Abstract
The main forms of childhood malnutrition occur predominantly in children <5 years of age living in low-income and middle-income countries and include stunting, wasting and kwashiorkor, of which severe wasting and kwashiorkor are commonly referred to as severe acute malnutrition. Here, we use the term ‘severe malnutrition’ to describe these conditions to better reflect the contributions of chronic poverty, poor living conditions with pervasive deficits in sanitation and hygiene, a high prevalence of infectious diseases and environmental insults, food insecurity, poor maternal and fetal nutritional status and suboptimal nutritional intake in infancy and early childhood. Children with severe malnutrition have an increased risk of serious illness and death, primarily from acute infectious diseases. International growth standards are used for the diagnosis of severe malnutrition and provide therapeutic end points. The early detection of severe wasting and kwashiorkor and outpatient therapy for these conditions using ready-to-use therapeutic foods form the cornerstone of modern therapy, and only a small percentage of children require inpatient care. However, the normalization of physiological and metabolic functions in children with malnutrition is challenging, and children remain at high risk of relapse and death. Further research is urgently needed to improve our understanding of the pathophysiology of severe malnutrition, especially the mechanisms causing kwashiorkor, and to develop new interventions for prevention and treatment.
Tackling malnutrition is a major global health priority that is relevant to several of the United Nations’ Sustainable Development Goals (SDGs) and is highlighted directly in Goal 2 (‘Zero hunger’), which aims to “end hunger, achieve food security and improved nutrition and promote sustainable agriculture1”. Several forms of malnutrition are recognized, including stunting, which is characterized by reduced linear growth, wasting (including moderate wasting and severe wasting, also known as marasmus), which is characterized by low body tissue mass and other physiological abnormalities (FIG. 1), and kwashiorkor, which is characterized by diffuse peripheral oedema. The current classification of malnutrition is based on body size or the presence of oedema, which does not indicate the aetiology or precise nutritional deficits in an individual. Accordingly, this classification can effectively screen and identify malnutrition, but it does not address each and every specific nutrient deficiency a child might have or the biological variability among children. Such differences have no effect on current empirical management strategies that aim to address the predominant macronutrient and micronutrient deficiencies and treat possible infections.
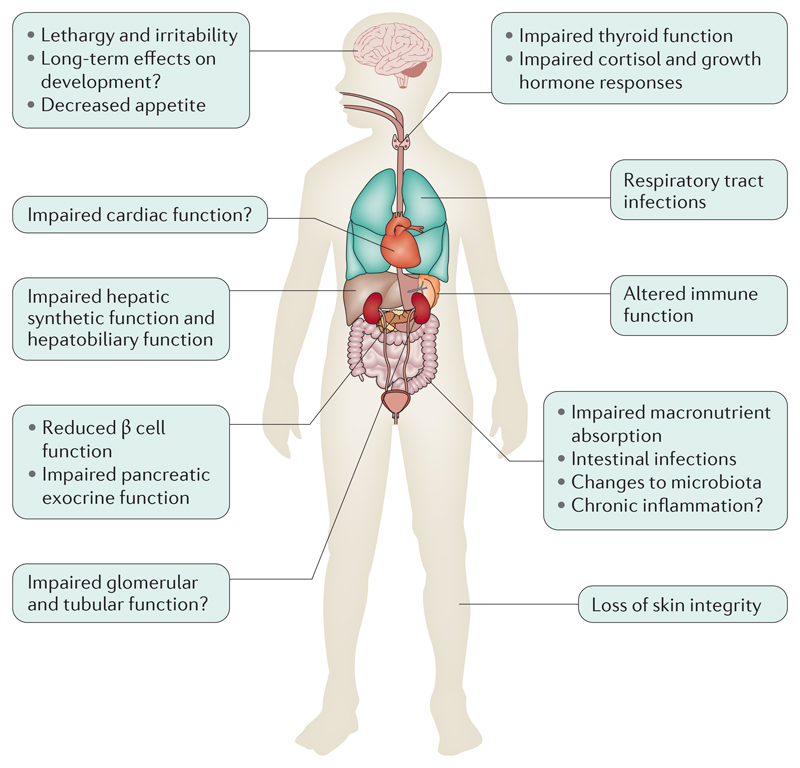
Figure 1. Organ system involvement in severe malnutrition.
Severe malnutrition can affect several organ systems. The functional impairments in these systems have been characterized, but the underlying mechanisms have not been fully elucidated.
The term ‘severe acute malnutrition’ has replaced ‘protein–energy malnutrition’, which was used to describe children with severe wasting and kwashiorkor (also known as nutritional oedema). In this Primer, we focus on and use the composite term ‘severe malnutrition’ to refer to severe wasting and kwashiorkor and to better reflect the longstanding nature of the combined infectious and environmental insults that can occur in such cases. It is important to emphasize the multifactorial aetiology of severe malnutrition and its strong association with mortality2, as well as the frequent coexistence of different types of malnutrition in the same child over time3 and that such concurrence further exacerbates mortality4. Indeed, most children with severe wasting or kwashiorkor are also stunted5–9. In addition, we concentrate on the largest and most vulnerable group, children <5 years of age, reflecting SDGs Goal 2.2, which aims to “…by 2030 end all forms of malnutrition, including achieving by 2025 the internationally agreed targets on stunting and wasting in children under five years of age…1”. Other populations can be malnourished, such as pregnant women (which has implications for fetal growth)10, elderly individuals11,12 and those with disabilities13,14, but discussing these populations is beyond the scope of this Primer.
Epidemiology
Regions with a high prevalence of severe malnutrition often also have high childhood mortality, with underlying malnutrition having a major role in the risk of death. The global estimates of the prevalence of severe malnutrition vary between agencies and according to the methodology used (BOX 1).
Box 1. Estimating the epidemiology of malnutrition.
Global data regarding the epidemiology of malnutrition must be understood with reference to methodological challenges and contextual factors. For example, wasting is often highly seasonal, and the number of cases peaks around the time of pre-harvest rains, when food is scarce and the burden of infectious diseases increases (for example, a higher prevalence of malaria and diarrhoeal diseases). In many instances, the interface of food insecurity (that is, a lack of access to a sufficient quantity of affordable, nutritious food), population displacement and drought also produce the conditions leading to famine.
In addition, although prevalence estimates from cross-sectional surveys are suitable for slowly changing conditions such as stunting, they are not suitable for capturing the rapidly changing nature of severe and moderate malnutrition, which have a fairly acute onset and rapid resolution. Malnutrition can also recur, which can be unrecorded or without contact with health services at each episode. Accordingly, incidence is the most informative epidemiological measure of acute malnutrition220, but it is rarely well documented owing to difficulties in ascertainment221. Addressing this limitation, prevalence-to-incidence conversion factors are sometimes used to determine the incidence of malnutrition, especially to estimate caseloads to plan treatment services222. Efforts are underway to improve surveillance systems for severe malnutrition.
The problems with the measurements used to diagnose malnutrition in surveys can also affect epidemiological estimates. For example, mid-upper arm circumference (MUAC) and the presence of oedema, which are both case-defining criteria, are not measured in many field surveys, resulting in underestimates of prevalence as these criteria do not necessarily overlap with those of wasting, which is defined on the basis of low weight-for-length measures223,224. Even when included, the presence of oedema might be missed in rapid surveys undertaken by poorly trained surveyors16.
Finally, infants <6 months of age with malnutrition might be missed in epidemiological surveys, either by design or because MUAC standards for this age group have not been established. Whether surveys of malnutrition in children <5 years of age include infants <6 months of age is not always clear.
Commonly used figures are from the WHO, UNICEF and World Bank Group Interagency estimates, which provide both levels and trends for child growth and malnutrition15 and are based on standard anthropometric indices. An estimated 155 million children <5 years of age were stunted in 2016, and 52 million were wasted, of whom 17 million were severely wasted15. The global burden of kwashiorkor remains uncertain due to its wide geographical variability and the failure to include assessments of oedema in most large nutritional surveys16; however some estimates from regions of southern and eastern Africa suggest that kwashiorkor accounts for 50–70% of cases of severe malnutrition5. Geographical variations in the prevalence of severe malnutrition have been observed (FIG. 2), which probably reflects differences in the distribution, causes and effects of multiple risk factors for malnutrition. These risk factors include social and environmental factors (such as poverty, poor education, limited health care access and contaminated environments)17–20 and living in areas with a high burden of infectious diseases, such as respiratory tract infections, diarrhoeal diseases21, HIV and turberculosis19,22. Other risks include dietary factors, such as acute and chronic food insecurity23, as occurs during famines, as well as suboptimal breastfeeding and suboptimal complementary feeding practices24.
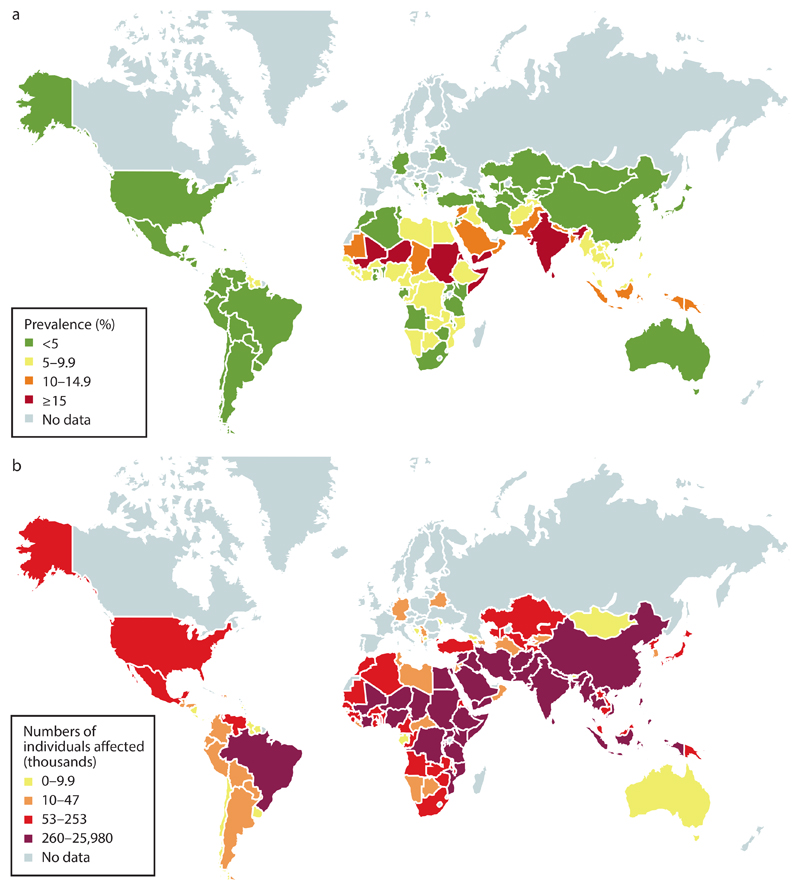
Figure 2. Prevalence of wasting.
The prevalence of wasting as a percentage of the population (part a) and the number of individuals (thousands; part b). Data from the Global Targets Tracking tool, version 3 (http://www.who.int/nutrition/trackingtool/en/; May 2017).
The other major global metrics initiative, the Global Burden of Disease study, estimates the number of deaths and disabilities directly related to protein–energy malnutrition. The 2015 Global Burden of Disease Study reported a global decline in the prevalence of severe malnutrition from 25.4 million in 1990 to 22.4 million in 2015 (REF. 25). However, 1990 might have represented a peak compared with previous years, during which figures were uncertain and HIV was less prevalent. This influence is especially relevant for Sub-Saharan Africa, where HIV and severe malnutrition are strongly linked22.
The 2015 Global Burden of Disease Study also reported that 174,000 deaths among children <5 years of age were directly due to protein–energy malnutrition25. Indeed, malnutrition, especially severe malnutrition, is associated with a high risk of death. The latest Lancet Nutrition series (published in 2013) estimated that 875,000 deaths were attributable to wasting (which was the cause of death of 12.6% of children <5 years of age), and 516,000 deaths were related to severe wasting (which was the cause of death of 7.4% of children <5 years of age)2. In ten longitudinal studies including ~54,000 child-years of follow-up and 1,300 deaths in children <5 years of age, all degrees of stunting, wasting and underweight were associated with an increased risk of death4. Mortality is higher in children with both wasting and stunting (who have a mortality hazard ratio (HR) of 12.3) than in those who have either wasting or stunting (a mortality HR of 2.3 for those with wasting only)4. The causes of death in individuals with malnutrition include infectious diseases (such as diarrhoeal diseases, pneumonia, measles and malaria) and metabolic disturbances (such as hypoglycaemia and refeeding syndrome (see Management))26,27. TABLE 1 summarizes the known HRs for deaths due to major childhood infectious diseases associated with various types of malnutrition2.
Table 1. Anthropometric measures and risk of death in children <5 years of age.
| All deaths HR (95% CI) |
Pneumonia deaths HR (95% CI) |
Diarrhoeal deaths HR (95% CI) |
Measles deaths HR (95% CI) |
Deaths due to other infectious diseases HR (95% CI) |
|
|---|---|---|---|---|---|
| Height-for-age or length-for-age Z-score | |||||
| <–3 | 5.5 (4.6, 6.5) | 6.4 (4.2, 9.8) | 6.3 (4.6, 8.7) | 6.0 (3.0, 12.0) | 3.0 (1.6, 5.8) |
| –3 to <–2 | 2.3 (1.9, 2.7) | 2.2 (1.4, 3.4) | 2.4 (1.7, 3.3) | 2.8 (1.4, 5.6) | 1.9 (1.0, 3.6) |
| –2 to <–1 | 1.5 (1.2, 1.7) | 1.6 (1.0, 2.4) | 1.7 (1.2, 2.3) | 1.3 (0.6, 2.6) | 0.9 (0.5, 1.9) |
| ≥–1 (control) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Weight-for-length Z-score | |||||
| <–3 | 11.6 (9.8, 13.8) | 9.7 (6.1, 15.4) | 12.3 (9.2, 16.6) | 9.6 (5.1, 18.0) | 11.2 (5.9, 21.3) |
| –3 to <–2 | 3.4 (2.9, 4.0) | 4.7 (3.1, 7.1) | 3.4 (2.5, 4.6) | 2.6 (1.3, 5.1) | 2.7 (1.4, 5.5) |
| –2 to <–1 | 1.6 (1.4, 1.9) | 1.9 (1.3, 2.8) | 1.6 (1.2, 2.1) | 1.0 (0.6, 1.9) | 1.7 (1.0, 2.8) |
| ≥–1 (control) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
Adapted from Black et al.2. CI, confidence interval; HR, hazard ratio.
Mechanisms/pathophysiology
Determinants of severe malnutrition
The loss of muscle and fat tissue that characterizes wasting can be caused by inadequate protein and energy intake resulting from food insecurity, poor diet and disease. However, severe malnutrition is rarely caused by a single factor and generally arises from an interplay between social, political and economic factors, the presence of chronic infections and inflammation (both in the gut and systemically). In some circumstances, gender issues, such as a lack of female empowerment, are important drivers of malnutrition28. Children with severe malnutrition are common in conditions of population displacement, conflict and food shortage29, which worsens the effects of many of the risk factors for malnutrition and are associated with ineffective remedial strategies.
Underlying mechanisms
Since the initial descriptions of severe malnutrition, studies aiming to understand the mechanisms and organ-specific and metabolic pathophysiology of severe weight deficits and oedema have been carried out. Historical comparisons are difficult given the changing definitions of malnutrition over time30 and the wide range of clinical manifestations that reflects different pathologies.
