Abstract
Background
Neoadjuvant chemotherapy (NAC) for breast cancer increases breast-conserving surgery (BCS) rates, but many women opt for mastectomy with contralateral prophylactic mastectomy (CPM). Here we evaluate factors associated with CPM use in women undergoing mastectomy post-NAC.
Methods
A retrospective institutional NAC database review identified women with clinical stage I-III, unilateral invasive breast cancer undergoing UM or CPM mastectomy from 9/2013–12/2017. Clinical/pathologic characteristics, imaging, and presence of contraindications to BCS post-NAC were compared, with subset analysis of BCS candidates. Multivariable analysis adjusted for potential confounders.
Results
569 women underwent mastectomy after NAC, 297(52%) UM and 272(48%) CPM. On univariable analysis, younger age, BRCA+, lower pre-NAC clinical stage, pCR, and axillary surgery extent were associated with CPM (all p<0.01). Favorable post-NAC clinical factors of no residual palpable disease, clinically negative nodes, complete response on breast imaging, and no post-NAC contraindication to BCS were also associated with CPM(all p<0.01).
On multivariable analysis, young age (OR 0.93,95%CI 0.91–0.95), lower pre-NAC stage (OR 0.51,95% CI 0.34–0.77), and no contraindication to BCS(OR 3.12, 95%CI 2.02–4.82) were significantly associated with CPM.
Among the 203(35%) women who had no contraindications to BCS post-NAC, 145(71%) underwent CPM. BRCA+ and family history were reasons more frequently cited for mastectomy among CPM than UM(p<0.001).
Conclusions
CPM was performed in 48% of women undergoing mastectomy after NAC; younger women with earlier-stage cancers were more likely to undergo CPM. While increased use of CPM in women with more favorable disease is medically appropriate, our findings indicate a lost opportunity for use of BCS.
Keywords: breast cancer, mastectomy, breast-conserving surgery, neoadjuvant chemotherapy, contralateral prophylactic mastectomy
INTRODUCTION
The survival of women undergoing breast conserving surgery after neoadjuvant chemotherapy (NAC) is equivalent to those undergoing mastectomy.1,2 Contemporary uses of NAC include downstaging of disease in the breast and axilla; NAC decreases the use of axillary dissection for those with nodal metastases at presentation and increases eligibility for breast-conserving surgery (BCS). Clinical trials have reported improved pathologic complete response rates (pCR) with modern chemotherapy regimens without a corresponding increase in BCS rates.3,4 Although more than 40% of women who receive NAC become eligible for BCS, about 20–30% still choose mastectomy.5,6 Additionally, rates of contralateral prophylactic mastectomy (CPM) in women receiving NAC are increasing, consistent with what has been observed nationally in patients having primary surgery.7–10
While CPM is appropriate to consider in women with genetic predisposition, CPM has not been demonstrated to improve overall or disease-free survival for women with unilateral invasive breast cancer, regardless of stage.10–14 The incidence of contralateral breast cancer has decreased in the era of modern systemic therapy, and current estimates of incidence are less than 0.5% per year.15–17 Given that the lifetime risk of a contralateral breast cancer is low, the women with the greatest likelihood of benefit from CPM might be young women with early-stage breast cancers at low risk of death from the index malignancy.18–20 However, the majority of patients undergoing NAC are not early stage at presentation, given the goals of pre-surgical systemic therapy, but may have an excellent prognosis if pathologic complete response is obtained.6
A number of factors associated with use of CPM at initial diagnosis have been reported; however, limited data on factors associated with CPM in women receiving NAC are available. The purpose of our study was to identify patient and tumor factors associated with use of CPM after NAC, and to determine whether response to NAC is associated with the decision to choose CPM.
METHODS
We performed an institutional review board-approved retrospective review of an institutional, prospective NAC database to identify women with clinical stage I-III, unilateral invasive breast cancer receiving NAC and undergoing unilateral (UM) or bilateral (CPM) mastectomy from 9/2013–12/2017.
