Abstract
Diabetic cardiomyopathy (DCM) is one of the cardiovascular complications of diabetes mellitus independent of hypertension, coronary disease, and other heart diseases. The development of DCM is multifactorial and hard to detect at an early stage. Long non-coding RNA metastasis-associated lung adenocarcinoma transcript 1 (Malat1) is emerging as a regulator of DCM, the underlying mechanism of its role in DCM has not been elaborated yet. In this study, we established a mouse DCM model via streptozocin injection as evidenced by cell hypertrophy and cell apoptosis of myocardial tissue, and found that Malat1 expression was upregulated in the myocardium in DCM mice. Meanwhile, elevated expression of pro-apoptotic factors p53, p21, cleaved caspase 3, cleaved caspase 9 and BAX, and down-regulation of anti-apoptotic BCL-2 were observed in DCM myocardium. We further investigated the effect of Malat1 on cardiomyocytes under high glucose condition by silencing Malat1 with its specific short-hairpin RNA. Like in vivo, expression of Malat1 in cardiomyocytes was notably raised, remarkable cell apoptosis and changes in apoptosis-related factors were also observed following high glucose treatment. Besides, we validated that Malat1 acted as a sponge of miR-181a-5p. Inhibition of miR-181a-5p could, at least partially, abolish Malat1 knockdown-induced alteration in cardiomyocytes. In addition, p53, a critical regulator of apoptosis, was validated to be a downstream target of miR-181a-5p. In summary, our findings reveal that Malat1 knockdown attenuates high glucose-induced cardiomyocyte apoptosis via releasing miR-181a-5p, and this mechanism may provide us with new diagnosis target of DCM.
Keywords: cardiomyocyte apoptosis, diabetic cardiomyopathy, metastasis-associated lung adenocarcinoma transcript 1, miR-181a-5p, p53
Introduction
Diabetes mellitus (DM) is a metabolic disease featured by a high blood glucose level over a long period. DM is one of the largest global health emergencies of the 21st century owing to the extension of average life and increased obesity rate [5]. The prevalence of DM-related heart disease is in rapid and becomes a serious public health risk. The cardiovascular complications are the leading cause of diabetic patients, and studies show that the rate of heart failure is five times higher in diabetic women and two times higher in diabetic men [15, 36]. There are three major diabetic heart diseases: coronary artery disease (CAD), cardiac autonomic neuropathy (CAN), and diabetic cardiomyopathy (DCM) [29]. The terminology “diabetic cardiomyopathy” was first distinctly proposed in 1972 when heart failure was found in four diabetic patients [32]. DCM is specific cardiomyopathy in diabetic patients which is independent of coronary disease, hypertension, alcohol, and other structural heart diseases. It is characterized by left ventricular hypertrophy, interstitial fibrosis, cardiac microangiopathy and contractile dysfunction [25]. However, as the occurrence of DCM is multifactorial, there is no efficient and specific method for DCM diagnosis. Besides, DCM may remain asymptomatic for many years and accompany with other complications including obesity, hypertension, and vasculopathy, which makes the diagnosis of DCM even harder [19, 23]. Accordingly, exploring the underlying mechanism is of utmost importance for the diagnosis and treatment of DCM.
Long noncoding RNAs (lncRNAs) are defined as non-protein coding transcripts longer than 200 bp [13]. LncRNA metastasis-associated lung adenocarcinoma transcript 1 (Malat1), also known as noncoding nuclear-enriched abundant transcript 2 (Neat2), Linc00047, Ncrn00047 and Hcn, is one of the first identified and most comprehensively studied lncRNAs [12]. Malat1 was first discovered in non-small cell lung cancer and highly conserved among 33 mammalian species [14]. Since then, accumulating studies revealed its pivotal role in a variety of physiological processes. Malat1 is involved in molecular modification such as alternative splicing, transcriptional regulation and post-transcriptional regulation [20, 35, 37]. Malat1 is also implicated in various pathological processes including cancer development and progression, neurological disorders and, particularly, diabetes mellitus-related complications [2, 11, 30]. The expression of Malat1 is significantly up-regulated in rats with DM compared to control. Cardiomyocyte apoptosis is remarkably reduced after Malat1 knockdown and left ventricular function is consequently improved in diabetic rats [39].
MicroRNAs (miRNAs) are another group of endogenous non-coding RNA that usually containing approximately 22 nucleotides [1]. MiRNAs participate in multiple pathways and processes owing to its low complementarity requirement between the sequences of miRNAs and their targets. MicroRNA miR-181a-5p was first found to be involved in carcinogenesis, and aberrant miR-181a-5p expression was responsible for abnormal cellular functions in cancers [18, 33]. A recent study revealed that miR-181a-5p was significantly down-regulated in diabetic patients, diabetic rats, and high-glucose treated cardiomyocytes and that p53 and p21 acted as its target genes [31]. Besides, lncRNA could specifically bind to miR-181a-5p predicted on Starbase (http://starbase.sysu.edu.cn/index.php), which implies that Malat1 may serve as a sponge of miR-181a-5p. Thus we hypothesize that Malat1 is involved in DCM by regulating miR-181a-5p.
In this study, we examined the expression of Malat1 and miR-181a-5p in mice with DCM. Then we investigated the effect of MLAT1 and miR-181a-5p on cardiomyocyte apoptosis. Furthermore, we validated the correlation between Malat1 and miR-181a-5p.
Materials and Methods
Animal model establishment
Healthy C57BL/6J mice, 6–8 weeks old, were purchased from HKF (Shanghai, China). Mice were randomly divided into four groups: Control-4 weeks, DCM-4 weeks, Control-8 weeks, and DCM-8 weeks (n=6/group). Mice were intraperitoneally injected with 100 mg/kg streptozocin (STZ) (Aladdin, Shanghai, China) or the same volume normal saline (Dubang, Shenzhen, China) once a day for two days. A blood glucose level higher than 300 mg/dl demonstrated that the mouse diabetic model was successfully established. Mice were euthanized at week 4 and 8 following the STZ injection, and myocardial tissues were ultra-cryopreserved for subsequent detection. All animal experiments were carried out following the guideline for the care and use of laboratory animals and approved by Mudanjiang Medical College.
