Abstract
Hyperpolarization-activated cyclic nucleotide-gated potassium channel 1 (HCN1) contribute to spontaneous rhythmic activity in different tissues, including the heart and brain. Deficiency in HCN1 function is associated with sick sinus syndrome in mice and epilepsy in humans. We recently developed Hcn1-deficient rats and found that they exhibit absence epilepsy. While rearing Hcn1-deficient rats, we noticed loose muscle tension and abnormal gait. We therefore evaluated the muscle strength and motor functions of Hcn1-deficient rats. When subjected to the wire hang test, Hcn1-deficient rats fell down more easily than control F344 rats. Grip strength of Hcn1-deficient rats was significantly smaller than F344 rats. In the inclined plane test, they exhibited a smaller maximum angle. In the rotarod test, the latency to fall was shorter for Hcn1-deficient rats than F344 rats. In the footprint analysis, Hcn1-deficient rats exhibited smaller step length and wider step width than F344 rats. Instead of poor motor coordination ability and muscle weakness, Hcn1-deficient rats exhibited normal electromyograms, muscle histology, and deep tendon reflex. These findings suggest that HCN1 channels contribute to motor coordination and muscle strength, and that the muscle weakness of Hcn1-deficient rats results from the involvement not of the peripheral but of the central nervous system.
Keywords: hyperpolarization-activated cyclic nucleotide-gated potassium channel 1 (HCN1), motor coordination, muscle strength, rat
Introduction
Hyperpolarization-activated cyclic nucleotide-gated potassium channels (HCN) underlie an inward cationic current Ih, which is activated by hyperpolarization rather than depolarization [1]. These channels also contribute to spontaneous rhythmic activity in both the heart and brain [22]. HCN1 is one of four subunits (HCN1–4) that make up HCN channels and mainly expresses in the central nervous system (CNS) [15, 21] and in cardiomyocytes [2]. In Hcn1-deficient mice, functional defects have been shown in both the heart and brain. For example, Hcn1-deficient mice exhibited profound motor learning and memory deficits in swimming and elevated-speed rotarod tasks [14] and are more susceptible to kainic acid-induced seizures [7]. They also exhibited sick sinus syndrome, which is characterized by bradycardia and sinus dysrhythmia [5].
Recently, we developed Hcn1-deficient rats by transcription activator-like effector nuclease mutagenesis [12]. Neither Hcn1 transcript nor HCN1 protein could be detected in the CNS. The cortical and hippocampal pyramidal neurons of these rats displayed a significant reduction in Ih, a pronounced hyperpolarizing shift of the resting membrane potential, and increased input resistance. Thus, the functions of HCN1 channels are lost in Hcn1-deficient rats. Hcn1-deficient rats are more susceptible to pentylenetetrazol-induced seizures, which suggests that CNS neurons are activated more easily. Hcn1-deficient rats also exhibited spontaneous absence seizures that were accompanied by spike-and-wave discharges (SWDs) and behavioral arrest. These findings indicated that a deficiency in HCN1 function caused absence epilepsy in rats [12].
In this study, we evaluate the muscle strength and motor functions of Hcn1-deficient rats, because they exhibited abnormal gait.
Materials and Methods
Ethical use of animals
All animal experiments were approved by the Animal Research Committees of Kyoto University and were conducted according to their regulations on animal experimentation.
Animals
Male Hcn1-knockout rats (F344-Hcn1em1Kyo) with body weights between 250–350 g at 12–15 weeks of age were obtained from the National Bio Resource Project for the Rat (Kyoto, Japan). Male F344/NSlc rats (250–350 g) at 12–15 weeks of age were purchased from Japan SLC, Inc. (Shizuoka, Japan) and were used as control animals.
Wire hang test
A rope was stretched horizontally 50 cm above floor level and each rat was allowed to grasp the rope with its forelimbs. Body positions were examined with a six-point scale system: 0, falling off; 1, hanging on the rope by two forelimbs; 2, same as 1 but attempting to climb on the rope; 3, same as 1 plus one or both hind limbs; 4, same as 3 plus tail wrapped around the rope; and 5, escaping. Latency to fall was also measured. The test was performed three times for each animal. The maximum score and duration achieved among the three trials was recorded.
