Abstract
The liver is the central hub of xenobiotic metabolism and consequently the organ most prone to cosmetic- and drug-induced toxicity. Failure to detect liver toxicity or to assess compound clearance during product development is a major cause of postmarketing product withdrawal, with disastrous clinical and financial consequences. While small animals are still the preferred model in drug development, the recent ban on animal use in the European Union created a pressing need to develop precise and efficient tools to detect human liver toxicity during cosmetic development. This article includes a brief review of liver development, organization, and function and focuses on the state of the art of long-term cell culture, including hepatocyte cell sources, heterotypic cell–cell interactions, oxygen demands, and culture medium formulation. Finally, the article reviews emerging liver-on-chip devices and discusses the advantages and pitfalls of individual designs. The goal of this review is to provide a framework to design liver-on-chip devices and criteria with which to evaluate this emerging technology.
Keywords: liver, organ on chip, microphysiological systems, human on chip, in vitro models
1. Introduction
The liver is the largest internal organ of the human body, accounting for 2% of the weight of an adult and around 5% of the weight of a neonate (1, 2). More than 500 functions are ascribed to the liver; it serves as an endocrine organ secreting albumin and urea into the blood, an exocrine organ secreting bile into the intestine, and a storage organ for glycogen and triglycerides. The liver also serves as a central hub for the regulation of carbohydrate, lipid, and amino acid metabolism (3), and this function is strongly affected by circadian rhythm (4). Due to this metabolic capacity, the liver is tasked with inactivating toxins, clearing nanoparticles, and removing xenobiotics absorbed by the intestine, skin, or lungs (5–8), making it susceptible to acute or accumulative injury due to exposure to pharmaceutical or cosmetic ingredients. In fact, more than 900 drugs, chemicals, toxins, and herbs have been reported to cause liver injury. Drug-induced liver injury is the most common reason cited for withdrawal of an approved drug, costing the pharmaceutical industry an estimated US$2 billion annually. Data analysis carried out during the SEURAT-1 Research Initiative (9) suggested that cosmetic ingredients can similarly affect the liver, particularly in chronic exposure models (10).
Small animal models are the regulatory-accepted method to assess drug-induced injury and evaluate pharmacokinetic parameters. However, differences in genetics, metabolic processes, and physiological parameters between humans and rodents limit the predictive value of such models (11, 12). In addition, the recent introduction of European cosmetics regulation EC 1223/2009 (13) has banned the marketing of cosmetic products containing ingredients that have been tested on animals. Therefore, there is a pressing need to develop in vitro human models of liver function and toxicity. In this review, we provide an overview of the state of the art of in vitro liver models, describing major advantages and disadvantages of each. We discuss liver development, architecture, and function as they relate to these culture techniques. Finally, we review current opportunities and major challenges in integrating cell culture, bioreactor design, and microtechnology to develop new systems for novel applications in cosmetic and pharmaceutical development.
2. Liver Development and Biology
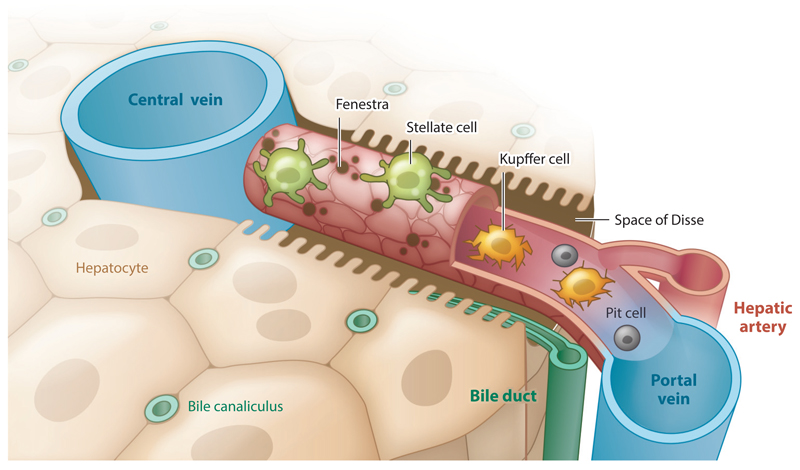
The liver forms as a bud from the thickening of the foregut. As the bud grows, it invades the mesenchyme of the septum transversum (14–16). The proliferating endodermal cells of the hepatic portion, called hepatoblasts, migrate toward a network of capillaries emanating from the umbilical and vitelline veins. In doing so, the hepatic cords envelop the capillaries, giving rise to parenchymal plates and liver sinusoids (1, 17). Studies suggest that early endothelial cells promote hepatic differentiation and migration in both liver and pancreas (18, 19). The liver basic structural unit is the hepatic sinusoid, a specialized capillary of fenestrated liver sinusoidal endothelial cells (LSECs) averaging 10 μm in diameter and 275 μm in length (Figure 1) (2, 20). Blood flows through the sinusoid at an average linear flow rate of 144 μm/s for a mean residence time of 1.9 s. Importantly, compounds absorbed by the intestine divide through the liver extensive vasculature but encounter only a single sinusoid before leaving the liver. Thus, hepatocytes need to carry out more than 500 functions in under 2 s, requiring substantial metabolic function and a streamlined processing line. The sinusoid delivers approximately 2,000 nmol/mL of oxygen to the surrounding shell of hepatocytes as the partial pressure drops from 70 to 25 mm Hg (20). In total, the sinusoids deliver oxygen at a rate of 72 nmol/(min·106 cells). Primary hepatocytes, appropriately cultured in vitro, consume oxygen at lower rates ranging from 54 to 18 nmol/(min·106 cells) (21).
Figure 1.
Schematic of the hepatic sinusoid. The sinusoid is a fenestrated capillary, 275 μm in length, composed of sinusoidal endothelial cells (red). The liver-resident pericytes, called stellate cells (green), wrap the capillary. Resident macrophages, called Kupffer cells (yellow), and natural killer cells, called pit cells (gray), roam the inside of the vessel.
Between the hepatocytes and the LSECs is the space of Disse, a thin, 1.4-μm-wide reticular basement membrane composed of fibronectin, laminin, collagen IV, and collagen I (2, 22). The hepatocytes surrounding the sinusoid extend numerous microvilli into the space of Disse and beyond and are therefore in direct contact with the blood (1). It is thought that the gradient of oxygen, growth factors, and hormones that forms along the sinusoid is responsible for the zonation of hepatic function (Table 1), although some studies suggest that endothelial-produced Wnt signaling is responsible for metabolic zonation (2, 23, 24).
Table 1. Metabolic zonation of the liver.
| Periportal | Perivenous | |
|---|---|---|
| Oxygen | 60–70 mm Hg | 25–35 mm Hg |
| Hormones | High | Low |
| Glucose | Gluconeogenesis | Glycolysis |
| CYP450 | Low | High |
| Lipid | β-Oxidation | Liponeogenesis |
| Nitrogen | Ureagenesis | Glutamine synthesis |
One example of the zonation of hepatic function is ammonia detoxification (25, 26). Ammonia is removed by the urea cycle along the length of the sinusoid, except for the last few cells near the central vein (i.e., perivenous cells), which convert ammonia to glutamine (25). Glutamine synthesis occurs at much lower ammonia concentrations than urea synthesis, allowing the perivenous hepatocytes to remove the remaining molecules of ammonia that could not be converted to urea in time. Therefore, perivenous hepatocytes act as a scrubber, reducing the amount of ammonia in the circulation. Glutamine eventually returns to the liver via the systemic circulation. At this point, periportal hepatocytes expressing glutaminase release ammonia from glutamine, leading to more efficient urea synthesis (25).
Sinusoid zonation is not limited to the metabolic function of hepatocytes. The sinusoid architecture and cellular composition also vary from the periportal to the perivenous region (26). Variations include the composition of the extracellular matrix (ECM) along the sinusoid (27), cell morphology, gene expression, and cell activity (28). For example, liver sinusoidal cells have larger fenestrae in the periportal region, but smaller and more numerous fenestrae close to the central vein (29). In addition, the liver’s resident macrophages (Kupffer cells) are more concentrated near the portal triads and differ metabolically according to their lobular location (30, 31). Table 2 presents the cellular composition of the liver.
Table 2. Cellular composition of the liver.
| Cell type | Diameter (μm) | Volume (% total) | Number (% total) | Type | Density (g/mL) |
|---|---|---|---|---|---|
| Hepatocytes | 20–25 | 77.8% | 65% | Epithelial | 1.10–1.15 |
| Sinusoidal endothelial cells | 7–11 | 2.8% | 16% | Endothelial | 1.061–1.080 |
| Kupffer cells | 10–13 | 2.1% | 12% | Macrophages | 1.076 |
| Stellate cells | 11–12 | 1.4% | 8% | Fibroblasts | 1.05 |
| Pit cells | 15–18a | Minor | Minor | Lymphocytes | 1.065–1.070a |
Pit cells have not been well characterized, as they are sparsely found, yet these cells are similar in size and characteristics to other large granular lymphocytes.
Liver parenchymal tissue is composed of hepatocytes, which are large (20- to 25-μm-diameter) cuboidal epithelial cells (2). Hepatocytes are arranged in plates that form tight junctions among themselves. Unlike other epithelial cells, hepatocytes do not form a sheet bounding a large apical lumen, but rather enclose small tubular apical spaces between adjacent hepatocytes, called bile canaliculi. Each hepatocyte features two basal surfaces on opposite sides of the cell body, bounding the space of Disse, through which albumin secretion and lipoprotein uptake take place (2). Hepatic architecture and sinusoidal organization are essential for proper liver function (Figure 1). Loss of liver architecture due to trauma or disease, such as fibrosis, leads to loss of tissue function (32).
3. Maintenance of Complex Liver Tissue
The need to preserve relevant tissue organization and function led many research groups to develop in situ models of liver function. One example is the isolated perfused rat liver, in which the organ remains intact and is nourished by perfusing the portal vein with media following ligation of the hepatic artery (33). This technique preserves heterotypic cell–cell interactions, as well as the organ’s three-dimensional (3D) architecture and function (33). Isolated liver perfusion has been used to study metabolic changes during injury and disease (34), protein turnover (35), toxic response (36, 37), and liver synthetic function (38, 39). However, isolated livers perfused in this way can only be maintained for a few hours, and only a single experiment can be carried out on each liver, leading to significant variance in the results (33).