Cardiomyocyte isolation and viral infection
Cardiomyocytes were isolated as previously described [24]. In short, the heart of C57BL/6J mouse was carefully removed from thorax under a sterile environment and washed with PBS twice. Cardiac tissue was minced using curved scissors and digested with digestion buffer containing 1 mg/ml collagenase II, 1 mg/ml albumin and 25 µM CaCl2. Digested tissues were then filtered with 140 µm screen mesh, cardiomyocytes were then cultured with serum-free Dulbecco’s modified Eagle medium (DMEM) containing 0.2% albumin, 2 mM L-carnitine, 5 mM creatine, 5mM taurine, 0.1 µM insulin, 0.1 nM triiodothyronine and 10 mM 2,3-butanedione monoxime in a humidified incubator with 5% CO2 at 37°C. The Malat1 short hairpin RNA (shMalat1) was inserted into lentivirus shuttle plasmid Tet-pLKO-puro between AgeI and EcoRI. Anti-miR-181a-5p sequence was also inserted into Tet-pLKO-puro. In order to investigate the effect of Malat1 silencing on cardiomyocyte apoptosis, cardiomyocytes were infected with LV-shMalat1 (virus titer = 1 × 108 Tu/ml) and treated with 30 mM glucose for 48 h after infection. To further investigate the effect of miR-181a-5p inhibition on cardiomyocyte apoptosis, cardiomyocytes were co-infected with LV-shMalat1 and LV-anti-miR-181a-5p, and treated with 30 mM glucose for 48 h after infection. Sequences used in this study were as follow:
Malat1 shRNA: 5’-ccggcccGATTGAAGCTAGCAATCAAttcaagagaTTGATTGCTAGCTTCAATCttttt-3’ (sense); NC shRNA: 5’-ccggcccTTCTCCGAACGTGTCACGTttcaagagaACGTGACACGTTCGGAGAAttttt-3’ (sense).
Quantitative real-time PCR
Total RNAs of myocardial tissues and cardiomyocytes were extracted and reversely transcribed into cDNA using M-MLV reverse transcriptase (Tiangen, Beijing, China) following the manufacturer’s manual. Quantitative real-time PCR was performed using SYBR Green mix (Solarbio, Beijing, China) and data were analyzed using the 2-ΔΔCT method. U6 snRNA was used as an internal control for miR-181a-5p and Gapdh was used as a control for Malat1 and p53. Stem-loop RT primers for miRNAs and real-time PCR primers used in this study were as follows:Mmu-miR-181a-5p specific stem-loop primer: 5’-GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACACTCAC-3’; U6 snRNA specific stem-loop primer: 5’-GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACAAAAATATGG-3’; Mmu-miR-181a-5p-Forward: 5’-CGGCAACATTCAACGCTGT-3’; Mmu-miR-181a-5p-Reverse: 5’-GTGCAGGGTCCGAGGTATTC-3’; U6 snRNA-Forward: 5’-CGCAAGGATGACACGCAAAT-3’; U6 snRNA-Reverse: 5’-GTGCAGGGTCCGAGGTATTC-3’; LncRNA Malat1-Forward: 5’-TTTGCGGGTGTTGTAGGTTT-3’; lncRNA Malat1-Reverse: 5’-ACAGGAGTGAGGCTTGTGGT-3’; P53-Forward: 5’-GGGCATGAACCGCCGACCTA-3’; p53-Reverse: 5’-GGCAGGCACAAACACGAACC-3’; GAPDH-Forward: 5’-TGTTCCTACCCCCAATGTGTCCGTC-3’; GAPDH-Reverse: 5’-CTGGTCCTCAGTGTAGCCCAAGATG-3’.
Western blot
Tissues and cells were lysed with RIPA lysis buffer containing 1 mM PMSF (Solarbio) and protein concentration was quantified using a BCA kit (Solarbio). Proteins were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membranes (Millipore, Billerica, MA, USA). PVDF membranes were then blocked with 5% skim milk (Sangon Biotech, Shanghai, China) and incubated with one of the primary antibodies overnight at 4°C. After three-times PBS washing, PVDF membranes were incubated with certain secondary antibody for 60 min at 37°C. Primary antibodies used in this study were as follows: p53 antibody (1:2,000, Proteintech, Hangzhou, China), p21 antibody (1:1,000, Abcam, Cambridge, UK), cleaved caspase 3 antibody (1:1,000, CST, Framingham, MA, USA), cleaved caspase 9 antibody (1:1,000, CST), BCL-2 antibody (1:2,000, Proteintech), BAX antibody (1:5,000, Proteintech), and GAPDH antibody (1:10,000, Proteintech). HRP-conjugated goat anti-rabbit lgG (1:3,000, Solarbio) and HRP-conjugated goat anti-mouse lgG (1:3,000, Solarbio) were used as secondary antibodies.
Hematoxylin-eosin staining
Mouse myocardial tissues were embedded with paraffin and sliced into 5-µm sections, the paraffin sections were then deparaffinized and stained with hematoxylin and eosin successively. The morphological change of mouse myocardial tissues was photographed under a microscope (×400 magnification).
TdT mediated X-dUTP nicked labeling (TUNEL) assay
Cell apoptosis was detected via TUNEL staining. For mouse myocardial tissues, paraffin sections of 5 µm were deparaffinized and permeated with 0.1% Triton X-100. Afterward, sections were labeled with In Situ Cell Death Detection Kit (Roche, Basel, Switzerland), stained with hematoxylin and washed with gradient ethanol. The apoptosis of myocardial tissues was observed under a microscope (×400 magnification). For mouse cardiomyocytes, cell slides were permeated with 0.1% Triton X-100 and labeled with In Situ Cell Death Detection Kit at 37°C for 1 h. Then cell slides were stained with DAPI (Beyotime, Shanghai, China) for 5 min at room temperature and sealed with anti-fluorescence quenching reagent (Solarbio), typical pictures were captured under a microscope (×200 magnification).
