect of the analyzed treatments. Another obvious limitation is the moderate quality of the data and the scarce evidence of the analyzed articles, since they include prospective and descriptive observational studies without including any clinical trial, which may be due to the shortage of literature on this research topic. However, all references were submitted to an assessment of their methodological quality in order to identify their limitations and biases.
5. Conclusions
In this review, 3 diagnostic and assessment scales of the IWS with sedatives and/or narcotic analgesics in clinically ill paediatric patients have been identified: WAT-1, SOS, and OBWS. All these tools are validated and have good predictive and diagnostic qualities. WAT-1 and OBWS have similar psychometric properties and are more effective than the SOS in the detection of the IWS. On the other hand, nurses are essential in the diagnosis of the IWS and they perceived the OBWS as a useful tool in the detection and assessment of this phenomenon. The use of validated IWS valuation scales in paediatrics’ clinical practice should be encouraged, and the nursing staff should be trained in their usage.
Regarding IWS treatments related to prolonged administration of hypnotics and opiates in hospitalized children in critical care units, clonidine proved to be effective in preventing, palliating, and decreasing the intensity of withdrawal symptoms from both opioids and hypnotics, especially by subsiding sympathetic activation. However, it may cause rebound arterial hypertension as an adverse effect, although this is rare.
On the other hand, enteral and parenteral methadone reduce the risk of IWS produced by opioids, facilitate the discontinuation, and alleviate withdrawal manifestations after the suspension. Dexmedetomidine was not only efficient in reducing the opioid withdrawal symptoms, even in patients with cardiac transplantation. In addition, it facilitated the withdrawal of these analgesics while maintaining a hemodynamic stability. Finally, phenobarbital is efficient in alleviating and reducing the intensity of opioids and BZD withdrawal symptoms, that are resistant to other therapies.
To conclude, the literature on paediatric IWS assessment tools and the pharmacological treatment of this syndrome is scarce. Consequently, future research on these issues is necessary to develop a more complete comparison of the effectiveness of reviewed scales and treatments. In the same way, a more effective management and assessment of the IWS could be achieved, as well as the determination of its prevalence in the paediatric population in a more reliable way.
Author contributions
Conceptualization: Jennihe Alejandra Ávila-Alzate, Santiago Martínez-Isasi.
Data curation: Jennihe Alejandra Ávila-Alzate, Macarena Romero-Martín, Daniel Fernández-García.
Formal analysis: Jennihe Alejandra Ávila-Alzate, Macarena Romero-Martín, Daniel Fernández-García.
Investigation: Juan Gómez-Salgado, Santiago Martínez-Isasi, Daniel Fernández-García.
Methodology: Jennihe Alejandra Ávila-Alzate, Juan Gómez-Salgado, Macarena Romero-Martín, Santiago Martínez-Isasi, Daniel Fernández-García.
Project administration: Jennihe Alejandra Ávila-Alzate.
Resources: Juan Gómez-Salgado, Macarena Romero-Martín, Daniel Fernández-García.
Supervision: Jennihe Alejandra Ávila-Alzate, Juan Gómez-Salgado, Santiago Martínez-Isasi.
Validation: Juan Gómez-Salgado, Santiago Martínez-Isasi.
Visualization: Jennihe Alejandra Ávila-Alzate, Macarena Romero-Martín, Santiago Martínez-Isasi.
Writing – original draft: Jennihe Alejandra Ávila-Alzate, Macarena Romero-Martín, Daniel Fernández-García.
Writing – review & editing: Juan Gómez-Salgado, Santiago Martínez-Isasi.
Supplementary Material
Footnotes
Abbreviations: APD = acute pulmonary disease, BSS = Behavioural State Scale, BT = bacterial tracheitis, BZD = benzodiazepines, CASPe = Critical Appraisal Skills Programme España, CENTRAL = Cochrane Central Register of Controlled Trials. CINAHL = Cumulative Index to Nursing & Allied Health Literature, CNS = central nervous system, DBP = diastolic blood pressure, DXD = dexmedetomidine, DZM = diazepam, ECMO = extracorporeal oxygenation membrane, FNT = fentanyl, GA = gestational Age, GI = gastrointestinal, HI = head injury, HR = heart Rate, ICI = Interclass Correlation Index, IQR = interquartile range, IVR = intravenous route, IWS = Iatrogenic Withdrawal Syndrome, LILACS = Latin American and Caribbean Literature in Health Sciences, LR = likehood ratio, LRZ = lorazepam, MDZ = midazolam, MeSH = Medical Subject Headings, MV = mechanical ventilation, NMB = neuromuscular blockers, NRS = Numerical Rating Scale, NWS = Neonatal Withdrawal Score, OBWS = Opioid and Benzodiazepine Withdrawal Score, OR = oral route, PB = phenobarbital, PH = pulmonary haemorrhage, PHT = pulmonary hypertension, PICU = Paediatric Intensive Care Units, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, PWI = Paediatric Withdrawal Inventory, RMF= Remifentanil, ROC = receiver operating characteristic, RR = respiratory rate, SBP = systolic blood pressure, SCC = Spearman Correlation Coefficient, SFT = sufentanyl, SOS = Sophia Observation Withdrawal Symptoms, Ttm = treatment, UMSS = University of Michigan Sedation Score, WAT = Withdrawal Assessment Tool.