In our database, tumor size, clinical tumor stage, clinical nodal stage, degree of clinical response on imaging and exam, and candidacy for BCS were prospectively recorded both before receipt of NAC and after NAC completion. All patients had neoadjuvant chemotherapy, and excluded the use of neoadjuvant endocrine therapy. Final pathology was recorded as a complete pathologic response (pCR), which, for this study, was defined as the presence of invasive disease or not, and did not include residual ductal carcinoma in situ (DCIS). The reasons for mastectomy and contraindications to BCS post-NAC were also prospectively recorded. Contraindications to BCS criteria were uniform across all surgeons and included inflammatory breast cancer, multicentric disease, contraindications to radiation therapy, and extensive disease at presentation. Patient preference for mastectomy in those eligible for BCS was prospectively recorded by the surgeon, but a specific reason for unilateral versus bilateral mastectomy was not collected. Women who had known BRCA mutations, and those who were not known mutation carriers but who had > 1 first-degree relative or > 2 second-degree relatives with breast cancer, or any family history of ovarian cancer, were considered to be high risk for contralateral cancer.
Median and interquartile range (IQR), and frequency and percentage were reported for continuous and categorical variables, respectively. To test for differences in clinicopathologic features between women undergoing UM and CPM, the Kruskal-Wallis test was used for continuous variables and Fisher’s exact test was used for categorical variables. Logistic regression was used to test for an interaction between surgery year and mastectomy group with respect to response to NAC, and a p-value < 0.1 would be considered a significant interaction effect. Multivariable logistic regression for associations with CPM versus UM included factors determined a priori. Complete pathologic response was not used in our multivariable model, as it is not a known factor prior to surgery. A p-value < 0.05 was considered statistically significant. All statistical analyses were conducted using R software version 3.5.0 (R Core Development Team, Vienna, Austria).
RESULTS
We identified 569 women with unilateral breast cancer receiving NAC who underwent mastectomy for surgical therapy. 297 (52%) underwent UM, while 272 (48%) underwent CPM (therapeutic mastectomy and contralateral prophylactic mastectomy). Rates of UM and CPM were similar across the years of the study period (p = 0.73).
Clinical and Pathologic Factors
On univariable analysis, the two groups differed in multiple clinical and pathologic features (Table 1). Women who underwent CPM were younger (median 44 years of age [IQR 37, 50.2] vs. median 53 years of age [IQR 45, 63], p < 0.001), had lower overall stage (p < 0.001), and were more likely to have triple negative or HER2/neu positive tumors (p < 0.001). Of the entire study population, 320 (56.2%) women had genetic testing, and 56 women had deleterious BRCA mutations—14 women with a BRCA variation of unknown significance (VUS). 250 women had negative genetic testing. All results were available prior to surgery. 14 patients had a VUS, of whom 50% had unilateral surgery and 50% had bilateral. This was in contrast to those who tested negative, where those who were negative had unilateral (42%) and bilateral mastectomy (58%). Too few VUS were identified to allow an adequate comparison. Women with BRCA mutations were significantly more likely to select CPM (96.4% CPM versus 3.6% UM, p < 0.001).
TABLE 1.
Patient and Disease Characteristics by Mastectomy Group
P-values are from the Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables. Numbers are median (interquartile range) for continuous variables and number (percent) for categorical variables.