Dual-luciferase assay
To investigate the correlation between lncRNA Malat1 and miR-181a-5p, the wildtype and mutant type of Malat1 fragments were inserted into pmirGLO (Promega, Madison, WI, USA) between Nhe I and Sal I, abbreviated as Malat1-WT and Malat1-MUT. 293T cells were co-transfected with Malat1 plasmids and miR-181a-5p/NC mimics. To investigate the regulation of miR-181a-5p on p53, the wildtype and mutant type of p53 3’-UTR were inserted into pmirGLO between Nhe I and Sal I, abbreviated as p53-WT and p53-MUT. 293T cells were co-transfected with p53 3’-UTR and miR-181a-5p/Nonspecific control microRNA mimic, respectively. Cells were lysed and the binding activities were evaluated by firefly luciferase activity/renilla luciferase activity.
Statistical analysis
GraphPad Prism 7 was used to perform the data analysis. All results were presented as means ± SD and compared using Student’s t-test (two groups) or One-way ANOVA (three or more groups). A P value<0.05 was considered as statistically significant.
Results
Mouse DCM model was successfully established
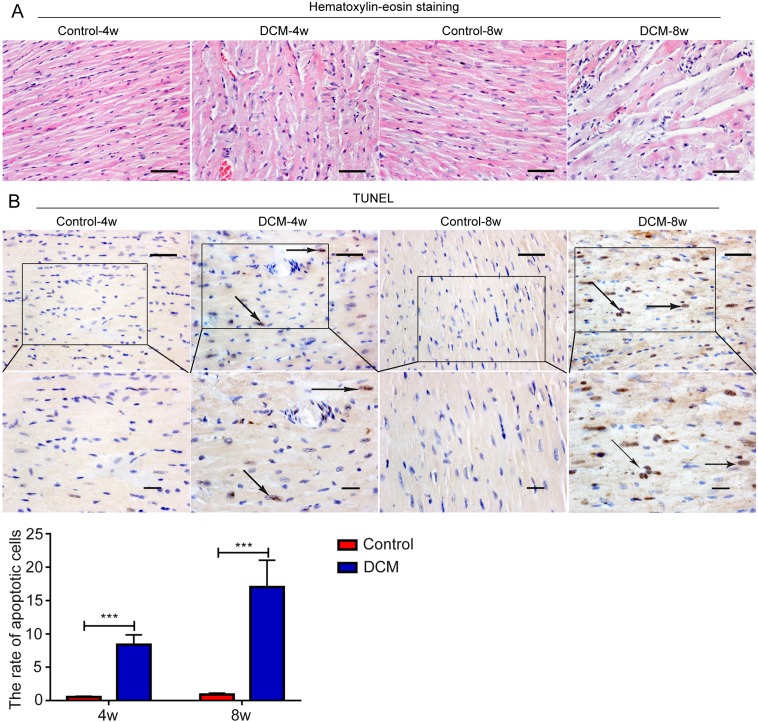
By hematoxylin-eosin staining, we found that myocardial fibers in control mice were arranged neatly, and showed no cell swelling. Myocardial fibers of mice 4 weeks after STZ injection showed obvious disordered arrangement, cell hypertrophy and hyperchromatic nucleus. These characteristics were more notable at week 8, accompanied by cardiomyocyte necrosis and a large area of light staining (Fig. 1A). Then we detected the apoptosis in myocardial tissues using TUNEL assay. Cardiomyocytes in the control heart were arranged orderly and cell apoptosis was hardly detected. Four weeks post-STZ injection, cardiomyocytes were arranged disorderly, partial apoptotic cells were detected, which was more significant at week 8 (Fig. 1B). These results suggest that we successfully established the mouse diabetic cardiomyopathy model.
Fig. 1.
Animal model of diabetic cardiomyopathy was successfully established. (A) Morphological change of mouse myocardial tissue was detected by hematoxylin-eosin staining. (B) The apoptosis of mouse myocardial tissue was assessed by TdT mediated X-dUTP nicked labeling assay. Bar=50 µm.
The expression of Malat1, miR-181a-5p and apoptosis-related factors in mice with DCM
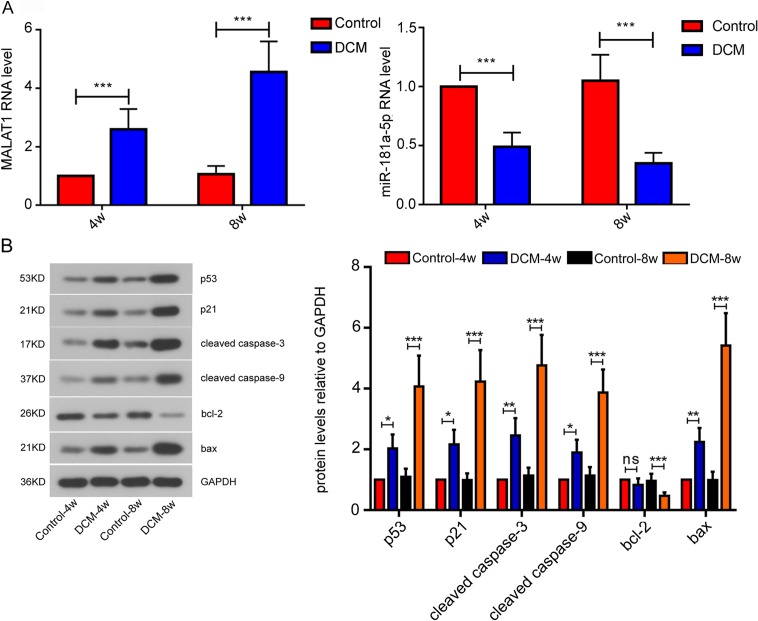
We analyzed the expression changes of lncRNA Malat1 and miR-181a-5p in mouse myocardial tissues by real-time PCR. Malat1 was up-regulated at week 4 following STZ injection compared to control, which was even more significant at week 8. On the contrary, the RNA level of miR-181a-5p was remarkably decreased at week 4 after STZ treatment and the decrease was exacerbated at week 8 (Fig. 2A). Then we evaluated the expression of apoptosis-related factors by western blot. We found that the protein levels of p53, p21, cleaved caspase 3, cleaved caspase 9 and BAX were remarkably elevated at week 4 post-STZ injection in contrast with control, which were more significant at week 8. On the contrary, the expression of BCL-2 was significantly suppressed at week 8 after STZ injection in contrast with control (Fig. 2B). These results indicate that STZ injection induces cardiomyocyte apoptosis.