Wasting
Our knowledge of the mechanisms and metabolic changes associated with wasting comes mainly from the literature on long-term starvation and cachexia (that is, wasting induced by a chronic illness)31. During short-term starvation (that is, up to several days of fasting), free fatty acids (FFAs) and ketone bodies are primarily oxidized using available fat stores from adipose tissue, and myofibrillar proteins can be broken down into amino acids, which can be converted into glucose (through gluconeogenesis). After several days of starvation (when body fat has been depleted), myofibrillar proteins are extensively broken down to maintain essential metabolic processes. The short-term regulation of macronutrient oxidation and synthesis depends on insulin and glucagon, whereas the long-term regulation of these processes is mediated by other hormones, such as growth hormone, thyroid hormones, catecholamines and corticosteroids.
In addition, cytokine release in cachexia, especially the release of tumour necrosis factor (TNF), IL-1 and IL-6, can negatively influence body composition through a reduction in appetite and food intake, and direct catabolic effects on skeletal muscle and adipose tissue32,33. Increased activation of the ubiquitin–proteasome pathway is the main process that degrades myofibrillar proteins in cachectic conditions31,34. Autophagy has also been implicated in muscle wasting31 and ongoing autophagy can be detrimental for muscle cells by removing cellular components important for muscle metabolism and contraction, such as mitochondria. However, the specific roles of inflammation, proteasomes and autophagy in severe wasting in children in low-income and middle-income countries have not been studied in detail.
Kwashiorkor
Despite longstanding knowledge of kwashiorkor, the underlying pathophysiological mechanisms are poorly understood. In her earliest report, Cecily Williams documented that children with kwashiorkor in Ghana were fed mostly a monotonous corn diet that was deficient in essential amino acids such as lysine and tryptophan35. However, few studies have identified any specific nutritional deficiencies associated with the development of kwashiorkor, and studies typically have not revealed major differences in food group intake between children who developed kwashiorkor compared with those who did not or with those who developed wasting36,37. Despite numerous hypotheses, the aetiology of oedema, which is the hallmark of kwashiorkor, remains undefined. In animal models, some features of kwashiorkor, such as hypoalbuminaemia, can be induced by a diet low in protein and high in monosaccharides and disaccharides38,39, but oedema is rarely observed. In addition, the degree of hypoalbuminaemia and recovery upon nutritional management in children with kwashiorkor correlates poorly with the degree of oedema or the speed of its resolution40. Thus, controversy remains regarding the contribution of factors other than hypoalbuminaemia to the development of oedema in individuals with kwashiorkor38,41.
Infections
Children with severe malnutrition are highly susceptible to life-threatening infections2,42, which is a consequence of a secondary immunodeficiency. Indeed, the presentation of children with severe malnutrition to medical services might be due to a serious infection rather than the presence of malnutrition alone. Several potential mechanisms of immune dysfunction exist in individuals with malnutrition43,44.
Skin, respiratory and gastrointestinal mucosal barrier integrity are often impaired in children with malnutrition, and the effects of this impairment are compounded by chronic subclinical enteric dysfunction in close association with alterations to gut microbiota45,46. Children with severe malnutrition have increased levels of markers of systemic immune activation, such as the pro-inflammatory cytokines TNF, IL-1, IL-6 and IL-12, which alter the growth hormone–insulin-like growth factor 1 (IGF1) axis and result in wasting and linear growth failure. Alterations in this axis are also observed in other childhood inflammatory conditions, such as juvenile arthritis and inflammatory bowel disease47. Systemic immune activation in severe malnutrition can be caused by acute and chronic infection, inflammatory enteropathy, translocation of microbial components from the gut lumen or mucosa into the circulation48,49 and dysregulated immune responses50.
Other alterations in the immune system in severe malnutrition include T cell dysfunction and reductions in neutrophil microbicidal activity51, the number of dendritic cells, antigen priming and presentation52 and protein levels in the complement cascade44. Thymic atrophy, T cell hyporesponsiveness and impaired T cell proliferation could result from chronic immune activation and/or the metabolic demands of T cells for glucose, amino acids and nutritionally mediated regulatory hormones, such as leptin50,53,54. Although some abnormalities in the immune system can resolve with nutritional rehabilitation55, the reversal of susceptibility to infection and reduction in immune activation have not been well characterized.
Altered metabolism
Severe malnutrition can also affect many aspects of macronutrient metabolism and endocrine function56,57 (FIG. 3). In contrast to severe wasting, which is associated with a starvation-induced response of subcutaneous fat loss and muscle wasting, kwashiorkor has been speculated to be associated with a maladaptive metabolic response; a high-carbohydrate, low-protein diet is thought to lead to a continuous glycolytic response and protein catabolism that is inadequate to meet the amino acid requirements necessary to maintain essential protein synthesis pathways58. Indeed, protein breakdown has been shown to be lower in children with kwashiorkor than in children with severe wasting and in those in a recovered state59,60. In addition, reduced concentrations of essential and some nonessential amino acids have been reported, and these deficits were even more pronounced in children with kwashiorkor61.
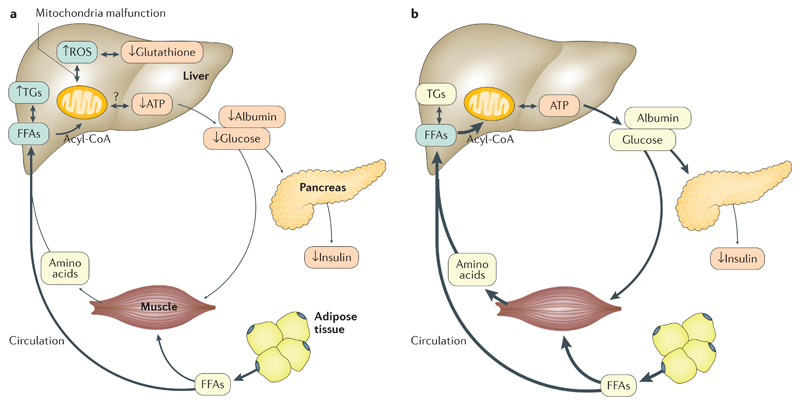
Figure 3. Metabolic changes in severe malnutrition.
Reduced secretion of insulin contributes to a catabolic state in both kwashiorkor (part a) and severe wasting (part b). In severe wasting, the reduced secretion of insulin leads to a lipolytic and proteolytic response and the release of free fatty acids (FFAs) and amino acids into the bloodstream. FFAs are taken up by muscle tissue for oxidation. Both FFAs and amino acids are also partially taken up by the liver and are used for ATP production and the synthesis of essential proteins and glucose. In kwashiorkor, this adaptive response is disturbed, which causes a reduced release of FFAs from adipose tissue and amino acids from muscle tissue. Mitochondrial damage in the liver is associated with increased reactive oxygen species (ROS) production and reduced glutathione. Arrow thickness represents the amount of metabolites in that pathway. TGs, triglycerides.
Lipid metabolism is also differentially affected in kwashiorkor and severe wasting. Adipocyte lipolysis is a tightly regulated process, with a central role for insulin in inhibiting hormone-sensitive lipase. Lipolysis is stimulated during starvation (that is, when insulin levels are low)62,63, and lipolysis has been shown to be increased in a small cohort of children with severe malnutrition compared with a control cohort62. However, another study did not show elevated lipolysis in children with severe malnutrition at hospital admission compared with those in a state of nutritional recovery, although reduced FFA (palmitate) flux and oxidation were noted in children with kwashiorkor compared with children with severe wasting63. One caveat of this study is that lipolysis was assessed when children were in the semifasted state, when insulin levels were likely low and lipolysis was stimulated. Thus, how lipolysis is altered in the postprandial state is unknown. A recent study identified high acylcarnitine levels in the early period of hospital admission for severe malnutrition regardless of the presence of oedema, suggesting preferential fatty acid oxidation64.
Glucose homeostasis is also disrupted in children with severe malnutrition; hypoglycaemia is common, although frequent or continuous glucose measurement studies have not yet been carried out to characterize this fully56,65. Kwashiorkor is associated with reduced endogenous glucose production compared with production in children with severe wasting or in healthy children, likely contributing to the development of hypoglycaemia66. This finding is consistent with the hypothesis that kwashiorkor is associated with a maladaptive metabolic response58. In addition, glucose clearance from the blood and uptake by tissues, which is regulated by insulin, are affected; several studies have reported impaired glucose clearance in both kwashiorkor and severe wasting67–69. A striking feature that seems to contribute to impaired glucose clearance is the blunted pancreatic endocrine response, with insulin sensitivity likely unaffected67. However, despite the reports of impaired glucose clearance in metabolic studies, hyperglycaemia has not been specifically reported in children with severe malnutrition (likely due to glucose levels usually being checked only when patients are symptomatic for hypoglycaemia), and its prevalence is unknown.
The mechanisms of impaired pancreatic function in children with severe malnutrition are poorly understood, but studies in preclinical models have implicated a more-polarized membrane potential, changes in cAMP-dependent protein kinase catalytic subunit-α protein levels and a reduced ability to increase intracellular Ca2+ levels in response to glucose70–72. In addition, no clear data are available regarding the pancreatic glucagon response (which is responsible for stimulating glucose production) in children with severe malnutrition. Glucagon concentrations have been reported to be either mildly reduced during the acute phase of malnutrition compared with levels at recovery or elevated in children with severe wasting compared with controls66,73, but stimulation tests or systematic glucagon response measurements during hypoglycaemia have not been carried out.
In general, cortisol levels in children with severe malnutrition are similar to levels in children without malnutrition or are increased, which is likely related to stress and indicates that the hypothalamic–pituitary–adrenal axis is preserved in children with severe malnutrition and is probably not responsible for the development of hypoglycaemia64,74,75. Most studies have shown a reduction in thyroid function in children with severe malnutrition, but the clinical relevance of this remains unclear76,77. In addition, leptin concentrations are low in children with severe malnutrition, reflecting the extent of adipose tissue loss. Leptin has a direct role in immune function, metabolism and appetite regulation, and its levels are inversely associated with mortality64.
Oxidative stress
Oxidative stress has also been associated with severe malnutrition and, in particular, with kwashiorkor. Indeed, children with severe malnutrition have reduced levels of antioxidants, including vitamin E and glutathione, compared with children without malnutrition, and this reduction is more-pronounced in children with kwashiorkor78–82. Although a reduced intake of antioxidants likely contributes to the lower levels observed in children with malnutrition, this is also likely, in part, related to a reduced synthesis of certain antioxidants, such as glutathione59. An imbalance between levels of reactive oxygen species and antioxidants has been postulated to have a role in the pathophysiology of kwashiorkor80, although a large randomized trial of an antioxidant mixture (containing riboflavin, vitamin E, selenium and N-acetylcysteine) in Malawi failed to show decreased rates of kwashiorkor83, despite a pilot study suggesting a potential beneficial treatment effect78. An imbalance between reactive oxygen species production and detoxification by peroxisomes results in mitochondrial damage, which ultimately reduces ATP production and impairs cellular function in the liver. Mitochondrial dysfunction and ATP depletion together with specific nutrient deficiencies might influence the response to an intercurrent infection and contribute to the development of multi-organ failure84–87; this process needs further characterization.
Cardiac function and haemodynamics
Cohort studies of small numbers of children with severe malnutrition have reported cardiac muscle atrophy and decreased cardiac output88, especially in children with kwashiorkor89. However, other studies have reported a normal cardiac output when corrected for body surface area90,91, with impairments observed only in the most severely ill children, which was probably related to sepsis. The largest study to date excluded severely ill children and showed no major differences in cardiac function between hospitalized children with severe malnutrition and children without malnutrition at an average of 4 days after admission91. The mechanisms leading to cardiac atrophy have not been well studied, although one study used a mouse model of calorie restriction to show that this was related to a proportional decrease in different subcellular components of cardiomyocytes92.
Hepatic function
Severe malnutrition, in particular kwashiorkor, is associated with changes in hepatic metabolic function. A striking feature of kwashiorkor is the presence of hepatic steatosis (that is, fatty liver)35. One study suggested that the hepatic steatosis in kwashiorkor is not related to the impaired secretion of lipids (in the form of very low-density lipoproteins) by the liver93. In addition, an increase in fatty acid release from adipose tissue that could be taken up by the liver has been observed in children with kwashiorkor in some62,94, but not all63, studies, which might be related to whether the analyses were performed when the participants were in the fasted state. Impaired hepatic lipid oxidation might also explain the hepatic steatosis observed in children with kwashiorkor63. This lipid oxidation is difficult to assess in children, but some data from post-mortem samples and animal models suggest that mitochondrial function, which is mainly responsible for hepatic lipid oxidation, is impaired66,84,95,96. Indeed, impaired mitochondrial function would be expected to affect hepatic synthetic pathways, and reduced glucose synthesis in children with kwashiorkor was shown to be correlated with mitochondrial activity66. Other alterations in the livers of children with kwashiorkor include reduced albumin synthesis97, although whether other hepatic synthetic processes (such as the production of coagulation factors) are altered has not been studied.
Enteropathy
Although much interest surrounds the potential association of stunting with enteropathy98, intestinal dysfunction also accompanies severe malnutrition. Indeed, diarrhoea is common in children with malnutrition and is associated with poor clinical outcomes99–101. Several factors might contribute to secretory and osmotic diarrhoea in individuals with malnutrition, including intestinal infections and inflammation102. In addition, poor nutrient digestion resulting from impaired hepatobiliary and pancreatic exocrine function could contribute to nutrient malabsorption and diarrhoea103–106. Malnutrition leads to small intestinal villous blunting, thereby reducing intestinal absorptive capacity, including impaired monosaccharide and disaccharide absorption, which could contribute to osmotic diarrhoea107.
Children with severe malnutrition have distinct alterations in their intestinal microbiota that can affect intestinal inflammation and function, as well as the growth of the child108. In addition, the faecal microbiota of children with kwashiorkor in Malawi was immature, with a reduced diversity, compared with the microbiota of their twins without malnutrition109. Interestingly, mice that received transplantation of the faecal microbiota from children with kwashiorkor had a more profound weight loss than mice transplanted with microbiota from the twin without malnutrition, and this weight loss was associated with signs of disturbed metabolic pathways. In a separate birth cohort in Bangladesh, the presence of an immature faecal microbiota was linked with wasting110. Another study comparing the microbiomes between children with kwashiorkor and children with wasting found a more-modest difference in microbiome composition111. A potential limitation of studying the microbiota in children with severe malnutrition is the use of antibiotic treatment before stool collection, which profoundly affects microbiota composition. Interest is growing regarding the interaction between the bacterial microbiome, the virome112 and eukaryotic organisms, but the functional consequences of microbiota alterations in children with severe malnutrition have yet to be well characterized.
Renal function
Studies assessing renal function (determined by glomerular filtration rate) in children with severe malnutrition have been limited. Given the frequency of diarrhoea and dehydration in these children, the pre-renal contribution to reduced glomerular filtration might have a substantial role. Low glomerular filtration rates in children with malnutrition and dehydration have been reported76, and subsequent studies have reported impaired glomerular filtration rates and signs of tubular dysfunction with reduced urine osmolality, but the clinical relevance of low glomerular filtration remains unclear113.
Brain function
Severe malnutrition is associated with acutely altered cerebral function and behavioural changes; children with kwashiorkor have cerebral atrophy114,115 and irritability, and children with severe wasting are often apathetic, with slowed movements and impaired speech. However, the underlying mechanisms of these behavioural changes are poorly understood. Although the association of severe malnutrition and growth in early life and development has been well documented, few studies have focused on long-term developmental effects. Impaired development after an episode of severe malnutrition has been reported in children116, and psychosocial interventions have been shown to improve their development117. However, differing case definitions and treatment strategies make it challenging to disentangle the direct effects of severe malnutrition from the effects of other risk factors and adversities that these children typically experience. Additional studies are needed, particularly to understand the long-term effects on different domains of development. In contrast to severe malnutrition, the cognitive deficits associated with stunting are well described. Indeed, for every 10% increase in the prevalence of stunting, the proportion of children reaching the end of primary school has been estimated to drop by 7.9%118,119.