Grip strength test
The grip strength was measured with a digital force gauge (A&D Co., Ltd., Tokyo, Japan) attached to a piece of grid. Each rat was held by the base of its tail and allowed to grasp the grid with its forelimbs. The rat was slowly moved backwards until it released the grid. The test was performed three times for each animal and the greatest force (N) among the three trials was recorded.
Inclined plane test
The inclined plane test was performed using a sliding apparatus described by Rivlin and Tator [19]. Each rat was placed on a sliding plate covered with cardboard. The angle of the sliding plate was gradually increased by one degree per two seconds. The maximum angle was determined at the moment when the rat could no longer support its body position. The test was performed five times for each animal. The greatest angle measured among the five trials was recorded.
Balance beam test
The balance beam test was performed as described previously with slight modifications [26]. Four 1.2-m-long wooden planks of different widths (4.5 cm, 3.0 cm, 2.0 cm, 1.5 cm) were elevated about 30 cm above floor level. We placed rats on the end of the plank and put them through the test three times for each plank width. The narrowest plank on which each rat could walk from end to end without falling was recorded and scores were assigned as follows: 1 for 4.5 cm, 2 for 3.0 cm, 3 for 2.0 cm, and 4 for 1.5 cm.
Rotarod test
The rotarod test was performed with a rotarod apparatus (O’hara & Co., Ltd., Tokyo, Japan). Before the test, rats were trained to walk on the rotating rod at 5 rpm. Thirty min after the training, rats were tested on the rotating rod at 10 rpm fixed speed for 300 s. If rats fell down before 300 s, the time was recorded. The test was performed three times and the maximum time among the three trials was recorded.
Footprint analysis
Rats were placed in a 9-cm-wide and 100-cm-long corridor of which the floor was covered with white absorbing paper. Rats were first trained to explore the corridor before their hind paws were dipped in ink. Two trials were performed for each animal. Distances between two subsequent feet on y-axis (length) and on x-axis (width) were measured [25].
Electromyogram (EMG)
EMG was recorded from the musculus gastrocnemius and musculus triceps brachii. Rats were anesthetized with isoflurane to permit EMG electrode implantation. Thirty-gauge concentric needle electrodes (Ambu, Kopenhagen, Danmark) were implanted into the muscle. Twenty-seven-gauge needles were inserted subcutaneously on the back and used as ground electrodes. After recovery from anesthesia, EMG activity was recorded. The knee was bent to record the gastrocnemius muscular EMG. The forelimb was stretched to record the triceps brachii muscular EMG. EMG activity was amplified (MEG-6108; Nihonkohden, Tokyo, Japan) and observed with a thermal alley recorder (RTA-1100; Nihonkohden). The recorded signals were analyzed using PowerLab ML845 software (AD Instruments, Bella Vista, Austria).
Histology
Histological analysis was performed as described previously [13]. The musculus gastrocnemius and musculus triceps brachii were collected from 12-week-old Hcn1-deficient and F344 rats. Tissue samples were fixed in 10% neutral-buffered formalin and embedded in paraffin. Sections were cut at 4 µm and stained with hematoxylin and eosin.
Deep tendon reflex test
Rats were postured in the supine position. The knee was bent to 90 degrees and the ankle was kept in the neutral position. Deep tendon reflex was evoked by tapping the tendon of the tibias anterior muscle with a wooden pencil hammer. Deep tendon reflexes were scored as previously described [18]: 0 for no reflex, 1 for somewhat diminished, 2 for normal reflex, 3 for brisker than average, and 4 for hyperactive, associated with clonus.
Statistical analysis
Data were expressed as mean ± SEM. Statistical significance of differences between groups was determined by Student’s t-test with Microsoft Office Excel 2007 (Microsoft Corp., Redmond, WA, USA). In the wire hang score test and balance beam test, Wilcoxon rank sum test was conducted with exactRanktests package of R3.3.3 (R project Contributors) [24]. In the deep tendon reflex test, Welch’s two sample t-test was conducted with R3.3.3. A P value less than 0.05 was considered statistically significant.
