Abstract
Introduction
SYSVAC is an online bibliographic database of systematic reviews and systematic review protocols on vaccines and immunisation compiled by the London School of Hygiene & Tropical Medicine and hosted by the World Health Organization (WHO) through their National Immunization Technical Advisory Groups (NITAG) resource centre (www.nitag-resource.org). Here the development of the database and a bibliometric review of its content is presented, describing trends in the publication of policy-relevant systematic reviews on vaccines and immunisation from 2008 to 2016.
Materials and methods
Searches were conducted in seven scientific databases according to a standardized search protocol, initially in 2014 with the most recent update in January 2017. Abstracts and titles were screened according to specific inclusion criteria. All included publications were coded into relevant categories based on a standardized protocol and subsequently analysed to look at trends in time, topic, area of focus, population and geographic location.
Results
After screening for inclusion criteria, 1285 systematic reviews were included in the database. While in 2008 there were only 34 systematic reviews on a vaccine-related topic, this increased to 322 in 2016. The most frequent pathogens/diseases studied were influenza, human papillomavirus and pneumococcus. There were several areas of duplication and overlap.
Discussion
As more systematic reviews are published it becomes increasingly time-consuming for decision-makers to identify relevant information among the ever-increasing volume available. The risk of duplication also increases, particularly given the current lack of coordination of systematic reviews on vaccine-related questions, both in terms of their commissioning and their execution. The SYSVAC database offers an accessible catalogue of vaccine-relevant systematic reviews with, where possible access or a link to the full-text.
Conclusions
SYSVAC provides a freely searchable platform to identify existing vaccine-policy-relevant systematic reviews. Systematic reviews will need to be assessed adequately for each specific question and quality.
Keywords: Vaccine, Immunization, Systematic review, Bibliometric
1. Introduction
The global landscape of immunisation has changed considerably during the past two decades. New and considerably more expensive vaccines are becoming increasingly available in high-income countries (HIC) while adoption patterns are accelerating in low- and middle-income countries (LMIC). In LMIC this has been aided by substantial donor support, such as funds from Gavi, the Vaccine Alliance, for both strengthening the Expanded Programme for Immunisation (EPI) and for adopting new and underutilised vaccines [1]. However, decision-makers in both HIC and LMIC face an array of questions about which vaccines to prioritise given their limited budgets. WHO recommends that national vaccine policy is guided by National Immunisation Technical Advisory Groups (NITAGs) [2]. However, NITAGs also face difficulties in assimilating an ever-increasing amount of information. Hence, the need for collating and synthesising the available evidence to support decision-making in vaccine-related policy.
During the past decade, the number of scientific research articles and systematic reviews on vaccines has risen substantially. Consequently, there is a need for tools to filter this evidence and present it on an accessible platform. Systematic reviews are a particularly efficient means of summarising evidence for decision-makers because they use clear, transparent methods for combining evidence from multiple studies. This means decision-makers do not need to identify, appraise and synthesise findings from numerous individual studies themselves [3]. Systematic reviews aim to answer specific questions in order to minimise bias and present pre-filtered evidence for researchers and decision-makers [4,5].
At present, systematic reviews on vaccine-related questions are not coordinated, either in terms of commissioning or dissemination. Unless decision-makers specifically commission a review, there is currently no process to ensure that proposed systematic review topics respond to their information needs, which may differ from one decision-maker to another. This not only leads to gaps in knowledge if particular questions are neglected, but also to duplication and overlap. Therefore, many NITAGs commission reviews to inform them, which leads to duplication [6–11]. At present, there is no common understanding of what vaccine-relevant systematic reviews have, or have not, been conducted. It is therefore unclear where duplication is a risk, or which areas have been neglected. Ideally, NITAGs should be able to ensure prior to commissioning that no similar reviews are planned, ongoing or have been published.
To date there is no singe repository where decision-makers can find systematic reviews conducted on topics relevant to vaccination policy. Identifying reviews on a specific topic requires time, skills in literature searching and access to academic databases. To facilitate this, the London School of Hygiene and Tropical Medicine, with funding from the National Institute for Health Research (NIHR), has created a database of vaccine policy-relevant systematic reviews (including both completed reviews and protocols) (SYSVAC). The database is hosted by the World Health Organization (WHO), who took over the NITAG resource centre (www.nitag-resource.org) from the Agence de Médicine Préventive (AMP) and is updated quarterly.
