Abstract
Spontaneous hhy mice show hydrocephalus and subcortical heterotopia, and a mutation in the Ccdc85c gene has been identified. To contribute to the comparison of the role of Ccdc85c in different species, we established a Ccdc85c KO rat and investigated its pathological phenotypes. Ccdc85c KO rats were produced by genomic engineering using transcription activator-like effector nuclease (TALEN). The KO rats had an approximately 350-bp deletion in Ccdc85c and lacked CCDC85C protein expression. The KO rats showed non-obstructive hydrocephalus, subcortical heterotopia, and intracranial hemorrhage. The KO rats had many pathological characteristics similar to those in hhy mice. These results indicate that CCDC85C plays an important role in cerebral development in rats, and the function of CCDC85C in the cerebrum are similar in rats and mice.
Keywords: Ccdc85c, hydrocephalus, rat, subcortical heterotopia
Introduction
The hemorrhagic hydrocephalus (hhy) mouse is a spontaneous mutant with non-obstructive hydrocephalus, subcortical heterotopia and frequent brain hemorrhage [11]. A previous study [16] revealed that the hhy homozygous mouse has a mutation in the coiled-coil domain containing 85c (Ccdc85c) gene and lacks protein expression of CCDC85C. The lack of CCDC85C expression in hhy homozygous mice results in abnormal migration of radial glia, the formation of subcortical heterotopia, and ependymal agenesis, leading eventually to hydrocephalus. Thus, the function of CCDC85C is considered to be closely correlated with the maintenance of neural progenitor cells and neurogenesis in the central nervous system.
To definitively determine the function of Ccdc85c, different species of animal model with the Ccdc85c gene knocked out (Ccdc85c KO) would be useful. Many studies have reported that genetically engineered rats show a phenotype closer to humans than do mice, such as Adenomatous polyposis coli (Apc) KO rats and DNA-PKcs (Prkdc) KO rats [2, 15]. Therefore, analyzing gene function in multiple species is important in clarifying the universal and species-specific functions of the causative gene.
In this study, we established Ccdc85c KO rats and investigated their pathological phenotypes to compare them with those of hhy mice.
Materials and Methods
Establishment of Ccdc85c knockout rats by TALEN
Ccdc85c-deficient rats were established by genetic engineering using a highly active variant of transcription activator-like effector nuclease (TALEN), Platinum TALEN [20]. Platinum Ccdc85c TALEN, designed against rat Ccdc85c (NC_005105.4; NCBI), was constructed as previously described [20]. Briefly, DNA-binding repeats of TALEN were assembled using the two-step Golden Gate cloning method to bind the sequences 5’-TGGCGAAGCCCCCGGCG-3’ (left) and 5’-TCCGACGCCGCCGCCGC-3’ (right). The mRNA of left and right Platinum Ccdc85c TALEN was synthesized and injected into F344 rat zygotes. Platinum Ccdc85c TALEN-injected embryos were transferred into the oviducts of two pseudopregnant female rats. F0 rats were mated with control F344 rats. Then F1 male rats were mated with F1 female rats in each mutant lineage. On the basis of the clinical symptoms of the F2 rats, we chose lines to continue breeding. F2 rats and their offspring were used for genotyping, sequencing, histopathology and immunohistochemistry.
All rats were maintained under specific-pathogen-free conditions in a room with a controlled temperature and a 12-h light-dark cycle at the Animal Facility of Osaka Prefecture University. Food and water were provided ad libitum. All rats were handled according to the Guidelines for Animal Experimentation of Osaka Prefecture University.
Genotyping and sequencing
Genotyping was performed by polymerase chain reaction (PCR) and the mutation site in Ccdc85c was identified by direct sequencing. Genomic DNA was extracted from tail or ear biopsies of rats using a KAPA Express Extract kit (Nippon Genetics, Tokyo, Japan). PCR was performed with Tks GflexTM DNA Polymerase (Takara, Shiga, Japan). The primer sequences were 5’-CTGCCCAATCAGACCTGTG-3’ (forward primer; AABR07065498; Ensembl) and 5’-AGCACCAGCTCTTTCAGCTC-3’ (reverse primer; XM_001076332.2; NCBI). Since the reference genome of the rat is incorrect at the Ccdc85c locus, the sequence upstream of the start codon was obtained from AABR07065498, and the sequence after the start codon from XM_001076332.2. The PCR conditions were 1 cycle of 94°C for 1 min and 28 cycles of 98°C for 10 s, 60°C for 15 s, and 68°C for 30 s. PCR products were analyzed by electrophoresis in 3% agarose gels. The PCR products from each genomic DNA were directly sequenced. Sequence analysis was performed at Macrogen Japan Co. (Kyoto, Japan).