UM unilateral mastectomy, CPM contralateral prophylactic mastectomy, ER estrogen receptor, PR progesterone receptor, Vus variation of unknown significance, pCR pathologic complete response, ALND axillary lymph node dissection, BCS breast-conserving surgery, NAC neoadjuvant chemotherapy
*Not all patients received imaging, which is why these numbers do not add up to the total number of patients
| Variable | Unilateral mastectomy (UM) (n = 297) | Bilateral mastectomy (CPM) (n = 272) | p-value |
|---|---|---|---|
| Age | 53 (45, 63) | 44 (37, 50.2) | < .001 |
| Tumor stage | < .001 | ||
| T1/Tis/Tx | 8 (15.4) | 44 (84.6) | |
| T2 | 127 (46.5) | 146 (53.5) | |
| T3 | 91 (57.2) | 68 (42.8) | |
| T4 | 71 (83.5) | 14 (16.5) | |
| Overall stage | < .001 | ||
| 1 | 1 (5.6) | 17 (94.4) | |
| 2 | 141 (44.1) | 179 (55.9) | |
| 3 | 155 (67.1) | 76 (32.9) | |
| Tumor subtype | < .001 | ||
| ER−, PR−, HER2− | 56 (39.4) | 86 (60.6) | |
| ER−, PR−, HER2+ | 54 (63.5) | 31 (36.5) | |
| ER/PR+, HER2− | 125 (59.2) | 86 (40.8) | |
| ER/PR+, HER2+ | 62 (47.3) | 69 (52.7) | |
| BRCA status | < .001 | ||
| Negative | 105 (42) | 145 (58.0) | |
| Positive | 2 (3.6) | 54 (96.4) | |
| Vus | 7 (50) | 7 (50) | |
| Not tested | 183 | 66 | |
| pCR (invasive) | 0.006 | ||
| No | 213 (56.3) | 165 (43.7) | |
| Yes | 84 (44) | 107 (56) | |
| Axillary surgery | < .001 | ||
| Sentinel lymph node alone | 104 (38.8) | 164 (61.2) | |
| Completion ALND | 193 (64.1) | 108 (35.9) | |
| Candidate for BCS post-NAC | < .001 | ||
| Borderline | 2 (100) | 0 (0) | |
| Contraindication | 237 (65.1) | 127 (34.9) | |
| Candidate | 58 (28.6) | 145 (71.4) | |
| Residual palpable disease | < .001 | ||
| No | 173 (45.6) | 206 (54.4) | |
| Yes | 124 (65.3) | 66 (34.7) | |
| Clinical axillary nodal status | 0.005 | ||
| Negative | 260 (50.3) | 257 (49.7) | |
| Positive | 37 (71.2) | 15 (28.8) | |
| Persistent abnormality on any imaging (Mammo and/or MRI) post NAC* | < .001 | ||
| No | 25 (33.3) | 50 (66.7) | |
| Yes | 199 (59.9) | 133 (40.1) |
Multiple preoperative clinical factors were associated with use of CPM on univariable analysis. Women who were candidates for BCS (n = 145/203 [71.4%], p < 0.001), had a complete clinical response on imaging and exam (n = 206/379 [54.4%], p < 0.001), were clinically node negative (n = 257/517 [49.7%], p = 0.005), and had a complete radiographic response (i.e., no abnormality on imaging after NAC, n = 50/75 [66.7%], p < 0.001) were more likely to undergo CPM (Table 1). While pathologic response is unknown before surgery, patients who had a pCR on final pathology (n = 107/191 [56%], p = 0.006) were more likely to undergo CPM. Patients who had a pCR as well as a complete imaging response were more likely to have CPM and less likely to need an axillary node dissection. Overall, axillary node dissection was performed in 64.1% of the women who had unilateral mastectomy as compared to 35.9% of the women who had bilateral mastectomy (p < .001, Table 1). In a subset of 41 patients who remained node positive after neoadjuvant chemotherapy and went on to have an axillary node dissection, the majority 32 (78%) had unilateral mastectomy and only 9 (22%) had bilateral mastectomy.
On multivariable analysis, we found increased age at surgery and higher-stage disease (stage 3 versus stages 1/2) were significantly associated with decreased odds of CPM. Candidacy for BCS post-NAC was significantly associated with increased odds of CPM. Molecular subtype, residual palpable disease, and clinically positive axillary nodes were not significant independent predictors of CPM on multivariable analysis (Table 2). An additional multivariable analysis was performed on the subset of women (n = 407) who had imaging response on mammography and/or MRI after NAC. In this subset, the absence of imaging abnormality was associated with CPM use in addition to the factors identified in the multivariable analysis for the entire group (Table 3).
TABLE 2.
Multivariable Associations Between Patient and Disease Factors, and Mastectomy Group
OR odds ratio, CI confidence interval, ER estrogen receptor, PR progesterone receptor, BCT breast-conserving therapy, NAC neoadjuvant chemotherapy
| Factor | OR (95% CI) | p-value |
|---|---|---|
| Age at surgery | 0.93 (0.91–0.95) | < .001 |
| Stage | 0.001 | |
| 1 or 2 | 1.00 | |
| 3 | 0.51 (0.34–0.77) | |
| Subtype | 0.109 | |
| ER−, PR−, HER2− | 1.00 | |
| ER−, PR−, HER2+ | 0.49 (0.25–0.93) | |
| ER/PR+, HER2− | 0.6 (0.36–1) | |
| ER/PR+, HER2+ | 0.74 (0.42–1.31) | |
| Candidate for BCT post-NAC | 3.12 (2.02–4.82) | < .001 |
| Residual palpable disease | 0.71 (0.46–1.09) | 0.115 |
| Positive clinical axillary nodes | 0.85 (0.42–1.73) | 0.662 |
TABLE 3.