In an effort to perform multiple experiments on each isolated liver, several groups developed precision-cut liver slices (40–42). Such slices can be made as thin as 0.2 mm, corresponding to eight cell layers, and can be cultured for up to 72 h in a high-oxygen environment (41, 43). Recent research integrating liver slices into microfluidic devices demonstrated the detection of unstable drug metabolites using high-performance liquid chromatography (44) and increased tissue survival to a few days in culture (45). The main advantage of liver slices is that they preserve the heterotypic interactions and architecture of the liver, while enabling a series of experiments on a single organism, greatly reducing variability (42). However, as noted above, liver slices cannot be maintained for more than a few days in culture.
Recent research on 3D bioprinting offers the ability to produce liver-like architecture from the ground up. Organovo (San Diego, CA) developed bioprinted cocultures containing primary human hepatocytes, stellate cells, and liver endothelial cells in a 24-well plate format. The company reported sustained function for 28 to 42 days in vitro, but the reported values for albumin production appear to be 30- to 70-fold below what should be expected for primary cells, namely 20–30 μg/(day·106 cells) (46, 47). More recently, Ma et al. (48) bioprinted human induced pluripotent stem cell (iPSC)-derived hepatocytes, endothelial cells, and adipose-derived cells in a hydrogel matrix that mimics the hexagonal architecture of the liver. The engineered liver tissue could be maintained for 2 to 8 days, similarly showing low values of albumin production of approximately 2–8 μg/(day·106 cells) (48).
4. Hepatocyte Source
The most commonly used hepatocyte isolation technique was introduced in 1976 by Seglen (49). The isolation relies on in situ collagenase perfusion, followed by mechanical segregation of the tissue and a purification step based on cell density (49). This technique allows for high viability (>90%) and, due to the high density of hepatocytes (1.13 g/cm3), a relatively pure population (>95%). One can routinely obtain 5–10% of the liver mass in hepatocytes, approximately 108 cells for rat livers and more than 109 cells for human livers (50). These freshly isolated human hepatocytes maintain a high level of function and gene expression when held in suspension for up to 6 h (51). Although it is fairly difficult to obtain freshly isolated human hepatocytes, as rejected donor grafts are available only sporadically, cells isolated from humanized Fah−/− NOD/SCID mice (52) can now be purchased from Yecuris (Portland, OR). However, primary human hepatocytes isolated from such FRG® (Fah−/−/Rag2−/−/II2rg−/−) mice have a limited life span in culture, similar to standard isolations.
The most common alternative to freshly isolated cells is cryopreserved hepatocytes, which are available from multiple vendors. The function of cryopreserved hepatocytes can vary widely on the basis of their source material and freezing process, resulting in batches of cells with minimal cytochrome P450 (CYP450) functionality or nonadherent populations. Vendors label specific batches as adherent (i.e., plateable) and provide information about the cell’s metabolic function. Plating efficiency is critical to function, as it can range from 50% to 80%. Low cell density leads to rapid loss of function; therefore, a high initial seeding density of approximately 120,000 cells/cm2 is preferred. The most common technique to culture primary hepatocytes is to seed the cells on a single layer of collagen I. Standard hepatic culture medium contains fetal bovine serum, corticosteroids (i.e., hydrocortisone, dexamethasone), and insulin (53). Several serum-free media formulations, such as hepatocyte maintenance medium from Lonza (Basel, Switzerland), are available commercially and described in detail in the literature (54). Albumin is a major carrier protein for xenobiotics and lipids. Thus, appropriate medium formulations must include at least 3.75 g/L of the protein, which is found at concentrations ranging from 35 to 50 g/L in plasma. Primary hepatocytes cultured in serum-free medium can maintain function for up to 3 to 5 days in culture. Properly cultured primary hepatocytes should have an oxygen consumption rate (OCR) of 20–50 nmol O2/(min·106 cells), CYP450 activity measured by 7-benzyloxy-4-trifluoromethylcoumarin (BFC) clearance of approximately 10–15 pmol/(min·mg protein), and an albumin production rate of 20– 30 μg/(day·106 cells) (Table 3).
Table 3. Functional characteristics of common liver cell models.
| Basal OCR [nmol/(min·106 cells)]b | BFC clearance rate [pmol/(min·mg protein)] | Albumin synthesis rate [μg/(day·106 cells)] | |
|---|---|---|---|
| HepG2/C3A | 2 | 0.2 | 2 |
| hPSC-derived hepatocytesa | 4.5 | 3 | 12 |
| HepaRG | 5 | 7 | 10 |
| E6/E7low Upcyte hepatocytes | 8 | 6.24 | 7 |
| Primary hepatocytes | 24 | 12 | 25 |
hPSCs include embryonic and induced cells.
Measured on a SeaHorse FluxAnalyzer.
Abbreviations: BFC, 7-benzyloxy-4-trifluoromethylcoumarin; hPSC, human pluripotent stem cell; OCR, oxygen consumption rate.
Unfortunately, the process of immortalizing human hepatocytes rapidly leads to loss of liver-specific function. A cancer cell line that offers a semblance of function is the HepG2/C3A derivative. HepG2/C3A cells rapidly expand in culture, with a doubling time of 18–20 h, and can be growth arrested using 1% dimethyl sulfoxide (DMSO) or spheroid formation. The resulting cells show measurable CYP450 activity, but two to five orders of magnitude lower than that of primary hepatocytes. Furthermore, even under the best conditions, HepG2/C3A cells are still primarily glycolytic and dedifferentiated, showing an OCR of 2 nmol O2/(min·106 cells), CYP450 activity measured by BFC clearance of approximately 0.1–0.3 pmol/(min·mg protein), and an albumin production rate of 2–3 μg/(day·106 cells) (Table 3). HepG2/C3A cells can remove ammonia but do not have a functional urea cycle.
One functional alternative is the HepaRG cell line, derived from a tumor of hepatic progenitor cells originating from a patient with hepatitis C viral infection (55). HepaRG cells have a doubling time of 24 h and can be induced to differentiate into a mixed population of hepatocytes and cholangiocytes following 28-day exposure to 1.7% DMSO (56). The resulting hepatocytes show a remarkable level of liver-specific function, on par with the lower bound of cryopreserved hepatocytes. The HepaRG cell line can be obtained under license or purchased as a mixture of hepatocytes and cholangiocytes from Biopredic International (Saint-Grégoire, France). HepaRG cells show an OCR of 4–6 nmol O2/(min·106 cells), CYP450 activity measured by BFC clearance of approximately 5–9 pmol/(min·mg protein), and an albumin production rate of 8–12 μg/(day·106 cells) (Table 3).
Recently, Levy et al. (57) introduced a method to conditionally expand human hepatocytes using low-level expression of human papillomavirus proteins E6 and E7. The induction released hepatocytes from cell cycle arrest, permitting the cells to expand under oncostatin M (OSM) stimulation. These Upcyte® hepatocytes showed a doubling time of 33 to 49 h, and were induced to differentiate into hepatocytes within 4 days of OSM removal (57). The authors generated a diverse library of lines that showed liver-specific function on par with that of cryopreserved hepatocytes. Drug toxicity across 18 compounds showed R2 > 0.98. Upcyte® hepatocytes can be obtained under license or purchased from Upcyte Technologies (Hamburg, Germany). Upcyte® hepatocytes show an OCR of 7–9 nmol O2/(min·106 cells), CYP450 activity measured by BFC clearance of approximately 6–7 pmol/(min·mg protein), and an albumin production rate of 6–9 μg/(day·106 cells) (Table 3). Overall, HepaRG and Upcyte® hepatocytes show similar levels of function, although the latter enable experiments to be conducted with shorter preparation and less variability, whereas the former are rapidly becoming the standard for cholestasis. Recently, Corning (Tewksbury, MA) developed a similar line of functional hepatocytes, termed HepatoCells, that promise similar levels of function.
Human pluripotent stem cell (hPSC)-derived hepatocytes have significant potential to replace primary cells with a similar level of metabolic function and lower batch-to-batch variability. However, the CYP450 function of commercially available hPSC-derived hepatocytes (from, e.g., Cellular Dynamics International, Madison, WI) is often two to five orders of magnitude below that of primary cells. Recent research by the Nahmias and Hay groups showed hPSC-derived hepatocytes with elevated levels of function (58) using microbiome-derived cues and recombinant laminins, respectively (58, 59). The Nahmias group (58) showed drug toxicity across nine compounds with R2 > 0.94, as well as accurate prediction of steatosis, cholestasis, and apoptosis. The cells can be maintained for 5–7 days post differentiation and show an OCR of 3–5 nmol O2/(min·106 cells), CYP450 activity measured by BFC clearance of 2–4 pmol/(min·mg protein), and an albumin production rate of approximately 12 μg/(day·106 cells) (Table 3). However, fetal properties are maintained, as these cells express α-fetoprotein and fetal CYP450 enzymes at much higher levels than those of primary hepatocytes.
5. Long-term Culture
Methods to stabilize the metabolic function of hepatocytes have been developed over the past 30 years and are in widespread use. One type of 3D support that stabilizes hepatocyte polarization and metabolic function is the collagen “sandwich” configuration developed by the Yarmush lab (60–63). The addition of heparan sulfate proteoglycan to the top collagen layer allows the expression of basal surface markers and gap-junction proteins, which are not observed in the standard collagen sandwich configuration (63–65). While the collagen sandwich produces a flat morphology, supporting high-content screening, the technique is cumbersome to perform, as improper handling or gelation causes the top layer to detach. Similarly, shear forces can remove the top gel layer, making the sandwich configuration difficult to use in microfluidic devices.
In contrast, hepatocyte spheroid formation is rapidly gaining popularity, as multiple tools to standardize size and aggregation kinetics have recently become available. Hepatocytes rapidly aggregate when cultured on a soft protein matrix (e.g., Matrigel) or nonadherent surfaces, producing spheroids of various sizes (65–68). Hepatocyte spheroids maintain a high level of hepatic structural polarity, forming distinct apical, lateral, and basal domains (69). The liver-specific function of hepatic spheroids is also enhanced, showing high levels of albumin production (67), urea secretion, and CYP450 activity (70, 71). While traditional methods to produce spheroids did not allow control over size, producing a mixture of large spheroids with necrotic cores, this is no longer the case. GravityPLUS™ (InSphero, Schlieren, Switzerland) is a 96-well plate that streamlines hanging drop aggregation, allowing the production of hepatocyte spheroids with 250-μm diameter and 10% variability (72). AggreWell™ (Stem Cell Technologies, Vancouver, Canada) is a competing technology that produces 4,700 spheroids per well with minimal variability. Liver organoids can be considered a heterotypic variant of 3D spheroids. Organoids are composed of both parenchymal and nonparenchymal cell types (73). For example, IntestiCult™ (Stem Cell Technologies) and MimEX™ (R&D Systems, Minneapolis, MN) could be used to establish liver organoids from commercially available fresh, cryopreserved, or iPSC-derived parenchymal and nonparenchymal cells.