How to cite this article: Ávila-Alzate JA, Gómez-Salgado J, Romero-Martín M, Martínez-Isasi S, Navarro-Abal Y, Fernández-García D. Assessment and treatment of the withdrawal syndrome in paediatric intensive care units: Systematic review. Medicine. 2020;99:5(e18502).
This research received no external funding
University of Valencia, University Hospital of León, University of Huelva, Universidad Espíritu Santo, University of Sevilla, Universidad de Santiago de Compostela.
The authors declare no conflict of interest.
Supplemental Digital Content is available for this article.
References
- [1].Burastero M, Telechea H, González S, et al. Incidencia del síndrome de abstinencia en niños críticamente enfermos. Arch Pediatr Urug 2017;88:6–11. [Google Scholar]
- [2].Mencía S, Botrán M, López-Herce J, et al. Grupo de Estudio de Sedoanalgesia de la SECIP. Manejo de la sedoanalgesia y de los relajantes musculares en las unidades de cuidados intensivos pediátricos españolas. An Pediatr 2011;74:396–404. [DOI] [PubMed] [Google Scholar]
- [3].Dreyfus L, Javouhey E, Denis A, et al. Implementation and evaluation of a paediatric nurse-driven sedation protocol in a paediatric intensive care unit. Ann Intensive Care 2017;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kudchadkar SR, Yaster M, Punjabi NM. Sedation, sleep promotion, and delirium screening practices in the care of mechanically ventilated children: a wake-up call for the pediatric critical care community. Crit Care Med 2014;42:1592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Curley MAQ, Wypij D, Watson RS, et al. Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomized clinical trial. JAMA 2015;313:379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tobias JD. Tolerance, withdrawal, and physical dependency after long-term sedation and analgesia of children in the pediatric intensive care unit. Crit Care Med 2000;28:2122–32. [DOI] [PubMed] [Google Scholar]
- [7].Fernández-Carrión F, Gaboli M, González-Celador R, et al. Síndrome de abstinencia en Cuidados Intensivos Pediátricos. Incidencia y factores de riesgo. Med Intensiva 2013;37:67–74. [DOI] [PubMed] [Google Scholar]
- [8].Hammer GB. Sedation and analgesia in the pediatric intensive care unit following laryngotracheal reconstruction. Paediatr Anaesth 2009;19:166–79. [DOI] [PubMed] [Google Scholar]
- [9].Hronová K, Pokorná P, Posch L, et al. Sufentanil and midazolam dosing and pharmacogenetic factors in pediatric analgosedation and withdrawal syndrome. Physiol Res 2016;65:S463–72. [DOI] [PubMed] [Google Scholar]
- [10].Grant MJ, Balas MC, Curley MAQ. Defining sedation-related adverse events in the pediatric intensive care unit. Heart Lung 2013;42:171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ista E, van Dijk M, Gamel C, et al. Withdrawal symptoms in children after long-term administration of sedatives and/or analgesics: a literature review. "Assessment remains troublesome". Intensive Care Med 2007;33:1396–406. [DOI] [PubMed] [Google Scholar]
- [12].Best KM, Boullata JI, Curley MAQ. Risk factors associated with iatrogenic opioid and benzodiazepine withdrawal in critically ill pediatric patients: A systematic review and conceptual model. Pediatr Crit Care Med 2015;16:175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Playfor S, Jenkins I, Boyles C, et al. Consensus guidelines on sedation and analgesia in critically ill children. Intensive Care Med 2006;32:1125–36. [DOI] [PubMed] [Google Scholar]
- [14].Anand KJS, Willson DF, Berger J, et al. Tolerance and withdrawal from prolonged opioid use in critically Ill children. Pediatrics 2010;125:e1208–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Best KM, Wypij D, Asaro LA, et al. Patient, process and system predictors of iatrogenic withdrawal syndrome in critically ill children∗. Crit Care Med 2017;45:e7–15. [DOI] [PubMed] [Google Scholar]
- [16].Fisher D, Grap MJ, Younger JB, et al. Opioid withdrawal signs and symptoms in children: Frequency and determinants. Heart Lung 2013;42:407–13. [DOI] [PubMed] [Google Scholar]
- [17].Honey BL, Benefield RJ, Miller JL, et al. α2-receptor agonists for treatment and prevention of iatrogenic opioid abstinence syndrome in critically ill patients. Ann Pharmacother 2009;43:1506–11. [DOI] [PubMed] [Google Scholar]
- [18].Urrútia G, Bonfill X. Declaración PRISMA: una propuesta para mejorar la publicación de revisiones sistemáticas y metaanálisis. Med Clin 2010;135:507–11. [DOI] [PubMed] [Google Scholar]
- [19].Cabello JB. Plantilla para ayudarte a entender estudios de cohortes. In CASPe. Guías CASPe de Lectura Crítica de la Literatura Médica. Alicante: CASPe; cuaderno II; 2005;23–7. [Google Scholar]