Multivariable Associations Between Patient and Disease Factors, and Mastectomy Group Among Those with Data on Imaging Response (n = 407)
OR odds ratio, CI confidence interval, ER estrogen receptor, PR progesterone receptor, BCT breast-conserving therapy, mammo mammogram, NAC neoadjuvant chemotherapy
| Factor | OR (95% CI) | p-value |
|---|---|---|
| Age at surgery | 0.92 (0.9–0.95) | < .001 |
| Stage | 0.005 | |
| 1/2 | 1.00 | |
| 3 | 0.49 (0.3–0.81) | |
| Subtype | 0.014 | |
| ER−, PR−, HER2− | 1.00 | |
| ER−, PR−, HER2+ | 0.32 (0.14–0.72) | |
| ER/PR+, HER2− | 0.46 (0.25–0.86) | |
| ER/PR+, HER2+ | 0.74 (0.38–1.43) | |
| Candidate BCT post-chemo | 2.6 (1.56–4.34) | < .001 |
| Residual palpable disease | 0.58 (0.34–0.98) | 0.043 |
| Positive clinical axillary nodes | 1.23 (0.53–2.84) | 0.627 |
| Persistent abnormality on any imaging (mammo and/or MRI) post NAC | 0.004 | |
| No | 1.00 | |
| Yes | 0.39 (0.21–0.74) |
Factors Associated with Surgical Choice
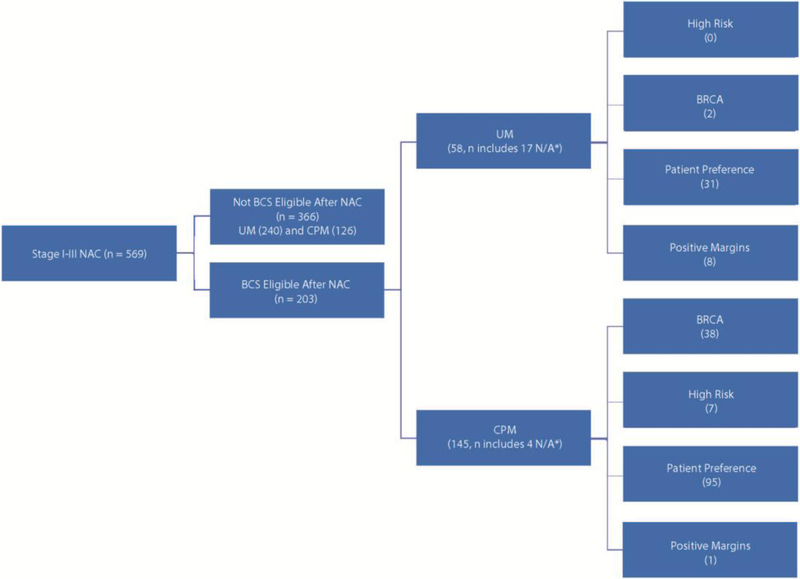
Fig. 1 shows the overall factors associated with surgical choice in the study group. In the subset of women who were eligible for BCS, we conducted an analysis of clinical factors and association with surgeon-recorded reason for mastectomy. In patients who were not eligible for BCS, independent reason for mastectomy (other than ineligibility for BCS) was not recorded. The pre-selected variables included BRCA gene mutation, high-risk family history, and patient preference as reasons cited for CPM choice. For women undergoing CPM, surgeons cited BRCA mutation or family history as the most common reason for surgical therapy choice (p < 0.001). Patient preference was most common variable among a subset of 203 patients who were candidates for BCS post-NAC but who elected to undergo mastectomy (Table 4). Of note, only a minority of women (n = 9, 1.6%) underwent mastectomy after an initial attempt at BCS that resulted in positive margins.
Fig. 1. Reason for mastectomy in patients who were eligible for breast-conserving surgery (BCS).