Fig. 2.
Expression of long noncoding RNA Malat1, miR-181a-5p and apoptosis-related factors in mouse myocardial tissue. (A) The level of lncRNA Malat1 and miR-181a-5p were measured by quantitative real-time PCR. (B) Protein levels of apoptosis-related factors were evaluated by western blot assay. All data were presented as mean ± SD. ns: not significant; *P<0.05; **P<0.01; ***P<0.001.
Malat1 acts as a sponge of miR-181a-5p
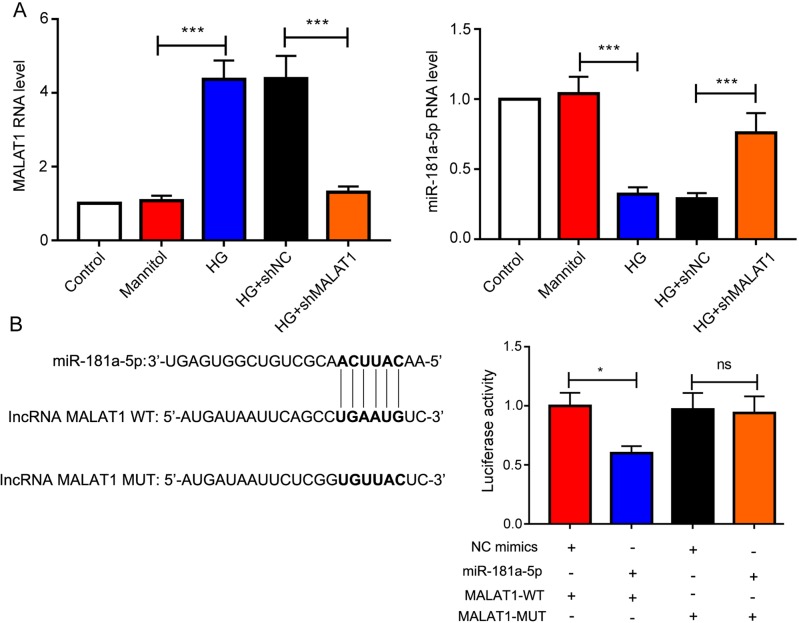
Mouse cardiomyocyte treated with high glucose (HG) showed significant higher Malat1 expression compared to mannitol group, and the expression of Malat1 was successfully knocked down by shMalat1. Like in vivo, the expression of miR-181a-5p showed the contrary trend to that of Malat1. The level of miR-181a-5p was downregulated following HG treatment but elevated after Malat1 knockdown (Fig. 3A). These results inspired us to investigate the relationship between Malat1 and miR-181a-5p. LncRNA Malat1 could specifically bind to miR-181a-5p predicted on StarBase (http://starbase.sysu.edu.cn/index.php), and we validated their interaction by performing the dual-luciferase assay. The binding site of miR-181a-5p on Malat1 was shown in Fig. 3B Cells co-transfected with Malat1-WT and miR-181a-5p mimics showed significantly weaker luciferase activity in contrast with that co-transfected with Malat1-WT and NC mimics. On the contrary, after Malat1 mutation, no obvious difference of luciferase activity was observed between cells transfected with NC mimics and miR-181a-5p mimics (Fig. 3B).
Fig. 3.
Long noncoding RNA Malat1 acts as a sponge of miR-181a-5p. (A) The levels of lncRNA Malat1 and miR-181a-5p in mouse cardiomyocytes after Malat1 silencing were measured by quantitative real-time PCR. (B) The specific binding site of miR-181a-5p on Malat1 was shown, and the correlation between Malat1 and miR-181a-5p was analyzed by dual-luciferase assay, ns: not significant; *P<0.05; *** P<0.001.
Malat1 knockdown abrogates cardiomyocyte apoptosis induced by high glucose
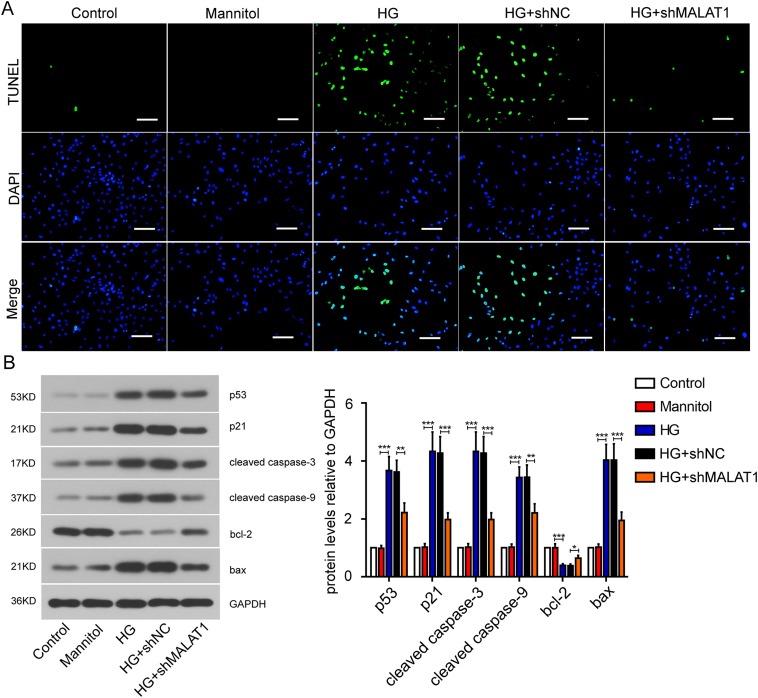
We investigated the effect of Malat1 on cardiomyocyte apoptosis via TUNEL assay. Obvious cardiomyocyte apoptosis was observed post the high glucose treatment. It was noteworthy that Malat1 silencing abolished the apoptosis induced by high glucose (Fig. 4A). High glucose treatment significantly up-regulated the pro-apoptosis proteins including p53, p21, cleaved caspase 3, cleaved caspase 9 and BAX, yet inhibited the expression of BCL-2. Malat1 silencing could alleviate the expression changes of these proteins induced by high glucose (Fig. 4B). These results indicate that Malat1 silencing can suppress cardiomyocyte apoptosis.