Other pathophysiological alterations
Cellular Na+/K+-ATPase pumps that maintain fluid, electrolyte and substrate levels might be impaired in children with severe malnutrition. An increased membrane permeability was found in leukocytes from children with kwashiorkor, and reduced Na+/K+-ATPase activity has been observed in children with severe wasting, potentially as part of an adaptation to reduce energy expenditure120,121.
Diagnosis, screening and prevention
Diagnosis
Although case definitions of severe malnutrition for epidemiological, biological and clinical purposes focus on anthropometry122 (BOX 2), malnutrition is a functional problem and has been defined as a state resulting from lack of uptake or intake of nutrition leading to altered body composition, decreased body cell mass leading to diminished physical and mental function and impaired clinical outcome from disease123. Anthropometry is used to assess severe malnutrition because the functional deficits are difficult to measure directly. Anthropometry is therefore a proxy and not a direct measure of malnutrition. Importantly, no ‘gold standard’ measurement for the diagnosis of severe malnutrition exists. Different anthropometric measures assess different aspects and durations of the underlying functional deficit, and some relate more closely to body composition and clinical outcomes than others.
Box 2. Anthropometric criteria for nonmicronutrient malnutrition in children 6–59 months of age*.
Global acute malnutrition‡
Any of the following:
Weight-for-height Z-score <−2
Mid-upper arm circumference (MUAC) <125 mm
The presence of nutritional oedema
Moderate acute malnutrition‡,§
Either of the following:
Weight-for-height Z-score ≥−3 to <−2
MUAC ≥115 mm to <125 mm in the absence of nutritional oedema
Severe wasting‡,‖
Either of:
Weight-for-height Z-score <−3
MUAC <115 mm in the absence of nutritional oedema
Kwashiorkor‡
Any weight-for-height Z-score
Any MUAC
The presence of nutritional oedema
Severe acute malnutrition
Severe wasting and/or kwashiorkor
Moderate underweight¶
Weight-for-age Z-score ≥−3 to <−2
Severe underweight¶
Weight-for-age Z-score <−3
Moderate stunting¶
Height-for-age Z-score ≥−3 to <−2
Severe stunting¶
Height-for-age Z-score <−3
For younger children, typically those <2 years age, length is commonly measured instead of height, giving weight-for-length or length-for-age values. Z-scores refer to the number of standard deviations away from a reference population median. For example, a Z-score of −1 equates to 1 standard deviation below the 2006 WHO Growth Standard (http://www.who.int/childgrowth/en). *See REF. 131. ‡Acute conditions. §Diagnosed according to weight-for-length or weight-for-height and/or MUAC criteria; also referred to as ‘moderate wasting.’ ‖Diagnosed according to weight-for-length or weight-for-height and/or MUAC criteria. ¶Chronic conditions.
Anthropometry
The accepted diagnostic criteria for severe malnutrition have changed over time. Initially, the measurement of weight-for-age (that is, comparing the weight of the child with the weight of age-matched children in a reference population) was used, and severe malnutrition was defined as <60% of the reference weight124. This definition was later refined with the addition of oedema as an additional criterion to diagnose kwashiorkor30. To focus on the highest risk group of the most acutely malnourished children rather than on those with a low weight-for-age who were mainly stunted, weight-for-height was later used. Weight-for-height compares the weight of the child to the weight of well-nourished children of the same height (length is used if children are <2 years old). The WHO recommended expressing measurements as weight-for-height Z-scores (WHZs), that is, the number of standard deviations below or above the reference median value125. The main growth references that are used for the diagnosis of severe malnutrition are those derived from the 2006 WHO Multi-Country Growth Reference Study, which describes how children ‘should’ grow under optimal health, environmental and nutritional conditions126. Severe acute malnutrition is defined by the WHO as a WHZ of >3 standard deviations below the reference value for median weight-for-height or the presence of bilateral pitting oedema127.
Interestingly, a review of all studies on the relationship between anthropometric indices and mortality concluded that WHZs are the least effective predictor of mortality128 and that unadjusted mid-upper arm circumference (MUAC) was more-discriminatory for assessing mortality in several countries in Africa and Asia129. The association between MUAC and mortality might be due to the preferential selection of children who are younger, have both stunting and wasting and are particularly at risk130. A MUAC of <115 mm was introduced as an independent diagnostic criterion for severe acute malnutrition in children aged 6–59 months by the WHO in 2013 (REF. 131) (BOX 2). Although the use of MUAC for diagnosis is limited as this measurement does not identify the same children with malnutrition as WHZs122, following the development of community-based management programmes that are effective in preventing malnutrition-associated deaths, MUAC has been used more frequently. An alternative is to increase the MUAC cut-off value up to 120 mm to pragmatically define severe malnutrition. Although the additional children diagnosed with severe malnutrition on the basis of an increased MUAC value are not the same as those identified by WHZs, this approach can better identify children with a higher risk of death than an approach using both MUAC and a WHZ132.
Identification of oedema
Oedema is a sign of kwashiorkor and is assessed by pressing firmly down on the third to fourth tarsal bones on the dorsal aspect of the child’s foot for 3–5 seconds and then looking for pitting oedema for 2–3 seconds. Oedema is graded according to the location on the body; grade 1+ indicates oedema limited to the feet and lower legs, 2+ indicates oedema that is present in the arms and grade 3+ indicates oedema of the face. The presence of symmetric, bilateral oedema of the feet in a vulnerable child in a high-risk population, regardless of the child’s anthropometric measurements, is generally sufficient to diagnose kwashiorkor.
Infants <6 months of age
The diagnosis of severe malnutrition in infants <6 months of age presents special challenges. These include the practical difficulties of undertaking anthropometric measurements on small infants133 and that many individuals assume malnutrition is rare at this age and so do not take measurements at all134. However, the burden of malnutrition in this age group is high135. The WHO currently recommends using a weight-for-length Z-score (WLZ) of <−3 to identify infants with severe malnutrition, but only because of the paucity of evidence for other criteria. Few prospective studies have examined the discriminatory performance of different anthropometric measures against the risk of death, but both weight-for-age and MUAC have been suggested for infants >60 days old136. Similar to what has been found in older children, MUAC identifies a slightly different group of infants but has superior performance for predicting mortality than WLZs for populations in Gambia and Kenya137,138. A MUAC of <110 mm for infants >60 days of age has been proposed based on a clinical trial in Kenya, which confirms that this measurement can identify infants with a very high risk of death6,138.
Screening
In practice, given the challenges of measuring WHZs frequently or at a large scale in a community, screening programmes tend to use a MUAC of <115 mm to assess severe malnutrition in children 6 months to 5 years of age, and the assessment of oedema is used to diagnose kwashiorkor (FIG. 4). Indeed, the best approach for screening for severe malnutrition is to measure MUAC frequently, ideally every month or every time a child is unwell. All anthropometric indices are better at identifying high-risk children in the short term, that is, in the month following the measure128, and this applies to MUAC as well139. A promising approach to screening for severe malnutrition is to provide mothers with MUAC tapes that have a cut-off value of 115 mm and show them how to detect malnutrition using this measurement6,140. This technique is simple enough to be taught rapidly140 and enables malnutrition to be detected early (before the onset of complications), hence reducing the need for initial inpatient treatment6. However, the use of MUAC tapes to screen for severe malnutrition still has substantial room for global expansion, which has been delayed owing to the lack of guidelines, until recently, by normative bodies such as the WHO and UNICEF. This delay has contributed to the poor uptake of this practice by local practitioners and professional bodies.
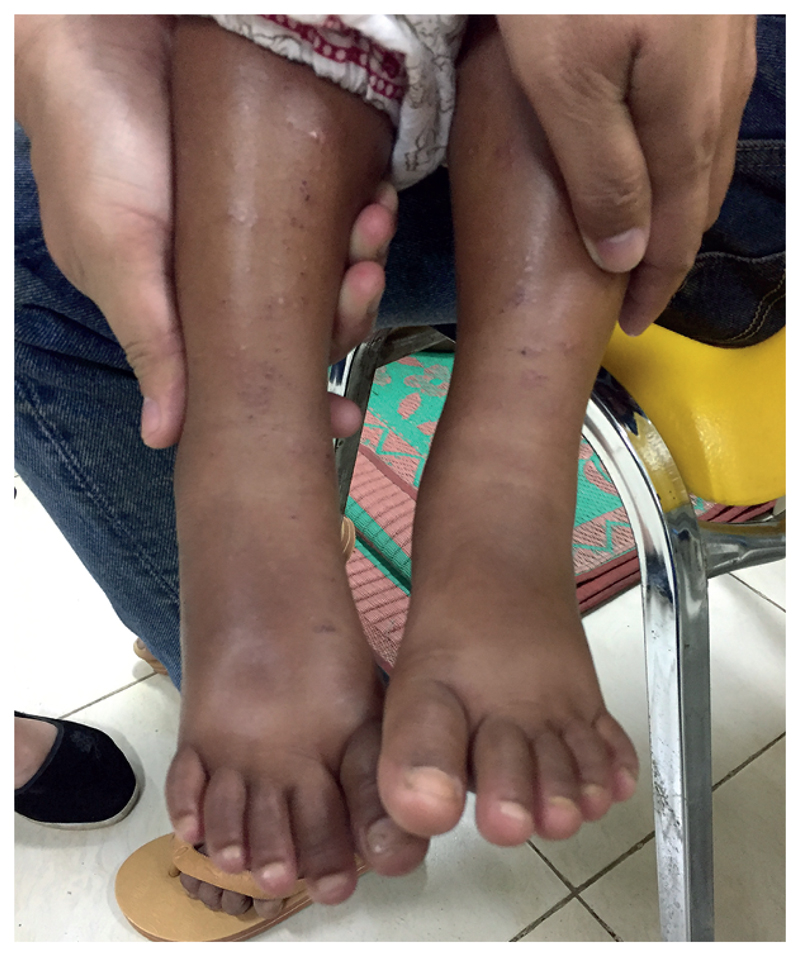
Figure 4. Assessment of oedema.
Oedematous swelling of the lower extremities with skin changes in a child with kwashiorkor.
Prevention
Given the high morbidity and mortality associated with severe malnutrition, preventing it is one of the most important goals in global health. Nevertheless, prevention remains elusive in most impoverished and humanitarian settings, and it requires programmes that address maternal and child malnutrition holistically. Many countries have reduced the overall prevalence of severe malnutrition and stunting through the reduction of inequities141–143. In most instances, this achievement has required a combination of economic growth, the use of public sector programmes focused on reducing inequities and investments in nutrition-sensitive (those that directly affect nutrition, such as breastfeeding and complementary feeding support programmes) and nutrition-specific (those that indirectly affect nutrition, such as improved agriculture and social safety nets) interventions. Indeed, no single intervention has effectively reduced the rates of severe malnutrition or stunting for individual children144,145, and packages of public health approaches are needed. These packages should include improved access to clean water, sanitation and hygiene, improved agricultural productivity to minimize food insecurity, timely universal vaccinations, early and efficient access to primary health facilities and care for acute illnesses (for example, pneumonia, diarrhoea, measles and malaria), an emphasis on exclusive and continued breastfeeding and attention to effective complementary feeding. Each approach is likely to affect malnutrition generally and, in turn, affect the rates of severe malnutrition146.
Despite several countries reducing the rates of malnutrition in general, inconsistent effects of vertical programmes on childhood malnutrition have been reported. Indeed, the largest and most comprehensive multicentre trial testing the use of small quantities of lipid-containing nutrient supplements did not show significant benefits regarding stunting or wasting in Malawi147 and showed only modest benefits in Ghana148,149. The same intervention did have a better effect on stunting and wasting in Burkina Faso150, but notably, this trial also included treatment for diarrhoea and malaria. These data emphasize the importance of interventions beyond nutritional therapy alone in at-risk populations.
At the level of the individual, a comprehensive prevention package should begin with teenage girls and young women before pregnancy and should include comprehensive health education and the use of nutritional interventions aimed at optimizing their overall physical health, nutritional status and psychosocial readiness for pregnancy and childbirth151. During pregnancy, women should be provided with evidence-based prenatal and perinatal support, including micronutrient and macronutrient supplementation, treatment for infectious diseases (including prophylaxis and treatment for acute infections) and early and frequent education regarding neonatal nutrition and care152; this strategy is believed to improve the health status of both the mother and the infant.
Optimizing macronutrient intake during infancy to prevent the development of severe malnutrition begins with the prompt initiation of breastfeeding, followed by exclusive breastfeeding for the first six months of life and the subsequent progressive introduction of nutritious and diverse complementary foods, with continued breastfeeding for ≥2 years153. In addition, context-specific micronutrient programmes can be used and might include vitamin A, iron, multiple micronutrient powders or small quantities of lipid-containing nutrient supplements. Additionally, zinc supplements can be used in children with acute diarrhoea. All children <5 years of age living in at-risk communities should undergo frequent growth monitoring, with the commencement of treatment for acute malnutrition when necessary. They should also receive all recommended immunizations and have ready access to essential interventions and therapeutics when required (including oral rehydration salts for diarrhoea, antibiotics for pneumonia and antimalarials, as well as antiretroviral therapy and opportunistic infection prophylaxis for children who have or have been exposed to HIV infection)154. Early and comprehensive intervention for children with moderate malnutrition is important for limiting the incidence of severe malnutrition as these children are assumed to have an increased risk of progression to severe malnutrition155.
Management
The goals of management of severe malnutrition are to prevent short-term mortality, achieve sustained nutritional recovery to reduce susceptibility to life-threatening infections and to support neurocognitive development. Despite their striking clinical differences, the treatment recommendations for kwashiorkor and severe wasting have several common elements (BOX 3).
Box 3. Management of severe acute malnutrition.
The management of children with severe malnutrition has several components:
Therapeutic feeding with macronutrients and micronutrients to achieve homeostasis and overcome the nutrient deficits
The immediate identification and management of life-threatening infections and complications such as sepsis and metabolic derangements
The treatment of subclinical infections and underlying conditions, such as HIV or tuberculosis
The cognitive stimulation and emotional support of the child and psychosocial support for their parents or caregivers
Linkage to medical, nutritional and social services during therapy and recovery
The current management of severe malnutrition has evolved over several decades and is mostly based on expert opinion and a few observational studies and not on data from modern randomized clinical trials156,157. Management is based on a series of WHO guidelines and training programmes, which are practical and have been widely adopted. Indeed, the management guidelines have become so engrained in clinical practice that it might be considered unethical to carry out randomized controlled trials except to show equivalence or superiority to the current standard of care. Notably, many of the principles of management have emerged from emergencies, and translating these to low-income and middle-income countries, in which severe malnutrition is a daily burden on health systems, remains a challenge158. In general, management includes the use of therapeutic foods along with antibiotics to treat any underlying infections. Management can be undertaken in communities for children with uncomplicated severe malnutrition (that is, children who are clinically stable and do not display clinical features of complications, especially coincidental infections).
Therapeutic foods
Therapeutic foods, such as ready-to-use therapeutic foods (RUTFs) and F-75 and F-100 milks, were designed for nutritional and metabolic stabilization and rehabilitation, and their use aims to address anticipated calorific needs and treatment stage-appropriate protein, electrolyte and micronutrient requirements, in addition to initially limiting exposure to nutrients that could be harmful to metabolically unstable children or those with infections, such as sodium and iron. However, the precise nutrient requirements of children with severe malnutrition and the bioavailability of these therapeutic foods are not well established and might vary by setting and the presence of comorbidities in some patients.
The formulations of F-75 and F-100 were derived and optimized based on metabolic studies of hospitalized children with severe malnutrition, with the aim of providing the micronutrients and macronutrients that are likely to be deficient in a child with severe malnutrition. F-75 is used for initial refeeding and F-100 is effective in facilitating rapid catch-up weight gain but might not correct all of the metabolic disturbances in children with severe malnutrition.