Histopathology
For histopathological analysis, homozygous (Ccdc85c KO) rats at four weeks of age (4W), wild type (WT) at 4W and 17W, and heterozygous (hetero) rats at 17W were used. The rats were euthanized by isoflurane and the visceral organs removed and fixed in 10% neutral buffered formalin. The fixed tissues were processed by the routine method, embedded in paraffin wax, cut into 3-µm sections and stained with hematoxylin and eosin (HE). In addition, the duodenum was removed to test for the expression of CCDC85C protein immunohistochemically, embedded in TISSU MOUNT® (Chiba Medical, Saitama, Japan), rapidly frozen and stored at −80°C to await the preparation of frozen sections as previously described [25]. In our previous study [25], we found that the expression of CCDC85C protein decreased with the development of the organs, whereas the intestine continued to express CCDC85C protein even at postnatal day 60 (P60). Therefore, the expression of CCDC85C protein was evaluated in the duodenum.
Production of rabbit polyclonal anti-rat CCDC85C antibody
A segment of rat CCDC85C consisting of the C-terminal 187 residues (serine-119 to leucine-305) was expressed as a Glutathione S-transferase (GST)-fusion protein in Escherichia coli DH5α (XM_006240553.1, XP_006240615.1; NCBI). Using this protein, a rabbit polyclonal anti-rat CCDC85C antibody was produced at Eve Bio-Science Co., Ltd. (Wakayama, Japan) as previously described [25].
Immunohistochemistry
For immunofluorescence staining, 10-µm frozen sections of duodenum were cut on a cryostat and air-dried at room temperature for 1 h. The tissue sections were fixed in Zamboni’s solution (0.21% picric acid, 2% paraformaldehyde) for 15 min at room temperature. The sections were then washed with 0.3% Triton X-100 in phosphate-buffered saline for 15 min, treated with 10% normal goat serum (Sigma, St. Louis, MO, USA) for 30 min, and incubated with rabbit polyclonal antibody against rat CCDC85C (1:100,000) at 4°C overnight. The sections were incubated with Alexa488-labeled anti-rabbit IgG secondary antibody (1:1,000; Life Technologies, Carlsbad, CA, USA) for 45 min at room temperature and coverslipped with mounting medium containing DAPI (Vector Laboratories, Burlingame, CA, USA). Signals were detected with a confocal imaging system (C1Si; Nikon, Tokyo, Japan).
Results
Establishment of Ccdc85c knockout rats
Nine F0 rats were obtained from two female rats. By direct sequencing, it was found that there were different mutations in each of the nine rats at the TALEN targeting site (data not shown). Three F0 rats died before mating; the cause of death in these rats could not be determined. In the observation of the general symptoms of the F2 rats, some rats derived from one rat line showed an enlarged head macroscopically. Rats of this lineage were maintained for use in further experiments.
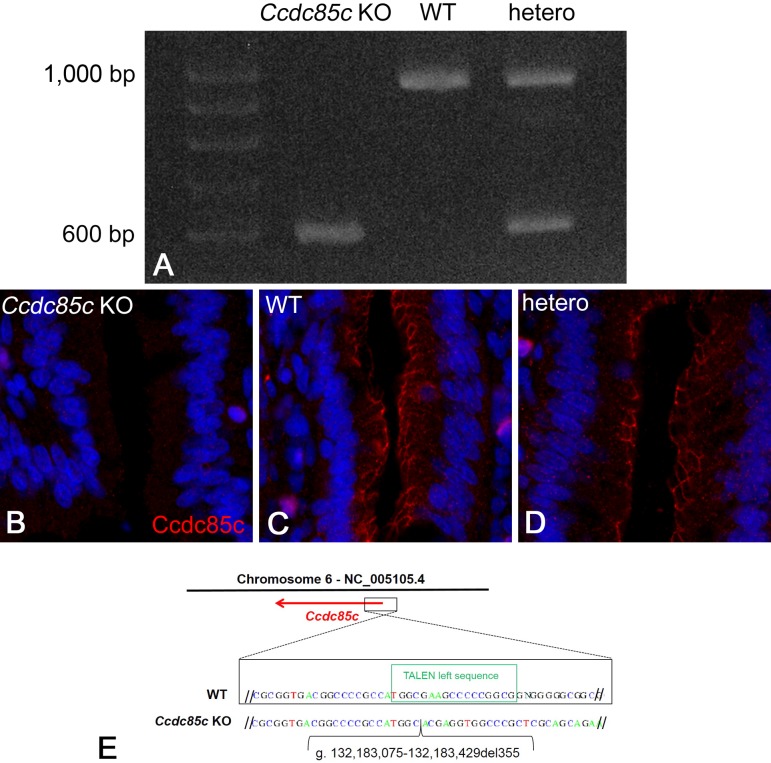
In the PCR for genotyping, PCR products from unaffected rats showed two patterns: one band at 942 bp, and two bands at 942 bp and around 600 bp (Fig. 1A). Unaffected rats with one band were judged to be WT and unaffected rats with two bands were judged to be heterozygous. In contrast, the PCR products from rats with an enlarged head were around 600 bp, and were therefore about 350 bp shorter than the 942-bp product from WT rats (Fig. 1A). Thus, affected rats were judged to be homozygous. Genotyping revealed that only homozygous rats exhibited macroscopic abnormality.