6. Heterotypic Cell–Cell Interactions
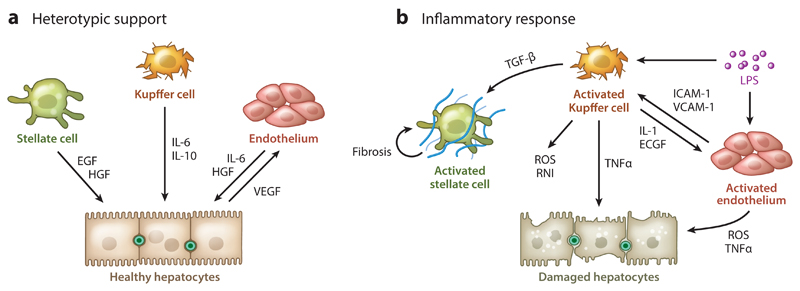
The liver nonparenchymal cell population (see the sidebar) plays an important role in supporting hepatocyte function and mediating pathological challenges such as inflammation (Figure 2). Kupffer cells are the liver’s resident macrophages, which play an important role as scavengers, ingesting aged erythrocytes, bacteria, various endogenous toxins, as well as in iron metabolism (1, 6). Kupffer cells have been implicated in drug toxicity and ischemic injury, becoming activated in response to a foreign stimulus such as lipopolysaccharide (LPS) or protein adducts and secreting reactive oxygen and nitrogen species that aggravate hepatic damage (74–76). Cryopreserved Kupffer cells can be purchased directly from Thermo Fisher Scientific (Waltham, MA), but they will activate over the first few days of culture, producing a mild inflammatory state upon long-term culture. Short-term cocultures of hepatocytes and Kupffer cells, in direct contact (77), in spheroids (72), or separated using a culture insert (78), can mimic certain aspects of drug toxicity (8). For example, Nguyen et al. (79) showed LPS-induced CYP450 inhibition, while Messner et al. (72) showed increased sensitivity to trovafloxacin.
Figure 2.
(a) Supportive and (b) inflammatory interactions between hepatocytes and nonparenchymal cells in the liver. Abbreviations: ECGF, endothelial cell growth factor; EGF, epidermal growth factor; HGF, hepatocyte growth factor; ICAM, intercellular adhesion molecule; IL, interleukin; LPS, lipopolysaccharide; RNI, reactive nitrogen intermediates; ROS, reactive oxygen species; TGF, transforming growth factor; TNF, tumor necrosis factor; VCAM, vascular cell adhesion molecule; VEGF, vascular endothelial growth factor.
Stellate cells are the liver’s resident pericytes, storing vitamin A and controlling sinusoid perfusion. Stellate cells are distinctively different from the liver’s resident fibroblasts, which decorate the larger blood vessels. Activation of stellate cells during an inflammatory cascade leads to a rapid loss of function as the cells differentiate into fibroblasts, leading to increased collagen deposition and fibrosis. Unfortunately, stellate cell activation occurs immediately upon plating and is driven in part by mechanical stimulus (80). Thus, commercially available cryopreserved stellate cells (from, e.g., Zen-Bio, ScienCell, or iXcells Biotechnologies) represent liver fibroblasts and cannot be used to study fibrosis. A notable exception is recent research by Leite et al. (80), who used a proprietary medium formulation to preserve inactivated stellate cells in order to study methotrexate induction of fibrosis. More recently, iPSC-derived stellate cells were cocultured and maintained in an inactivated state to study fibrosis (81).
Importantly, although fibroblasts cannot be used to study fibrosis, they do support long-term hepatocyte function. The mouse 3T3-J2 cell line is notably efficient in maintaining hepatocyte liver-specific function through a combination of cell–cell contacts (e.g., T/N-cadherin, decorin) and short-acting diffusible substances (82, 83). Rodent hepatocytes seeded at low densities on fibroblast feeder layers can proliferate in vitro (84), while micropatterned human hepatocytes can do so under small-molecular stimulation (85). Notably, other nonparenchymal cells, such as cholangiocytes, can similarly support long-term function.
Sinusoidal endothelial cells are the liver’s resident microvascular endothelial cells that share many functional similarities with macrophages. Sinusoidal endothelial cells are active scavengers, secreting cytokines upon inflammatory activation. Unfortunately, sinusoidal cell activation occurs rapidly in culture, and the cells dedifferentiate to a CD299−/CD31+ microvascular phenotype unless cocultured directly with hepatocytes (86). Cryopreserved sinusoidal endothelial cells sold by Creative Bioarray are actually CD31+ endothelial cells from larger blood vessels. Pioneering research by Morin & Normand (87) and Goulet et al. (88) has shown that liver sinusoidal endothelial cells stabilize hepatic urea and albumin secretion for up to a month in culture. Sinusoidal endothelial cells secrete hepatocyte growth factor (HGF) and interleukin-6 in response to hepatocyte-secreted vascular endothelial growth factor, protecting hepatocytes from toxic damage (Figure 2) (89). Primary hepatocytes cultured on soft gels specifically migrate toward endothelial capillaries in response to endothelial-secreted HGF (73). Microscale patterning of endothelial cells using laser guidance (90, 91) allowed researchers to align hepatocytes in a sinusoid-like structure, suggesting that endothelial cells might impart structural information to the developing and regenerating liver (92). More recent research showed that liver sinusoidal endothelial cells, but not fibroblasts, induce basal surface expression of the low-density lipoprotein receptor in hepatocytes (86). While albumin is often considered the ubiquitous carrier of hydrophobic drugs, a significant amount of lipid-soluble drug can also be transported in lipoproteins (93, 94). Thus, hepatocyte basal surface polarity is an important parameter to evaluate in emerging in vitro models.
7. Role of Oxygen in Hepatocyte Culture
Oxygen is an important component of the hepatic microenvironment, mediating cellular metabolism, differentiation, and growth (23, 95). Energy production in primary hepatocytes is highly dependent on oxidative phosphorylation, as each cell contains more than 1,500 mitochondria, which consume oxygen at a rate of 24 to 72 nmol/(min·106 cells) (96, 97). In order to supply hepatocytes with this amount of oxygen in vivo, the liver is connected to the highly oxygenated arterial circulation in addition to the portal circulation (98). During in vitro culture, oxygen is supplied by diffusion from the air–liquid interface. This oxygen diffusion limits the density of hepatocytes that can be seeded in culture or grown in a bioreactor (99).
Under ambient conditions, oxygen partial pressure of 21% or 160 mm Hg results in a dissolved concentration of 220 nmol/mL oxygen in culture medium or plasma. Therefore, without external supply through diffusion or flow, 1 × 106 hepatocytes will consume all available oxygen in 1 mL of culture medium within 3 to 9 min. Oxygen transport by diffusion across a distance of 1 mm can barely support the OCR of primary hepatocytes, 24 nmol/(min·106cells), limiting cellular function. A solution reported by Kidambi et al. (51) was to increase partial pressure to 95% oxygen, demonstrating a two-fold increase in CYP450 activity and albumin production under serum-free conditions. Using this configuration, the Nahmias group demonstrated similar clearance rates to in vivo for rapid- and slow-clearing drugs such as antipyrine and carbamazepine with R2 = 0.92 (51). A different solution, reported by Khetani & Bhatia (100), was to limit hepatocyte density while confining the cells to micropatterns, maintaining local high density of homotypic interactions. With hepatocyte absolute density cut by threefold, diffusion can supply a higher OCR of up to 72 nmol/(min·106 cells). These solutions to supply oxygen are especially important during the initial phase of cellular spreading, when the oxygen uptake rate is 40% to 300% higher than the value observed during the stable phase of culture (96, 101). This initial demand for oxygen makes seeding of hepatocytes in various bioreactors and microfluidic devices very challenging (53, 97). The small liquid volume in which cells are seeded stores very little oxygen, while flow cannot be used to deliver more oxygen until the cells have adhered. Therefore, many devices and reactors are seeded in a static open configuration, which is hermetically closed and perfused only after cellular adhesion (95).
Perfusion offers an alternative solution for oxygen delivery, but the relatively low oxygen-carrying capacity of culture medium requires relatively high perfusion rates of 0.1 to 0.3 mL/min to support 1 × 106 primary human hepatocytes or 3–4 × 106 HepaRG cells. At these perfusion rates, shear forces can damage hepatocytes, while any metabolite the cells produce would be extensively diluted unless the medium is rapidly reoxygenated and recirculated in the system. One solution is to add an oxygen carrier. Hepatocytes cultured in a “sandwich” of oxygen-carrying collagen showed a significant increase in albumin secretion and CYP450 activity (102). Oxygen carriers such as emulsified fluorocarbon and red blood cells have also been used in a number of bioartificial liver studies and were shown to similarly increase hepatic survival and function (103–105).
8. Impact of Culture Medium Formulation
Typical hepatocyte culture media contain much higher levels of hormones compared with human plasma. For example, insulin is used at levels approximately 104 times the physiological value. These formulations were developed in the early days of hepatocyte culture and have not received as much scrutiny as other aspects of hepatic tissue culture (106). It is likely that some of the requirements for hormonal and other supplements in the culture medium may be relaxed in newer hepatocyte culture systems, which provide more in vivo cues from complex ECM and cell–cell interactions. Data from the literature suggest that collagen-sandwiched hepatocytes can be placed in media containing physiologically relevant hormone levels—at least for a few days—in order to observe metabolic responses to stress hormones (107). Supraphysiological levels of hormones can also lead to paradoxical responses when cultured hepatocytes are placed in animal or human plasma, which is clearly a more physiologically relevant fluid than culture medium. Previous studies showed that rat hepatocytes become severely fatty and lose hepatic functions when transferred from culture medium to plasma (108) but that plasma-induced intracellular lipid accumulation can be eliminated if culture medium containing low insulin levels is used prior to exposure to plasma (109).