This paper presents a bibliometric analysis of the reviews included in this database. Bibliometric analysis aims to quantitatively characterise the literature, rather than to examine its findings [12]. The objectives of this paper are: (i) to describe the development of the SYSVAC database, (ii) to provide an overview of the vaccine-related systematic review literature by describing the trends in time, topic, area of focus, population and geographic location of published systematic reviews relevant to vaccine policy published between 1 January 2008 and 31 December 2016.
2. Material and methods
In the remaining text the word systematic review will be used for both completed systematic reviews as well as systematic review protocols.
2.1. Development of the SYSVAC database
Systematic reviews on vaccine- and immunization-related topics were identified through searches carried out in MEDLINE, Embase, the Cochrane Library (systematic reviews and Health Technology Assessments only), Scopus, Web of Science, Global Health and the PROSPERO International prospective register of systematic reviews [13]. PROSPERO is unique in that it includes the description of not only completed but also ongoing and planned systematic reviews. The final search was conducted in January 2017. Search terms specific to vaccines and immunisation were combined with filters designed to retrieve systematic reviews. The entries were restricted to a publication date from 1 January 2000 to 31 January 2017. Vaccine-related search terms were adapted to each database from the filters used in the National Institute of Health and Care Excellence Guidelines PH21 [14]. Search filters specific to systematic reviews were adapted from the Canadian Agency for Drugs and Technologies in Health strategy for searches on the Ovid platform [15] in the initial search. However, for the updates, the more specific BMJ search filter was used for Medline and Embase [16] and adapted for the other databases. The reason for this change was that the updates aimed to retrieve the more recent systematic reviews only, so a broader filter was no longer needed. In contrast to the early days of systematic reviewing, when a range of terms may have been used to describe the method, there is now a greater consensus on how to present, report and describe systematic reviews so omitting less specific terms does not lead to a loss in sensitivity. Wherever possible, searches were limited to title, keyword and abstract fields and to research involving human subjects. The full list of search terms used for each database can be found in supplementary file 1.
For each set of search results, duplicate references were removed and titles and abstracts were screened using predefined inclusion criteria. To be included in the database, articles had to meet the following criteria: (1) be a systematic review; (2) focus on human subjects; (3) focus on preventive vaccines, rather than vaccines for treatment or immunotherapy; (4) have a publication date on or after 1st January 2008 (see supplementary file 1 for more details). In addition, the analysis presented here is restricted to reviews published on or before the 31st of December 2016. (5) Not focus on vaccine development; and (6) not be withdrawn. Full texts of the papers were not retrieved during the screening. All systematic reviews meeting the inclusion criteria were combined into one Endnote database (Thomson Reuters 2014) and duplicates were removed.
Because of the size of the database, all entries were indexed using keywords for ease of categorisation. A keyword coding protocol was developed, pre-tested and refined by two researchers (SF and HB), with input from staff at AMP. Keywords enabled entries to be assigned to categories in five areas: (1) pathogen or disease, e.g. varicella; (2) target population, e.g. adolescents, pregnant women; (3) geographical location according to WHO regions [17]; (4) country income group according to the World Bank list of economies published July 2015 [18] and (5) topic area, e.g. immunology. An exhaustive list of all keywords can be found in supplementary file 1. Keywords were not mutually exclusive and articles could be classified with multiple keywords or none from each category. Any queries during the screening and coding process were resolved by consensus between two researchers (SF and HB).
2.2. Data analysis
The database content was exported from Endnote X7 to Excel 2016. Data classifications were checked and cleaned. The database was analysed by year of publication, journal and keywords. Trends over time were explored for the disease/pathogen keyword category. As a comparison to general biomedical research output, we explored the number of records published in PubMed per year in general and records containing “systematic review” in title or abstract. Further we examined annual numbers of systematic reviews registered in Prospero (a systematic review register launched in 2011) in general and records containing ‘vaccin*’ or ‘immuni*’ in title/abstract or keywords.
For the main disease/pathogen and topic areas, titles and abstracts were compared to ascertain any two or more reviews on identical or overlapping topics.