RUTFs were developed to contain the same nutritional content as the F-100 therapeutic milk formula but in a dehydrated form that can be administered in the home environment with no preparation or cooking required and minimal risk of contamination159. RUTFs have no mandatory formulation but do have minimum micronutrient and macronutrient concentrations and quality control and microbiological safety standards, which are outlined by WHO and UNICEF160. The predominant formulation of RUTFs consists of peanut paste, milk powder, sugar, oil and micronutrients. The high cost of RUTFs is largely due to their milk powder component, which might be only partially overcome by using local production methods as this is also associated with high costs, including the costs of constructing and maintaining appropriate production facilities and importing ingredients, as well as high local taxes. Trials investigating the use of RUTFs without milk powder have suggested an inferior effect on child growth161,162. The essential polyunsaturated fatty acid (PUFA) composition of RUTFs is not optimal8,163, but a more-favourable composition, such as a reduced ω-6 PUFA content or increased long-chain ω-3 PUFA content, presents challenges for shelf life; the effects of these compositions on growth and neurodevelopment are uncertain.
As RUTFs provide individuals with nutrients at levels that are nearly impossible to reach without supplementation164, the direct assessment of the effect of RUTFs on weight gain compared with the effect of a local diet alone has not been carried out. However, children treated with RUTFs have a higher proportion of recovery and weight gain than children who received a local diet supplemented with additional vitamins and minerals7,165.
Despite the benefits associated with use of therapeutics foods, a rapid increase in macronutrient and micronutrient intake can lead to some negative health consequences, including diarrhoea (due to carbohydrate malabsorption)166,167 and refeeding syndrome (BOX 4). The risk of refeeding syndrome might be reduced when children consume therapeutic foods on demand based on their appetite rather than being force-fed using a nasogastric tube168,169. However, no randomized trials testing the formulations, amounts or durations of feeding of F-75 or F-100 have been published.
Box 4. Refeeding syndrome.
Refeeding syndrome can contribute to poor clinical outcomes in children with severe malnutrition. Rapidly increased nutrient intake can cause a switch from a catabolic to an anabolic state, which can lead to a surge in insulin secretion, acute hypoglycaemia and the transport of extracellular electrolytes into cells. This electrolyte flux can lead to dangerously low blood concentrations of potassium, magnesium and phosphate, which can result in lethargy, seizures, muscle weakness, impaired cardiac function and respiratory failure225.
The precise incidence of refeeding syndrome is unknown, which is related to the lack of a precise definition and the fact that, in most settings, the accurate measurement of electrolytes and other biomarkers cannot be carried out. In addition, insufficient intake of electrolytes and their loss through the gastrointestinal and renal systems can affect electrolyte concentrations, making it challenging to relate abnormal plasma levels to refeeding syndrome. A recent study in Uganda reported hypophosphataemia in 76% of children with severe malnutrition 2 days after admission, which was associated with mortality168.
Interestingly, refeeding syndrome influenced the development of the main milk-based refeeding formulation used for the stabilization of patients (F-75), which is characterized by a low protein content and reduced energy content (mostly from carbohydrates) compared with F-100, and contains phosphate, potassium, magnesium, vitamin A and zinc, among other micronutrients. However, as F-75 provides individuals with an energy source that is mostly from carbohydrates, this leads to increased glucose levels and, potentially, a surge in insulin secretion and a high demand for phosphorylated glycolytic intermediates, which could precipitate severe hypophosphataemia. Whether reducing calorie intake or energy from carbohydrates during the initial hospitalization of patients with severe malnutrition would reduce the incidence and severity of refeeding syndrome is currently unknown.
Antibiotics
Current guidelines recommend empiric antibiotics for all children with severe malnutrition because of concerns that although some children might not initially present with signs of infection, they might deteriorate suddenly without fever or other warning signs170,171. No prospective clinical trials showing the need for antibiotics in children with complicated severe malnutrition have been published. However, there are reports of in vitro nonsusceptibility to β-lactam antibiotics and gentamicin, the recommended first-line agents, although the implications of these findings in terms of outcomes have yet to be tested in clinical trials. Interpreting the results of these studies is challenging owing to biases; these studies are usually conducted in tertiary centres and/or do not distinguish community-acquired from hospital-acquired infections172.
For outpatient management of children with uncomplicated, severe malnutrition, the use of routine antibiotic therapy has been questioned because of the cost and the risk of enhancing antimicrobial resistance. In one study in Malawi, children with severe malnutrition receiving amoxicillin or cefdinir had 36% and 44% reductions in mortality, respectively, and they had faster recoveries than children who received placebo5. Of note, this area had a high prevalence of kwashiorkor compared with other centres. In another study in Niger, mortality was unchanged in children with uncomplicated severe wasting who had not been previously treated for malnutrition and who were given oral amoxicillin compared with placebo in an outpatient feeding programme attached to a referral hospital173. However, amoxicillin treatment was associated with a faster recovery and a 14% reduction in the proportion of children who were referred to hospital in another study173. Collectively, evidence from these trials supports the use of empiric antibiotics in outpatient management172,174, although further research on other types and severities of malnutrition, other care settings, the effects on antimicrobial resistance, the potential use of point-of-care biomarkers and costs are needed.
Other interventions
Profuse watery diarrhoea, hypovolaemia and shock are common in children with severe malnutrition and are associated with high mortality, despite current management guidelines99,175. The optimal composition and rate of administration of intravenous or oral fluid therapy, or other supportive therapies, in settings that do not have access to advanced paediatric intensive care is unclear and controversial176 as only two small trials have been conducted to study individuals with severe malnutrition177,178. Another large trial indicated that aggressive fluid management in children without severe malnutrition and diarrhoea, but with some signs of shock, is harmful179, which is likely to be the case in children with malnutrition. Studies that distinguish shock caused by sepsis from hypovolaemia caused by diarrhoea in children with severe malnutrition have not been carried out, but they are needed to inform management strategies that could be implemented in hospital settings.
Other features of severe malnutrition include chronic systemic and intestinal inflammation, infection and environmental enteric dysfunction (that is, inflammation, reduced absorption and reduced barrier function in the small intestine)49. Mortality risk is more closely related to markers of systemic rather than intestinal inflammation102, although bacterial intestinal translocation and aberrant immune function might contribute to both systemic and intestinal inflammation. Trials assessing the use of oral prebiotics or probiotics among children with severe malnutrition have not shown effects on inpatient mortality outcomes, but they do suggest subsequent benefits for growth9,180, and in the future, the use of prebiotics and probiotics are likely to be better directed by improved knowledge of microbiota ontogeny and function112,181. Gut-protective therapies targeting inflammation, microbial translocation, malabsorption, intraluminal nutrient processing and normalization of the intestinal microbiota might yield novel and effective interventions. Other potential strategies for reducing malnutrition-associated mortality and improving growth include the use of anti-inflammatory agents182 and gut-trophic nutrients, including alanyl-glutamine176,183 and other dipeptides.
Uncomplicated severe malnutrition
Until the 2000s, all children with severe malnutrition were usually managed as inpatients with milk-based therapeutic foods and empiric broad-spectrum parenteral antibiotics, which assumed that these patients always had nutritional and metabolic complications and serious, sub-patent infections127. However, management has been revolutionized by the detection of children with uncomplicated malnutrition, who can be safely treated in the community via a proactive, public health-based approach to care. This is made possible by community screening that uses MUAC assessments and by feeding children a RUTF at a dose of 175 kcal/kg per day129,184.
The effectiveness of RUTFs in decreasing the mortality of children with severe malnutrition cannot be overstated. Early RUTF trials reported a lower mortality for children treated with RUTFs in their community than for those previously treated in hospitals. Following these trials, community treatment programmes rapidly expanded, which dramatically improved treatment coverage to reach previously untreated children. Following from this, many community-based programmes have shown lower mortality rates compared with historical data for hospital-based treatment outcomes. Accordingly, the development of community-based programmes has largely occurred in the absence of formal randomized controlled trials (comparing children treated with RUTFs with untreated children), as carrying out these trials would be unethical. For the same reasons, the effectiveness of hospital-based treatment in reducing mortality has never been formally established.
Decentralized services closer to patients’ homes can avoid the costs and risks of inpatient care, including nosocomial infections185,186, and has enabled uncomplicated severe malnutrition (generally >90% of all children with severe malnutrition) to be treated in the community184 (FIG. 5). Outcomes of children with uncomplicated severe malnutrition treated in their community are markedly better than of children with complicated malnutrition of similar anthropometric status187,188.
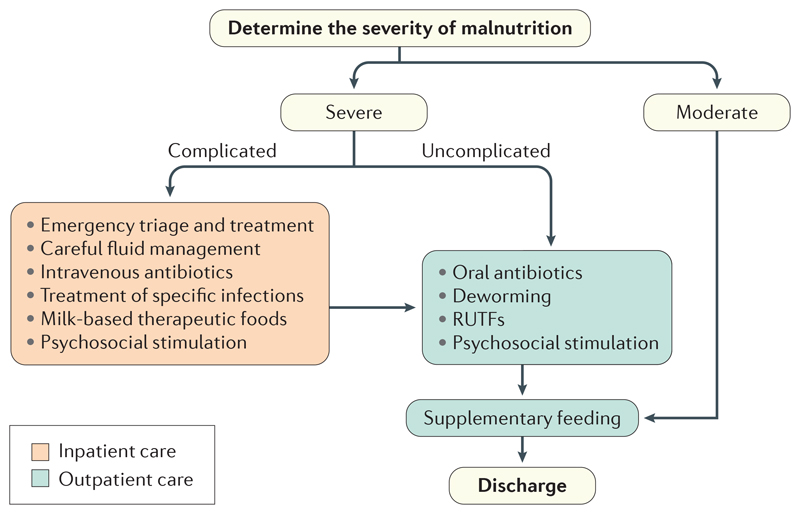
Figure 5. Overview of management of severe and moderate malnutrition.
The first step in the treatment of malnutrition is to determine the severity. Moderate malnutrition is typically treated with the use of supplementary foods, which are administered in addition to the child’s normal diet. Uncomplicated severe malnutrition is usually treated with ready-to-use therapeutic foods (RUTFs), which are given instead of the child’s normal diet, in addition to a course of oral antibiotics and along with other treatments, such as deworming and treatment for other non-life-threatening infections. The management of complicated severe malnutrition requires admission to an inpatient facility with adequate paediatric emergency care facilities and a nutritional rehabilitation care programme. Children with complicated malnutrition can be discharged from inpatient facilities when serious complications have resolved, and subsequently entered into outpatient management programmes. Management includes the use of therapeutic foods and antibiotics and the treatment of other infections. After the completion of treatment for severe malnutrition, children are transitioned from RUTFs to their home diet, which is a potential point of faltering. However, aside from standardized supplementary feeding, strategies to decrease this risk have not been assessed in trials.
Complicated severe malnutrition
Children with complicated severe malnutrition (that is, children with a more-severe disease who are sick) are managed as inpatients so that life-threatening complications (such as infections) can be addressed along with nutritional and metabolic deficits. The aim of nutritional management is to address disturbed carbohydrate and energy metabolism, which affects blood glucose levels and body temperature regulation67,189, as well as the distribution of fluid and electrolytes. Regular feeding with, initially, less energy-dense food aims to prevent and treat hypoglycaemia, reduce sodium levels, and increase potassium and phosphate levels. The administration of hyperosmolar and intravenous fluids is avoided whenever possible190.
Therapeutic feeding is cautiously started with milk-based food (specifically, 130 ml/kg per day of F-75, which provides 97.5 kcal/kg per day) that initially contain low levels of protein and sodium and high levels of potassium, which is designed to match the child’s reduced metabolic capacity. Initial feeding aims to improve metabolism, gut motility and nutrient absorption, and clinical improvements during this phase manifest as improved appetite and reduced oedema (if present). When appetite is restored and the signs of infectious complications improve, children transition to RUTFs or milk-based foods with higher energy and protein levels (such as F-100 at 130–200 ml/kg per day which provides 130–200 kcal/kg per day) to gain weight. No other food should be given, with the exception of breast milk, which should be given before therapeutic foods are administered to keep stimulating the maternal milk supply. Additional micronutrients (such as zinc for diarrhoea) beyond what are included in the therapeutic food are generally unnecessary without specific signs of a deficiency190.
Under current WHO recommendations, children are discharged from inpatient care when their serious complications have resolved rather than after they have met specific anthropometric targets, and they continue therapeutic feeding and supplementary feeding (for moderate malnutrition) as outpatients151 (FIG. 6). The transition of patients from the hospital setting to home requires ensuring that the parent or caregiver understands the treatment phases and what is expected from them in addition to enrolling them in available nutritional, medical and social services.
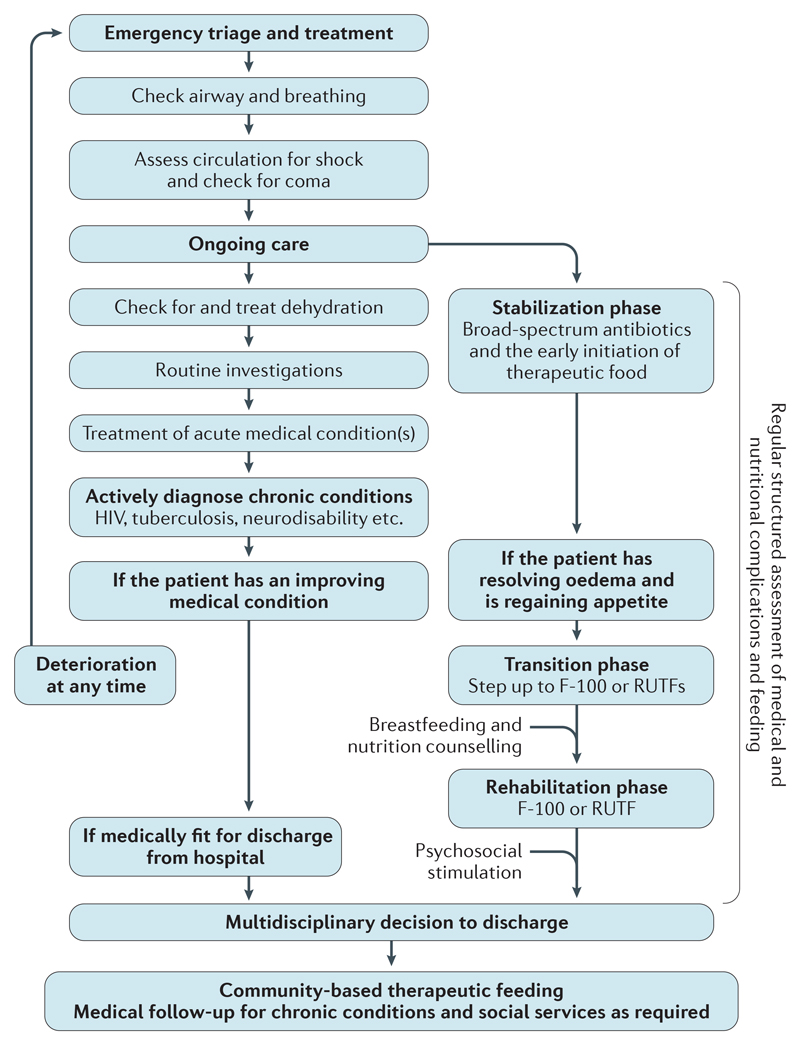
Figure 6. Detailed management of complicated severe malnutrition.