Fig. 4.
Effect of Malat1 silencing on mouse cardiomyocyte apoptosis. (A) Apoptosis of mouse cardiomyocytes after Malat1 silencing was detected by TdT mediated X-dUTP nicked labeling assay (Bar=100 µm). (B) Expression of apoptosis-related factors after Malat1 silencing was measured by western blot assay. All data were presented as mean ± SD. *P<0.05; **P<0.01; ***P<0.001.
The effect of miR-181a-5p on cardiomyocyte apoptosis
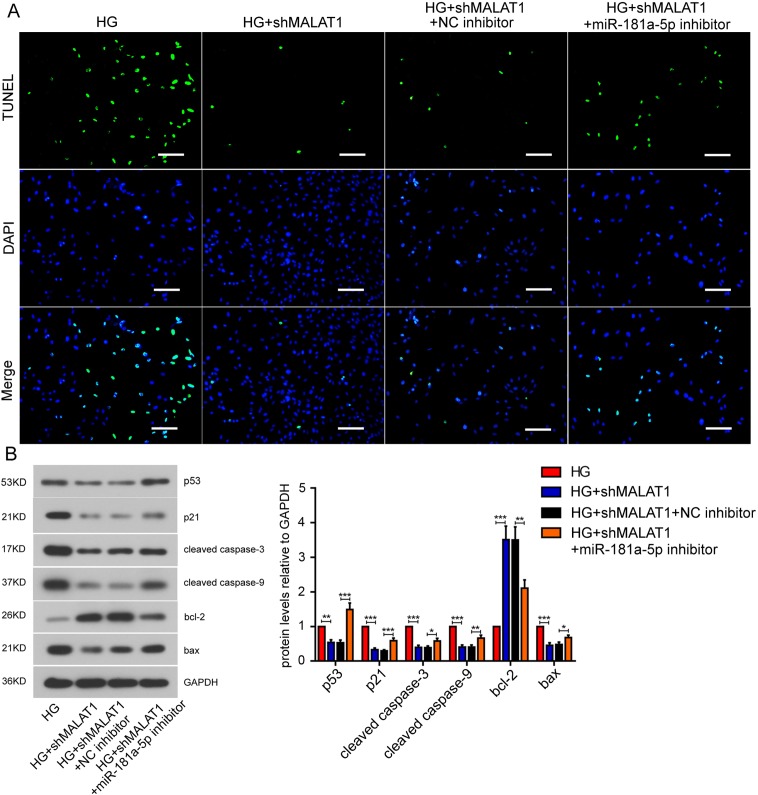
Owing to the interaction between Malat1 and miR-181a-5p, we further investigated the effect of miR-181a-5p on cardiomyocyte apoptosis. Like we previously described, Malat1 knockdown abrogated the high glucose-induced cardiomyocyte apoptosis. Then we suppressed the miR-181a-5p expression with anti-miR-181a-5p viral particles, and apoptotic cells re-appeared (Fig. 5A). Malat1 silencing down-regulated the expression of p53, p21, cleaved caspase 3, cleaved caspase 9 and BAX, but elevated the protein level of BCL-2. All these expression changes were remarkably reversed after miR-181a-5p inhibition (Fig. 5B). These results suggest that miR-181a-5p is involved in Malat1-mediated cardiomyocyte apoptosis.
Fig. 5.
Effect of miR-181a-5p on mouse cardiomyocytes apoptosis. (A) Apoptosis of mouse cardiomyocytes after miR-181a-5p inhibition was assessed by TdT mediated X-dUTP nicked labeling assay (Bar=100 µm). (B) Protein levels of apoptosis-related factors after miR-181a-5p inhibition were detected by western blot assay. All data were presented as mean ± SD. *P<0.05; **P<0.01; ***P<0.001.
P53 is a downstream target of miR-181a-5p
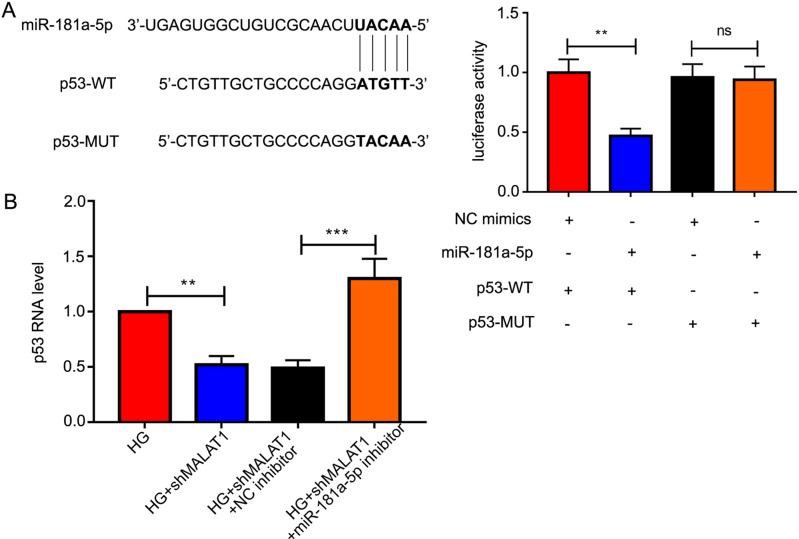
The binding site of miR-181a-5p and p53 was shown in Fig. 6A Cells co-transfected with p53-WT and miR-181a-5p mimics showed notably weaker luciferase activity compared to that co-transfected with p53-WT and NC mimics. There was no difference of luciferase activity between cells co-transfected with p53-MUT + NC mimics and that co-transfected with p53-MUT + miR-181a-5p mimics (Fig. 6A). Furthermore, the mRNA level of p53 was remarkably elevated after miR-181a-5p inhibition, demonstrating that p53 is a downstream target of miR-181a-5p.