3. Results
3.1. Search results
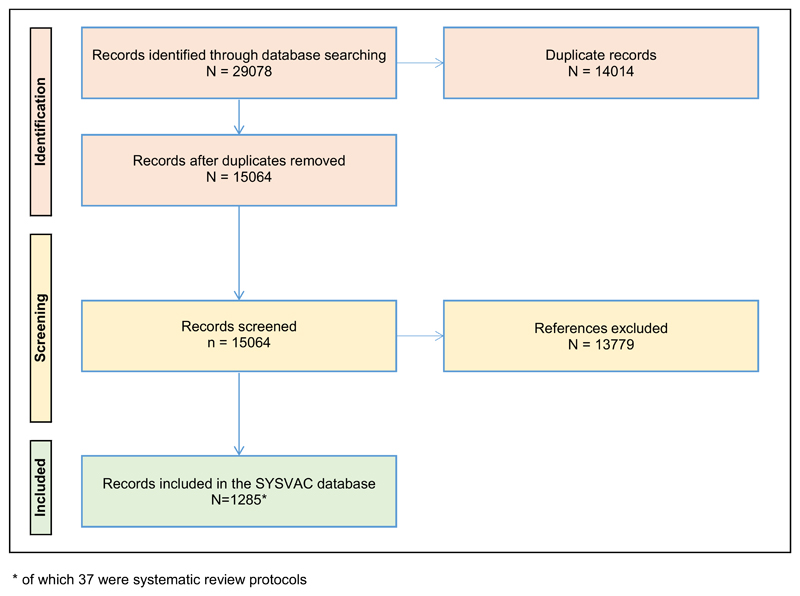
All searches combined identified 29,078 references. After removing duplicates, 15,064 records remained and after screening, 1285 unique systematic reviews and protocols were included in the database and analysis. The full details are shown in the PRISMA flow diagram in Fig. 1.
Fig. 1.
Flow of references from initial search to inclusion in database.
3.2. Most relevant journals
The 1285 unique systematic reviews were published in 398 separate academic journals as well as the Cochrane databases, most commonly “Vaccine” (N = 153) and the “Cochrane systematic review database” (N = 82).
3.3. Trends over time
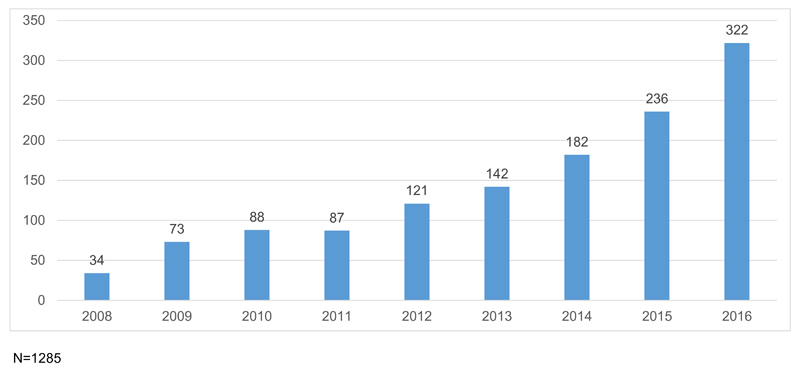
The number of systematic reviews identified per year on vaccine-related topics increased steadily from 34 in 2008 to 322 in 2016 (see Fig. 2), a more than ninefold increase. During the same period the total number of articles indexed on PubMed increased by 50% from 835,946 in 2008 to 1,255,235 in 2016. PubMed records containing “systematic review” in either the title or abstract increased 5-fold from 3456 in 2008 to 17,691 in 2016. The number of reviews (on any topic) registered on Prospero increased by almost 30 times from 284 in 2011 to 8162 in 2016. However, vaccine-related review registrations lagged behind this general trend, with only three identified in 2011 and 53 in 2016 – an almost 18-fold increase (for more details, see supplementary file 2.1, Tables S1 and S2).
Fig. 2.
Number of systematic reviews on vaccination/immunization by year.