Fig. 1.
PCR products from genomic DNA of Ccdc85c(A). Ccdc85c KO rats have an approximately 350-bp deletion in Ccdc85c. Immunofluorescence staining for CCDC85C protein in the duodenum (B–D). CCDC85C is expressed in the epithelial cells in wild type (WT) (C) and heterozygous (D) rats at 17W. CCDC85C expression was not observed in Ccdc85c KO rats at four weeks of age (4W) (B). Scale bar, 25 µm. Sequences of the WT and Ccdc85c KO rat PCR products (E). A 355-bp genomic sequence encompassing exon 1 is deleted in Ccdc85c KO rats (g. 132,183,075-132,183,429del355; NCBI NC_005105.4).
To test for CCDC85C protein expression, we performed immunofluorescence staining for CCDC85C in the duodenum using a polyclonal antibody against rat CCDC85C protein. Expression of CCDC85C at the apical junctions of epithelial cells was detected in unaffected rats (Figs. 1C and D) but not in rats with an enlarged head (Fig. 1B). On the basis of PCR genotyping and immunofluorescence analysis, we consider the affected rats to be Ccdc85c KO rats.
To identify the mutations of the TALEN targeting site in Ccdc85c, PCR products from this site were directly subjected to DNA sequencing analysis. We found that a 355-bp genomic sequence encompassing exon 1 was deleted in affected rats (g. 132,183,075–132,183,429del355; NCBI NC_005105.4) (Fig. 1E).
At the time of finalizing the data used in this paper, 73 rats had been obtained, among them 12 Ccdc85c KO, 19 WT and 42 hetero rats. Thus, the incidence of Ccdc85c KO rats was 16.4% and that of unaffected rats was 83.6%. This segregation ratio is lower than the expected 1:3 ratio (χ2=2.85; P=0.09). Prior to genotyping, young rats in poor condition were killed by the mother.
Pathological phenotypes in Ccdc85c KO rats
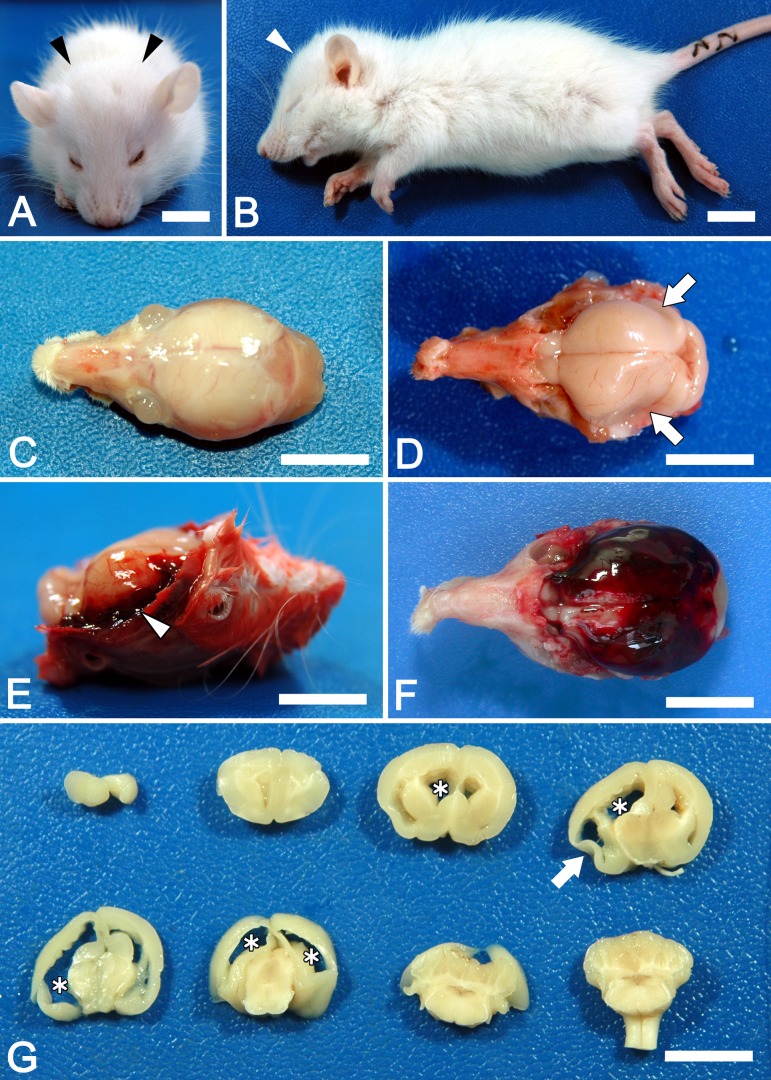