Fig. 6.
P53 is a downstream target gene of miR-181a-5p. (A) The specific binding site of miR-181a-5p on p53 was displayed, and the correlation between miR-181a-5p and p53 was assessed by dual-luciferase assay. (B) The mRNA level of p53 after miR-181a-5p inhibition was measured by quantitative real-time PCR. All data were presented as mean ± SD. ns: not significant; **P<0.01; ***P<0.001.
Discussion
Cardiovascular-related complications are responsible for approximately 65% of diabetic death [29]. Among which DCM leads to diastolic dysfunction and further systolic dysfunction, and eventually greatly increases the risk of heart failure [10]. Myocardial fibrosis and hypertrophy, as well as cardiomyocyte apoptosis, are vital contributors in the onset and progression of DCM [9]. In this study, we established a mouse DCM model via intraperitoneal injection of STZ according to a previous study [34]. Clear hypertrophy of myocardial fibers and myocardial necrosis were detectable at week 8 following STZ injection. TUNEL assay further showed that STZ treatment led to substantial cardiomyocyte apoptosis. Taken all these together, we built an appropriate model for investigations of DCM.
The major pathological characteristics of DM patients include hyperglycemia, hyperlipidemia, and inflammation, among which hyperglycemia is widely believed to be the leading cause of DCM pathogenesis [28]. Sustained hyperglycemia leads to a variety of metabolic changes in cardiomyocytes including oxidative stress through the development of reactive oxygen species (ROS), which could further induce the apoptosis and DNA damage of cardiomyocytes [3, 8]. Hyperglycemia-induced cardiomyocytes apoptosis is regulated by the mitochondrial apoptotic pathway, as evidenced by the release of cytochrome c and activation of caspase 3 [3]. Furthermore, p53, a well-known tumor suppressor, is involved in the development of oxidative damage-induced cardiomyopathy through regulating p21 [26]. P53 also interacts with p21 to inhibit cell proliferation and induce cell apoptosis via activating BAX [16]. In particular, the expression of p53 is significantly up-regulated in diabetic mice and leads to cardiac dysfunction, whereas cardiac impairment is abolished in p53 knock-out mice [27]. In this study, the elevated expression of p53, p21, cleaved caspase 3, cleaved caspase 9, and pro-apoptotic BAX, and down-regulation of anti-apoptotic BCL-2 were detected in the myocardium of STZ-induced DCM mice, indicating that mitochondrial apoptotic pathway mediated apoptosis occurred in diabetic myocardial tissue. Similar cell apoptosis was also observed in high glucose-treated cardiomyocytes, indicating that hyperglycemia could result in cardiomyocyte apoptosis in vitro.
LncRNAs are involved in various physiological processes via binding to DNA/RNA/protein or changing the localization/affinity of protein [6]. They also regulate gene expression via decoying specific microRNAs. Increasing evidence indicates that abnormal expression of lncRNAs is implicated in cardiovascular complications of diabetes [7]. Malat1, highly conserved among various mammals, is emerging as a regulator of DCM. For instance, Malat1 is notably up-regulated in myocardial tissue of diabetic rats. Knockdown of Malat1 significantly improved the left ventricular end-diastolic diameter (LVEDD) and left ventricular end-systolic diameter (LVESD), indicating that Malat1 silencing could abrogate the cardiac impairment of diabetic rats [38]. Besides, cardiomyocyte apoptosis was reduced and cardiac function was improved in diabetic rats after Malat1 knockdown [39]. Our results, in accord with previous studies, demonstrated that Malat1 was up-regulated in DCM mice and high glucose-treated cardiomyocytes. Knockdown of Malat1 via shMalat1 significantly alleviated apoptosis induced by high glucose as evidenced by expression changes of apoptosis-related proteins. MiR-181a-5p was found to be involved in regulating apoptosis of endothelial cells [4]. MiR-181a-5p also promotes invasion of hepatocellular carcinoma by p53 signaling pathway [21]. Our study first validated the interaction between Malat1 and miR-181a-5p, whose expression levels were opposite in both diabetic myocardial tissues and high glucose-treated cardiomyocytes. Inhibition of miR-181a-5p could partially reverse the anti-apoptotic effect of shMalat1. In particular, miR-181a-5p is down-regulated in DCM rats developed by high-fat diet and low-dose STZ, and decreased miR-181a-5p promotes cell apoptosis via regulating the p53-p21 pathway [31, 40]. Increasing evidence revealed that aberrant microRNA expression is associated with diabetic cardiovascular complications. For instance, cardiomyocyte hypertrophy induced by diabetes showed close relationship with down-regulation of miR-150, miR-133a and miR-30c, among which elevated miR-30c expression could alleviate the cardiomyocyte hypertrophy [22]. MiR-30d, miR-133a, miR-206, and miR-1 are involved in diabetes-induced cardiomyocyte apoptosis as well as mitochondrial dysfunction[22]. For clinical application, a previous clinical study showed that the expression of seven microRNAs is remarkably up-regulated in diabetic patients in contrast with pre-diabetes patients and susceptible individuals [17]. DCM is hard to detect at the early stage of the disease, thus the serum levels of microRNAs could act as a sensitive and efficient indicator for the cardiovascular complication of diabetes [17].
In conclusion, our study demonstrates that Malat1 promotes cardiomyocyte apoptosis by preventing miR-181a-5p from binding to p53. Malat1-miR-181a-5p-p53 might serve as a therapeutic target for DCM.
Conflict of Interest
None.
Acknowledgments
This study was supported by grants from the Natural Science Foundation of Heilongjiang Province (No. H2018069), the Scientific Research Projects for Heilongjiang Provincial Universities (No. 2017-KYYWFMY-0678 and 2018-KYYWFMY-0078), the Scientific Research Project of Health and Family Planning Commission of Heilongjiang Province (No. 2017-313), and the National Natural Science Foundation of China (No. 81870977).