3.4. Disease/pathogen areas
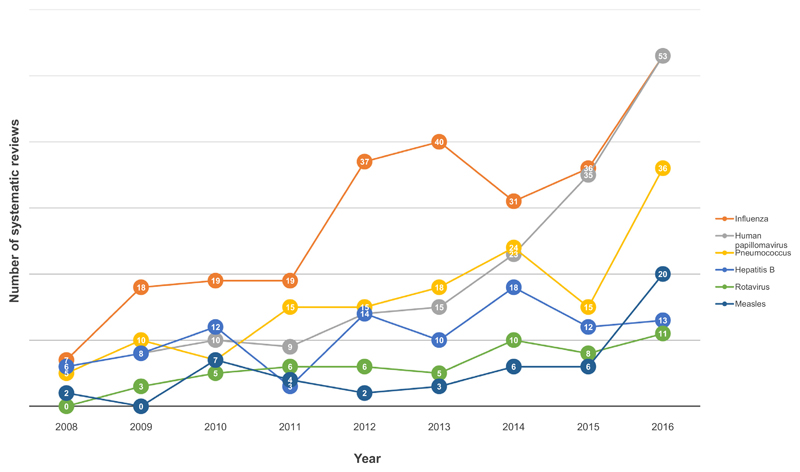
The database was analysed for 48 specific disease/pathogen areas ranging alphabetically from Adenovirus to Zika virus (see supplementary file 1). Table 1 shows the number of systematic reviews for each disease/pathogen. Out of 1285 systematic reviews, 312 (24%) were conducted on a general immunisation topic, while 973 (76%) focused on disease/pathogen specific topics, of which 859 (88%) concentrated on one disease/pathogen area and 114 (12%) on two or more. For six disease/pathogen areas at least 50 systematic reviews were identified: influenza (N = 260; 20%), human papilloma virus (HPV) (N = 173; 13%), pneumococcus (N = 145; 11%), hepatitis B (N = 96; 7%), rotavirus (N = 54; 4%) and measles (N = 50; 4%). Fig. 3 shows the trend over time for these six disease/pathogen areas, all of which exhibit a general upwards trend in the annual number of systematic reviews. The increase in numbers of systematic reviews was steepest for influenza, HPV and pneumococcus. Annual numbers of systematic reviews for hepatitis B seem to have plateaued in the past two years. On the other hand, there appears to have been a sharp increase in measles systematic reviews in 2016. For influenza, there is a peak in the years 2012 and 2013, shortly after the 2009/2010 H1N1 pandemic; the time lag may reflect the time taken to commission, conduct and publish systematic reviews.
Table 1. Number of systematic reviews by disease/pathogen.
| Disease/pathogen specific estimates | ||
|---|---|---|
| Disease/pathogen area | Quantity | % of total |
| Influenza | 260 | 20% |
| Human papillomavirus | 173 | 13% |
| Pneumococcus | 145 | 11% |
| Hepatitis B | 96 | 7% |
| Rotavirus | 54 | 4% |
| Measles | 50 | 4% |
| Pertussis | 49 | 4% |
| Tetanus toxoid | 41 | 3% |
| Varicella | 37 | 3% |
| Tuberculosis | 35 | 3% |
| Rubella | 33 | 3% |
| Haemophilus influenzae type b (Hib) | 32 | 2% |
| Poliomyelitis | 29 | 2% |
| Herpes zoster | 26 | 2% |
| Diptheria | 25 | 2% |
| Mumps | 23 | 2% |
| Combination vaccine | 19 | 1% |
| Hepatitis A | 18 | 1% |
| Meningococcal infection | 16 | 1% |
| Cholera | 11 | 1% |
| Yellow fever | 10 | 1% |
| Dengue | 8 | 1% |
| Malaria, Rabies | 7 | 1% |
| Smallpox | 6 | <1% |
| Escherichia coli, HIV (each) | 5 | <1% |
| Japanese encephalitis | 4 | <1% |
| Hepatitis C, Leishmaniasis, Salmonella (each) | 3 | <1% |
| Adenovirus, Anthrax, Coxiella burnetii (Q fever), Ebola, Shigella, Tick-borne encephalitis, Typhoid (each) | 2 | <1% |
| Campylobacter, Enterovirus vaccine (hand foot and mouth), Herpes simplex, Mycobacterium leprae, Mycobacterium vaccae, Pseudomonas aeruginosa, Respiratory Syncytial Virus (RSV), Streptococcus group B (each) | 1 | <1% |
| Summary estimates | ||
| All systematic reviews | 1285 | 100% |
| On general vaccination/immunization topic | 312 | 24% |
| On disease-specific topic | 973 | 76% |
| On one disease/pathogen area | 859 | 67% |
| On two or more disease/pathogen area | 114 | 9% |
Fig. 3.
Shows the trend over time and the annual number of systematic reviews for disease/pathogen areas for which ≥ 50 systematic reviews were identified.