9. Dynamic Flow Cultures
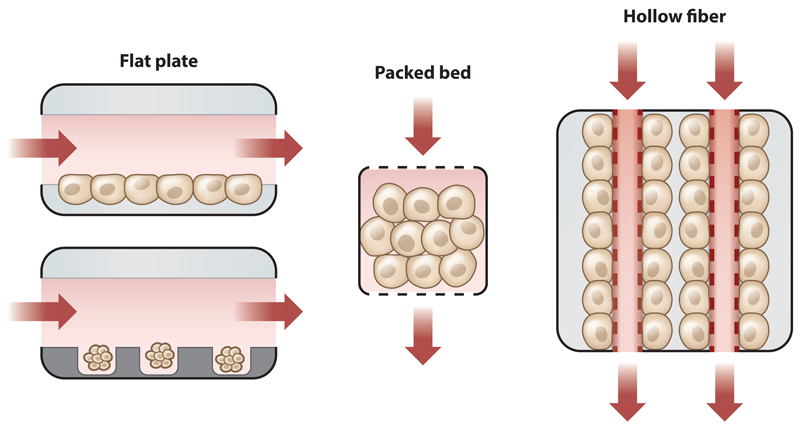
Microfluidics is an engineering field that deals with the behavior and manipulation of fluids on the small, submillimeter scale that predominates in tissues. This ability allows for the fabrication of microdevices that can potentially mimic the physiological microenvironment (110). In the liver-on-chip platform, this fluid control should enable the development of metabolic zonation, as cells can be exposed to a gradient of oxygen and hormones mimicking in vivo conditions. Notably, despite dozens of minor variations, there are only three overarching types of bioreactors (Figure 3): a flat-plate design, a packed-bed design, and a hollow-fiber design.
Figure 3.
Schematic representation of three different bioreactor types and perfusion strategies. (Left) In flat-plate bioreactors, flow crosses over cells or microtissues seeded on the bottom plate. Cells in a flat-plate design can be exposed to shear forces (e.g., HμREL, TissUSE, Hesperos) or protected in microwells (e.g., Tissue Dynamics). (Middle) In packed-bed bioreactors (e.g., CN BioInnovation), flow passes through cell aggregates. (Right) In hollow-fiber bioreactors (e.g., Charité 3D bioreactor), flow passes through fibers, while the cells are embedded between the fibers.
The flat-plate bioreactor is a simple design that can be machined without the need for microfabrication (95). Using this design, Tilles et al. (95) demonstrated that hepatocyte function is significantly reduced when cells are exposed to shear rates exceeding 5 dyn/cm2 (0.5 Pa). Although reducing shear flow limits mechanical damage, it also reduces oxygen delivery, making it difficult to culture a high density of primary hepatocytes in this design (95). One strategy is to add an oxygen carrier (e.g., emulsified fluorocarbon) to the medium, reducing the overall flow to more physiological rates of approximately 2 dyn/cm2. An alternative strategy is to protect hepatocytes from shear forces by seeding cells in microfabricated groves (111), microwells (112), or specially designed niches (113). When microwell depth is carefully matched to sinusoid length, a consumption gradient can develop within, mimicking the environment of metabolic zonation (112). Allen & Bhatia (23) showed differential PEPCK (phosphoenolpyruvate carboxykinase) and CYP2B1/2 expression consistent with metabolic zonation in flat-plate bioreactors. This consumption gradient is impossible to achieve in systems that rely on rocking motion to produce gravity-driven flow because the gradient keeps changing (114, 115).
In packed-bed bioreactors, hepatocyte aggregates are perfused in an environment that permits 3D organization (116, 117). Designs include hepatocytes cultured on polyvinyl formal resin (118), hepatocytes entrapped in alginate particles (119), or hepatocyte aggregates packed between silica beads (120). An interesting design, based on research by Linda Griffith’s lab and commercialized by CN Bio Innovations (Garden City, UK), is a microfabricated array bioreactor (121). At the heart of the bioreactor is a silicon scaffold perforated with a regular array of square holes and seated atop a microporous filter. Wells are 300 μm wide and 235 μm high, designed for sinusoid length (121, 122). Primary hepatocytes are mixed with nonparenchymal cells (Kupffer cells and sinusoidal endothelium) and self-assemble into functional micro-organs that maintain gene expression and metabolic function for weeks in culture (118–120, 123). While it is not clear that flow through packed-bed bioreactors offers a significant advantage compared with flow over microwell bioreactors (112), both designs should support the development of metabolic zonation. Unfortunately, the 3D nature of the micro-organ in both designs makes optical imaging of the hepatic aggregates difficult. Recent research by Schepers et al. (124) overcame this limitation by trapping iPSC-derived liver organoids horizontally in a 500-μm design.
Hollow-fiber bioreactors represent the most complex design, in which cells aggregate around fibers delivering oxygen and nutrients, acting as an artificial perfused vasculature. Large hollow-fiber cartridges were used in the original bioartificial liver designs (e.g., Algenix, HepatAssist, HepaLife). The most advanced cross-flow design, developed by the Gerlach group (125, 126), showed 3D organization of mouse and human liver cells. Recently, the Andersson group (127) reported a miniaturized version of a similar design. While the hollow-fiber design permits the culture of high-density tissue, it requires a significant mass of cells for initial seeding (approximately 108), making it a fairly expensive single-use device (127).
10. Current Liver-on-Chip Technology
Several liver-on-chip technologies have recently become commercially available and offer competing microdevices for the study of drug metabolism and toxicity. Static models focus mainly on maintenance of primary hepatocyte cultures, reducing the variability and time limitations related with traditional toxicity assays. For example, InSphero offers 3D InSight™ 3D spheroids composed of primary hepatocytes and Kupffer cells (80). This model allows function to be maintained for more than 28 days in vitro. Similarly, Organovo produces ExVive™, a bioprinted liver-like tissue composed of primary hepatocytes and nonparenchymal cells. This model also allows function to be maintained for more than 28 days in vitro (47). In accordance with these models, Ascendance Biotechnology (Medford, MA), recently purchased by BioIVT (Boston, MA), licensed micropatterned hepatocyte technology developed by Sangeeta Bhatia’s lab. Primary hepatocytes are patterned on 0.5-mm adhesive islands and cocultured with mouse 3T3-J2 fibroblasts (100). This model allows function to be maintained for more than 50 days in vitro.
Perfused liver-on-chip models are also widely available. HμREL (North Brunswick, NJ), founded in 2005, licensed the Shuler lab’s original human-on-chip patents as well as the Nahmias lab’s tissue culture configurations (128). The HμREL devices offer a simple flat-plate bioreactor design that uses cryopreserved primary hepatocytes and recirculates flow using an external peristaltic pump and polytetrafluoroethylene tubing to minimize nonspecific absorption, which is common in reperfusion systems. The configuration enables accurate prediction of drug clearance rates, but because of the lack of scaffolds, the hepatocytes within this system are subject to high direct shear forces. Using a microfluidic gradient generator, Kang et al. (129) extended this flat-plate design to create artificial zone-specific niches for periportal and perivenous hepatocytes with tangential flow. Emulate (Boston, MA), founded in 2013, licensed the organ-on-chip designs developed by Don Ingber’s lab and is also working on a liver-on-chip device that will have a flat-bed design in which hepatocytes and sinusoidal endothelium are confined to separate channels.
TissUse (Berlin, Germany), founded in 2010 by Uwe Marx, offers devices that support two or four different tissues in a closed-circuit perfusion device. TissUse devices circulate medium through an integrated silicone peristaltic pump. The flat-plate liver chamber is seeded with HepaRG cells (130) or preformed organoids composed of HepaRG and stellate cells (131, 132). Cells are perfused at a pumping frequency of 0.3–0.8 Hz, resulting in direct shear stress of 2.6–7 dyn/cm2. As shear stress above 2 dyn/cm2 can damage cells, loss of hepatic function is expected. Indeed, the device shows a 10-fold drop in CYP3A4 expression upon perfusion (see figure 5d of Reference 131) and albumin production of 4.8 ng/(day·106 HepaRG cells)—approximately 1,000-fold lower than expected (see figure 4b of Reference 132). Notably, the high surface area and silicone components of the microfluidic pump layer suggest that TissUse could have high levels of nonspecific absorption, making it difficult to predict the drug clearance rates of slow-metabolizing compounds such as antipyrine or carbamazepine.
CN Bio Innovations, founded in 2009, licensed several technologies, including the seminal LiverChip packed-bed design developed by the Griffith lab (133). The current design, named PhysioMimix, uses cocultures of primary hepatocytes and nonparenchymal cells in filtered microwells. Medium is continuously recirculated between two chambers through an integrated silicone peristaltic pump. Oxygen sensors are located above the cells to monitor gross changes in cellular viability. While the surface area and silicone components suggest significant nonspecific absorption, the high metabolic function and long-term function of the primary cells (134) indicate that measurement of drug clearance is possible.
Hesperos (Orlando, FL), founded in 2015 by James Hickman and Mike Shuler, is developing a pump-free multiorgan-on-chip device. The liver chamber is a flat-plate design utilizing HepG2/C3A cells. Medium is moved back and forth by tilting the device, utilizing gravity to create flow. The pump-free technology, also used by the Mimetas OrganoPlate (135), reduces operational complexity and cost by eliminating pumps but is inherently limited to slow flow rates, with a physiological shear rate of 0.25 dyn/cm2 (114). Unfortunately, oscillating flow does not support metabolic zonation, as oxygen and hormone gradients fluctuate (114, 115). In addition, low oxygen transport appears to affect HepG2/C3A survival, with 29 ± 17% of the cells dying within 14 days (114). Albumin production appears stable at approximately 1.6 μg/(day·106 cells) (see figure 2a of Reference 114).
Tissue Dynamics (Jerusalem, Israel) is a recent entry to the field, founded in 2017 by Yaakov Nahmias. The company developed a multisensor platform that enables monitoring of tissue function and physiological stress by tracking the dynamics of fuel efficiency and rate of function changes (112, 136). The current design uses cocultures of E6/E7low Upcyte hepatocytes (57) and microvascular endothelial cells in microwells. Medium is perfused on top of the wells, which protect the cells from shear forces, creating an oxygen and hormone gradient that reportedly leads to the formation of metabolic zonation (112). As medium is not recirculated, nonspecific absorption is minimized, and the organoids reach a metabolic steady state. This metabolic steady state enabled integration of the system with optical lifetime-based oxygen microprobes embedded within the organoids (137), as well as electrochemical sensors in the device outflow for glucose, lactate, and glutamine (136). The Tissue Dynamics device was first used to reveal new mechanisms of toxicity explaining the idiopathic effects of acetaminophen (137) and the idiosyncratic effect of troglitazone (112). More recently, the Nahmias group partnered with L’Oréal to show the effects of subtoxic long-term exposure to valproate, suggesting a new mechanism of action (136). Other research groups have since followed suit, integrating multiple sensors into multiorgan-on-chip platforms (138).