Results
Wire hang, grip strength, and inclined plane tests
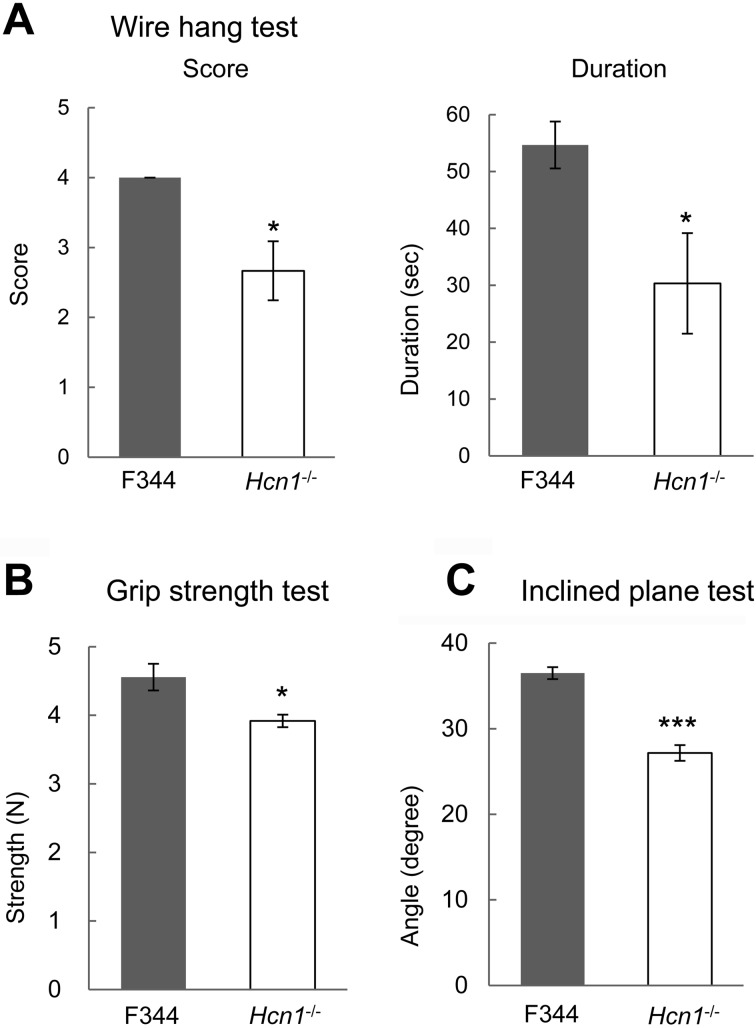
To assess muscle strength, we performed wire hang and grip strength tests. Hcn1-deficient rats were unable to wrap their tails around the rope and fell down more easily from the rope than control F344 rats. The average maximum score and latency to fall of Hnc1-deficient rats (n=6) were significantly smaller than those of F344 rats (n=6): score, 2.67 ± 0.422 vs. 4.00 ± 0.00, P=0.0152; latency, 30.3 ± 8.83 vs. 54.7 ± 4.13, P=0.0317 (Fig. 1A). The average maximum grip strength of the forepaws of Hcn1-deficient rats (n=10) was significantly smaller than that of F344 rats (n=10): 3.92 ± 0.0909 N vs. 4.56 ± 0.194 N, P=0.0125 (Fig. 1B). In the inclined plane test, the average maximum angle of Hnc1-deficient rats (n=6) was significantly smaller than that of F344 rats (n=6): 27.2 ± 0.925 degrees vs. 36.5 ± 0.697 degrees, P<0.001 (Fig. 1C).
Fig. 1.
Hcn1-deficient rats exhibited weaker muscle strength. A: Wire hang test (n=6, each). B: Grip strength test (n=6, each). C: Inclined plane test (n=6, each). Results for Hcn1-deficient rats are shown as white bars, while those for F344 rats are shown as black bars. *: P<0.05, ***: P<0.001.