The challenges associated with the management of children with complicated severe malnutrition require the integration of structured, high-quality paediatric care and nutritional support, which must happen in parallel and in a multidisciplinary manner. Paediatric care focuses on the identification and treatment of the most immediately life-threatening problems, as laid out in the WHO Emergency Triage and Treatment guidelines. Nutritional support focuses first on helping to restore physiological processes to a normal state and then on achieving rehabilitation. Initially, use of the ‘ABCD’ mnemonic can help focus attention on the assessment and maintenance of the airway (A) and breathing (B), circulation (C) and disability (coma, convulsion and dehydration (D)). Children with complicated severe malnutrition remain exceptionally vulnerable during treatment and can deteriorate rapidly without clear prior warning signs. Accordingly, monitoring and frequent re-evaluation are essential. When prevalent, comorbidities such as tuberculosis and HIV should be actively tested for. If community-based management of uncomplicated severe malnutrition is available, the decision to discharge children from hospital is based on their appetite and the resolution of complications rather than on anthropometric parameters, and should be decided by nutritional and clinical staff. The treatment process in hospital and after discharge should be explained to parents and caregivers, and they should know what help is available to them and what is expected of them. RUTFs, ready-to-use therapeutic foods.
Children with complicated severe malnutrition are usually admitted to hospital because of severe infections and typically have a fatality rate of 12% to >20%168,191. HIV infection is present in 15–29% of children hospitalized with severe malnutrition in Sub-Saharan Africa and is associated with a threefold increased risk of inpatient mortality168,192,193. Studies aiming to reduce mortality in children with complicated severe malnutrition have included increased nursing support, systematic feeding or reductions in intravenous fluid administration and have been met with limited success194–196, presenting a clear need for research to improve care22,176.
Infants <6 months of age
Infants <6 months of age with severe malnutrition are fed with breast milk and infant formula, or diluted F-100 milk, often by using a technique called ‘supplementary suckling’. A key aim of treatment is to establish or reestablish effective exclusive breastfeeding wherever possible. These infants also receive empiric antibiotics134. Further research is needed to determine the efficacy of this strategy and other components of the management of these infants197.
Quality of life
Children with severe malnutrition typically live in settings of pervasive poverty, low female literacy, food insecurity, inadequate water and sanitation and overstretched health systems198. Acute management interventions for the treatment of severe malnutrition do not fundamentally alter these underlying risks. Most children with acute malnutrition are also severely stunted at presentation7,173, which indicates that they have chronic ill health and malnutrition. Several studies in Malawi, Kenya, Gambia and Bangladesh have shown that anthropometric recovery is not sufficient as children have a high rate of death following discharge from inpatient care for severe malnutrition, predominantly from pneumonia and diarrhoea6,199. In addition, mortality is increased in children following treatment in community-based nutrition programmes184,200. The reasons for this likely contains a home environment that includes risk factors for malnutrition, an undiagnosed chronic illness and that attending health care visits for episodes of illness or ongoing therapeutic feeding might be limited in certain areas200,201. In addition, some children with severe malnutrition might have a pre-existing vulnerability (such as a mild immune deficit that would cause minimal health problems in a resource-rich setting with a low prevalence of infectious diseases) that results in a cycle of illness and malnutrition and a high degree of vulnerability to infection202. Certainly, not all siblings who grow up in the same family are equally affected by the same home environment203.
During treatment and recovery from severe malnutrition, weight and MUAC usually increase rapidly, but height does not, and growth is typically sufficient only to reach the baseline height-for-age Z-score173,203. Children who have been treated for severe malnutrition typically have less lean mass (that is, fat-free mass) than children without malnutrition in the same community162,204,205. Indeed, children in Malawi had lower lean tissue and were more severely stunted at an average of 7 years after hospitalization for severe malnutrition, than controls, but had a similar head circumference and respiratory and cardiometabolic function204. In addition, children with prior severe malnutrition have weaker hand grip and lower exercise tolerance than children who have not had malnutrition204,206. This ‘thrifty’ growth pattern indicates that severe malnutrition in early childhood might predispose an individual to noncommunicable diseases later in life. However, this predisposition is difficult to assess as stunting and other insults might have been present before severe malnutrition developed in the children enrolled in these studies. Although the effect of in utero exposure to malnutrition and its risk factors leading to a wide range of noncommunicable diseases later in life is established207,208, the critical window or risk for these effects and the consequent effect of infant and child malnutrition on noncommunicable diseases later in life is still unclear209.
Severe malnutrition and stunting are associated with delayed child neurodevelopment, behavioural problems, lower school achievement and reduced adult earning potential2,210. The use of psychosocial stimulation, such as play and giving positive feedback training to mothers or prolonged home visiting programmes after infants or young children with severe wasting or kwashiorkor have been discharged from the hospital, has been shown to significantly improve infant development scores in the short term, as well as educational achievement and IQ into adolescence116,211,212. Several micronutrients are potentially essential for cognitive development, including PUFAs, thiamine, choline, vitamin D, folate, iron, copper, selenium and iodine213, and deficiencies in these might vary among children with severe malnutrition according to the child’s age, location and the cause of malnutrition. However, supplementation with these micronutrients has not undergone clinical trials with neurodevelopmental end points in the context of severe malnutrition.
Further studies are needed to assess the optimal duration of treatment with therapeutic foods, factors limiting lean (muscle, organ and connective) tissue synthesis (including the role of high-quality dietary protein), how zinc and other micronutrients can modulate lean tissue deposition, the benefits of psychosocial stimulation and physical activity and the long-term effects of malnutrition on health risks in adolescence and adulthood204.
Outlook
Although this Primer has highlighted many of the negative consequences of severe malnutrition, it is important to note that our understanding of the epidemiology and management of severe malnutrition has improved. Indeed, community-based management of severe malnutrition has revolutionized treatment programmes and has enabled large numbers of children to receive effective nutritional and clinical care in various settings. Children with severe malnutrition and a high risk of death can be easily identified, and many of them can be successfully treated at the community level by parents with assistance from community health workers, in addition to outreach services.
The use of appropriate prevention and management interventions for severe malnutrition could prevent 61% of cases of wasting and almost 350,000 child deaths annually214, and several major international initiatives, such as No Wasted Lives215, are working towards ambitious targets to achieve this. Although progress towards lowering childhood mortality is ongoing, more work is still required. Indeed, as more children survive episodes of severe malnutrition, and as poverty decreases in many countries, new challenges will emerge. For example, helping children to survive an episode of severe malnutrition is no longer enough. Following the Global Strategy for Women’s, Children’s and Adolescents’ Health (2016–2030), we must also help these children to thrive214. To do this, curative and preventive services must be transformed. More effort is needed in previously neglected populations, such as infants <6 months of age216 and children with underlying disabilities217 and other identified vulnerabilities. In addition, focus is needed on the prevention of relapse and readmission, although how this can be achieved is uncertain. One large trial investigating the use of prophylactic co-trimoxazole for high-risk children following discharge from hospital in Kenya showed negative results for reducing the risk of relapse and death6. However, other broad-spectrum antibiotics or other therapies that could support a prolonged period of immune system recovery might be beneficial. In addition, improved social support and social safety nets to address food security are other areas to explore. Other research gaps regarding severe malnutrition are described in BOX 5 (REFS 218,219).
Box 5. Selected research priorities in severe malnutrition.
Determining the burden of severe malnutrition based on its incidence
Determining the causes of death associated with malnutrition, including infection and impaired energy metabolism
Determining the pathophysiology of kwashiorkor and its implications for management
Determining the mechanisms of functional immune impairment and the association with nutritional rehabilitation
Determining the long-term effect on body composition, metabolism and neurodevelopment of severe malnutrition and its treatment, with or without preterm and/or low birth rates
Devising preventive strategies that work on a large scale and are low in cost
Developing strategies for the integration of protocols to prevent severe malnutrition in fragile and humanitarian settings
Improving the management of acute malnutrition in infants <6 months of age197
Improving risk stratification to help identify which patients need inpatient care and those that need empiric antimicrobials, and determining which antimicrobials should be used
Simplifying treatment protocols and developing protocols that can be used at the community level with minimal supervision
Developing new formulations of ready-to-use therapeutic foods that optimize several nutritional parameters to maximize patient survival, weight gain, correction of metabolic perturbations, and long-term cognitive, physical and immunological development
An improved understanding of several areas of the pathophysiology of severe malnutrition and the efficacy of potential interventions might be able to save the lives of these high-risk children. For example, understanding the pathophysiology of kwashiorkor could lead to phenotype-specific therapeutic interventions; this requires research that uses modern tools for assessing metabolism and other physiological functions. In addition, although much of the previous research on the pathophysiology of severe malnutrition has been informative and hypothesis-generating, the research does not commonly meet today’s standards for quality in terms of participant selection, the avoidance of bias and sample size. Going forward, the testing of well-founded hypotheses in appropriately designed randomized trials with the use of biomarkers, information on mechanisms and sound clinical measures is required.
The overarching priority is to make severe malnutrition a thing of the past in countries in Africa and Asia, as is the case in Europe and North America today. Achieving the SDGs for health and nutrition will be possible only through addressing severe malnutrition, and to do this, we must recognize that reducing the burden of morbidity and mortality associated with severe childhood malnutrition is a global moral imperative. To eradicate severe malnutrition will require political will, investment in peace building and reducing man-made and natural disasters, which are issues that should unite the world.
Acknowledgements
The authors are grateful to Grace Belayneh for her thoughtful assistance in the coordination of this work and facilitation of communication among the writing group.
Footnotes
Author contributions
Introduction (Z.A.B.); Epidemiology (M.K. and Z.A.B.); Mechanisms/pathophysiology (R.H.B. and J.A.B.); Diagnosis, screening and prevention (A.B., I.T. and M.K.); Management (J.A.B. and I.T.); Quality of life (J.A.B.); Outlook (Z.A.B., M.K. and A.B.); Overview of Primer (Z.A.B.).
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.United Nations. Sustainable development goal 2. United Nations; 2015. https://sustainabledevelopment.un.org/sdg2. [Google Scholar]
- 2.Black RE, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [This is an important global review of the epidemiology, major risk factors and consequences of malnutrition.] [DOI] [PubMed] [Google Scholar]
- 3.Nandy S, Miranda JJ. Overlooking undernutrition? Using a composite index of anthropometric failure to assess how underweight misses and misleads the assessment of undernutrition in young children. Soc Sci Med. 2008;66:1963–1966. doi: 10.1016/j.socscimed.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald CM, et al. The effect of multiple anthropometric deficits on child mortality: meta-analysis of individual data in 10 prospective studies from developing countries. Am J Clin Nutr. 2013;97:896–901. doi: 10.3945/ajcn.112.047639. [This review of data from ten prospective cohorts summarized the observed association of major anthropometric deficits and the additive effects of infections on risks of death in childhood.] [DOI] [PubMed] [Google Scholar]
- 5.Trehan I, et al. Antibiotics as part of the management of severe acute malnutrition. N Engl J Med. 2013;368:425–435. doi: 10.1056/NEJMoa1202851. [This landmark study demonstrated the beneficial impact on overall mortality and recovery of administering antibiotics to children treated for severe malnutrition.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berkley JA, et al. Daily co-trimoxazole prophylaxis to prevent mortality in children with complicated severe acute malnutrition: a multicentre, double-blind, randomised placebo-controlled trial. Lancet Glob Health. 2016;4:e464–e473. doi: 10.1016/S2214-109X(16)30096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhandari N, et al. Efficacy of three feeding regimens for home-based management of children with uncomplicated severe acute malnutrition: a randomised trial in India. BMJ Glob Health. 2016;1:e000144. doi: 10.1136/bmjgh-2016-000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh JC, et al. High-oleic ready-to-use therapeutic food maintains docosahexaenoic acid status in severe malnutrition. J Pediatr Gastroenterol Nutr. 2015;61:138–143. doi: 10.1097/MPG.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerac M, et al. Probiotics and prebiotics for severe acute malnutrition (PRONUT study): a double-blind efficacy randomised controlled trial in Malawi. Lancet. 2009;374:136–144. doi: 10.1016/S0140-6736(09)60884-9. [DOI] [PubMed] [Google Scholar]
- 10.Katz J, et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet. 2013;382:417–425. doi: 10.1016/S0140-6736(13)60993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Pols-Vijlbrief R, Wijnhoven HA, Schaap LA, Terwee CB, Visser M. Determinants of protein-energy malnutrition in community-dwelling older adults: a systematic review of observational studies. Ageing Res Rev. 2014;18:112–131. doi: 10.1016/j.arr.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Fritsch P. Nutrition interventions for older people in emergencies: HelpAge International. HelpAge International; 2013. http://nutritioncluster.net/wp-content/uploads/sites/4/2015/06/Nutrition-FINAL.pdf. [Google Scholar]