11. Minimum Requirements for Liver-on-Chip Microdevices
Given the complexity of the human liver, it should come as no surprise that no commercially available liver-on-chip technology can faithfully reproduce liver function. However, several companies are coming close to achieving this goal. We propose to define minimum requirements for a liver-on-chip model:
Metabolic function of source cells. The ability of an organ model to mimic native function is only as good as its source. We propose that the liver on chip should demonstrate albumin production of at least 7 μg/(day·106 cells), an OCR of at least 5 nmol/(min·106 cells), BFC metabolism of at least 5 pmol/(min·mg), and CYP3A4 induction of at least fivefold following exposure to 20 μM rifampicin. These criteria currently exclude HepG2/C3A and most hPSC-derived hepatocytes, as these cells are glycolytic rather than oxidative and show low synthetic and metabolic activity. HepaRG and Upcyte hepatocytes currently satisfy these criteria, while emerging protocols and cell lines could permit other cell populations to do so as well.
Physiological design parameters. The ability of the liver module to function depends on limiting its exposure to shear forces and on the presence of sufficient oxygen and nutrients. In general, unless hepatocytes are protected from shear in microwells (112) or traps (124), the device design must show shear forces lower than 5 dyn/cm2; setting an upper bound on flow rates. In addition, as the OCR of hepatocytes should be at least 5 nmol/(min·106 cells) and the oxygen solubility in medium is 220 nmol/mL, the device design must be able to create flow rates higher than 22 μL/(min·106 cells) defining the lower bound on flow rates in a physiological system. This lower bound to the flow rate can be relaxed if the medium contains an oxygen carrier.
Nonspecific absorption and drug clearance. Proteins, lipids, and xenobiotics adsorb to silicone and plastics, reducing their concentration as a function of time by as much as 50% in 24 h, even in the absence of cells (51). The high ratio of surface area to volume in microfluidic devices, medium recirculation, and the use of integrated silicone peristaltic pumps could rapidly eliminate hydrophobic compounds, even in the absence of cells. Thus, the ability to measure drug metabolite formation is good, but not sufficient to calculate clearance rates. To demonstrate utility in physiologically based pharmacokinetic modeling, liver-on-chip devices need to demonstrate a measurable difference in the elimination of hydrophobic drugs such as carbamazepine and antipyrine in both the presence and absence of cells.
Toxicological endpoints. Most pharmaceutical and cosmetic compounds have toxic effects at high concentration, even in the absence of physiological effect. The main question is whether a liver-on-chip device can mimic the in vivo response to the drug through similar mechanistic and toxicological endpoints. There are four major toxicological endpoints that should be demonstrated: apoptosis/necrosis, steatosis/phospholipidosis, cholestasis, and fibrosis. While specificity can vary, acetaminophen is a good model for apoptosis that can be evaluated using a terminal TUNEL (deoxynucleotidyl transferase dUTP nick-end labeling) assay. Valproate is a good model for steatosis that can be evaluated using the LipidTox (Thermo Fisher) assay. Troglitazone is a good model for cholestasis that can be evaluated through accumulation of CDFDA [5(6)-carboxy-2′,7′-dichlorofluorescein diacetate] or similar markers. Finally, methotrexate is a useful model for fibrosis induction evaluation through stellate cell activation and collagen deposition (81).
12. Conclusions
Many of the insights discussed in this review are the result of efforts to develop bioartificial liver systems (BALs) over the past 30 years (99, 139). While knowledge gained from BAL development should have been utilized by the organ-on-chip community, a generation gap and a lack of communication between clinical and engineering disciplines led to limited development (140). In this context, the recent European ban on cosmetic testing in animals invigorated the field, driving researchers, industry, and regulatory agencies to invest in the development of liver-on-chip devices. However, after studying many of the available technologies, we believe that the ability to use data generated from currently available liver-on-chip microdevices is still in question. It is not clear how reliable the information is, or how it should be utilized in physiologically based pharmacokinetic modeling or safety assessments. We hope that the protocols and recommendations described above enable companies to improve on their devices, leading to results that will allow toxicologists to identify and model mechanisms of toxicity.
Nonparenchymal Cells of the Liver.
Liver sinusoidal endothelial cells (LSECs). Liver sinusoids are composed of highly specialized microvascular endothelial cells, which play an important role in lipid metabolism, coagulation, cellular growth, differentiation, immunity, and inflammatory response. LSEC membranes contain numerous open pores called fenestra, which range from 100 to 1,000 nm in diameter and are thought to act as a sieve for blood-borne particles. LSECs are highly phagocytic scavenger cells that express major histocompatibility complex classes I and II. LSECs are usually purified from the nonparenchymal fraction of the liver by centrifugal elutriation or by two-step Percoll gradient separation. L-SIGN/CD299 is a specific marker for LSECs. Although LSECs do not proliferate in culture, a tumor cell line (SK-HEP-1) and an immortalized cell line (TMNK-1) are available. Cryopreserved liver endothelial cells are available from Creative Bioarray (Shirley, NY).
Kupffer cells. Kupffer cells are the liver-resident macrophages, thought to originate from the bone marrow. Kupffer cells ingest and degrade old erythrocytes, bacteria, and various endotoxins and play an important role in iron metabolism. Due to similarities in density and size, it is difficult to distinguish Kupffer cells from LSECs. However, relatively pure populations can be purified using centrifugal elutriation. The ED2 antibody is specific for Kupffer cells. Kupffer cells do not proliferate, and rapidly activate in culture. A mouse Kupffer cell line is available (KC13-2). Cryopreserved Kupffer cells are available from Thermo Fisher.
Hepatic stellate cells (HSCs). Stellate cells (Ito cells, fat-storing cells) are vitamin A–storing pericytes that decorate the liver’s sinusoids. HSCs are the main matrix-producing cells in the liver and play an important role in regeneration, differentiation, and inflammation. HSCs activate during liver fibrosis, increasing collagen and DNA synthesis and acquiring a myofibroblast-like phenotype. The low density of the HSCs allows for simple purification using density centrifugation. CD95 and Desmin II are specific markers for HSCs in the liver. HSCs become rapidly activated in culture. An immortalized rat liver stellate cell line (HSC-T6) is commercially available. Activated stellate cells proliferate in culture and are offered as cryopreserved cells by Zen-Bio (Research Triangle Park, NC), ScienCell (Carlsbad, CA), and iXcells Biotechnologies (San Diego, CA).
Acknowledgments
The writing of this review was supported by the European Research Council project OCLD (grant 681870), the Israeli Ministry of Health (grant 3-13971), a generous gift from the Nikoh Foundation, and L’Oréal Research and Innovation.
Footnotes
Disclosure Statement
Y.N. is the founder and chief scientific officer of Tissue Dynamics. G.O. is an employee of L’Oréal Research and Innovation.
Literature Cited
- 1.Zakim D, Boyer T. Hepatology: A Textbook of Liver Disease. Philadelphia: Saunders; 1996. [Google Scholar]
- 2.Desmet VJ. Organizational principles. In: Arias IM, editor. The Liver: Biology and Pathobiology. Philadelphia: Lippincott, Williams & Wilkins; 2001. pp. 3–15. [Google Scholar]
- 3.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–47. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 4.Mauvoisin D, Wang J, Jouffe C, Martin E, Atger F, et al. Circadian clock–dependent and –independent rhythmic proteomes implement distinct diurnal functions in mouse liver. PNAS. 2014;111:167–72. doi: 10.1073/pnas.1314066111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nedredal GI, Elvevold KH, Ytrebo LM, Olsen R, Revhaug A, Smedsrod B. Liver sinusoidal endothelial cells represent an important blood clearance system in pigs. Comp Hepatol. 2003;2:1. doi: 10.1186/1476-5926-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willekens FLA, Werre JM, Kruijt JK, Roerdinkholder-Stoelwinder B, Groenen-Döpp YAM, et al. Liver Kupffer cells rapidly remove red blood cell–derived vesicles from the circulation by scavenger receptors. Blood. 2005;105:2141–45. doi: 10.1182/blood-2004-04-1578. [DOI] [PubMed] [Google Scholar]
- 7.Behnia K, Bhatia S, Jastromb N, Balis U, Sullivan S, et al. Xenobiotic metabolism by cultured primary porcine hepatocytes. Tissue Eng. 2000;6:467–79. doi: 10.1089/107632700750022125. [DOI] [PubMed] [Google Scholar]
- 8.Grattagliano I, Portincasa P, Palmieri VO, Palasciano G. Overview on the mechanisms of druginduced liver cell death. Ann Hepatol. 2002;1:162–68. [PubMed] [Google Scholar]
- 9.Jennings P, Schwarz M, Landesmann B, Maggioni S, Goumenou M, et al. SEURAT-1 liver gold reference compounds: a mechanism-based review. Arch Toxicol. 2014;88:2099–133. doi: 10.1007/s00204-014-1410-8. [DOI] [PubMed] [Google Scholar]
- 10.Casale T, Caciari T, Rosati MV, Biagi M, De Sio S, et al. Liver function in workers exposed of the cosmetics industry. Ann Ig. 2013;25:519–27. doi: 10.7416/ai.2013.1952. [DOI] [PubMed] [Google Scholar]
- 11.Martignoni M, Groothuis GM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol. 2006;2:875–94. doi: 10.1517/17425255.2.6.875. [DOI] [PubMed] [Google Scholar]