Balance beam and rotarod tests
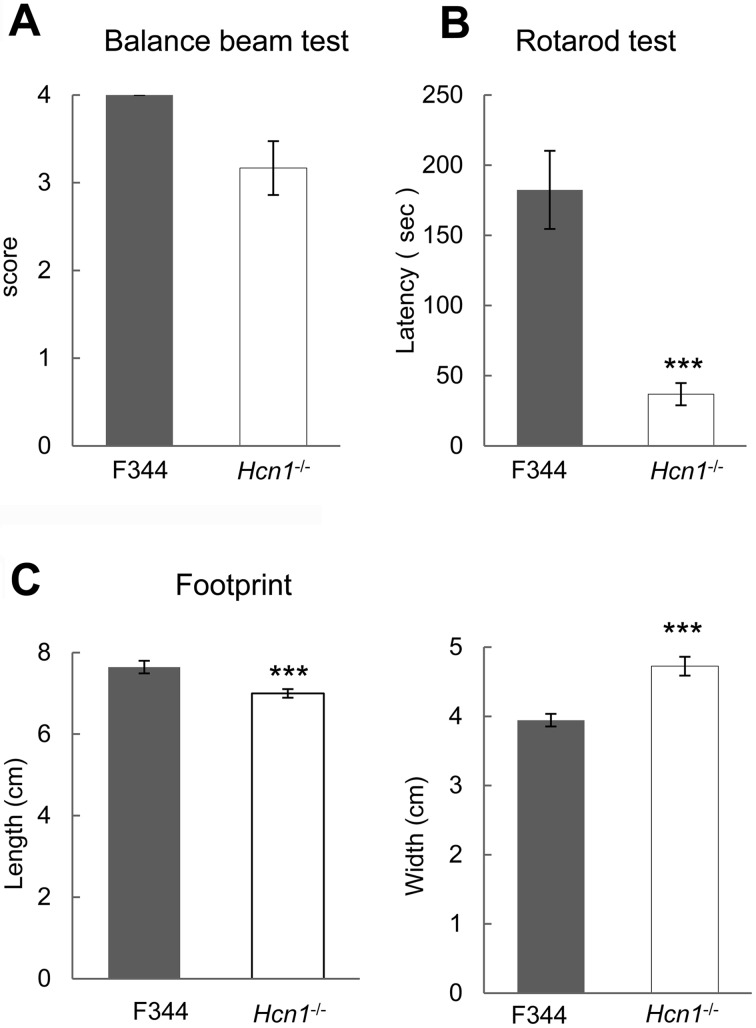
To assess motor ability, we performed balance beam and rotarod tests. In the balance beam test, F344 rats could walk across a 1.5-cm plank, while Hcn1-deficient rats often could not walk the same plank. The average maximum score of Hcn1-deficient rats (n=6), however, did not differ significantly from that of F344 rats (n=6): 3.17 ± 0.307 vs. 4.00 ± 0.00, P=0.0606 (Fig. 2A). In rotarod test, rats were tested on a rotarod at a constant speed of 10 rpm. The average maximum latency to fall of Hcn1-deficient rats (n=6) was significantly shorter than F344 rats (n=6): 36.8 ± 7.96 s vs. 182 ± 27.8 s, P<0.001 (Fig. 2B).
Fig. 2.
Hcn1-deficient rats exhibited impaired motor ability. A: Balance beam test (n=6, each). B: Hcn1-deficient rats exhibited shorter latency to fall from the rod than F344 rats (n=6, each). C: Footprint analysis. Results for Hcn1-deficient rats are shown as white bars, while those for F344 rats are shown as black bars (n=9, each). ***: P<0.001.
Footprint analysis
Footprint analysis showed smaller step length and wider step width in Hcn1-deficient rats (n=9) compared with F344 rats (n=9): length, 7.00 ± 0.107 cm vs. 7.64 ± 0.154 cm, P<0.001; width, 4.73 ± 0.136 cm vs. 3.94 ± 0.0911 cm, P<0.001 (Fig. 2C).