- 13.Gladstone M, et al. Assessment of neurodisability and malnutrition in children in Africa. Semin Pediatr Neurol. 2014;21:50–57. doi: 10.1016/j.spen.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Kerac M, et al. The interaction of malnutrition and neurologic disability in Africa. Semin Pediatr Neurol. 2014;21:42–49. doi: 10.1016/j.spen.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 15.United Nations Children’s Fund, World Health Organization & World Bank Group. Joint child malnutrition estimates 2017. WHO; 2017. http://apps.who.int/gho/data/node.wrapper.nutrition-2016. [Google Scholar]
- 16.Frison S, Checchi F, Kerac M. Omitting edema measurement: how much acute malnutrition are we missing? Am J Clin Nutr. 2015;102:1176–1181. doi: 10.3945/ajcn.115.108282. [This article presented a secondary data analysis of 852 nutrition cross-sectional survey data sets of children 6–59 months of age and showed that the prevalence of oedematous malnutrition varied from 0% to 32.9% and that many children with oedema did not have wasting.] [DOI] [PubMed] [Google Scholar]
- 17.Islam MA, Rahman MM, Mahalanabis D. Maternal and socioeconomic factors and the risk of severe malnutrition in a child: a case–control study. Eur J Clin Nutr. 1994;48:416–424. [PubMed] [Google Scholar]
- 18.Owor M, Tumwine JK, Kikafunda JK. Socio-economic risk factors for severe protein energy malnutrition among children in Mulago Hospital, Kampala. East Afr Med J. 2000;77:471–475. doi: 10.4314/eamj.v77i9.46691. [DOI] [PubMed] [Google Scholar]
- 19.Saloojee H, De Maayer T, Garenne ML, Kahn K. What’s new? Investigating risk factors for severe childhood malnutrition in a high HIV prevalence South African setting. Scand J Public Health Suppl. 2007;69:96–106. doi: 10.1080/14034950701356435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mishra K, Kumar P, Basu S, Rai K, Aneja S. Risk factors for severe acute malnutrition in children below 5 y of age in India: a case–control study. Indian J Pediatr. 2014;81:762–765. doi: 10.1007/s12098-013-1127-3. [DOI] [PubMed] [Google Scholar]
- 21.Ricci JA, Becker S. Risk factors for wasting and stunting among children in Metro Cebu, Philippines. Am J Clin Nutr. 1996;63:966–975. doi: 10.1093/ajcn/63.6.966. [DOI] [PubMed] [Google Scholar]
- 22.Trehan I, O’Hare BA, Phiri A, Heikens GT. Challenges in the management of HIV-infected malnourished children in sub-Saharan Africa. AIDS Res Treat. 2012;2012 doi: 10.1155/2012/790786. 790786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maxwell D, et al. Emergency Food Security Interventions. Humanitarian Practice Network; 2008. [Google Scholar]
- 24.Tolboom JJ, Ralitapole-Maruping AP, Kabir H, Molatseli P, Anderson J. Severe protein energy malnutrition in Lesotho, death and survival in hospital, clinical findings. Trop Geogr Med. 1986;38:351–358. [PubMed] [Google Scholar]
- 25.Institute for Health Metrics and Evaluation. Global Burden of Disease Results Tool. GHDx. 2015 http://ghdx.healthdata.org/gbd-results-tool?params=querytool-permalink/d34931ea04210ce4039fbf42ec0a62c8. [Google Scholar]
- 26.Pelletier DL, Frongillo EA, Jr, Schroeder DG, Habicht JP. The effects of malnutrition on child mortality in developing countries. Bull World Health Organ. 1995;73:443–448. [PMC free article] [PubMed] [Google Scholar]
- 27.Caulfield LE, de Onis M, Blossner M, Black RE. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr. 2004;80:193–198. doi: 10.1093/ajcn/80.1.193. [DOI] [PubMed] [Google Scholar]
- 28.Sethuraman K, Lansdown R, Sullivan K. Women’s empowerment and domestic violence: the role of sociocultural determinants in maternal and child undernutrition in tribal and rural communities in South India. Food Nutr Bull. 2006;27:128–143. doi: 10.1177/156482650602700204. [DOI] [PubMed] [Google Scholar]
- 29.Kinyoki DK, et al. Conflict in Somalia: impact on child undernutrition. BMJ Global Health. 2017;2:e000262. doi: 10.1136/bmjgh-2016-000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Classification of infantile malnutrition. Lancet. 1970;2:302–303. [No authors listed.] [PubMed] [Google Scholar]
- 31.Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14:58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- 32.Argiles JM, Busquets S, Lopez-Soriano FJ. Cytokines in the pathogenesis of cancer cachexia. Curr Opin Clin Nutr Metab Care. 2003;6:401–406. doi: 10.1097/01.mco.0000078983.18774.cc. [DOI] [PubMed] [Google Scholar]
- 33.Grant RW, Stephens JM. Fat in flames: influence of cytokines and pattern recognition receptors on adipocyte lipolysis. Am J Physiol Endocrinol Metab. 2015;309:E205–E213. doi: 10.1152/ajpendo.00053.2015. [DOI] [PubMed] [Google Scholar]
- 34.Bowen TS, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle. 2015;6:197–207. doi: 10.1002/jcsm.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams CD. A nutritional disease of childhood associated with a maize diet. Arch Dis Child. 1933;8:423–433. doi: 10.1136/adc.8.48.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laditan AA, Reeds PJ. A study of the age of onset, diet and the importance of infection in the pattern of severe protein-energy malnutrition in Ibadan, Nigeria. Br J Nutr. 1976;36:411–419. doi: 10.1079/bjn19760096. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan J, Ndekha M, Maker D, Hotz C, Manary MJ. The quality of the diet in Malawian children with kwashiorkor and marasmus. Matern Child Nutr. 2006;2:114–122. doi: 10.1111/j.1740-8709.2006.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coulthard MG. Oedema in kwashiorkor is caused by hypoalbuminaemia. Paediatr Int Child Health. 2015;35:83–89. doi: 10.1179/2046905514Y.0000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coward DG, Whitehead RG. Experimental protein-energy malnutrition in baby baboons. Attempts to reproduce the pathological features of kwashiorkor as seen in Uganda. Br J Nutr. 1972;28:223–237. doi: 10.1079/bjn19720029. [DOI] [PubMed] [Google Scholar]
- 40.Golden MH, Golden BE, Jackson AA. Albumin and nutritional oedema. Lancet. 1980;1:114–116. doi: 10.1016/s0140-6736(80)90603-0. [DOI] [PubMed] [Google Scholar]
- 41.Golden MH. Oedematous malnutrition. Br Med Bull. 1998;54:433–444. doi: 10.1093/oxfordjournals.bmb.a011699. [DOI] [PubMed] [Google Scholar]
- 42.Scrimshaw NS. Historical concepts of interactions, synergism and antagonism between nutrition and infection. J Nutr. 2003;133:316S–321S. doi: 10.1093/jn/133.1.316S. [DOI] [PubMed] [Google Scholar]
- 43.Bourke CD, Berkley JA, Prendergast AJ. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol. 2016;37:386–398. doi: 10.1016/j.it.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rytter MJ, Kolte L, Briend A, Friis H, Christensen VB. The immune system in children with malnutrition — a systematic review. PLoS ONE. 2014;9:e105017. doi: 10.1371/journal.pone.0105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmed T, et al. An evolving perspective about the origins of childhood undernutrition and nutritional interventions that includes the gut microbiome. Ann NY Acad Sci. 2014;1332:22–38. doi: 10.1111/nyas.12487. [This review summarized some of the major insights and hypotheses into how the interplay between microbial, environmental and host factors contribute to severe malnutrition and how this knowledge might be used to develop new therapies against malnutrition.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Assa A, et al. Vitamin D deficiency promotes epithelial barrier dysfunction and intestinal inflammation. J Infect Dis. 2014;210:1296–1305. doi: 10.1093/infdis/jiu235. [DOI] [PubMed] [Google Scholar]
- 47.Wong SC, et al. Growth and the growth hormone–insulin like growth factor 1 axis in children with chronic inflammation: current evidence, gaps in knowledge, and future directions. Endocr Rev. 2016;37:62–110. doi: 10.1210/er.2015-1026. [DOI] [PubMed] [Google Scholar]
- 48.Guerrant RL, et al. Biomarkers of environmental enteropathy, inflammation, stunting, and impaired growth in children in Northeast Brazil. PLoS ONE. 2016;11:e0158772. doi: 10.1371/journal.pone.0158772. [This study of systemic biomarkers of enteropathy and growth predictors in 375 children 6–26 months of age in northeast Brazil demonstrated how environmental enteric dysfunction contributes to growth stunting and predisposes children to severe malnutrition.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Owino V, et al. Environmental enteric dysfunction and growth failure/stunting in global child health. Pediatrics. 2016;138:e20160641. doi: 10.1542/peds.2016-0641. [DOI] [PubMed] [Google Scholar]
- 50.Naylor C, Petri WA., Jr Leptin regulation of immune responses. Trends Mol Med. 2016;22:88–98. doi: 10.1016/j.molmed.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 51.Takele Y, et al. Malnutrition in healthy individuals results in increased mixed cytokine profiles, altered neutrophil subsets and function. PLoS ONE. 2016;11:e0157919. doi: 10.1371/journal.pone.0157919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hughes SM, et al. Dendritic cell anergy results from endotoxemia in severe malnutrition. J Immunol. 2009;183:2818–2826. doi: 10.4049/jimmunol.0803518. [DOI] [PubMed] [Google Scholar]
- 53.Hoang T, Agger EM, Cassidy JP, Christensen JP, Andersen P. Protein energy malnutrition during vaccination has limited influence on vaccine efficacy but abolishes immunity if administered during Mycobacterium tuberculosis infection. Infect Immun. 2015;83:2118–2126. doi: 10.1128/IAI.03030-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saucillo DC, Gerriets VA, Sheng J, Rathmell JC, Maciver NJ. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J Immunol. 2014;192:136–144. doi: 10.4049/jimmunol.1301158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palacio A, Lopez M, Perez-Bravo F, Monkeberg F, Schlesinger L. Leptin levels are associated with immune response in malnourished infants. J Clin Endocrinol Metab. 2002;87:3040–3046. doi: 10.1210/jcem.87.7.8636. [DOI] [PubMed] [Google Scholar]
- 56.Kerpel-Fronius E, Kaiser E. Hypoglycaemia in infantile malnutrition. Acta Paediatr Scand Suppl. 1967;172:119–+. doi: 10.1111/j.1651-2227.1967.tb15286.x. [DOI] [PubMed] [Google Scholar]
- 57.Golden M. The effects of malnutrition in the metabolism of children. Trans R Soc Trop Med Hyg. 1988;82:3–6. doi: 10.1016/0035-9203(88)90245-3. [DOI] [PubMed] [Google Scholar]
- 58.Whitehead RG, Alleyne GA. Pathophysiological factors of importance in protein-calorie malnutrition. Br Med Bull. 1972;28:72–79. doi: 10.1093/oxfordjournals.bmb.a070897. [DOI] [PubMed] [Google Scholar]
- 59.Jahoor F, Badaloo A, Reid M, Forrester T. Protein metabolism in severe childhood malnutrition. Ann Trop Paediatr. 2008;28:87–101. doi: 10.1179/146532808X302107. [DOI] [PubMed] [Google Scholar]
- 60.Jahoor F, Badaloo A, Reid M, Forrester T. Protein kinetic differences between children with edematous and nonedematous severe childhood undernutrition in the fed and postabsorptive states. Am J Clin Nutr. 2005;82:792–800. doi: 10.1093/ajcn/82.4.792. [DOI] [PubMed] [Google Scholar]
- 61.Jahoor F, Badaloo A, Reid M, Forrester T. Sulfur amino acid metabolism in children with severe childhood undernutrition: methionine kinetics. Am J Clin Nutr. 2006;84:1400–1405. doi: 10.1093/ajcn/84.6.1400. [DOI] [PubMed] [Google Scholar]
- 62.Lewis B, Wittmann W, Krut LH, Hansen JD, Brock JF. Free fatty acid flux through plasma in protein malnutrition of infants. Clin Sci. 1966;30:371–375. [PubMed] [Google Scholar]
- 63.Badaloo AV, Forrester T, Reid M, Jahoor F. Lipid kinetic differences between children with kwashiorkor and those with marasmus. Am J Clin Nutr. 2006;83:1283–1288. doi: 10.1093/ajcn/83.6.1283. [DOI] [PubMed] [Google Scholar]
- 64.Bartz S, et al. Severe acute malnutrition in childhood: hormonal and metabolic status at presentation, response to treatment, and predictors of mortality. J Clin Endocrinol Metab. 2014;99:2128–2137. doi: 10.1210/jc.2013-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wharton B. Hypoglycaemia in children with kwashiorkor. Lancet. 1970;1:171–173. doi: 10.1016/s0140-6736(70)90408-3. [DOI] [PubMed] [Google Scholar]
- 66.Bandsma RH, et al. Mechanisms behind decreased endogenous glucose production in malnourished children. Pediatr Res. 2010;68:423–428. doi: 10.1203/PDR.0b013e3181f2b959. [DOI] [PubMed] [Google Scholar]
- 67.Spoelstra MN, et al. Kwashiorkor and marasmus are both associated with impaired glucose clearance related to pancreatic β-cell dysfunction. Metabolism. 2012;61:1224–1230. doi: 10.1016/j.metabol.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 68.Becker DJ, Pimstone BL, Hansen JD, MacHutchon B, Drysdale A. Patterns of insulin response to glucose in protein-calorie malnutrition. Am J Clin Nutr. 1972;25:499–505. doi: 10.1093/ajcn/25.5.499. [DOI] [PubMed] [Google Scholar]
- 69.Baig HA, Edozien JC. Carbohydrate metabolism in kwashiorkor. Lancet. 1965;2:662–665. doi: 10.1016/s0140-6736(65)90397-1. [DOI] [PubMed] [Google Scholar]
- 70.Reis MA, et al. Glucose-induced insulin secretion is impaired and insulin-induced phosphorylation of the insulin receptor and insulin receptor substrate-1 are increased in protein-deficient rats. J Nutr. 1997;127:403–410. doi: 10.1093/jn/127.3.403. [DOI] [PubMed] [Google Scholar]
- 71.Ferreira F, et al. Decreased insulin secretion in islets from rats fed a low protein diet is associated with a reduced PKAα expression. J Nutr. 2004;134:63–67. doi: 10.1093/jn/134.1.63. [DOI] [PubMed] [Google Scholar]
- 72.Soriano S, et al. Reduced insulin secretion in protein malnourished mice is associated with multiple changes in the β-cell stimulus-secretion coupling. Endocrinology. 2010;151:3543–3554. doi: 10.1210/en.2010-0008. [DOI] [PubMed] [Google Scholar]
- 73.Robinson HM, Seakins A. Fasting pancreatic glucagon in Jamaican children during malnutrition and subsequent recovery. Pediatr Res. 1982;16:1011–1015. doi: 10.1203/00006450-198212000-00008. [DOI] [PubMed] [Google Scholar]
- 74.Jaya Rao KS, Srikantia SG, Gopalan C. Plasma cortisol levels in protein-calorie malnutrition. Arch Dis Child. 1968;43:365–367. doi: 10.1136/adc.43.229.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paisley RB, Angers M, Frenk S. Plasma cortisol levels in malnourished children with and without superimposed acute stress. Arch Dis Child. 1973;48:714–716. doi: 10.1136/adc.48.9.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kerpel-Fronius E. Pathophysiology of Infantile Malnutrition: Protein-Energy Malnutrition and Failure to Thrive. Akademiai Kiado; 1983. [Google Scholar]
- 77.Kalk WJ, et al. Thyroid hormone and carrier protein interrelationships in children recovering from kwashiorkor. Am J Clin Nutr. 1986;43:406–413. doi: 10.1093/ajcn/43.3.406. [DOI] [PubMed] [Google Scholar]
- 78.Becker K, et al. Effects of antioxidants on glutathione levels and clinical recovery from the malnutrition syndrome kwashiorkor — a pilot study. Redox Rep. 2005;10:215–226. doi: 10.1179/135100005X70161. [DOI] [PubMed] [Google Scholar]
- 79.Fechner A, et al. Antioxidant status and nitric oxide in the malnutrition syndrome kwashiorkor. Pediatr Res. 2001;49:237–243. doi: 10.1203/00006450-200102000-00018. [DOI] [PubMed] [Google Scholar]
- 80.Golden MH, Ramdath D. Free radicals in the pathogenesis of kwashiorkor. Proc Nutr Soc. 1987;46:53–68. doi: 10.1079/pns19870008. [DOI] [PubMed] [Google Scholar]
- 81.Sive AA, Subotzky EF, Malan H, Dempster WS, Heese HD. Red blood cell antioxidant enzyme concentrations in kwashiorkor and marasmus. Ann Trop Paediatr. 1993;13:33–38. doi: 10.1080/02724936.1993.11747622. [DOI] [PubMed] [Google Scholar]
- 82.Manary MJ, Leeuwenburgh C, Heinecke JW. Increased oxidative stress in kwashiorkor. J Pediatr. 2000;137:421–424. doi: 10.1067/mpd.2000.107512. [DOI] [PubMed] [Google Scholar]
- 83.Ciliberto H, et al. Antioxidant supplementation for the prevention of kwashiorkor in Malawian children: randomised, double blind, placebo controlled trial. BMJ. 2005;330:1109. doi: 10.1136/bmj.38427.404259.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Zutphen T, et al. Malnutrition-associated liver steatosis and ATP depletion is caused by peroxisomal and mitochondrial dysfunction. J Hepatol. 2016;65:1198–1208. doi: 10.1016/j.jhep.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 85.Brooks SE, Doherty JF, Golden MH. Peroxisomes and the hepatic pathology of childhood malnutrition. West Indian Med J. 1994;43:15–17. [PubMed] [Google Scholar]
- 86.Ruggieri AJ, Levy RJ, Deutschman CS. Mitochondrial dysfunction and resuscitation in sepsis. Crit Care Clin. 2010;26:567–575. doi: 10.1016/j.ccc.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leite HP, de Lima LF. Metabolic resuscitation in sepsis: a necessary step beyond the hemodynamic? J Thorac Dis. 2016;8:E552–E557. doi: 10.21037/jtd.2016.05.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Webb JG, Kiess MC, Chan-Yan CC. Malnutrition and the heart. CMAJ. 1986;135:753–758. [PMC free article] [PubMed] [Google Scholar]