3.5. Topic area
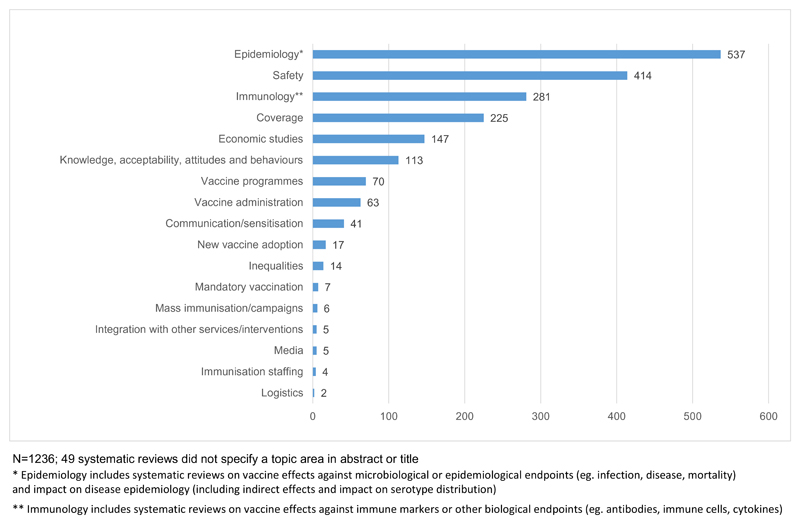
Fig. 4 shows the number of systematic reviews by topic area. A large number focused on epidemiology (N = 537, 42%), safety/pain (N = 414, 32%) and immunology (N = 281, 22%). These topic areas were followed by coverage (N = 225, 18%), economic studies (N = 147, 11%) and knowledge, attitude, behaviour & acceptability (N = 113, 9%).
Fig. 4.
Number of systematic reviews by topic area.
3.6. Geography and country income
Most reviews did not focus on a specific region of the world (N = 1096, 85%). Out of the 189 (15%) articles with a geographical focus, 171 (90%) concentrated on one specific region and 18 (10%) on more than one specific region. Of the 189 systematic reviews with a geographical restriction, the commonest target was Europe with 61 (32%) articles, followed by Western Pacific and the Americas with 45 (24%) and 51 (27%) articles, respectively. Lastly, a smaller number of reviews focused on Africa and South-East Asia (both N = 24, 13%), as well as the Eastern Mediterranean region (N = 16, 8%) (see Fig. S1 in supplementary file 2).
The majority of reviews (N = 1061, 83%) were not restricted to a specific country income group. Of the 224 (17%) reviews that had this focus, 145 (65%) concentrated on one and 79 (35%) on more than one country income group, with 121 (54%) focusing on middle-income countries, 105 (47%) on high-income countries and 83 (37%) on low-income countries (see Fig. S2 in supplementary file 2).
3.7. Population focus
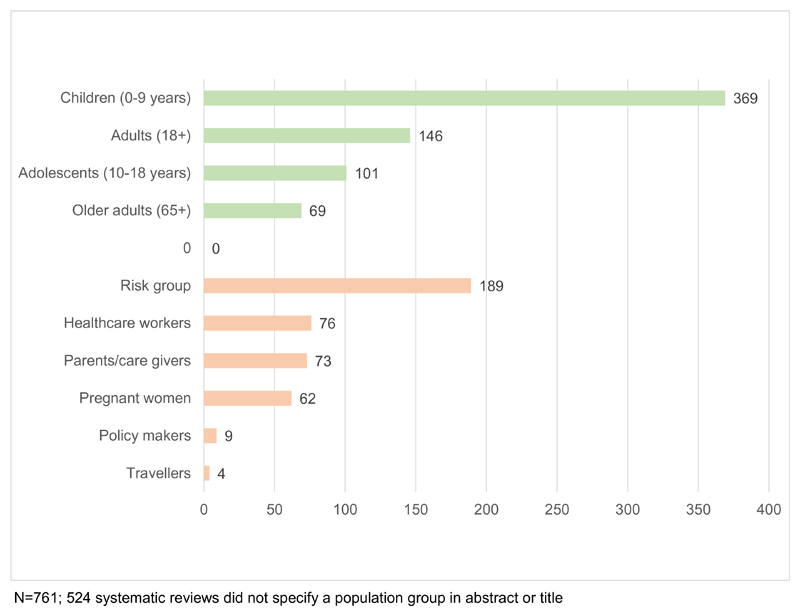
Reviews focused on different age groups and specific populations of interest. A total of 524 (41%) reviews did not specify any focal population group, while 504 (39%) focused on one group, and 257 (20%) on more than one particular population group. Fig. 5 shows that the most common focal age group was 0–9 years (N = 369, 29%). Eleven percent (N = 146) of reviews focused on adults, 8% (N = 101) on 10–18 year olds and 5% (N = 69) on older adults aged 65 or more. Other focal populations included risk groups (N = 189, 15%), health care workers (N = 76, 6%), parents/care givers (N = 73, 6%), and pregnant women (N = 62, 5%).