EMG, histology, and deep tendon reflex
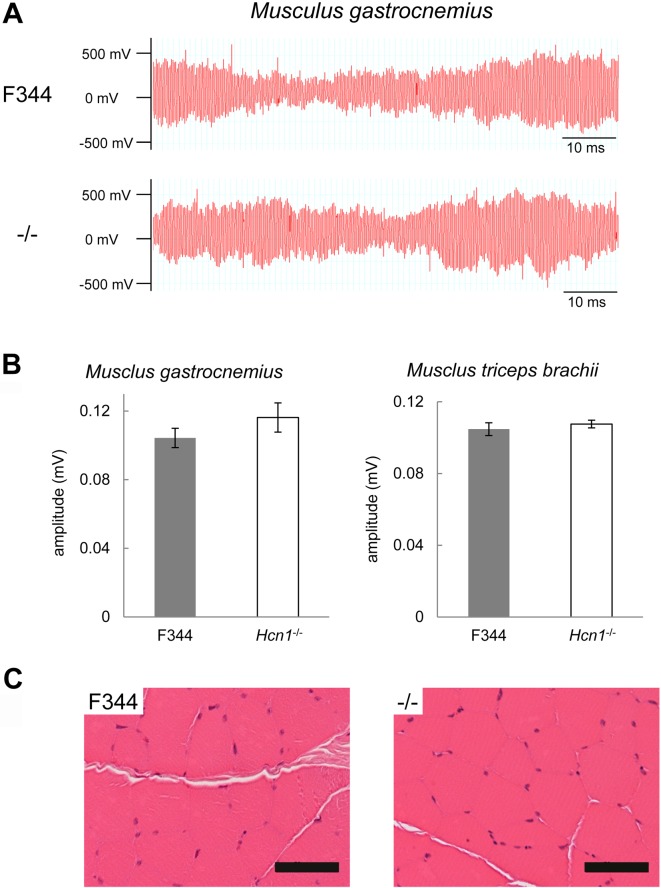
We performed EMG and histological analyses on Hcn1-deficient rats as they exhibited weaker muscle strength. Hcn1-deficient rats exhibited similar EMGs as F344 rats (Fig. 3A). Maximum action potentials were not significantly different in the musculus gastrocnemius and triceps brachii between Hcn1-deficient rats (n=5) and F344 rats (n=5). For the musculus gastrocnemius, 0.116 ± 0.00850 vs. 0.104 ± 0.00565, P=0.277; for the musculus triceps brachii, 0.108 ± 0.00217 vs. 0.105 ± 0.00352, P=0.511 (Fig. 3B).
Fig. 3.
Electromyogram and histology of muscles of Hcn1-deficient rats. A: Electromyograms of the musculus gastrocnemius of F344 (upper) and Hcn1-deficient rats (lower). B: Maximum action potential in the musculus gastrocnemius (left) and the musculus triceps brachii (right). Filled and open bars represent F344 (n=5) and Hcn1-deficient rats (n=5), respectively. C: Histology of the musculus triceps brachii in F344 (left) and Hcn1-deficient rats (right). Bar: 50 µm.
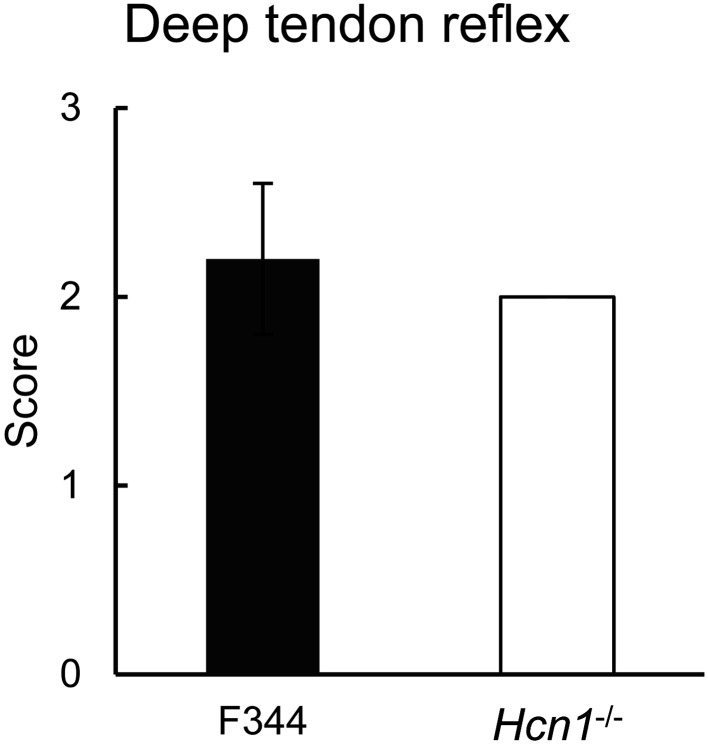
In terms of histopathology, Hcn1-deficient rats exhibited no apparent abnormal alternation in the muscles (Fig. 3C). Hcn1-deficient rats (n=5) exhibited normal or symmetrical deep tendon reflexes similar with F344 rats (n=5): 2.0 ± 0.0 vs. 2.2 ± 0.4, P=0.37 (Fig. 4).