In Ccdc85c KO rats, enlargement of the head was observed from around P14 and became prominent at P30 (Figs. 2A and B). Ccdc85c KO rats showed difficulty in walking and most of them died around P30. Necropsy revealed that all 12 Ccdc85c KO rats had hydrocephalus (enlarged cranium with thin cranial bones; Fig. 2C), accumulation of cerebrospinal fluid, attenuated cerebral parenchyma (Figs. 2D and G) and severe dilatation of the lateral ventricles (Fig. 2G). Intracranial hemorrhage was observed in 4 (33%) out of 12 Ccdc85c KO rats, which were dead or moribund (Figs. 2E and F). In contrast, WT and hetero rats did not show any clinical symptoms or pathological abnormality.
Fig. 2.
Macroscopic phenotype of Ccdc85c KO rats at four weeks of age (4W). Ccdc85c KO rats showed enlarged heads (A and B, arrowheads). Hydrocephalus causes skull thinning (C) and attenuated cerebral parenchyma (D, arrowheads). Intracranial hemorrhage is observed in some Ccdc85c KO rats (E, arrowhead: hemorrhage at temporal region, F: severe hemorrhagic case; blood clot covered the whole cerebrum). Dilated lateral ventricles (asterisks) and attenuated cerebral parenchyma (arrow) are observed in coronally sliced brain (G). Scale bars, 1 cm.
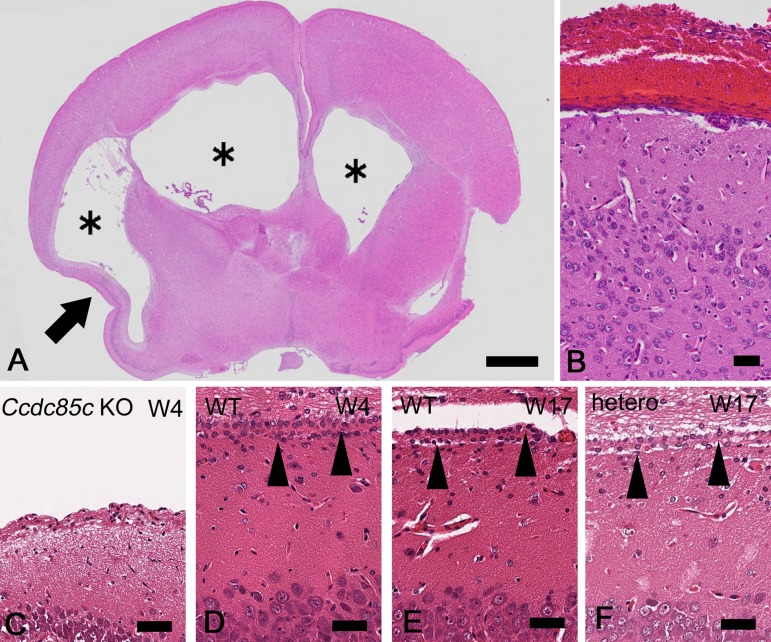
Histopathologically, brains of Ccdc85c KO rats at 4W exhibited dilated lateral ventricles (Fig. 3A) lacking most of the ependymal cells (Fig. 3C), attenuated cerebral parenchyma (Fig. 3A) and subcortical heterotopia (Figs. 4A and B). In Ccdc85c KO rats, hemorrhage was observed in the meninges (Fig. 3B). Dilatation of the lateral ventricles was marked, while the third and fourth ventricles and aqueduct were not affected. Neither causative stenosis nor obstruction in the ventricular system was detected. Ependymal cells were not present at the dorsal or lateral position of the lateral ventricles. No abnormality was observed in the choroid plexus. The subcortical heterotopia of Ccdc85c KO rats was broadly distributed and located at the dorsal position of the lateral ventricles (Fig. 4A). These histopathological features of Ccdc85c KO rats are similar to those of hhy mice.
Fig. 3.