References
- 1.Bartel D.P.2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297. doi: 10.1016/S0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- 2.Bernard D., Prasanth K.V., Tripathi V., Colasse S., Nakamura T., Xuan Z., Zhang M.Q., Sedel F., Jourdren L., Coulpier F., Triller A., Spector D.L., Bessis A.2010. A long nuclear-retained non-coding RNA regulates synaptogenesis by modulating gene expression. EMBO J. 29: 3082–3093. doi: 10.1038/emboj.2010.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai L., Li W., Wang G., Guo L., Jiang Y., Kang Y.J.2002. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes 51: 1938–1948. doi: 10.2337/diabetes.51.6.1938 [DOI] [PubMed] [Google Scholar]
- 4.Carmona A., Guerrero F., Buendia P., Obrero T., Aljama P., Carracedo J.2017. Microvesicles Derived from Indoxyl Sulfate Treated Endothelial Cells Induce Endothelial Progenitor Cells Dysfunction. Front. Physiol. 8: 666. doi: 10.3389/fphys.2017.00666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiang J.L., Kirkman M.S., Laffel L.M., Peters A.L., Type 1 Diabetes Sourcebook Authors. 2014. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care 37: 2034–2054. doi: 10.2337/dc14-1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chien H.Y., Chen C.Y., Chiu Y.H., Lin Y.C., Li W.C.2016. Differential microRNA Profiles Predict Diabetic Nephropathy Progression in Taiwan. Int. J. Med. Sci. 13: 457–465. doi: 10.7150/ijms.15548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das A., Samidurai A., Salloum F.N.2018. Deciphering Non-coding RNAs in Cardiovascular Health and Disease. Front. Cardiovasc. Med. 5: 73. doi: 10.3389/fcvm.2018.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du X., Matsumura T., Edelstein D., Rossetti L., Zsengellér Z., Szabó C., Brownlee M.2003. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J. Clin. Invest. 112: 1049–1057. doi: 10.1172/JCI18127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falcão-Pires I., Leite-Moreira A.F.2012. Diabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Fail. Rev. 17: 325–344. doi: 10.1007/s10741-011-9257-z [DOI] [PubMed] [Google Scholar]
- 10.Gilca G.E., Stefanescu G., Badulescu O., Tanase D.M., Bararu I., Ciocoiu M.2017. Diabetic Cardiomyopathy: Current Approach and Potential Diagnostic and Therapeutic Targets. J. Diabetes Res. 2017: 1310265. doi: 10.1155/2017/1310265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutschner T., Hämmerle M., Diederichs S.2013. MALAT1 -- a paradigm for long noncoding RNA function in cancer. J. Mol. Med. (Berl.) 91: 791–801. doi: 10.1007/s00109-013-1028-y [DOI] [PubMed] [Google Scholar]
- 12.Hutchinson J.N., Ensminger A.W., Clemson C.M., Lynch C.R., Lawrence J.B., Chess A.2007. A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genomics 8: 39. doi: 10.1186/1471-2164-8-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iyer M.K., Niknafs Y.S., Malik R., Singhal U., Sahu A., Hosono Y., Barrette T.R., Prensner J.R., Evans J.R., Zhao S., Poliakov A., Cao X., Dhanasekaran S.M., Wu Y.M., Robinson D.R., Beer D.G., Feng F.Y., Iyer H.K., Chinnaiyan A.M.2015. The landscape of long noncoding RNAs in the human transcriptome. Nat. Genet. 47: 199–208. doi: 10.1038/ng.3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji P., Diederichs S., Wang W., Böing S., Metzger R., Schneider P.M., Tidow N., Brandt B., Buerger H., Bulk E., Thomas M., Berdel W.E., Serve H., Müller-Tidow C.2003. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 22: 8031–8041. doi: 10.1038/sj.onc.1206928 [DOI] [PubMed] [Google Scholar]
- 15.Kannel W.B., McGee D.L.1979. Diabetes and cardiovascular disease. The Framingham study. JAMA 241: 2035–2038. doi: 10.1001/jama.1979.03290450033020 [DOI] [PubMed] [Google Scholar]
- 16.Kim E.M., Jung C.H., Kim J., Hwang S.G., Park J.K., Um H.D.2017. The p53/p21 Complex Regulates Cancer Cell Invasion and Apoptosis by Targeting Bcl-2 Family Proteins. Cancer Res. 77: 3092–3100. doi: 10.1158/0008-5472.CAN-16-2098 [DOI] [PubMed] [Google Scholar]
- 17.Kong L., Zhu J., Han W., Jiang X., Xu M., Zhao Y., Dong Q., Pang Z., Guan Q., Gao L., Zhao J., Zhao L.2011. Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: a clinical study. Acta Diabetol. 48: 61–69. doi: 10.1007/s00592-010-0226-0 [DOI] [PubMed] [Google Scholar]
- 18.Lashine Y.A., Seoudi A.M., Salah S., Abdelaziz A.I.2011. Expression signature of microRNA-181-a reveals its crucial role in the pathogenesis of paediatric systemic lupus erythematosus. Clin. Exp. Rheumatol. 29: 351–357. [PubMed] [Google Scholar]