- 12.Blais EM, Rawls KD, Dougherty BV, Li ZI, Kolling GL, et al. Reconciled rat and human metabolic networks for comparative toxicogenomics and biomarker predictions. Nat Commun. 2017;8 doi: 10.1038/ncomms14250. 14250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Parliament, Council of the European Union. Regulation (EC) no. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off J Eur Union. 2009;342:59–209. [Google Scholar]
- 14.Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284:1998–2003. doi: 10.1126/science.284.5422.1998. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, et al. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 16.Lemaigre F, Zaret KS. Liver development update: new embryo models, cell lineage control, and morphogenesis. Curr Opin Genet Dev. 2004;14:582–90. doi: 10.1016/j.gde.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Sosa-Pineda B, Wigle JT, Oliver G. Hepatocyte migration during liver development requires Prox1. Nat Genet. 2000;25:254–55. doi: 10.1038/76996. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;297:559–63. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 19.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–67. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 20.McCuskey RS, Ekataksin W, LeBouton AV, Nishida J, McCuskey MK, et al. Hepatic microvascular development in relation to the morphogenesis of hepatocellular plates in neonatal rats. Anat Rec A. 2003;275:1019–30. doi: 10.1002/ar.a.10117. [DOI] [PubMed] [Google Scholar]
- 21.Nahmias Y, Kramvis Y, Barbe L, Casali M, Berthiaume F, Yarmush ML. A novel formulation of oxygen-carrying matrix enhances liver-specific function of cultured hepatocytes. FASEB J. 2006;20:2531–33. doi: 10.1096/fj.06-6192fje. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Hernandez A, Amenta PS. The extracellular matrix in hepatic regeneration. FASEB J. 1995;9:1401–10. doi: 10.1096/fasebj.9.14.7589981. [DOI] [PubMed] [Google Scholar]
- 23.Allen JW, Bhatia SN. Formation of steady-state oxygen gradients in vitro: application to liver zonation. Biotechnol Bioeng. 2003;82:253–62. doi: 10.1002/bit.10569. [DOI] [PubMed] [Google Scholar]
- 24.Planas-Paz L, Orsini V, Boulter L, Calabrese D, Pikiolek M, et al. The RSPO-LGR4/5-ZNRF3/RNF43 module controls liver zonation and size. Nat Cell Biol. 2016;18:467–79. doi: 10.1038/ncb3337. [DOI] [PubMed] [Google Scholar]
- 25.Haussinger D, Lamers WH, Moorman AF. Hepatocyte heterogeneity in the metabolism of amino acids and ammonia. Enzyme. 1992;46:72–93. doi: 10.1159/000468779. [DOI] [PubMed] [Google Scholar]
- 26.Jungermann K. Role of intralobular compartmentation in hepatic metabolism. Diabetes Metab. 1992;18:81–86. [PubMed] [Google Scholar]
- 27.Reid LM, Fiorino AS, Sigal SH, Brill S, Holst PA. Extracellular matrix gradients in the space of Disse: relevance to liver biology. Hepatology. 1992;15:1198–203. doi: 10.1002/hep.1840150635. [DOI] [PubMed] [Google Scholar]
- 28.Bouwens L, Bleser PD, Vanderkerken K, Geerts B, Wisse E. Liver cell heterogeneity: functions of non-parenchymal cells. Enzyme. 1992;46:155–68. doi: 10.1159/000468782. [DOI] [PubMed] [Google Scholar]
- 29.Braet F, Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol. 2002;1:1. doi: 10.1186/1476-5926-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morin O, Goulet F, Normand C. Liver sinusoidal endothelial cells: isolation, purification, characterization and interaction with hepatocytes. Revis Biol Cel. 1988;15:1–85. [PubMed] [Google Scholar]
- 31.Nguyen-Lefebvre AT, Horuzsko A. Kupffer cell metabolism and function. J Enzymol Metab. 2015;1:101. [PMC free article] [PubMed] [Google Scholar]
- 32.Bellan M, Castello LM, Pirisi M. Candidate biomarkers of liver fibrosis: a concise, pathophysiology-oriented review. J Clin Transl Hepatol. 2018;6:317–25. doi: 10.14218/JCTH.2018.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gores GJ, Kost LJ, LaRusso NF. The isolated perfused rat liver: conceptual and practical considerations. Hepatology. 1986;6:511–17. doi: 10.1002/hep.1840060331. [DOI] [PubMed] [Google Scholar]
- 34.Lee K, Berthiaume F, Stephanopoulos GN, Yarmush DM, Yarmush ML. Metabolic flux analysis of postburn hepatic hypermetabolism. Metab Eng. 2000;2:312–27. doi: 10.1006/mben.2000.0160. [DOI] [PubMed] [Google Scholar]
- 35.Tavill AS, East AG, Black EG, Nadkarni D, Hoffenberg E. Regulatory factors in the synthesis of plasma proteins by the isolated perfused rat liver. Ciba Found Symp. 1972;9:155–79. doi: 10.1002/9780470719923.ch9. [DOI] [PubMed] [Google Scholar]
- 36.Palmen NG, Evelo CT, Borm PJ, Henderson PT. Toxicokinetics of dimethylacetamide (DMAc) in rat isolated perfused liver. Hum Exp Toxicol. 1993;12:127–33. doi: 10.1177/096032719301200206. [DOI] [PubMed] [Google Scholar]
- 37.McKindley DS, Chichester C, Raymond R. Effect of endotoxin shock on the clearance of lidocaine and indocyanine green in the perfused rat liver. Shock. 1999;12:468–72. doi: 10.1097/00024382-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Gordon AH, Humphrey JH. Methods for measuring rates of synthesis of albumin by the isolated perfused rat liver. Biochem J. 1960;75:240–47. doi: 10.1042/bj0750240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burke WT. Urea synthesis in the isolated perfused rat liver. Biochem Biophys Res Commun. 1960;3:525–30. doi: 10.1016/0006-291x(60)90168-6. [DOI] [PubMed] [Google Scholar]
- 40.Zhao P, Kalhorn TF, Slattery JT. Selective mitochondrial glutathione depletion by ethanol enhances acetaminophen toxicity in rat liver. Hepatology. 2002;36:326–35. doi: 10.1053/jhep.2002.34943. [DOI] [PubMed] [Google Scholar]
- 41.Martin H, Sarsat JP, Lerche-Langrand C, Housset C, Balladur P, et al. Morphological and biochemical integrity of human liver slices in long-term culture: effects of oxygen tension. Cell Biol Toxicol. 2002;18:73–85. doi: 10.1023/a:1015379815897. [DOI] [PubMed] [Google Scholar]
- 42.Fisher RL, Ulreich JB, Nakazato PZ, Brendel K. Histological and biochemical evaluation of precision-cut liver slices. Toxicol Methods. 2001;11:59–79. [Google Scholar]
- 43.Lerche-Langrand C, Toutain HJ. Precision-cut liver slices: characteristics and use for in vitro pharmaco-toxicology. Toxicology. 2000;153:221–53. doi: 10.1016/s0300-483x(00)00316-4. [DOI] [PubMed] [Google Scholar]
- 44.van Midwoud PM, Merema MT, Verpoorte E, Groothuis GMM. Microfluidics enables small-scale tissue-based drug metabolism studies with scarce human tissue. J Assoc Lab Autom. 2011;16:468–76. doi: 10.1016/j.jala.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Schumacher K, Khong Y-M, Chang S, Ni J, Sun W, Yu H. Perfusion culture improves the maintenance of cultured liver tissue slices. Tissue Eng. 2007;13:197–205. doi: 10.1089/ten.2006.0046. [DOI] [PubMed] [Google Scholar]
- 46.Norona LM, Nguyen DG, Gerber DA, Presnell SC, LeCluyse EL. Editor’s highlight: Modeling compound-induced fibrogenesis in vitro using three-dimensional bioprinted human liver tissues. Toxicol Sci. 2016;154:354–67. doi: 10.1093/toxsci/kfw169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen DG, Funk J, Robbins JB, Crogan-Grundy C, Presnell SC, et al. Bioprinted 3D primary liver tissues allow assessment of organ-level response to clinical drug induced toxicity in vitro. PLOS ONE. 2016;11:e0158674. doi: 10.1371/journal.pone.0158674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma X, Qu X, Zhu W, Li Y-S, Yuan S, et al. Deterministically patterned biomimetic human iPSC–derived hepatic model via rapid 3D bioprinting. PNAS. 2016;113:2206–11. doi: 10.1073/pnas.1524510113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 50.Strain AJ. Isolated hepatocytes: use in experimental and clinical hepatology. Gut. 1994;35:433–36. doi: 10.1136/gut.35.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kidambi S, Yarmush R, Novik E, Chao PB, Yarmush ML, Nahmias Y. Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. PNAS. 2009;106:15714–19. doi: 10.1073/pnas.0906820106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, et al. Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Il2rg−/− mice. Nat Biotechnol. 2007;25:903–10. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yarmush ML, Toner M, Dunn JC, Rotem A, Hubel A, Tompkins RG. Hepatic tissue engineering. Development of critical technologies. Ann N Y Acad Sci. 1992;665:238–52. doi: 10.1111/j.1749-6632.1992.tb42588.x. [DOI] [PubMed] [Google Scholar]
- 54.Hamilton GA, Westmorel C, George AE. Effects of medium composition on the morphology and function of rat hepatocytes cultured as spheroids and monolayers. In Vitro Cell Dev Biol Anim. 2001;37:656–67. doi: 10.1290/1071-2690(2001)037<0656:eomcot>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 55.Gripon P, Rumin S, Urban S, Le Seyec J, Glaise D, et al. Infection of a human hepatoma cell line by hepatitis B virus. PNAS. 2002;99:15655–60. doi: 10.1073/pnas.232137699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anthérieu S, Chesné C, Li R, Camus S, Lahoz A, et al. Stable expression, activity, and inducibility of cytochromes P450 in differentiated HepaRG cells. Drug Metab Dispos. 2010;38:516–25. doi: 10.1124/dmd.109.030197. [DOI] [PubMed] [Google Scholar]
- 57.Levy G, Bomze D, Heinz S, Ramachandran SD, Noerenberg A, et al. Long-term culture and expansion of primary human hepatocytes. Nat Biotechnol. 2015;33:1264–71. doi: 10.1038/nbt.3377. [DOI] [PubMed] [Google Scholar]
- 58.Avior Y, Levy G, Zimerman M, Kitsberg D, Schwartz R, et al. Microbial-derived lithocholic acid and vitamin K2 drive the metabolic maturation of pluripotent stem cells–derived and fetal hepatocytes. Hepatology. 2015;62:265–78. doi: 10.1002/hep.27803. [DOI] [PubMed] [Google Scholar]