- [20].Cabello JB. Plantilla para ayudarte a entender un Estudio de Casos y Controles. In CASPe. Guías CASPe de Lectura Crítica de la Literatura Médica. Alicante: CASPe; cuaderno II; 2005;13–9. [Google Scholar]
- [21].Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Glob Adv Health Med 2013;2:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Franck LS, Scoppettuolo LA, Wypij D, et al. Validity and generalizability of the Withdrawal Assessment Tool-1 (WAT-1) for monitoring iatrogenic withdrawal syndrome in pediatric patients. Pain 2012;153:142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Franck LS, Harris SK, Soetenga DJ, et al. The Withdrawal Assessment Tool - Version 1 (WAT-1): an assessment instrument for monitoring opioid and benzodiazepine withdrawal symptoms in pediatric patients. Pediatr Crit Care Med 2008;9:573–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Finkel JC, Johnson YJ, Quezado ZMN. The use of dexmedetomidine to facilitate acute discontinuation of opioids after cardiac transplantation in children. Crit Care Med 2005;33:2110–2. [DOI] [PubMed] [Google Scholar]
- [25].Ista E, de Hoog M, Tibboel D, et al. Psychometric evaluation of the sophia observation withdrawal symptoms scale in critically ill children. Pediatr Crit Care Med 2013;14:761–9. [DOI] [PubMed] [Google Scholar]
- [26].Weber GM, Smerling AJ, Saroyan JM. Pentobarbital withdrawal and treatment in an infant in the pediatric cardiac intensive care unit. J Clin Anesth 2013;25:62–5. [DOI] [PubMed] [Google Scholar]
- [27].Cho HH, O’Connell JP, Cooney MF, et al. Minimizing Tolerance and Withdrawal to Prolonged Pediatric Sedation: Case Report and Review of the Literature. J Intensive Care Med 2007;22:173–9. [DOI] [PubMed] [Google Scholar]
- [28].Siddappa R, Fletcher JE, Heard AMB, et al. Methadone dosage for prevention of opioid withdrawal in children. Paediatr Anaesth 2003;13:805–10. [DOI] [PubMed] [Google Scholar]
- [29].Lugo RA, MacLaren R, Cash J, et al. Enteral methadone to expedite fentanyl discontinuation and prevent opioid abstinence syndrome in the PICU. Pharmacotherapy 2001;21:1566–73. [DOI] [PubMed] [Google Scholar]
- [30].Lardieri AB, Fusco NM, Simone S, et al. Effects of clonidine on withdrawal from long-term dexmedetomidine in the pediatric patient. J Pediatr Pharmacol Ther 2015;20:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tobias JD. Dexmedetomidine to treat opioid withdrawal in infants following prolonged sedation in the pediatric ICU. J Opioid Manag 2006;2:201–5. [DOI] [PubMed] [Google Scholar]
- [32].Franck LS, Naughton I, Winter I. Opioid and benzodiazepine withdrawal symptoms in paediatric intensive care patients. Intensive Crit Care Nurs 2004;20:344–51. [DOI] [PubMed] [Google Scholar]
- [33].Keogh SJ, Long DA, Horn DV. Practice guidelines for sedation and analgesia management of critically ill children: a pilot study evaluating guideline impact and feasibility in the PICU. BMJ Open 2015;5:e006428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Harris J, Ramelet AS, Van Dijk M, et al. Clinical recommendations for pain, sedation, withdrawal and delirium assessment in critically ill infants and children: an ESPNIC position statement for healthcare professionals. Intensive Care Med 2016;42:972–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Domínguez KD, Lomako DM, Katz RW, et al. Opioid withdrawal in critically ill neonates. Ann Pharmacother 2003;37:473–7. [DOI] [PubMed] [Google Scholar]
- [36].Ista E, Van Dijk M, Gamel C, et al. Withdrawal symptoms in critically ill children after long-term administration of sedatives and/or analgesics: afirst evaluation. Crit Care Med 2008;36:2427–32. [DOI] [PubMed] [Google Scholar]
- [37].Duffett M, Koop A, Menon K, et al. Clonidine for the sedation of critically ill children: a systematic review. J Pediatr Intensive Care 2012;1:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Robertson RC, Darsey E, Fortenberry JD, et al. Evaluation of an opiate-weaning protocol using methadone in pediatric intensive care unit patients. Pediatr Crit Care Med 2000;1:119–23. [DOI] [PubMed] [Google Scholar]
- [39].Bowens CD, Thompson JA, Thompson MT, et al. A trial of methadone tapering schedules in pediatric intensive care unit patients exposed to prolonged sedative infusions. Pediatr Crit Care Med 2011;12:504–11. [DOI] [PubMed] [Google Scholar]
- [40].Johnson PN, Boyles KA, Miller JL. Selection of the initial methadone regimen for the management of iatrogenic opioid abstinence syndrome in critically ill children. Pharmacother 2012;32:148–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