Fig. 4.
Hcn1-deficient rats exhibited normal deep tendon reflexes similar with F344 rats. Average scores of deep tendon reflexes. Filled and open bars represent F344 (n=5) and Hcn1-deficient rats (n=5), respectively.
Discussion
In this study, we evaluated the muscle strength and motor functions of Hcn1-deficient rats. The behavioral tests that we performed revealed apparent muscle weakness and defects in motor coordination and balance in Hcn1-deficent rats. Instead of muscle weakness, however, we observed that Hcn1-deficient rats exhibited normal EMG, muscle histology, and deep tendon reflex.
Hcn1-deficient rats exhibited a smaller score in the wire hang and grip strength tests, in addition to lower posture maintenance compared with control rats in the inclined plane test. The wire hang and grip strength tests measure forelimb strength and coordination in small rodents [20, 23], while the inclined plane test determines limb motor function of laboratory animals [4]. Our findings therefore indicate that Hcn1-deficient rats suffer from reduced muscle strength and defects in motor function.
The balance beam and rotarod tests, as well as footprint analysis, are well established and widely used protocols for measuring motor coordination and balance in mice and rats [3]. Although Hcn1-deficient rats exhibited similar scores to control rats in the balance beam test, they demonstrated significantly lower performance in the rotarod test. Our footprint analysis revealed abnormal gait i.e. shorter step length and wider step width in Hcn1-deficient rats. These findings indicated that Hcn1-deficient rats had defects in motor coordination and balance and suggested that HCN1 channels contribute to muscle strength and motor coordination and balance.
Loose muscle tension, decreased muscle strength, and abnormal gait i.e. short step length and wide step width suggest that Hcn1-deficient rats suffer from hypotonia, a symptom of diminished skeletal muscle tone associated with decreased resistance of the muscles to passive stretching [10]. Hypotonia can be caused by abnormalities of the CNS, known as central hypotonia, or any element of the lower motoneuron, known as peripheral hypotonia [6, 9, 17]. There are several clinical tests to differentiate central from peripheral hypotonia. Among these tests, the deep tendon reflex test is simple but critical. The deep tendon reflex is absent in peripheral hypotonia, but is not noticeably affected in central hypotonia [6]. Hcn1-deficeint rats exhibited normal deep tendon reflexes similar to those of F344 rats. In addition, they exhibited normal EMG and muscle histology. We therefore considered the hypotonia observed in Hcn1-deficient rats to be central hypotonia.
Central hypotonia is associated with a lack of the cerebellum’s facilitatory efferent influence on the fusimotor system that innervates intrafusal muscle fibers [16]. HCN1 channels are expressed in cerebellar Purkinje cells and are thus the major determinant of Ih in these cells [14]. In addition, HCN1 channels are also expressed in the spinal cord and the medulla oblongata [11]. Given the important roles of the cerebellum and spinal cord in the neuromuscular system [8], it is likely that HCN1 channel dysfunction in these tissues may be involved largely in the observed hypotonia of Hcn1-deficient rats.
Hcn1-deficient rats exhibit absence epilepsy from 10 weeks of age. The average number of SWDs, a hallmark of absence epilepsy, was 5.2 and the average duration of SWDs was 2.1 s during the 1 h observation period at this age [12]. As the Hcn1-deficient rats used in this study were around 9–14 weeks of age, they were likely to exhibit absence seizures. Although there is the possibility that the absence seizures are associated with muscle weakness, the relatively short cumulative duration of the SWD i.e. 10.9 s/h in Hcn1-deficient rats suggests HCN1 channel dysfunction contributes to the muscle weakness without depending on absence seizures.