- 59.Cameron K, Tan R, Schmidt-Heck W, Campos G, Lyall Marcus J, et al. Recombinant laminins drive the differentiation and self-organization of hESC-derived hepatocytes. Stem Cell Rep. 2016;5:1250–62. doi: 10.1016/j.stemcr.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dunn JC, Tompkins RG, Yarmush ML. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991;7:237–45. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- 61.Dunn JC, Tompkins RG, Yarmush ML. Hepatocytes in collagen sandwich: evidence for transcriptional and translational regulation. J Cell Biol. 1992;116:1043–53. doi: 10.1083/jcb.116.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dunn JC, Yarmush ML, Koebe HG, Tompkins RG. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J. 1989;3:174–77. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- 63.Berthiaume F, Moghe PV, Toner M, Yarmush ML. Effect of extracellular matrix topology on cell structure, function, and physiological responsiveness: hepatocytes cultured in a sandwich configuration. FASEB J. 1996;10:1471–84. doi: 10.1096/fasebj.10.13.8940293. [DOI] [PubMed] [Google Scholar]
- 64.Moghe PV, Berthiaume F, Ezzell RM, Toner M, Tompkins RG, Yarmush ML. Culture matrix configuration and composition in the maintenance of hepatocyte polarity and function. Biomaterials. 1996;17:373–85. doi: 10.1016/0142-9612(96)85576-1. [DOI] [PubMed] [Google Scholar]
- 65.Moghe PV, Coger RN, Toner M, Yarmush ML. Cell–cell interactions are essential for maintenance of hepatocyte function in collagen gel but not on Matrigel. Biotechnol Bioeng. 1997;56:706–11. doi: 10.1002/(SICI)1097-0290(19971220)56:6<706::AID-BIT14>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 66.Schuetz EG, Li D, Omiecinski CJ, Muller-Eberhard U, Kleinman HK, et al. Regulation of gene expression in adult rat hepatocytes cultured on a basement membrane matrix. J Cell Physiol. 1988;134:309–23. doi: 10.1002/jcp.1041340302. [DOI] [PubMed] [Google Scholar]
- 67.Koide N, Shinji T, Tanabe T, Asano K, Kawaguchi M, et al. Continued high albumin production by multicellular spheroids of adult rat hepatocytes formed in the presence of liver-derived proteoglycans. Biochem Biophys Res Commun. 1989;161:385–91. doi: 10.1016/0006-291x(89)91609-4. [DOI] [PubMed] [Google Scholar]
- 68.Peshwa MV, Wu FJ, Sharp HL, Cerra FB, Hu W-S. Mechanistics of formation and ultrastructural evaluation of hepatocyte spheroids. In Vitro Cell Dev Biol Anim. 1996;32:197–203. doi: 10.1007/BF02722946. [DOI] [PubMed] [Google Scholar]
- 69.Abu-Absi SF, Friend JR, Hansen LK, Hu W-S. Structural polarity and functional bile canaliculi in rat hepatocyte spheroids. Exp Cell Res. 2002;274:56–67. doi: 10.1006/excr.2001.5467. [DOI] [PubMed] [Google Scholar]
- 70.Wu FJ, Friend JR, Remmel RP, Cerra FB, Hu W-S. Enhanced cytochrome P450 IA1 activity of self-assembled rat hepatocyte spheroids. Cell Transplant. 1999;8:233–46. doi: 10.1177/096368979900800304. [DOI] [PubMed] [Google Scholar]
- 71.Landry J, Bernier D, Ouellet C, Goyette R, Marceau N. Spheroidal aggregate culture of rat liver cells: histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J Cell Biol. 1985;101:914–23. doi: 10.1083/jcb.101.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Messner S, Agarkova I, Moritz W, Kelm JM. Multi–cell type human liver microtissues for hepatotoxicity testing. Arch Toxicol. 2013;87:209–13. doi: 10.1007/s00204-012-0968-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nahmias Y, Schwartz RE, Wei-Shou H, Verfaillie CM, Odde DJ. Endothelium-mediated hepatocyte recruitment in the establishment of liver-like tissue in vitro. Tissue Eng. 2006;12:1627–38. doi: 10.1089/ten.2006.12.1627. [DOI] [PubMed] [Google Scholar]
- 74.Neyrinck A, Eeckhoudt SL, Meunier CJ, Pampfer S, Taper HS, et al. Modulation of paracetamol metabolism by Kupffer cells: a study on rat liver slices. Life Sci. 1999;65:2851–59. doi: 10.1016/s0024-3205(99)00554-8. [DOI] [PubMed] [Google Scholar]
- 75.Jaeschke H, Farhood A. Neutrophil and Kupffer cell–induced oxidant stress and ischemia– reperfusion injury in rat liver. Am J Physiol Gastrointest Liver Physiol. 1991;260:G355–62. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 76.James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31:1499–506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- 77.Hoebe KH, Witkamp RF, Fink-Gremmels J, Miert ASV, Monshouwer M. Direct cell-to-cell contact between Kupffer cells and hepatocytes augments endotoxin-induced hepatic injury. Am J Physiol Gastrointest Liver Physiol. 2001;280:G720–28. doi: 10.1152/ajpgi.2001.280.4.G720. [DOI] [PubMed] [Google Scholar]
- 78.Milosevic N, Schawalder H, Maier P. Kupffer cell–mediated differential down-regulation of cytochrome P450 metabolism in rat hepatocytes. Eur J Pharmacol. 1999;368:75–87. doi: 10.1016/s0014-2999(98)00988-1. [DOI] [PubMed] [Google Scholar]
- 79.Nguyen TV, Ukairo O, Khetani SR, McVay M, Kanchagar C, et al. Establishment of a hepatocyte– Kupffer cell coculture model for assessment of proinflammatory cytokine effects on metabolizing enzymes and drug transporters. Drug Metab Dispos. 2015;43:774–85. doi: 10.1124/dmd.114.061317. [DOI] [PubMed] [Google Scholar]
- 80.Leite SB, Roosens T, El Taghdouini A, Mannaerts I, Smout AJ, et al. Novel human hepatic organoid model enables testing of drug-induced liver fibrosis in vitro. Biomaterials. 2016;78:1–10. doi: 10.1016/j.biomaterials.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 81.Coll M, Perea L, Boon R, Leite SB, Vallverdu J, et al. Generation of hepatic stellate cells from human pluripotent stem cells enables in vitro modeling of liver fibrosis. Cell Stem Cell. 2018;23:101–13. doi: 10.1016/j.stem.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 82.Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell–cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13:1883–900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 83.Khetani SR, Szulgit G, Rio JAD, Barlow C, Bhatia SN. Exploring interactions between rat hepatocytes and nonparenchymal cells using gene expression profiling. Hepatology. 2004;40:545–54. doi: 10.1002/hep.20351. [DOI] [PubMed] [Google Scholar]
- 84.Cho CH, Berthiaume F, Tilles AW, Yarmush ML. A new technique for primary hepatocyte expansion in vitro. Biotechnol Bioeng. 2008;101:345–56. doi: 10.1002/bit.21911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shan J, Schwartz RE, Ross NT, Logan DJ, Thomas D, et al. Identification of small molecules for human hepatocyte expansion and iPS differentiation. Nat Chem Biol. 2013;9:514–20. doi: 10.1038/nchembio.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nahmias Y, Casali M, Barbe L, Berthiaume F, Yarmush ML. Liver endothelial cells promote LDLR expression and the uptake of HCV-like particles in primary rat and human hepatocytes. Hepatology. 2006;43:257–65. doi: 10.1002/hep.21016. [DOI] [PubMed] [Google Scholar]
- 87.Morin O, Normand C. Long-term maintenance of hepatocyte functional activity in co-culture: requirements for sinusoidal endothelial cells and dexamethasone. J Cell Physiol. 1986;129:103–10. doi: 10.1002/jcp.1041290115. [DOI] [PubMed] [Google Scholar]
- 88.Goulet F, Normand C, Morin O. Cellular interactions promote tissue-specific function, biomatrix deposition and junctional communication of primary cultured hepatocytes. Hepatology. 1988;8:1010–18. doi: 10.1002/hep.1840080506. [DOI] [PubMed] [Google Scholar]
- 89.LeCouter J, Moritz DR, Li B, Phillips GL, Liang XH, et al. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299:890–93. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- 90.Nahmias YK, Odde DJ. Analysis of radiation forces in laser trapping and laser-guided direct writing applications. IEEE J Quantum Electron. 2002;38:131–41. [Google Scholar]
- 91.Nahmias YK, Gao BZ, Odde DJ. Dimensionless parameters for the design of optical traps and laser guidance systems. Appl Opt. 2004;43:3999–4006. doi: 10.1364/ao.43.003999. [DOI] [PubMed] [Google Scholar]
- 92.Nahmias Y, Schwartz RE, Verfaillie CM, Odde DJ. Laser-guided direct writing for threedimensional tissue engineering. Biotechnol Bioeng. 2005;92:129–36. doi: 10.1002/bit.20585. [DOI] [PubMed] [Google Scholar]
- 93.Yamamoto H, Takada T, Yamanashi Y, Ogura M, Masuo Y, et al. VLDL/LDL acts as a drug carrier and regulates the transport and metabolism of drugs in the body. Sci Rep. 2017;7 doi: 10.1038/s41598-017-00685-9. 633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Simon N, Dailly E, Combes O, Malaurie E, Lemaire M, et al. Role of lipoproteins in the plasma binding of SDZ PSC 833, a novel multidrug resistance–reversing cyclosporin. Br J Clin Pharmacol. 1998;45:173–75. doi: 10.1046/j.1365-2125.1998.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tilles AW, Baskaran H, Roy P, Yarmush ML, Toner M. Effects of oxygenation and flow on the viability and function of rat hepatocytes cocultured in a microchannel flat-plate bioreactor. Biotechnol Bioeng. 2001;73:379–89. doi: 10.1002/bit.1071. [DOI] [PubMed] [Google Scholar]
- 96.Balis UJ, Behnia K, Dwarakanath B, Bhatia SN, Sullivan SJ, et al. Oxygen consumption characteristics of porcine hepatocytes. Metab Eng. 1999;1:49–62. doi: 10.1006/mben.1998.0105. [DOI] [PubMed] [Google Scholar]
- 97.Foy BD, Rotem A, Toner M, Tompkins RG, Yarmush ML. A device to measure the oxygen uptake rate of attached cells: importance in bioartificial organ design. Cell Transplant. 1994;3:515–27. doi: 10.1177/096368979400300609. [DOI] [PubMed] [Google Scholar]
- 98.Lemasters JJ. Hypoxic, ischemic, and reperfusion injury to liver. In: Arias IM, editor. The Liver: Biology and Pathobiology. Philadelphia: Lippincott, Williams & Wilkins; 2001. pp. 257–79. [Google Scholar]
- 99.Yarmush ML, Toner M, Dunn JCY, Rotem A, Hubel A, Tompkins RG. Hepatic tissue engineering: development of critical technologies. Ann N Y Acad Sci. 1992;665:238–52. doi: 10.1111/j.1749-6632.1992.tb42588.x. [DOI] [PubMed] [Google Scholar]
- 100.Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat Biotechnol. 2007;26:120–26. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 101.Rotem A, Toner M, Bhatia S, Foy BD, Tompkins RG, Yarmush ML. Oxygen is a factor determining in vitro tissue assembly: effects on attachment and spreading of hepatocytes. Biotechnol Bioeng. 1994;43:654–60. doi: 10.1002/bit.260430715. [DOI] [PubMed] [Google Scholar]