Histopathological analysis of hydrocephalus in Ccdc85c KO rats. HE stained sections of cerebrum in Ccdc85c KO rats at four weeks of age (4W) (A–C) and unaffected rats at 4W [D: wild type (WT)] or at 17W (E: WT, F: hetero). Lateral ventricles are dilated in Ccdc85c KO rats (A, asterisks). Atrophied cerebral parenchyma is also observed (A, arrow). Hemorrhage is mainly located at meninges (B). Lateral ventricles are lined by ependymal cells in WT rat and hetero rat (D–F, arrowheads). There are no ependymal cells at the lateral ventricles in Ccdc85c KO rats (C). Scale bar of A, 1 mm, scale bar of B, 50 µm, scale bar of C–F, 25 µm.
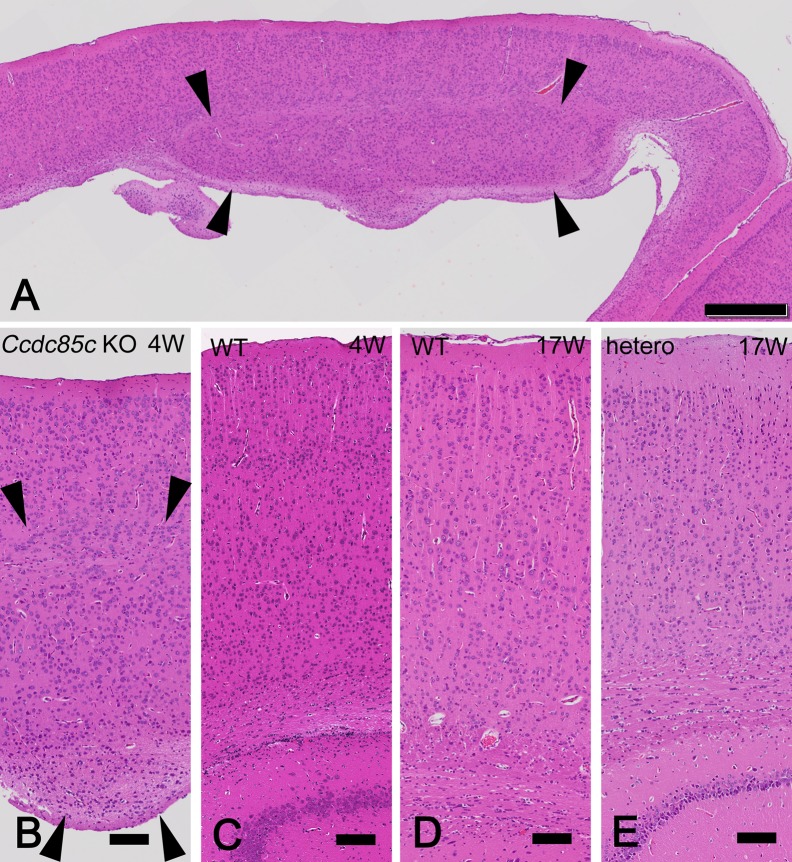
Fig. 4.
Histopathology of subcortical heterotopia in Ccdc85c KO rats. HE stained sections of cerebrum in Ccdc85c KO rats at four weeks of age (4W) (A and B) and unaffected rats at 4W [C: wild type (WT)] or at 17W (D: WT, E: hetero). Subcortical heterotopia is broadly formed at the dorsal surface of lateral ventricles in Ccdc85c KO rats (A and B, arrowheads). No lesion is observed at the cerebral parenchyma in WT and hetero rats (C–E). Scale bar of A, 500 µm, scale bars of B–E, 100 µm.
Discussion
Ccdc85c KO rats as a new model of hydrocephalus and subcortical heterotopia
In this study, we have successfully established Ccdc85c KO rats that had a 355-bp deletion in Ccdc85c accompanied by non-obstructive hydrocephalus, subcortical heterotopia without ependymal cells in the dorsal or lateral position of the ventricles, and occasional intracranial hemorrhage. These pathological characteristics are very similar to those of hhy mice [11]. HTX rats are known as a congenital hydrocephalus model [10], and hydrocephalus in HTX rats is caused by abnormal development of the subcommissural organ followed by closure of the cerebral aqueduct; however, the causative gene is unknown [8, 9, 22]. As a spontaneous cortical heterotopia model, telencephalic internal structural heterotopia (tish) rats have been reported [12]. Tish rats show subcortical band heterotopia, which is associated with seizures. Subcortical band heterotopia in tish rats is caused by dysregulation of the positioning, number and cell-cycle kinetics of neural progenitor cells [4]. In contrast to these rat models, Ccdc85c KO rats showed various pathological phenotypes simultaneously. Therefore Ccdc85c KO rats may serve as a novel, unique model of both congenital hydrocephalus and subcortical heterotopia.