In summary, Hcn1-deficient rats exhibit muscle weakness and defects in motor coordination and balance. As they exhibit normal deep tendon reflex, the cause of their muscle weakness appears to originate from the CNS. The muscle weakness itself may cause the motor coordination defects observed in Hcn1-deficient rats.
Acknowledgments
The authors would like to thank the National Bio Resource Project-Rat in Japan (http://www.anim.med.kyoto-u.ac.jp/nbr) for providing Hcn1-deficient rats (NBRP Rat No: 0821).
References
- 1.Biel M., Wahl-Schott C., Michalakis S., Zong X.2009. Hyperpolarization-activated cation channels: from genes to function. Physiol. Rev. 89: 847–885. doi: 10.1152/physrev.00029.2008 [DOI] [PubMed] [Google Scholar]
- 2.Calejo A.I., Reverendo M., Silva V.S., Pereira P.M., Santos M.A., Zorec R., Gonçalves P.P.2014. Differences in the expression pattern of HCN isoforms among mammalian tissues: sources and implications. Mol. Biol. Rep. 41: 297–307. doi: 10.1007/s11033-013-2862-2 [DOI] [PubMed] [Google Scholar]
- 3.Carter R.J., Morton J., Dunnett S.B.2001. Motor coordination and balance in rodents. Curr Protoc Neurosci. Chapter 8: Unit 8 12. [DOI] [PubMed] [Google Scholar]
- 4.Chang M.W., Young M.S., Lin M.T.2008. An inclined plane system with microcontroller to determine limb motor function of laboratory animals. J. Neurosci. Methods 168: 186–194. doi: 10.1016/j.jneumeth.2007.09.013 [DOI] [PubMed] [Google Scholar]
- 5.Fenske S., Krause S.C., Hassan S.I., Becirovic E., Auer F., Bernard R., Kupatt C., Lange P., Ziegler T., Wotjak C.T., Zhang H., Hammelmann V., Paparizos C., Biel M., Wahl-Schott C.A.2013. Sick sinus syndrome in HCN1-deficient mice. Circulation 128: 2585–2594. doi: 10.1161/CIRCULATIONAHA.113.003712 [DOI] [PubMed] [Google Scholar]
- 6.Harris S.R.2008. Congenital hypotonia: clinical and developmental assessment. Dev. Med. Child Neurol. 50: 889–892. doi: 10.1111/j.1469-8749.2008.03097.x [DOI] [PubMed] [Google Scholar]
- 7.Huang Z., Walker M.C., Shah M.M.2009. Loss of dendritic HCN1 subunits enhances cortical excitability and epileptogenesis. J. Neurosci. 29: 10979–10988. doi: 10.1523/JNEUROSCI.1531-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ko C.P.2001. Neuromuscular System. pp. 10595–10600. In: International Encyclopedia of the Social & Behavioral Sciences. (Smelser, N.J., and Baltes, P.B. eds.), Pergamon, Oxford. [Google Scholar]
- 9.Leyenaar J., Camfield P., Camfield C.2005. A schematic approach to hypotonia in infancy. Paediatr. Child Health 10: 397–400. doi: 10.1093/pch/10.7.397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lisi E.C., Cohn R.D.2011. Genetic evaluation of the pediatric patient with hypotonia: perspective from a hypotonia specialty clinic and review of the literature. Dev. Med. Child Neurol. 53: 586–599. doi: 10.1111/j.1469-8749.2011.03918.x [DOI] [PubMed] [Google Scholar]
- 11.Milligan C.J., Edwards I.J., Deuchars J.2006. HCN1 ion channel immunoreactivity in spinal cord and medulla oblongata. Brain Res. 1081: 79–91. doi: 10.1016/j.brainres.2006.01.019 [DOI] [PubMed] [Google Scholar]