- 102.Nahmias Y, Casali M, Barbe L, Berthiaume F, Yarmush ML. A novel formulation of oxygen-carrying matrix enhances liver specific function of cultured hepatocytes. FASEB J. 2006;20:2531–53. doi: 10.1096/fj.06-6192fje. [DOI] [PubMed] [Google Scholar]
- 103.King AT, Mulligan BJ, Lowe KC. Perfluorochemicals and cell culture. Nat Biotechnol. 1989;7:1037–42. [Google Scholar]
- 104.Gordon JE, Dare MR, Palmer AF. Engineering select physical properties of cross-linked red blood cells and a simple a priori estimation of their efficacy as an oxygen delivery vehicle within the context of a hepatic hollow fiber bioreactor. Biotechnol Prog. 2005;21:1700–7. doi: 10.1021/bp050204p. [DOI] [PubMed] [Google Scholar]
- 105.Rappaport C, Rensch Y, Abbasi M, Kempe M, Rocaboy C, et al. New perfluorocarbon system for multilayer growth of anchorage-dependent mammalian cells. Biotechniques. 2002;32:142–51. doi: 10.2144/02321rr03. [DOI] [PubMed] [Google Scholar]
- 106.Williams GM, Bermudez E, Scaramuzzino D. Rat hepatocyte primary cell cultures. III. Improved dissociation and attachment techniques and the enhancement of survival by culture medium. In Vitro. 1977;13:809–17. doi: 10.1007/BF02615128. [DOI] [PubMed] [Google Scholar]
- 107.Zupke CA, Stefanovich P, Berthiaume F, Yarmush ML. Metabolic effects of stress mediators on cultured hepatocytes. Biotechnol Bioeng. 1998;58:222–30. [PubMed] [Google Scholar]
- 108.Matthew HWT, Sternberg J, Stefanovich P, Morgan JR, Toner M, et al. Effects of plasma exposure on cultured hepatocytes: implications for bioartificial liver support. Biotechnol Bioeng. 1996;51:100–11. doi: 10.1002/(SICI)1097-0290(19960705)51:1<100::AID-BIT12>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 109.Chan C, Berthiaume F, Washizu J, Toner M, Yarmush ML. Metabolic pre-conditioning of cultured cells in physiological levels of insulin: generating resistance to the lipid-accumulating effects of plasma in hepatocytes. Biotechnol Bioeng. 2002;78:753–60. doi: 10.1002/bit.10275. [DOI] [PubMed] [Google Scholar]
- 110.Andersson H, van den Berg A. Microfabrication and microfluidics for tissue engineering: state of the art and future opportunities. Lab Chip. 2004;4:98–103. doi: 10.1039/b314469k. [DOI] [PubMed] [Google Scholar]
- 111.Park J, Berthiaume F, Toner M, Yarmush ML, Tilles AW. Microfabricated grooved substrates as platforms for bioartificial liver reactors. Biotechnol Bioeng. 2005;90:632–44. doi: 10.1002/bit.20463. [DOI] [PubMed] [Google Scholar]
- 112.Bavli D, Prill S, Ezra E, Levy G, Cohen M, et al. Real-time monitoring of metabolic function in liver-on-chip microdevices tracks the dynamics of mitochondrial dysfunction. PNAS. 2016;113:E2231–40. doi: 10.1073/pnas.1522556113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ledezma GA, Folch A, Bhatia SN, Yarmush ML, Toner M. Numerical model of fluid flow and oxygen transport in a radial-flow microchannel containing hepatocytes. J Biomech Eng. 1999;121:58–64. doi: 10.1115/1.2798043. [DOI] [PubMed] [Google Scholar]
- 114.Oleaga C, Bernabini C, Smith AST, Srinivasan B, Jackson M, et al. Multi-organ toxicity demonstration in a functional human in vitro system composed of four organs. Sci Rep. 2016;6 doi: 10.1038/srep20030. 20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen HJ, Miller P, Shuler ML. A pumpless body-on-a-chip model using a primary culture of human intestinal cells and a 3D culture of liver cells. Lab Chip. 2018;18:2036–46. doi: 10.1039/c8lc00111a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Strain AJ, Neuberger JM. A bioartificial liver—state of the art. Science. 2002;295:1005–9. doi: 10.1126/science.1068660. [DOI] [PubMed] [Google Scholar]
- 117.Griffith LG, Naughton G. Tissue engineering—current challenges and expanding opportunities. Science. 2002;295:1009–16. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 118.Ohshima N, Yanagi K, Miyoshi H. Packed-bed type reactor to attain high density culture of hepatocytes for use as a bioartificial liver. Artif Organs. 1997;21:1169–76. doi: 10.1111/j.1525-1594.1997.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 119.Murtas S, Capuani G, Dentini M, Manetti C, Masci G, et al. Alginate beads as immobilization matrix for hepatocytes perfused in a bioreactor: a physico-chemical characterization. J Biomater Sci Polym Ed. 2005;16:829–46. doi: 10.1163/1568562054255718. [DOI] [PubMed] [Google Scholar]
- 120.Li AP, Barker G, Beck D, Colburn S, Monsell R, Pellegrin C. Culturing of primary hepatocytes as entrapped aggregates in a packed bed bioreactor: a potential bioartificial liver. In Vitro Cell Dev Biol. 1993;29:A249–54. doi: 10.1007/BF02634192. [DOI] [PubMed] [Google Scholar]
- 121.Powers MJ, Domansky K, Kaazempur-Mofrad MR, Kalezi A, Capitano A, et al. A microfabricated array bioreactor for perfused 3D liver culture. Biotechnol Bioeng. 2002;78:257–69. doi: 10.1002/bit.10143. [DOI] [PubMed] [Google Scholar]
- 122.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–24. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 123.Powers MJ, Janigian DM, Wack KE, Baker CS, Stolz DB, Griffith LG. Functional behavior of primary rat liver cells in a three-dimensional perfused microarray bioreactor. Tissue Eng. 2002;8:499–513. doi: 10.1089/107632702760184745. [DOI] [PubMed] [Google Scholar]
- 124.Schepers A, Li C, Chhabra A, Seney BT, Bhatia S. Engineering a perfusable 3D human liver platform from iPS cells. Lab Chip. 2016;16:2644–53. doi: 10.1039/c6lc00598e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Monga SPS, Hout MS, Baun MJ, Micsenyi A, Muller P, et al. Mouse fetal liver cells in artificial capillary beds in three-dimensional four-compartment bioreactors. Am J Pathol. 2005;167:1279–92. doi: 10.1016/S0002-9440(10)61215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gerlach JC, Mutig K, Sauer IM, Schrade P, Efimova E, et al. Use of primary human liver cells originating from discarded grafts in a bioreactor for liver support therapy and the prospects of culturing adult liver stem cells in bioreactors: a morphologic study. Transplantation. 2003;76:781–86. doi: 10.1097/01.TP.0000083319.36931.32. [DOI] [PubMed] [Google Scholar]
- 127.Zeilinger K, Schreiter T, Darnell M, Söderdahl T, Lübberstedt M, et al. Scaling down of a clinical three-dimensional perfusion multicompartment hollow fiber liver bioreactor developed for extracorporeal liver support to an analytical scale device useful for hepatic pharmacological in vitro studies. Tissue Eng C. 2011;17:549–56. doi: 10.1089/ten.TEC.2010.0580. [DOI] [PubMed] [Google Scholar]
- 128.Kidambi S, Yarmush RS, Novik E, Chao P, Yarmush ML, Nahmias Y. Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. PNAS. 2009;106:15714–19. doi: 10.1073/pnas.0906820106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kang YB, Eo J, Mert S, Yarmush ML, Usta OB. Metabolic patterning on a chip: towards in vitro liver zonation of primary rat and human hepatocytes. Sci Rep. 2018;8 doi: 10.1038/s41598-018-27179-6. 8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guillouzo A, Corlu A, Aninat C, Glaise D, Morel F, Guguen-Guillouzo C. The human hepatoma HepaRG cells: a highly differentiated model for studies of liver metabolism and toxicity of xenobiotics. Chem Biol Interact. 2007;168:66–73. doi: 10.1016/j.cbi.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 131.Maschmeyer I, Lorenz AK, Schimek K, Hasenberg T, Ramme AP, et al. A four-organ chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip. 2015;15:2688–99. doi: 10.1039/c5lc00392j. [DOI] [PubMed] [Google Scholar]
- 132.Bauer S, Wennberg Huldt C, Kanebratt KP, Durieux I, Gunne D, et al. Functional coupling of human pancreatic islets and liver spheroids on-a-chip: towards a novel human ex vivo type 2 diabetes model. Sci Rep. 2017;7 doi: 10.1038/s41598-017-14815-w. 14620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Domansky K, Inman W, Serdy J, Dash A, Lim MH, Griffith LG. Perfused multiwell plate for 3D liver tissue engineering. Lab Chip. 2010;10:51–58. doi: 10.1039/b913221j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Long TJ, Cosgrove PA, Dunn RT, Stolz DB, Hamadeh H, et al. Modeling therapeutic antibody–small molecule drug–drug interactions using a three-dimensional perfusable human liver coculture platform. Drug Metab Dispos. 2016;44:1940–48. doi: 10.1124/dmd.116.071456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jang M, Neuzil P, Volk T, Manz A, Kleber A. On-chip three-dimensional cell culture in phaseguides improves hepatocyte functions in vitro. Biomicrofluidics. 2015;9 doi: 10.1063/1.4922863. 034113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ehrlich A, Tsytkin-Kirschenzweig S, Ioannidis K, Ayyash M, Riu A, et al. Microphysiological flux balance platform unravels the dynamics of drug induced steatosis. Lab Chip. 2018;18:2510–22. doi: 10.1039/c8lc00357b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Prill S, Bavli D, Levy G, Ezra E, Schmälzlin E, et al. Real-time monitoring of oxygen uptake in hepatic bioreactor shows CYP450-independent mitochondrial toxicity of acetaminophen and amiodarone. Arch Toxicol. 2016;90:1181–91. doi: 10.1007/s00204-015-1537-2. [DOI] [PubMed] [Google Scholar]
- 138.Zhang YS, Aleman J, Shin SR, Kilic T, Kim D, et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. PNAS. 2017;114:E2293–302. doi: 10.1073/pnas.1612906114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tsiaoussis J, Newsome PN, Nelson LJ, Hayes PC, Plevris JN. Which hepatocyte will it be? Hepatocyte choice for bioartifical liver support systems. Liver Transplant. 2001;7:2–10. doi: 10.1053/jlts.2001.20845. [DOI] [PubMed] [Google Scholar]
- 140.Ebrahimkhani MR, Neiman JAS, Raredon MSB, Hughes DJ, Griffith LG. Bioreactor technologies to support liver function in vitro. Adv Drug Deliv Rev. 2014;69/70:132–57. doi: 10.1016/j.addr.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]