NAC neoadjuvant chemotherapy, BCS breast-conserving surgery, UM unilateral mastectomy, CPM contralateral prophylactic mastectomy
*N/A: reason for mastectomy not recorded in database
TABLE 4.
Reasons for Mastectomy Among Patients Who Were Candidates for BCT Post-NAC
BCT breast-conserving therapy, NAC neoadjuvant chemotherapy, UM unilateral mastectomy, CPM contralateral prophylactic mastectomy, NA not available
*Analysis excludes patients for which no reason was recorded
| Clinically Recorded Reason for Choice of Mastectomy, n (%) | UM (n = 58) | CPM (n = 145) | p-value |
|---|---|---|---|
| < .001 | |||
| BRCA carrier | 2 (4.9) | 38 (27.0) | |
| High risk | 0(0) | 7(5.0) | |
| Patient preference | 31 (75.6) | 95 (67.4) | |
| Positive/close margins after BCT attempted | 8 (19.5) | 1 (0.7) | |
| NA* | 17 | 4 |
DISCUSSION
The trend of increased use of CPM for women with unilateral breast cancer is well reported,16,21 but there are limited data on the use of CPM in the neoadjuvant setting. Here we found that 48% of women undergoing mastectomy after NAC are also choosing CPM. We identified young age and early-stage disease as significant factors associated with use of CPM, a finding similar to other reported studies in the adjuvant setting.16,21
Women who are recommended to receive NAC have historically been higher stage, but current use of NAC in early-stage breast cancer is increasing.22 Neither NAC nor CPM have been demonstrated to improve survival, yet rates of both continue to increase. The benefits of NAC include both prognostic and predictive information for some tumor subtypes, and have potential to minimize surgical intervention. The marginal benefit of CPM may be highest in young women with early-stage cancer.12
Clinical response to NAC and tumor biology were associated with selection of CPM in univariable analysis. Thus, women who pre-NAC are likely to be candidates for BCS, are choosing CPM, and response to NAC may not be predictive of choice of surgical therapy. NAC has been consistently shown to downstage disease in the breast and increase the number of women eligible for breast conservation.5,16 Other authors have found that rates of CPM have been increasing in spite of increased use of NAC.8,9 In a review of the National Cancer Database, Pollom et al. identified an increased absolute rate of bilateral mastectomy from 8% to 13.1% between 2010 and 2014, and similarly identified that young age was associated with selection of bilateral mastectomy, in addition to insurance status, education, and socioeconomic status. Wapnir et al. also reported an increase in use of NAC, which doubled over the time course of their study, and CPM in patients who received NAC, again noting the age was significantly associated with use of CPM. These studies both report on cancer registries, and are limited in the detail of the decision making and specifics of clinical response available in our study.
If a woman is interested in CPM prior to initiation of therapy, downstaging disease in the breast may not avoid CPM. However, NAC should still be considered to downstage the axilla when appropriate.23 Patient-related factors such as age, socioeconomic status, race, and patient preference have consistently been associated with choice of CPM regardless of therapeutic approach.7,16 Recent guidelines have encouraged emphasis of the oncologic and survival equivalence of BCS, and have been endorsed as the standard of excellence in breast cancer care by the National Institutes of Health since 1991.11,24,25 Shared decision making, and strategies to communicate risk and treatment options to women need to be further investigated and promoted. Our findings in this study highlight the need to understand patient desire for CPM, and opportunities for education and intervention. Additionally, it highlights the potential to avoid NAC if surgical approach is unlikely to change, particularly in early-stage disease. There are no studies evaluating the impact of patient education or clinician counseling on choice of BCS compared to unilateral or bilateral mastectomies in patients undergoing NAC.
As expected, the use of CPM in BRCA mutation carriers and those with significant high-risk family history was high. While estimated mortality benefit even in this high-risk population is thought to be < 1%, CPM does reduce rates of contralateral breast cancer in this population in a meaningful way.12 However, the majority of women undergoing CPM (69%) did not have a known BRCA mutation or high-risk family history, indicating other reasons for their therapeutic decision making. There is no evidence that CPM improves disease-free or overall survival in patients who do not have BRCA mutations, as even in very young women, rates of contralateral breast cancer are < 1% per year.19
In the subset of patients undergoing mastectomy who were eligible for BCS after NAC, the majority selected CPM (71.4%). Patient preference was the primary reason for mastectomy for the majority of women undergoing CPM (67.4%). This suggests that response to NAC may not change patient preference for surgical approach, which could limit the use of NAC as a strategy to allow for increase in BCS and minimize surgical intervention beyond its benefit of nodal disease downstaging.