- 12.Nishitani A., Kunisawa N., Sugimura T., Sato K., Yoshida Y., Suzuki T., Sakuma T., Yamamoto T., Asano M., Saito Y., Ohno Y., Kuramoto T.2019. Loss of HCN1 subunits causes absence epilepsy in rats. Brain Res. 1706: 209–217. doi: 10.1016/j.brainres.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 13.Nishitani A., Tanaka M., Shimizu S., Kunisawa N., Yokoe M., Yoshida Y., Suzuki T., Sakuma T., Yamamoto T., Kuwamura M., Takenaka S., Ohno Y., Kuramoto T.2016. Involvement of aspartoacylase in tremor expression in rats. Exp. Anim. 65: 293–301. doi: 10.1538/expanim.16-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nolan M.F., Malleret G., Lee K.H., Gibbs E., Dudman J.T., Santoro B., Yin D., Thompson R.F., Siegelbaum S.A., Kandel E.R., Morozov A.2003. The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. Cell 115: 551–564. doi: 10.1016/S0092-8674(03)00884-5 [DOI] [PubMed] [Google Scholar]
- 15.Notomi T., Shigemoto R.2004. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J. Comp. Neurol. 471: 241–276. doi: 10.1002/cne.11039 [DOI] [PubMed] [Google Scholar]
- 16.O’Sullivan S.B.2019. Strategies to Improve Motor Function. pp. 361–399. In: Physical Rehabilitation. (O’Sullivan, S.B., Schmitz, T.J., and Fulk, G. eds.), F.A. Davis Company, Philadelphia. [Google Scholar]
- 17.Peredo D.E., Hannibal M.C.2009. The floppy infant: evaluation of hypotonia. Pediatr. Rev. 30: e66–e76. doi: 10.1542/pir.30-9-e66 [DOI] [PubMed] [Google Scholar]
- 18.Priebe M.M., Sherwood A.M., Thornby J.I., Kharas N.F., Markowski J.1996. Clinical assessment of spasticity in spinal cord injury: a multidimensional problem. Arch. Phys. Med. Rehabil. 77: 713–716. doi: 10.1016/S0003-9993(96)90014-3 [DOI] [PubMed] [Google Scholar]
- 19.Rivlin A.S., Tator C.H.1977. Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J. Neurosurg. 47: 577–581. doi: 10.3171/jns.1977.47.4.0577 [DOI] [PubMed] [Google Scholar]
- 20.Rogers D.C., Fisher E.M., Brown S.D., Peters J., Hunter A.J., Martin J.E.1997. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm. Genome 8: 711–713. doi: 10.1007/s003359900551 [DOI] [PubMed] [Google Scholar]
- 21.Santoro B., Chen S., Luthi A., Pavlidis P., Shumyatsky G.P., Tibbs G.R., Siegelbaum S.A.2000. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J. Neurosci. 20: 5264–5275. doi: 10.1523/JNEUROSCI.20-14-05264.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santoro B., Liu D.T., Yao H., Bartsch D., Kandel E.R., Siegelbaum S.A., Tibbs G.R.1998. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell 93: 717–729. doi: 10.1016/S0092-8674(00)81434-8 [DOI] [PubMed] [Google Scholar]
- 23.Smith J.P., Hicks P.S., Ortiz L.R., Martinez M.J., Mandler R.N.1995. Quantitative measurement of muscle strength in the mouse. J. Neurosci. Methods 62: 15–19. doi: 10.1016/0165-0270(95)00049-6 [DOI] [PubMed] [Google Scholar]
- 24.R Core team2017. R: A Language and Environment for Statistical Computing. Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org.
- 25.Teunissen C.E., Steinbusch H.W., Angevaren M., Appels M., de Bruijn C., Prickaerts J., de Vente J.2001. Behavioural correlates of striatal glial fibrillary acidic protein in the 3-nitropropionic acid rat model: disturbed walking pattern and spatial orientation. Neuroscience 105: 153–167. doi: 10.1016/S0306-4522(01)00164-6 [DOI] [PubMed] [Google Scholar]
- 26.von Euler M., Akesson E., Samuelsson E.B., Seiger A., Sundstrom E.1996. Motor performance score: a new algorithm for accurate behavioral testing of spinal cord injury in rats. Exp. Neurol. 137: 242–254. doi: 10.1006/exnr.1996.0023 [DOI] [PubMed] [Google Scholar]