Fig. 5.
Number of systematic reviews by population groups.
3.8. Repetition and overlap of reviews
There appeared to be a number of overlapping review topics. To highlight some of them, we looked at reviews on influenza and HPV in more detail. Among 260 systematic reviews looking at influenza, 17 focused on influenza vaccination during pregnancy and 13 of these looked at safety and adverse birth outcomes, ten at efficacy or effectiveness and two at coverage or determinants of coverage. In the year 2015, four systematic reviews were conducted on almost identical topics looking at the association between maternal influenza vaccination and negative birth outcomes, including stillbirth, spontaneous abortion, fetal death, preterm delivery and congenital malformation [19–22]. Thirty-six systematic reviews published between 2009 and 2016 looked at influenza vaccination among health care workers, 27 of which focused on uptake and its determinants as well as attitudes towards influenza vaccination and 17 on safety, efficacy or effectiveness outcomes. Out of the 173 reviews found on HPV, 28 reported on economic outcomes, with 16 synthesizing evidence on the cost-effectiveness of HPV vaccine when targeting females. There was also a substantial overlap of reviews in other topic areas of HPV vaccination, including epidemiology, immunology, safety, coverage and knowledge, attitudes & behaviour.
4. Discussion
The analyses presented in this paper show the substantial growth of systematic reviews on vaccine research during the past nine years, with annual numbers increasing more than ninefold from 2008 to 2016. Several factors may have contributed to this increase. Expansion in primary research may have augmented the number of potential systematic reviews. Over the past decade, a greater interest in vaccine research has developed, supported by more research funding [1,23], as well as broader availability of vaccines worldwide. Systematic reviews have become an increasingly popular method, with more being conducted in all fields, not just on vaccines. This may be the result of a wider recognition of the value of systematic reviews. While the annual number of records in PubMed containing the term “systematic review” has grown at a similar rate to the numbers in our database (130% and 145% average annual increase), the increase in output of general biomedical research was at a slower speed (106% average annual increase). The “Prospero” database, which registers systematic reviews, has seen an almost exponential increase (260% average annual increase) in numbers of annual records from 2011, the year of its inception, to 2016. However, it appears that vaccine-related records on Prospero increased at a slower rate (210% average annual increase), suggesting the recent popularity of systematic reviews as the primary driver of the annual increase in records on our database, rather than the more modest increase in vaccine-related literature. While there are now a large number of vaccine-relevant systematic reviews available or planned, it nevertheless seems that the field has a smaller secondary research output than other health-related topics.
As more systematic reviews are being conducted, the potential for duplication and overlap rises. We found numerous examples of reviews in very similar areas in the field of vaccination. Given the considerable time, effort and resources required to conduct a systematic review, such apparent duplication may be an inefficient use of resources. Before commencing a systematic review, as a first step to avoid duplication, reviewers should ideally confirm that there are no currently planned or completed systematic reviews on a similar topic using Prospero or SYSVAC. Thereafter, the commissioning NITAGs or the reviewers themselves should register their systematic review on Prospero at the start of their undertaking. A risk of duplication still exists for reviews that are planned or ongoing, but have not been registered on Prospero, because SYSVAC would only capture them in the next update following publication. More widespread use of Prospero and SYSVAC could improve efficiency, obviate duplication and potentially facilitate pooling of resources. Equally important to transparent planning of systematic reviews is choosing topics wisely and purposively. Decision-makers often lack crucial evidence required to support or reject policies. Systematic reviews can be of great use in the decision-making process, but without coordination and differentiation between researchers, funders and decision-makers, resources will not be used efficiently. Improving communication among the different stakeholders will increase efficiency. Key figures from different stakeholder groups could then develop a process to reach consensus about which review topics to pursue. A starting point would be to use the SYSVAC database and Prospero to identify which reviews have already been conducted or are planned. Such a process might open the possibility of pooling resources and collaborations among different groups. The involvement of decision-makers in topic selection should help ensure the topics are of use to them, which would ultimately benefit the people and communities being studied.
The SYSVAC database and this bibliometric analysis have a number of limitations. Firstly, while decision makers will easily be able to identify relevant systematic reviews on SYSVAC, they will still need to conduct a thorough quality assessment of each review, followed by a synthesis of all reviews applicable to their specific question. Currently, there are a number of tools available to appraise the quality of systematic reviews. The PRISMA [24] and AMSTAR [25] checklists are well-known and tested tools to assess methodological quality of systematic reviews. However, to appraise the quality or relevance of a systematic review a broader framework is needed. The US Agency for Health Care Research and Quality has proposed a five-step approach for identifying, appraising relevance and methodological quality, analysing and using existing systematic reviews [26]. In addition to following the guidelines and checklists mentioned above, it is essential to involve experts in the field to translate review results into policy.