- 89.Wharton BA, Howells GR, McCance RA. Cardiac failure in kwashiorkor. Lancet. 1967;2:384–387. doi: 10.1016/s0140-6736(67)92006-5. [DOI] [PubMed] [Google Scholar]
- 90.El-Sayed HL, Nassar MF, Habib NM, Elmasry OA, Gomaa SM. Structural and functional affection of the heart in protein energy malnutrition patients on admission and after nutritional recovery. Eur J Clin Nutr. 2006;60:502–510. doi: 10.1038/sj.ejcn.1602344. [DOI] [PubMed] [Google Scholar]
- 91.Silverman JA, et al. The effects of malnutrition on cardiac function in African children. Arch Dis Child. 2016;101:166–171. doi: 10.1136/archdischild-2015-309188. [DOI] [PubMed] [Google Scholar]
- 92.Gruber C, et al. Myocardial remodelling in left ventricular atrophy induced by caloric restriction. J Anat. 2012;220:179–185. doi: 10.1111/j.1469-7580.2011.01453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Badaloo A, Reid M, Soares D, Forrester T, Jahoor F. Relation between liver fat content and the rate of VLDL apolipoprotein B-100 synthesis in children with protein-energy malnutrition. Am J Clin Nutr. 2005;81:1126–1132. doi: 10.1093/ajcn/81.5.1126. [DOI] [PubMed] [Google Scholar]
- 94.Lewis B, Hansen JD, Wittman W, Krut LH, Stewart F. Plasma free fatty acids in kwashiorkor and the pathogenesis of the fatty liver. Am J Clin Nutr. 1964;15:161–168. doi: 10.1093/ajcn/15.3.161. [DOI] [PubMed] [Google Scholar]
- 95.Waterlow JC. Oxidative phosphorylation in the livers of normal and malnourished human infants. Proc R Soc Lond B Biol Sci. 1961;155:96–114. doi: 10.1098/rspb.1961.0059. [DOI] [PubMed] [Google Scholar]
- 96.Brooks SE, Goldon MH, Taylor E. Hepatic ultrastructure in children with protein-energy malnutrition. West Indian Med J. 1992;41:139–145. [PubMed] [Google Scholar]
- 97.Morlese JF, et al. Albumin kinetics in edematous and nonedematous protein-energy malnourished children. Am J Clin Nutr. 1996;64:952–959. doi: 10.1093/ajcn/64.6.952. [DOI] [PubMed] [Google Scholar]
- 98.Keusch GT, et al. Implications of acquired environmental enteric dysfunction for growth and stunting in infants and children living in low- and middle-income countries. Food Nutr Bull. 2013;34:357–364. doi: 10.1177/156482651303400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Irena AH, Mwambazi M, Mulenga V. Diarrhea is a major killer of children with severe acute malnutrition admitted to inpatient set-up in Lusaka, Zambia. Nutr J. 2011;10:110. doi: 10.1186/1475-2891-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maitland K, et al. Children with severe malnutrition: can those at highest risk of death be identified with the WHO protocol? PLoS Med. 2006;3:e500. doi: 10.1371/journal.pmed.0030500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Talbert A, et al. Diarrhoea complicating severe acute malnutrition in Kenyan children: a prospective descriptive study of risk factors and outcome. PLoS ONE. 2012;7:e38321. doi: 10.1371/journal.pone.0038321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Attia S, et al. Mortality in children with complicated severe acute malnutrition is related to intestinal and systemic inflammation: an observational cohort study. Am J Clin Nutr. 2016;104:1441–1449. doi: 10.3945/ajcn.116.130518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bartels RH, et al. Both exocrine pancreatic insufficiency and signs of pancreatic inflammation are prevalent in children with complicated severe acute malnutrition: an observational study. J Pediatr. 2016;174:165–170. doi: 10.1016/j.jpeds.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 104.Zhang L, et al. Impaired bile acid homeostasis in children with severe acute malnutrition. PLoS ONE. 2016;11:e0155143. doi: 10.1371/journal.pone.0155143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schneider RE, Viteri FE. Luminal events of lipid absorption in protein-calorie malnourished children; relationship with nutritional recovery and diarrhea. II. Alterations in bile acid content of duodenal aspirates. Am J Clin Nutr. 1974;27:788–796. doi: 10.1093/ajcn/27.8.788. [DOI] [PubMed] [Google Scholar]
- 106.Murphy JL, et al. Maldigestion and malabsorption of dietary lipid during severe childhood malnutrition. Arch Dis Child. 2002;87:522–525. doi: 10.1136/adc.87.6.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kvissberg MA, et al. Carbohydrate malabsorption in acutely malnourished children and infants: a systematic review. Nutr Rev. 2016;74:48–58. doi: 10.1093/nutrit/nuv058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Subramanian S, et al. Cultivating healthy growth and nutrition through the gut microbiota. Cell. 2015;161:36–48. doi: 10.1016/j.cell.2015.03.013. [This important review indicated that microbiota assembly is perturbed in children with malnutrition, resulting in persistent microbiota immaturity that is not rescued by current nutritional interventions and could be related to the pathogenesis and sequelae of malnutrition.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smith MI, et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science. 2013;339:548–554. doi: 10.1126/science.1229000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Subramanian S, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;510:417–421. doi: 10.1038/nature13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kristensen KH, et al. Gut microbiota in children hospitalized with oedematous and non-oedematous severe acute malnutrition in Uganda. PLoS Negl Trop Dis. 2016;10:e0004369. doi: 10.1371/journal.pntd.0004369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reyes A, et al. Gut DNA viromes of Malawian twins discordant for severe acute malnutrition. Proc Natl Acad Sci USA. 2015;112:11941–11946. doi: 10.1073/pnas.1514285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Klahr S, Alleyne GA. Effects of chronic protein-calorie malnutrition on the kidney. Kidney Int. 1973;3:129–141. doi: 10.1038/ki.1973.21. [DOI] [PubMed] [Google Scholar]
- 114.Gunston GD, Burkimsher D, Malan H, Sive AA. Reversible cerebral shrinkage in kwashiorkor: an MRI study. Arch Dis Child. 1992;67:1030–1032. doi: 10.1136/adc.67.8.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Atalabi OM, Lagunju IA, Tongo OO, Akinyinka OO. Cranial magnetic resonance imaging findings in kwashiorkor. Int J Neurosci. 2010;120:23–27. doi: 10.3109/00207450903315727. [DOI] [PubMed] [Google Scholar]
- 116.Grantham-McGregor S, Powell C, Walker S, Chang S, Fletcher P. The long-term follow-up of severely malnourished children who participated in an intervention program. Child Dev. 1994;65:428–439. [This landmark study demonstrated how children with severe malnutrition benefit from early and sustained psychosocial interventions in addition to nutritional rehabilitation.] [PubMed] [Google Scholar]
- 117.Nahar B, et al. Effects of psychosocial stimulation on growth and development of severely malnourished children in a nutrition unit in Bangladesh. Eur J Clin Nutr. 2009;63:725–731. doi: 10.1038/ejcn.2008.44. [DOI] [PubMed] [Google Scholar]
- 118.Grantham-McGregor S, et al. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369:60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Walker SP, et al. Child development: risk factors for adverse outcomes in developing countries. Lancet. 2007;369:145–157. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- 120.Patrick J, Golden M. Leukocyte electrolytes and sodium transport in protein energy malnutrition. Am J Clin Nutr. 1977;30:1478–1481. doi: 10.1093/ajcn/30.9.1478. [DOI] [PubMed] [Google Scholar]
- 121.Lang F. Mechanisms and significance of cell volume regulation. J Am Coll Nutr. 2007;26:613S–623S. doi: 10.1080/07315724.2007.10719667. [DOI] [PubMed] [Google Scholar]
- 122.World Health Organization Expert Committee. Physical status: the use and interpretation of anthropometry. Geneva: WHO; 1995. http://www.who.int/childgrowth/publications/physical_status/en/index.html. [PubMed] [Google Scholar]
- 123.Cederholm T, et al. Diagnostic criteria for malnutrition — an ESPEN Consensus Statement. Clin Nutr. 2015;34:335–340. doi: 10.1016/j.clnu.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 124.Gomez F, et al. Mortality in second and third degree malnutrition. J Trop Pediatr (Lond.) 1956;2:77–83. doi: 10.1093/oxfordjournals.tropej.a057419. [DOI] [PubMed] [Google Scholar]
- 125.Waterlow JC, et al. The presentation and use of height and weight data for comparing the nutritional status of groups of children under the age of 10 years. Bull World Health Organ. 1977;55:489–498. [PMC free article] [PubMed] [Google Scholar]
- 126.de Onis M, Garza C, Onyango AW, Martorell R. WHO child growth standards. Acta Paediatr. 2006;450:S1–S101. [Google Scholar]
- 127.World Health Organization. Management of Severe Malnutrition: A Manual for Physicians and Other Senior Health Workers. WHO; 1999. [Google Scholar]
- 128.Pelletier DL. The relationship between child anthropometry and mortality in developing countries: implications for policy, programs and future research. J Nutr. 1994;124:2047S–2081S. doi: 10.1093/jn/124.suppl_10.2047S. [DOI] [PubMed] [Google Scholar]
- 129.Myatt M, Khara T, Collins S. A review of methods to detect cases of severely malnourished children in the community for their admission into community-based therapeutic care programs. Food Nutr Bull. 2006;27:S7–S23. doi: 10.1177/15648265060273S302. [DOI] [PubMed] [Google Scholar]
- 130.Briend A, Khara T, Dolan C. Wasting and stunting — similarities and differences: policy and programmatic implications. Food Nutr Bull. 2015;36:S15–S23. doi: 10.1177/15648265150361S103. [This review highlighted the physiological, epidemiological and clinical similarities and differences between wasting and stunting, emphasizing that an integrated and thoughtful approach toward children with both was the most effective in decreasing morbidity and mortality.] [DOI] [PubMed] [Google Scholar]
- 131.World Health Organization & UNICEF. WHO Child Growth Standards and the Identification of Severe Acute Malnutrition in Infants and Children. WHO; 2009. [PubMed] [Google Scholar]
- 132.Briend A, Maire B, Fontaine O, Garenne M. Mid-upper arm circumference and weight-for-height to identify high-risk malnourished under-five children. Matern Child Nutr. 2012;8:130–133. doi: 10.1111/j.1740-8709.2011.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mwangome MK, Fegan G, Mbunya R, Prentice AM, Berkley JA. Reliability and accuracy of anthropometry performed by community health workers among infants under 6 months in rural Kenya. Trop Med Int Health. 2012;17:622–629. doi: 10.1111/j.1365-3156.2012.02959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.ENN/UCL/ACF. MAMI Project. Technical review: current evidence, policies, practices & programme outcomes. ENN. 2010 http://www.ennonline.net/mamitechnicalreview. [Google Scholar]
- 135.Kerac M, et al. Prevalence of wasting among under 6-month-old infants in developing countries and implications of new case definitions using WHO growth standards: a secondary data analysis. Arch Dis Child. 2011;96:1008–1013. doi: 10.1136/adc.2010.191882. [This analysis brought important attention to the growing need to address malnutrition in children <6 months of age.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lelijveld N, Kerac M, McGrath M, Mwangome M, Berkley JA. A Review of Methods to Detect Cases of Severely Malnourished Infants Less Than 6 Months for their Admission into Therapeutic Care. ENN; 2017. [Google Scholar]
- 137.Mwangome MK, Fegan G, Fulford T, Prentice AM, Berkley JA. Mid-upper arm circumference at age of routine infant vaccination to identify infants at elevated risk of death: a retrospective cohort study in the Gambia. Bull World Health Organ. 2012;90:887–894. doi: 10.2471/BLT.12.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mwangome M, et al. Diagnostic criteria for severe acute malnutrition among infants aged under 6 mo. Am J Clin Nutr. 2017;105:1415–1423. doi: 10.3945/ajcn.116.149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Briend A, Zimicki S. Validation of arm circumference as an indicator of risk of death in one to four year old children. Nutr Res. 1986;6:249–261. [Google Scholar]
- 140.Blackwell N, et al. Mothers Understand And Can do it (MUAC): a comparison of mothers and community health workers determining mid-upper arm circumference in 103 children aged from 6 months to 5 years. Arch Public Health. 2015;73:26. doi: 10.1186/s13690-015-0074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tam Y, Huicho L, Huayanay-Espinoza CA, Restrepo-Mendez MC. Remaining missed opportunities of child survival in Peru: modelling mortality impact of universal and equitable coverage of proven interventions. BMC Public Health. 2016;16:1048. doi: 10.1186/s12889-016-3668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Monteiro CA, et al. Narrowing socioeconomic inequality in child stunting: the Brazilian experience, 1974-2007. Bull World Health Organ. 2010;88:305–311. doi: 10.2471/BLT.09.069195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huicho L, et al. Factors behind the success story of under-five stunting in Peru: a district ecological multilevel analysis. BMC Pediatr. 2017;17:29. doi: 10.1186/s12887-017-0790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vaivada T, Gaffey MF, Das JK, Bhutta ZA. Evidence-based interventions for improvement of maternal and child nutrition in low-income settings: what’s new? Curr Opin Clin Nutr Metab Care. 2017;20:204–210. doi: 10.1097/MCO.0000000000000365. [DOI] [PubMed] [Google Scholar]
- 145.Bhutta ZA, et al. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet. 2013;382:452–477. doi: 10.1016/S0140-6736(13)60996-4. [This comprehensive review provided an essential data-driven overview of what interventions might be most cost-effective in decreasing the burden of maternal and child malnutrition globally and what the benefits might be on a global scale.] [DOI] [PubMed] [Google Scholar]
- 146.Nabwera HM, Fulford AJ, Moore SE, Prentice AM. Growth faltering in rural Gambian children after four decades of interventions: a retrospective cohort study. Lancet Glob Health. 2017;5:e208–e216. doi: 10.1016/S2214-109X(16)30355-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ashorn P, et al. Supplementation of maternal diets during pregnancy and for 6 months postpartum and infant diets thereafter with small-quantity lipid-based nutrient supplements does not promote child growth by 18 months of age in rural Malawi: a randomized controlled trial. J Nutr. 2015;145:1345–1353. doi: 10.3945/jn.114.207225. [DOI] [PubMed] [Google Scholar]
- 148.Adu-Afarwuah S, et al. Small-quantity, lipid-based nutrient supplements provided to women during pregnancy and 6 mo postpartum and to their infants from 6 mo of age increase the mean attained length of 18-mo-old children in semi-urban Ghana: a randomized controlled trial. Am J Clin Nutr. 2016;104:797–808. doi: 10.3945/ajcn.116.134692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Adu-Afarwuah S, et al. Lipid-based nutrient supplement increases the birth size of infants of primiparous women in Ghana. Am J Clin Nutr. 2015;101:835–846. doi: 10.3945/ajcn.114.091546. [DOI] [PubMed] [Google Scholar]
- 150.Hess SY, et al. Small-quantity lipid-based nutrient supplements, regardless of their zinc content, increase growth and reduce the prevalence of stunting and wasting in young Burkinabe children: a cluster-randomized trial. PLoS ONE. 2015;10:e0122242. doi: 10.1371/journal.pone.0122242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.World Health Organization. Essential Nutrition Actions: Improving Maternal, Newborn, Infant and Young Child Health and Nutrition. WHO; 2013. [PubMed] [Google Scholar]
- 152.World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. WHO; 2016. [PubMed] [Google Scholar]
- 153.Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2012;8:CD003517. doi: 10.1002/14651858.CD003517.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.World Health Organization. Towards a Grand Convergence for Child Survival and Health: A Strategic Revie of Options for the Future Building on Lessons Learnt from IMNCI. WHO; 2016. [Google Scholar]
- 155.Annan RA, Webb P, Brown R. Management of moderate acute malnutrition (MAM): Current Knowledge and Practice. CMAM Forum; 2014. [Google Scholar]