These pathological characteristics of Ccdc85c KO rats are very similar to those of hhy mice. In this study, all 12 Ccdc85c KO rats showed hydrocephalus and four of them also had intracranial hemorrhage. The frequency of intracranial hemorrhage (33%) was lower than in mice (87%) [11].The fact that the frequency of intracranial hemorrhage was lower than that of hydrocephalus in both species indicates that the intracranial hemorrhage followed the hydrocephalus. It is known that intracerebral hemorrhage causes secondary hydrocephalus in humans and subarachnoid hemorrhage model rats; however, hydrocephalus accompanied by hemorrhage has not previously been reported except in hhy mice [3, 5, 7, 11, 13, 16]. The intracranial hemorrhage in Ccdc85c KO animals is presumed to be secondary, but elucidating the pathogenesis of the hemorrhage in Ccdc85c mutants may contribute to novel findings about intracranial hemorrhage.
Pathogenesis of the lesions in Ccdc85c KO rats
This study leaves unclear the pathogenesis of the non-obstructive hydrocephalus in Ccdc85c KO rats. Because of the comparatively low frequency of intracranial hemorrhage (33%), we considered that hydrocephalus in Ccdc85c KO rats was not secondary to the intracranial hemorrhage. There were no ependymal cells in the dorsal or lateral position of the ventricles in the Ccdc85c KO rats. In hhy mice, this pathological change is conspicuous and is considered to be caused by ependymal agenesis [16]. It is known that ependymal cell abnormality causes hydrocephalus by inhibiting ependymal flow [6, 26]. Taken together, these findings support the hypothesis that hydrocephalus in Ccdc85c KO rats is directly related to the agenesis of ependymal cells. However, to demonstrate this, we should investigate whether or not the lack of ependymal cells in Ccdc85c KO rats is a primary change.
Ccdc85c KO rats showed subcortical heterotopia formation and ependymal cell agenesis. Subcortical heterotopia is characterized by the presence of neurons that do not complete their migration [21]. Cerebral cortex is a tissue in which orderly layers of laminated neurons are generated during development. This neuronal lamination in the cerebral cortex is known to be disrupted by abnormalities of various factors such as cell adhesion molecules, cell polarity molecules and signaling molecules [14, 17, 23, 28, 29]. Ependymal precursors are derived from radial glial cells that become neural stem cells on the ventricular surface [24]. In hhy mice, the agenesis of ependymal cells occurs in the paramedio-dorsal position of the subcortical areas, where subcortical heterotopia develops both in the Ccdc85c KO rats of the present study and in hhy mice. Recently, the proper development of medial wall ependymal cilia was shown to be important for normal brain development, and dysfunction of ependymal cells was suggested to cause an aqueductal disturbance, leading to hydrocephalus in Ccdc39 mutant mice [1]. Therefore our present results indicate that CCDC85C in rats as well as in hhy mice plays an important role in neurogenesis in the cerebrum of rats, and the lack of it causes abnormal neuronal migration.
In the previous study in rats [25], CCDC85C expression was especially strong in the small intestine, cerebrum, cerebellum and eye. Morphological maturation occurs in the small intestine by P42, the brain by P21, and the retina by P25 [18, 19, 27]. In the present study, Ccdc85c KO rats had abnormalities in the cerebrum but no malformations in the small intestine or cerebellum. By heterozygous mating, the observed value (16.4%) was lower than the expected frequency of homozygous pups (25%). Since neonatal rats in poor condition were killed by the mother, the actual incidence is expected to be close to 25%. Therefore, it is likely that the deficiency of CCDC85C protein was not lethal to the fetus. These results imply that the function of CCDC85C in organ maturation is more important in the cerebrum than in other organs.
Acknowledgments
Ccdc85c KO (F344-Ccdc85cem1Kyo) rats were deposited at National BioResource Project (NBRP)-Rat (NBRP Rat No. 0824). This work was supported by Grant-in-Aid for Scientific Research from Japan Society for Promotion of Science (JSPS; no. 15H04595) and Kenyuukai (The Veterinary Practitioners Association of Osaka Prefecture University). We thank Dr. Gerald E. Smyth for his English-language editing of the manuscript.