Secondly, while the present analysis identified a number of overlaps in review topics, it is unclear to what extent the methodology and results of these reviews are similar, as we only screened and coded based on the title and abstract. The user would need to assess this based on the specific question asked and scrutinize both methods and results of any reviews retrieved.
Decision-makers need to find systematic reviews easily if they are to use them. The SYSVAC database (www.nitag-resource.org) is a freely available online database of vaccine-relevant systematic reviews aimed at decision-makers as a resource for quick reference, information and guidance. It should increase the ease with which vaccine-relevant systematic reviews can be identified so that decisions can be based on the best available evidence to maximise population health outcomes.
5. Conclusions
From 2008 to 2016 the number of vaccine-related systematic reviews published annually increased by over 9 times. There appears to be large potential for duplication, which could be reduced if intentions to conduct a systematic review were registered prospectively and existing reviews were searched prior to new review commissioning. Decision-makers can use the SYSVAC database (www.nitag-resource.org) to search for systematic reviews on vaccine-related topics to inform their decision-making. The SYSVAC database could also be used as the focal point for the coordination of the commissioning of systematic reviews and the removal of duplication and inefficiency in evidence synthesis for vaccine policy.
Supplementary Material
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.vaccine.2018.02.049.
Acknowledgements
This work and the SYSVAC database were funded by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Immunisation at the London School of Hygiene and Tropical Medicine in partnership with Public Health England (PHE). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR the Department of Health and Social Care or Public Health England. We would like to thank the Agence de Médecine Préventive for their support in developing and refining the SYSVAC database and for the WHO’s willingness to host it. We are particularly grateful to Louise Henaff for her long-standing support in this process. Anthony Scott is supported by a fellowship from the Wellcome Trust (098532).
Footnotes
Conflict of interest
Anthony Scott is a member of the United Kingdom Joint Committee on Vaccination and Immunisation. All other authors declare no conflict of interest.
References
- [1].GAVI’s Mission statement. GAVI-the vaccine alliance. [accessed on 11 January 2017]; http://www.gavi.org/about/mission/
- [2].Global, regional, and national advisory committees on immunization. World Health Organization; [accessed on 14 March 2017]. http://www.who.int/immunization/policy/committees/en/ [Google Scholar]
- [3].Lavis J, Davies H, Oxman A, Denis JL, Golden-Biddle K, F E. Towards systematic reviews that inform health care management and policy-making. J Health Serv Res Policy. 2005;10(Suppl.1):35–48. doi: 10.1258/1355819054308549. [DOI] [PubMed] [Google Scholar]
- [4].Petticrew M, Roberts H. Systematic reviews in social sciences - a practical guide. John Wiley and Sons Ltd; 2005. [Google Scholar]
- [5].What is a systematic review? Cochrane Handbook. [accessed on 13 October 2016]; http://handbook.cochrane.org/chapter_1/1_2_2_what_is_a_systematic_review.htm.