- 19.Lee W.S., Kim J.2017. Diabetic cardiomyopathy: where we are and where we are going. Korean J. Intern. Med. (Korean. Assoc. Intern. Med.) 32: 404–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leucci E., Patella F., Waage J., Holmstrøm K., Lindow M., Porse B., Kauppinen S., Lund A.H.2013. microRNA-9 targets the long non-coding RNA MALAT1 for degradation in the nucleus. Sci. Rep. 3: 2535. doi: 10.1038/srep02535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S., Yao X., Zhang D., Sheng J., Wen X., Wang Q., Chen G., Li Z., Du Z., Zhang X.2018. Analysis of Transcription Factor-Related Regulatory Networks Based on Bioinformatics Analysis and Validation in Hepatocellular Carcinoma. BioMed Res. Int. 2018: 1431396. doi: 10.1155/2018/1431396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X., Liu S.2017. Role of microRNAs in the pathogenesis of diabetic cardiomyopathy. Biomed. Rep. 6: 140–145. doi: 10.3892/br.2017.841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorenzo-Almorós A., Tuñón J., Orejas M., Cortés M., Egido J., Lorenzo Ó.2017. Diagnostic approaches for diabetic cardiomyopathy. Cardiovasc. Diabetol. 16: 28. doi: 10.1186/s12933-017-0506-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo J., Hill B.G., Gu Y., Cai J., Srivastava S., Bhatnagar A., Prabhu S.D.2007. Mechanisms of acrolein-induced myocardial dysfunction: implications for environmental and endogenous aldehyde exposure. Am. J. Physiol. Heart Circ. Physiol. 293: H3673–H3684. doi: 10.1152/ajpheart.00284.2007 [DOI] [PubMed] [Google Scholar]
- 25.Miki T., Yuda S., Kouzu H., Miura T.2013. Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail. Rev. 18: 149–166. doi: 10.1007/s10741-012-9313-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mönkemann H., De Vriese A.S., Blom H.J., Kluijtmans L.A., Heil S.G., Schild H.H., Golubnitschaja O.2002. Early molecular events in the development of the diabetic cardiomyopathy. Amino Acids 23: 331–336. doi: 10.1007/s00726-001-0146-y [DOI] [PubMed] [Google Scholar]
- 27.Nakamura H., Matoba S., Iwai-Kanai E., Kimata M., Hoshino A., Nakaoka M., Katamura M., Okawa Y., Ariyoshi M., Mita Y., Ikeda K., Okigaki M., Adachi S., Tanaka H., Takamatsu T., Matsubara H.2012. p53 promotes cardiac dysfunction in diabetic mellitus caused by excessive mitochondrial respiration-mediated reactive oxygen species generation and lipid accumulation. Circ Heart Fail 5: 106–115. doi: 10.1161/CIRCHEARTFAILURE.111.961565 [DOI] [PubMed] [Google Scholar]
- 28.Nishikawa T., Edelstein D., Du X.L., Yamagishi S., Matsumura T., Kaneda Y., Yorek M.A., Beebe D., Oates P.J., Hammes H.P., Giardino I., Brownlee M.2000. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404: 787–790. doi: 10.1038/35008121 [DOI] [PubMed] [Google Scholar]
- 29.Pappachan J.M., Varughese G.I., Sriraman R., Arunagirinathan G.2013. Diabetic cardiomyopathy: Pathophysiology, diagnostic evaluation and management. World J. Diabetes 4: 177–189. doi: 10.4239/wjd.v4.i5.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puthanveetil P., Chen S., Feng B., Gautam A., Chakrabarti S.2015. Long non-coding RNA MALAT1 regulates hyperglycaemia induced inflammatory process in the endothelial cells. J. Cell. Mol. Med. 19: 1418–1425. doi: 10.1111/jcmm.12576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raut S.K., Singh G.B., Rastogi B., Saikia U.N., Mittal A., Dogra N., Singh S., Prasad R., Khullar M.2016. miR-30c and miR-181a synergistically modulate p53-p21 pathway in diabetes induced cardiac hypertrophy. Mol. Cell. Biochem. 417: 191–203. doi: 10.1007/s11010-016-2729-7 [DOI] [PubMed] [Google Scholar]
- 32.Rubler S., Dlugash J., Yuceoglu Y.Z., Kumral T., Branwood A.W., Grishman A.1972. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am. J. Cardiol. 30: 595–602. doi: 10.1016/0002-9149(72)90595-4 [DOI] [PubMed] [Google Scholar]
- 33.Seoudi A.M., Lashine Y.A., Abdelaziz A.I.2012. MicroRNA-181a - a tale of discrepancies. Expert Rev. Mol. Med. 14: e5. doi: 10.1017/S1462399411002122 [DOI] [PubMed] [Google Scholar]
- 34.Towner R.A., Smith N., Saunders D., Carrizales J., Lupu F., Silasi-Mansat R., Ehrenshaft M., Mason R.P.2015. In vivo targeted molecular magnetic resonance imaging of free radicals in diabetic cardiomyopathy within mice. Free Radic. Res. 49: 1140–1146. doi: 10.3109/10715762.2015.1050587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tripathi V., Ellis J.D., Shen Z., Song D.Y., Pan Q., Watt A.T., Freier S.M., Bennett C.F., Sharma A., Bubulya P.A., Blencowe B.J., Prasanth S.G., Prasanth K.V.2010. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol. Cell 39: 925–938. doi: 10.1016/j.molcel.2010.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z.V., Hill J.A.2015. Diabetic cardiomyopathy: catabolism driving metabolism. Circulation 131: 771–773. doi: 10.1161/CIRCULATIONAHA.115.015357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.West J.A., Davis C.P., Sunwoo H., Simon M.D., Sadreyev R.I., Wang P.I., Tolstorukov M.Y., Kingston R.E.2014. The long noncoding RNAs NEAT1 and MALAT1 bind active chromatin sites. Mol. Cell 55: 791–802. doi: 10.1016/j.molcel.2014.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang M., Gu H., Chen J., Zhou X.2016. Involvement of long noncoding RNA MALAT1 in the pathogenesis of diabetic cardiomyopathy. Int. J. Cardiol. 202: 753–755. doi: 10.1016/j.ijcard.2015.10.019 [DOI] [PubMed] [Google Scholar]
- 39.Zhang M., Gu H., Xu W., Zhou X.2016. Down-regulation of lncRNA MALAT1 reduces cardiomyocyte apoptosis and improves left ventricular function in diabetic rats. Int. J. Cardiol. 203: 214–216. doi: 10.1016/j.ijcard.2015.10.136 [DOI] [PubMed] [Google Scholar]
- 40.Zhu D.X., Zhu W., Fang C., Fan L., Zou Z.J., Wang Y.H., Liu P., Hong M., Miao K.R., Liu P., Xu W., Li J.Y.2012. miR-181a/b significantly enhances drug sensitivity in chronic lymphocytic leukemia cells via targeting multiple anti-apoptosis genes. Carcinogenesis 33: 1294–1301. doi: 10.1093/carcin/bgs179 [DOI] [PubMed] [Google Scholar]