References
- 1.Abdelhamed Z., Vuong S.M., Hill L., Shula C., Timms A., Beier D., Campbell K., Mangano F.T., Stottmann R.W., Goto J.2018. A mutation in Ccdc39 causes neonatal hydrocephalus with abnormal motile cilia development in mice. Development 145: dev154500. doi: 10.1242/dev.154500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amos-Landgraf J.M., Kwong L.N., Kendziorski C.M., Reichelderfer M., Torrealba J., Weichert J., Haag J.D., Chen K.S., Waller J.L., Gould M.N., Dove W.F.2007. A target-selected Apc-mutant rat kindred enhances the modeling of familial human colon cancer. Proc. Natl. Acad. Sci. USA 104: 4036–4041. doi: 10.1073/pnas.0611690104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burstein J., Papile L.A., Burstein R.1979. Intraventricular hemorrhage and hydrocephalus in premature newborns: a prospective study with CT. AJR Am. J. Roentgenol. 132: 631–635. doi: 10.2214/ajr.132.4.631 [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald M.P., Covio M., Lee K.S.2011. Disturbances in the positioning, proliferation and apoptosis of neural progenitors contribute to subcortical band heterotopia formation. Neuroscience 176: 455–471. doi: 10.1016/j.neuroscience.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasan D., Vermeulen M., Wijdicks E.F.M., Hijdra A., van Gijn J.1989. Management problems in acute hydrocephalus after subarachnoid hemorrhage. Stroke 20: 747–753. doi: 10.1161/01.STR.20.6.747 [DOI] [PubMed] [Google Scholar]
- 6.Ibañez-Tallon I., Pagenstecher A., Fliegauf M., Olbrich H., Kispert A., Ketelsen U.P., North A., Heintz N., Omran H.2004. Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Hum. Mol. Genet. 13: 2133–2141. doi: 10.1093/hmg/ddh219 [DOI] [PubMed] [Google Scholar]
- 7.Jing C., Zhang H., Shishido H., Keep R.F., Hua Y.2018. Association of brain CD163 expression and brain injury/hydrocephalus development in a rat model of subarachnoid hemorrhage. Front. Neurosci. 12: 313. doi: 10.3389/fnins.2018.00313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones H.C., Bucknall R.M.1988. Inherited prenatal hydrocephalus in the H-Tx rat: a morphological study. Neuropathol. Appl. Neurobiol. 14: 263–274. doi: 10.1111/j.1365-2990.1988.tb00887.x [DOI] [PubMed] [Google Scholar]
- 9.Jones H.C., Carter B.J., Depelteau J.S., Roman M., Morel L.2001. Chromosomal linkage associated with disease severity in the hydrocephalic H-Tx rat. Behav. Genet. 31: 101–111. doi: 10.1023/A:1010266110762 [DOI] [PubMed] [Google Scholar]
- 10.Kohn D.F., Chinookoswong N., Chou S.M.1981. A new model of congenital hydrocephalus in the rat. Acta Neuropathol. 54: 211–218. doi: 10.1007/BF00687744 [DOI] [PubMed] [Google Scholar]
- 11.Kuwamura M., Kinoshita A., Okumoto M., Yamate J., Mori N.2004. Hemorrhagic hydrocephalus (hhy): a novel mutation on mouse chromosome 12. Brain Res. Dev. Brain Res. 152: 69–72. doi: 10.1016/j.devbrainres.2004.05.006 [DOI] [PubMed] [Google Scholar]
- 12.Lee K.S., Schottler F., Collins J.L., Lanzino G., Couture D., Rao A., Hiramatsu K., Goto Y., Hong S.C., Caner H., Yamamoto H., Chen Z.F., Bertram E., Berr S., Omary R., Scrable H., Jackson T., Goble J., Eisenman L.1997. A genetic animal model of human neocortical heterotopia associated with seizures. J. Neurosci. 17: 6236–6242. doi: 10.1523/JNEUROSCI.17-16-06236.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lodhia K.R., Shakui P., Keep R.F.2006. Hydrocephalus in a rat model of intraventricular hemorrhage. Acta Neurochir. Suppl. (Wien) 96: 207–211. doi: 10.1007/3-211-30714-1_45 [DOI] [PubMed] [Google Scholar]
- 14.Masai I., Lele Z., Yamaguchi M., Komori A., Nakata A., Nishiwaki Y., Wada H., Tanaka H., Nojima Y., Hammerschmidt M., Wilson S.W., Okamoto H.2003. N-cadherin mediates retinal lamination, maintenance of forebrain compartments and patterning of retinal neurites. Development 130: 2479–2494. doi: 10.1242/dev.00465 [DOI] [PubMed] [Google Scholar]