- 156.Picot J, et al. The effectiveness of interventions to treat severe acute malnutrition in young children: a systematic review. Health Technol Assess. 2012;16:1–316. doi: 10.3310/hta16190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tickell KD, Denno DM. Inpatient management of children with severe acute malnutrition: a review of WHO guidelines. Bull World Health Organ. 2016;94:642–651. doi: 10.2471/BLT.15.162867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gross R, Webb P. Wasting time for wasted children: severe child undernutrition must be resolved in non-emergency settings. Lancet. 2006;367:1209–1211. doi: 10.1016/S0140-6736(06)68509-7. [DOI] [PubMed] [Google Scholar]
- 159.Murray E, Manary M. Home-based therapy for severe acute malnutrition with ready-to-use food. Paediatr Int Child Health. 2014;34:266–270. doi: 10.1179/2046905514Y.0000000135. [This review provided historical context on the evolution and widespread acceptance of RUTFs in making it possible to care for children with severe malnutrition in the community.] [DOI] [PubMed] [Google Scholar]
- 160.World Health Organization. Community-Based Management of Severe Acute Malnutrition. WHO; 2007. World Food Programme, United Nations System Standing Committee on Nutrition & United Nations Children’s Fund. [Google Scholar]
- 161.Oakley E, et al. A ready-to-use therapeutic food containing 10% milk is less effective than one with 25% milk in the treatment of severely malnourished children. J Nutr. 2010;140:2248–2252. doi: 10.3945/jn.110.123828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bahwere P, et al. Cereals and pulse-based ready-to-use therapeutic food as an alternative to the standard milk- and peanut paste-based formulation for treating severe acute malnutrition: a noninferiority, individually randomized controlled efficacy clinical trial. Am J Clin Nutr. 2016;103:1145–1161. doi: 10.3945/ajcn.115.119537. [DOI] [PubMed] [Google Scholar]
- 163.Jones KD, et al. Ready-to-use therapeutic food with elevated n-3 polyunsaturated fatty acid content, with or without fish oil, to treat severe acute malnutrition: a randomized controlled trial. BMC Med. 2015;13:93. doi: 10.1186/s12916-015-0315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ferguson EL, Briend A, Darmon N. Can optimal combinations of local foods achieve the nutrient density of the F100 catch-up diet for severe malnutrition? J Pediatr Gastroenterol Nutr. 2008;46:447–452. doi: 10.1097/MPG.0b013e318156cf5c. [DOI] [PubMed] [Google Scholar]
- 165.Manary MJ, Ndkeha MJ, Ashorn P, Maleta K, Briend A. Home based therapy for severe malnutrition with ready-to-use food. Arch Dis Child. 2004;89:557–561. doi: 10.1136/adc.2003.034306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bandsma RH, et al. Impaired glucose absorption in children with severe malnutrition. J Pediatr. 2011;158:282–287.e1. doi: 10.1016/j.jpeds.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 167.Nyeko R, Kalyesubula I, Mworozi E, Bachou H. Lactose intolerance among severely malnourished children with diarrhoea admitted to the nutrition unit, Mulago hospital, Uganda. BMC Pediatr. 2010;10:31. doi: 10.1186/1471-2431-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rytter MJ, et al. Risk factors for death in children during inpatient treatment of severe acute malnutrition: a prospective cohort study. Am J Clin Nutr. 2017;105:494–502. doi: 10.3945/ajcn.116.140822. [DOI] [PubMed] [Google Scholar]
- 169.Vemula P, Abela OG, Narisetty K, Rhine D, Abela GS. Potassium toxicity at low serum potassium levels with refeeding syndrome. Am J Cardiol. 2015;115:147–149. doi: 10.1016/j.amjcard.2014.09.038. [DOI] [PubMed] [Google Scholar]
- 170.Chisti MJ, Salam MA, Sharifuzzaman, Pietroni MA. Occult pneumonia: an unusual but perilous entity presenting with severe malnutrition and dehydrating diarrhoea. J Health Popul Nutr. 2009;27:808–812. doi: 10.3329/jhpn.v27i6.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jones KDJ, Berkley JA. Severe Acute Malnutrition and Infection. CMAM Forum; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Williams PCM, Berkley JA. Severe Acute Malnutrition Update: Current WHO Guidelines and the WHO Essential Medicine List for Children. WHO; 2016. [Google Scholar]
- 173.Isanaka S, et al. Routine amoxicillin for uncomplicated severe acute malnutrition in children. N Engl J Med. 2016;374:444–453. doi: 10.1056/NEJMoa1507024. [DOI] [PubMed] [Google Scholar]
- 174.Million M, Lagier JC, Raoult D. Meta-analysis on efficacy of amoxicillin in uncomplicated severe acute malnutrition. Microb Pathog. 2017;106:76–77. doi: 10.1016/j.micpath.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 175.Waterlow J. Treatment of children with malnutrition and diarrhoea. Lancet. 1999;354:1142. doi: 10.1016/S0140-6736(99)00329-3. [DOI] [PubMed] [Google Scholar]
- 176.Brewster DR. Inpatient management of severe malnutrition: time for a change in protocol and practice. Ann Trop Paediatr. 2011;31:97–107. doi: 10.1179/146532811X12925735813887. [DOI] [PubMed] [Google Scholar]
- 177.Akech S, et al. Volume expansion with albumin compared to gelofusine in children with severe malaria: results of a controlled trial. PLoS Clin Trials. 2006;1:e21. doi: 10.1371/journal.pctr.0010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Akech SO, Karisa J, Nakamya P, Boga M, Maitland K. Phase II trial of isotonic fluid resuscitation in Kenyan children with severe malnutrition and hypovolaemia. BMC Pediatr. 2010;10:71. doi: 10.1186/1471-2431-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Maitland K, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 180.Grenov B, et al. Effect of probiotics on diarrhea in children with severe acute malnutrition: a randomized controlled study in Uganda. J Pediatr Gastroenterol Nutr. 2017;64:396–403. doi: 10.1097/MPG.0000000000001515. [DOI] [PubMed] [Google Scholar]
- 181.Velly H, Britton RA, Preidis GA. Mechanisms of cross-talk between the diet, the intestinal microbiome, and the undernourished host. Gut Microbes. 2017;8:98–112. doi: 10.1080/19490976.2016.1267888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jones KDJ, et al. Mesalazine in the initial management of severely acutely malnourished children with environmental enteric dysfunction: a pilot randomized controlled trial. BMC Med. 2014;12:133. doi: 10.1186/s12916-014-0133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lima AA, et al. Effects of glutamine alone or in combination with zinc and vitamin A on growth, intestinal barrier function, stress and satiety-related hormones in Brazilian shantytown children. Clinics (Sao Paulo) 2014;69:225–233. doi: 10.6061/clinics/2014(04)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Trehan I, Manary MJ. Management of severe acute malnutrition in low-income and middle-income countries. Arch Dis Child. 2015;100:283–287. doi: 10.1136/archdischild-2014-306026. [DOI] [PubMed] [Google Scholar]
- 185.Aiken AM, et al. Risk and causes of paediatric hospital-acquired bacteraemia in Kilifi District Hospital, Kenya: a prospective cohort study. Lancet. 2011;378:2021–2027. doi: 10.1016/S0140-6736(11)61622-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bachmann MO. Cost-effectiveness of community-based treatment of severe acute malnutrition in children. Expert Rev Pharmacoecon Outcomes Res. 2010;10:605–612. doi: 10.1586/erp.10.54. [DOI] [PubMed] [Google Scholar]
- 187.Bahwere P, Mtimuni A, Sadler K, Banda T, Collins S. Long term mortality after community and facility based treatment of severe acute malnutrition: analysis of data from Bangladesh, Kenya, Malawi and Niger. J Public Health Epidemiol. 2012;4:215–225. [Google Scholar]
- 188.Shewade HD, et al. Effectiveness of indigenous ready-to-use therapeutic food in community-based management of uncomplicated severe acute malnutrition: a randomized controlled trial from India. J Trop Pediatr. 2013;59:393–398. doi: 10.1093/tropej/fmt039. [DOI] [PubMed] [Google Scholar]
- 189.Smith SE, Ramos RA, Refinetti R, Farthing JP, Paterson PG. Protein-energy malnutrition induces an aberrant acute-phase response and modifies the circadian rhythm of core temperature. Appl Physiol Nutr Metab. 2013;38:844–853. doi: 10.1139/apnm-2012-0420. [DOI] [PubMed] [Google Scholar]
- 190.World Health Organization. Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Illnesses with Limited Resources. WHO; 2013. [PubMed] [Google Scholar]
- 191.Chimhuya S, Kambarami RA, Mujuru H. The levels of malnutrition and risk factors for mortality at Harare Central Hospital-Zimbabwe: an observation study. Cent Afr J Med. 2007;53:30–34. doi: 10.4314/cajm.v53i5-8.62612. [DOI] [PubMed] [Google Scholar]
- 192.Fergusson P, Tomkins A, Kerac M. Improving survival of children with severe acute malnutrition in HIV-prevalent settings. Int Health. 2009;1:10–16. doi: 10.1016/j.inhe.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 193.Heikens GT, et al. Case management of HIV-infected severely malnourished children: challenges in the area of highest prevalence. Lancet. 2008;371:1305–1307. doi: 10.1016/S0140-6736(08)60565-6. [DOI] [PubMed] [Google Scholar]
- 194.Brewster DR, Manary MJ, Graham SM. Case management of kwashiorkor: an intervention project at seven nutrition rehabilitation centres in Malawi. Eur J Clin Nutr. 1997;51:139–147. doi: 10.1038/sj.ejcn.1600367. [DOI] [PubMed] [Google Scholar]
- 195.Bachou H, Tumwine JK, Mwadime RK, Ahmed T, Tylleskar T. Reduction of unnecessary transfusion and intravenous fluids in severely malnourished children is not enough to reduce mortality. Ann Trop Paediatr. 2008;28:23–33. doi: 10.1179/146532808X270644. [DOI] [PubMed] [Google Scholar]
- 196.Manary MJ, Brewster DR. Intensive nursing care of kwashiorkor in Malawi. Acta Paediatr. 2000;89:203–207. doi: 10.1080/080352500750028843. [DOI] [PubMed] [Google Scholar]
- 197.Angood C, et al. Research priorities to improve the management of acute malnutrition in infants aged less than six months (MAMI) PLoS Med. 2015;12:e1001812. doi: 10.1371/journal.pmed.1001812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lazzaroni S, Wagner N. Misfortunes never come singly: structural change, multiple shocks and child malnutrition in rural Senegal. Econ Hum Biol. 2016;23:246–262. doi: 10.1016/j.ehb.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 199.Chisti MJ, et al. Post-discharge mortality in children with severe malnutrition and pneumonia in Bangladesh. PLoS ONE. 2014;9:e107663. doi: 10.1371/journal.pone.0107663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chang CY, et al. Children successfully treated for moderate acute malnutrition remain at risk for malnutrition and death in the subsequent year after recovery. J Nutr. 2013;143:215–220. doi: 10.3945/jn.112.168047. [This trial demonstrated that children with moderate malnutrition who appear to achieve nutritional recovery still have high rates of morbidity and mortality in the year following discharge.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chuma J, Maina T. Catastrophic health care spending and impoverishment in Kenya. BMC Health Serv Res. 2012;12:413. doi: 10.1186/1472-6963-12-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Morgan G. What, if any, is the effect of malnutrition on immunological competence? Lancet. 1997;349:1693–1695. doi: 10.1016/S0140-6736(96)12038-9. [DOI] [PubMed] [Google Scholar]
- 203.Kerac M, et al. Follow-up of post-discharge growth and mortality after treatment for severe acute malnutrition (FuSAM study): a prospective cohort study. PLoS ONE. 2014;9:e96030. doi: 10.1371/journal.pone.0096030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lelijveld N, et al. Chronic disease outcomes after severe acute malnutrition in Malawian children (ChroSAM): a cohort study. Lancet Glob Health. 2016;4:e654–e662. doi: 10.1016/S2214-109X(16)30133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jackson AA. Protein requirements for catch-up growth. Proc Nutr Soc. 1990;49:507–516. doi: 10.1079/pns19900059. [DOI] [PubMed] [Google Scholar]
- 206.Benefice E, Fouere T, Malina RM, Beunen G. Anthropometric and motor characteristics of Senegalese children with different nutritional histories. Child Care Health Dev. 1996;22:151–165. [PubMed] [Google Scholar]
- 207.Fall C, Osmond C. Commentary: the developmental origins of health & disease: an appreciation of the life & work of Professor David, J.P. Barker, 1938–2013. Int J Epidemiol. 2013;42:1231–1232. doi: 10.1093/ije/dyt207. [DOI] [PubMed] [Google Scholar]
- 208.Gluckman PD, Hanson MA, Pinal C. The developmental origins of adult disease. Matern Child Nutr. 2005;1:130–141. doi: 10.1111/j.1740-8709.2005.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.DeBoer MD, et al. Early childhood growth failure and the developmental origins of adult disease: do enteric infections and malnutrition increase risk for the metabolic syndrome? Nutr Rev. 2012;70:642–653. doi: 10.1111/j.1753-4887.2012.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Martorell R. Improved nutrition in the first 1000 days and adult human capital and health. Am J Hum Biol. 2017;29:e22952. doi: 10.1002/ajhb.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hamadani JD, Huda SN, Khatun F, Grantham-McGregor SM. Psychosocial stimulation improves the development of undernourished children in rural Bangladesh. J Nutr. 2006;136:2645–2652. doi: 10.1093/jn/136.10.2645. [DOI] [PubMed] [Google Scholar]
- 212.Walker SP, Chang SM, Powell CA, Simonoff E, Grantham-McGregor SM. Effects of psychosocial stimulation and dietary supplementation in early childhood on psychosocial functioning in late adolescence: follow-up of randomised controlled trial. BMJ. 2006;333:472. doi: 10.1136/bmj.38897.555208.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Grantham-McGregor S. A review of studies of the effect of severe malnutrition on mental development. J Nutr. 1995;125:2233S–2238S. doi: 10.1093/jn/125.suppl_8.2233S. [DOI] [PubMed] [Google Scholar]
- 214.Every Woman Every Child. The global strategy for women’s, children’s and adolescents’ health (2016–2030) Every Woman Every Child. 2016 http://globalstrategy.everywomaneverychild.org/pdf/EWEC_globalstrategyreport_200915_FINAL_WEB.pdf.
- 215.Action Against Hunger. No wasted lives. Action Against Hunger. 2017 https://www.actionagainsthunger.org.uk/blog/no-wasted-lives. [Google Scholar]
- 216.Kerac M, Mwangome M, McGrath M, Haider R, Berkley JA. Management of acute malnutrition in infants aged under 6 months (MAMI): current issues and future directions in policy and research. Food Nutr Bull. 2015;36:S30–S34. doi: 10.1177/15648265150361S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Groce NE, Kerac M, Farkas A, Schultink W, Bieler RB. Inclusive nutrition for children and adults with disabilities. Lancet Glob Health. 2013;1:e180–181. doi: 10.1016/S2214-109X(13)70056-1. [DOI] [PubMed] [Google Scholar]
- 218.Angood C, Khara T, Dolan C, Berkley JA, WaSt Technical Interest, G Research priorities on the relationship between wasting and stunting. PLoS ONE. 2016;11:e0153221. doi: 10.1371/journal.pone.0153221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wazny K, et al. Setting global research priorities for integrated community case management (iCCM): results from a CHNRI (Child Health and Nutrition Research Initiative) exercise. J Glob Health. 2014;4 doi: 10.7189/jogh.04.020413. 020413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Garenne M, et al. Incidence and duration of severe wasting in two African populations. Public Health Nutr. 2009;12:1974–1982. doi: 10.1017/S1368980009004972. [DOI] [PubMed] [Google Scholar]
- 221.Hure A, Oldmeadow C, Attia J. Invited commentary: improving estimates of severe acute malnutrition requires more data. Am J Epidemiol. 2016;184:870–872. doi: 10.1093/aje/kww131. [DOI] [PubMed] [Google Scholar]
- 222.Emergency Nutrition Network. Caseload calculations (incidence rate) ENN. 2011 http://www.en-net.org/question/514.aspx.
- 223.Grellety E, Golden MH. Weight-for-height and mid-upper-arm circumference should be used independently to diagnose acute malnutrition: policy implications. BMC Nutr. 2016;2:10. [Google Scholar]
- 224.Briend A, et al. Low mid-upper arm circumference identifies children with a high risk of death who should be the priority target for treatment. BMC Nutr. 2016;2:63. [Google Scholar]
- 225.Friedli N, et al. Revisiting the refeeding syndrome: Results of a systematic review. Nutrition. 2017;35:151–160. doi: 10.1016/j.nut.2016.05.016. [This comprehensive review provided an excellent overview of refeeding syndrome.] [DOI] [PubMed] [Google Scholar]