- [6].Griffiths UK, Clark A, Gessner B, Miners A, Sanderson C, Sedyaningsih ER, et al. Dose-specific efficacy of Haemophilus influenzae type b conjugate vaccines: a systematic review and meta-analysis of controlled clinical trials. Epidemiol Infection. 2012;140:1343–55. doi: 10.1017/S0950268812000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jackson C, Mann A, Mangtani P, Fine P. Effectiveness of Haemophilus influenzae type b vaccines administered according to various schedules: systematic review and meta-analysis of observational data. Pediatric Infect Dis J. 2013;32:1261–9. doi: 10.1097/INF.0b013e3182a14e57. [DOI] [PubMed] [Google Scholar]
- [8].Low N, Redmond SM, Rutjes AW, Martinez-Gonzalez NA, Egger M, di Nisio M, et al. Comparing Haemophilus influenzae type b conjugate vaccine schedules: a systematic review and meta-analysis of vaccine trials. Pediatric Infect Dis J. 2013;32:1245–56. doi: 10.1097/INF.0b013e31829f0a7e. [DOI] [PubMed] [Google Scholar]
- [9].O’Loughlin RE, Edmond K, Mangtani P, Cohen AL, Shetty S, Hajjeh R, et al. Methodology and measurement of the effectiveness of Haemophilus influenzae type b vaccine: systematic review. Vaccine. 2010;28:6128–36. doi: 10.1016/j.vaccine.2010.06.107. [DOI] [PubMed] [Google Scholar]
- [10].Schonberger K, Kirchgassner K, Riedel C, von Kries R. Effectiveness of 2+1 PCV7 vaccination schedules in children under 2 years: a meta-analysis of impact studies. Vaccine. 2013;31:5948–52. doi: 10.1016/j.vaccine.2013.10.042. [DOI] [PubMed] [Google Scholar]
- [11].Scott P, Rutjes AW, Bermetz L, Robert N, Scott S, Lourenco T, et al. Comparing pneumococcal conjugate vaccine schedules based on 3 and 2 primary doses: systematic review and meta-analysis. Vaccine. 2011;29:9711–21. doi: 10.1016/j.vaccine.2011.07.042. [DOI] [PubMed] [Google Scholar]
- [12].Pritchard A. Statistical bibliography or bibliometrics. J Documentation. 1969;25(4):348–9. [Google Scholar]
- [13].PROSPERO – International prospective register of systematic reviews. Centre for reviews and dissemination – University of York; [accessed on 11 January 2017]. https://www.crd.york.ac.uk/PROSPERO/about.php?about=about. [Google Scholar]
- [14].NICE Guidelines [PH21] Reducing differences in the uptake of immunisations. National Institute for Health and Clinical Excellence (NICE); [accessed on 2 September 2014]. https://www.nice.org.uk/guidance/ph21. [Google Scholar]
- [15].Strings attached: CADTH’s database search filters. [accessed on 15 August 2014];Canadian agency for drugs and technologies in health strategy. https://www.cadth.ca/resources/finding-evidence/strings-attached-cadths-database-search-filters#syst.
- [16].Study design search filters. BMJ clinical evidence. [accessed on 15 August 2014]; http://clinicalevidence.bmj.com/x/set/static/ebm/learn/665076.html.
- [17].WHO member states regions. World Health Organization; [accessed on 5 March 2016]. http://www.who.int/about/regions/en/ [Google Scholar]
- [18].List of economies. World Bank. [accessed on 5 March 2016]; siteresources.worldbank.org/DATASTATISTICS/Resources/CLASS.XLS.
- [19].Bratton KN, Wardle MT, Orenstein WA, Omer SB. Maternal influenza immunization and birth outcomes of stillbirth and spontaneous abortion: a systematic review and meta-analysis. Clin Inf Dis: Official Publ Infect Dis Soc Am. 2015;60:e11–9. doi: 10.1093/cid/ciu915. [DOI] [PubMed] [Google Scholar]
- [20].Fell DB, Platt RW, Lanes A, Wilson K, Kaufman JS, Basso O, et al. Fetal death and preterm birth associated with maternal influenza vaccination: systematic review. BJOG. 2015;122:17–26. doi: 10.1111/1471-0528.12977. [DOI] [PubMed] [Google Scholar]
- [21].McMillan M, Porritt K, Kralik D, Costi L, Marshall H. Influenza vaccination during pregnancy: a systematic review of fetal death, spontaneous abortion, and congenital malformation safety outcomes. Vaccine. 2015;33:2108–17. doi: 10.1016/j.vaccine.2015.02.068. [DOI] [PubMed] [Google Scholar]
- [22].Polyzos KA, Konstantelias AA, Pitsa CE, Falagas ME. Maternal influenza vaccination and risk for congenital malformations: a systematic review and meta-analysis. Obstet Gynecol. 2015;126:1075–84. doi: 10.1097/AOG.0000000000001068. [DOI] [PubMed] [Google Scholar]
- [23].GAVI’s Institutional timeline. GAVI-The Vaccine Alliance. [accessed on 6 October 2016]; http://www.gavi.org/about/mission/institutional-timeline/
- [24].Preferred reporting items for systematic reviews and meta-analyses. [accessed on 1 February 2018]; ( http://www.prisma-statement.org/PRISMAStatement/Checklist.aspx)
- [25].AMSTAR - A MeaSurement tool to assess systematic reviews. [accessed on 1 February 2018]; https://amstar.ca/Amstar_Checklist.php.
- [26].Robinson KA, Whitlock EP, Oneil ME, Anderson JK, Hartling L, Dryden DM, et al. Integration of existing systematic reviews into new reviews: identification of guidance needs. Syst Rev. 2014;3:60. doi: 10.1186/2046-4053-3-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.