- 15.Mashimo T., Takizawa A., Kobayashi J., Kunihiro Y., Yoshimi K., Ishida S., Tanabe K., Yanagi A., Tachibana A., Hirose J., Yomoda J., Morimoto S., Kuramoto T., Voigt B., Watanabe T., Hiai H., Tateno C., Komatsu K., Serikawa T.2012. Generation and characterization of severe combined immunodeficiency rats. Cell Rep. 2: 685–694. doi: 10.1016/j.celrep.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 16.Mori N., Kuwamura M., Tanaka N., Hirano R., Nabe M., Ibuki M., Yamate J.2012. Ccdc85c encoding a protein at apical junctions of radial glia is disrupted in hemorrhagic hydrocephalus (hhy) mice. Am. J. Pathol. 180: 314–327. doi: 10.1016/j.ajpath.2011.09.014 [DOI] [PubMed] [Google Scholar]
- 17.Ouchi Y., Baba Y., Koso H., Taketo M.M., Iwamoto T., Aburatani H., Watanabe S.2011. β-Catenin signaling regulates the timing of cell differentiation in mouse retinal progenitor cells. Mol. Cell. Neurosci. 46: 770–780. doi: 10.1016/j.mcn.2011.02.010 [DOI] [PubMed] [Google Scholar]
- 18.Picut C.A., Coleman G.D.2016. Gastrointestinal Tract. pp. 127–171. In: Atlas of histology of the Juvenile rat. (Parker, G.A., and Picut, C.A. eds.), Academic Press, London. [Google Scholar]
- 19.Picut C.A., Brown D.L., Remick A.K.2016. Nervous System. pp. 45–87. In: Atlas of histology of the Juvenile rat. (Parker, G.A., and Picut, C.A. eds.), Academic Press, London. [Google Scholar]
- 20.Sakuma T., Ochiai H., Kaneko T., Mashimo T., Tokumasu D., Sakane Y., Suzuki K., Miyamoto T., Sakamoto N., Matsuura S., Yamamoto T.2013. Repeating pattern of non-RVD variations in DNA-binding modules enhances TALEN activity. Sci. Rep. 3: 3379. doi: 10.1038/srep03379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sisodiya S.M.2004. Malformations of cortical development: burdens and insights from important causes of human epilepsy. Lancet Neurol. 3: 29–38. doi: 10.1016/S1474-4422(03)00620-3 [DOI] [PubMed] [Google Scholar]
- 22.Somera K.C., Jones H.C.2004. Reduced subcommissural organ glycoprotein immunoreactivity precedes aqueduct closure and ventricular dilatation in H-Tx rat hydrocephalus. Cell Tissue Res. 315: 361–373. doi: 10.1007/s00441-003-0843-9 [DOI] [PubMed] [Google Scholar]
- 23.Sottocornola R., Royer C., Vives V., Tordella L., Zhong S., Wang Y., Ratnayaka I., Shipman M., Cheung A., Gaston-Massuet C., Ferretti P., Molnár Z., Lu X.2010. ASPP2 binds Par-3 and controls the polarity and proliferation of neural progenitors during CNS development. Dev. Cell 19: 126–137. doi: 10.1016/j.devcel.2010.06.003 [DOI] [PubMed] [Google Scholar]
- 24.Spassky N., Merkle F.T., Flames N., Tramontin A.D., García-Verdugo J.M., Alvarez-Buylla A.2005. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J. Neurosci. 25: 10–18. doi: 10.1523/JNEUROSCI.1108-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka N., Izawa T., Takenaka S., Yamate J., Kuwamura M.2015. Ccdc85C, a causative protein for hydrocephalus and subcortical heterotopia, is expressed in the systemic epithelia with proliferative activity in rats. Histol. Histopathol. 30: 823–832. [DOI] [PubMed] [Google Scholar]
- 26.Tissir F., Qu Y., Montcouquiol M., Zhou L., Komatsu K., Shi D., Fujimori T., Labeau J., Tyteca D., Courtoy P., Poumay Y., Uemura T., Goffinet A.M.2010. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat. Neurosci. 13: 700–707. doi: 10.1038/nn.2555 [DOI] [PubMed] [Google Scholar]
- 27.Walling B.E., Marit G.B.2016. The Eye and Harderian Gland. pp. 373–394. In: Atlas of histology of the Juvenile rat. (Parker, G.A., and Picut, C.A. eds.), Academic Press, London. [Google Scholar]
- 28.Wei X., Cheng Y., Luo Y., Shi X., Nelson S., Hyde D.R.2004. The zebrafish Pard3 ortholog is required for separation of the eye fields and retinal lamination. Dev. Biol. 269: 286–301. doi: 10.1016/j.ydbio.2004.01.017 [DOI] [PubMed] [Google Scholar]
- 29.Zheng M.H., Shi M., Pei Z., Gao F., Han H., Ding Y.Q.2009. The transcription factor RBP-J is essential for retinal cell differentiation and lamination. Mol. Brain 2: 38. doi: 10.1186/1756-6606-2-38 [DOI] [PMC free article] [PubMed] [Google Scholar]