We previously described the factors that influence the use of immediate reconstruction after NAC.26 Response to NAC was not a factor in patient decisions to undergo immediate reconstruction; however, the data suggested that the decision to undergo bilateral mastectomy was consistent with previously described patient factors (age, race, early stage).26 This study further confirms the need to better understand patient choice in surgical decisions.
Limitations of these data include how some of the data was recorded in our prospective database—i.e., “personal preference” as a reason for mastectomy choice does not provide further insight into decision making or discussions. In patients who were not candidates for breast conservation, preference for bilateral mastectomy compared to unilateral was not independently collected. Additionally, this dataset only captures women who had a mastectomy (unilateral or bilateral with CPM), and comparison with those who ultimately had BCS was not possible. Generalizations from these data are limited, as this study represents a single institutional experience.
Conclusions
In our study, CPM was performed in 48% of women who had a mastectomy after NAC, and younger women with earlier-stage disease were more likely to undergo CPM. These findings are similar to patients who undergo CPM in the adjuvant setting. While use of CPM in younger patients with earlier-stage disease is medically appropriate, our findings indicate a lost opportunity for use of BCS in this group. Further study into patient-related factors influencing surgical decisions is necessary to understand this trend.
Synopsis:
Here we evaluate factors associated with CPM use in women undergoing mastectomy post-NAC. We find that and that younger women with earlier-stage disease were more likely to undergo CPM—findings similar to patients who undergo CPM in the adjuvant setting.
ACKNOWLEDGEMENTS
The preparation of this manuscript was funded in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center, and this study was presented in poster format at the Society of Surgical Oncology 72nd Annual Cancer Symposium, March 27–30, 2019, San Diego, CA. Dr. Monica Morrow has received speaking honoraria from Genomic Health and Roche.
Disclosures: The preparation of this manuscript was funded in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center, and this study was presented in poster format at the Society of Surgical Oncology 72nd Annual Cancer Symposium, March 27–30, 2019, San Diego, CA. Dr. Monica Morrow has received speaking honoraria from Genomic Health and Roche.
REFERENCES
- 1.Mieog JS, van der Hage JA, van de Velde CJ. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev 2007. April 18(2):Cd005002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 2008. February 10;26(5):778–85. [DOI] [PubMed] [Google Scholar]
- 3.de Azambuja E, Holmes AP, Piccart-Gebhart M, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol 2014. September;15(10):1137–46. [DOI] [PubMed] [Google Scholar]
- 4.Guarneri V, Frassoldati A, Bottini A, et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II CHER-LOB study. J Clin Oncol 2012. June 1;30(16):1989–95. [DOI] [PubMed] [Google Scholar]
- 5.Golshan M, Cirrincione CT, Sikov WM, et al. Impact of neoadjuvant chemotherapy in stage II-III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: surgical results from CALGB 40603 (Alliance). Ann Surg 2015. September;262(3):434–9; discussion 8–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golshan M, Cirrincione CT, Sikov WM, et al. Impact of neoadjuvant therapy on eligibility for and frequency of breast conservation in stage II-III HER2-positive breast cancer: surgical results of CALGB 40601 (Alliance). Breast Cancer Res Treat 2016. November;160(2):297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kantor O, Ajmani G, Wang CH, Datta A, Yao K. The Shifting Paradigm for Breast Cancer Surgery in Patients Undergoing Neoadjuvant Chemotherapy. Ann Surg Oncol 2018. January;25(1):164–72. [DOI] [PubMed] [Google Scholar]
- 8.Pollom EL, Qian Y, Chin AL, et al. Rising rates of bilateral mastectomy with reconstruction following neoadjuvant chemotherapy. Int J Cancer 2018. December 15;143(12):3262–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wapnir IL, Kurian AW, Lichtensztajn DY, Clarke CA, Gomez SL. Rising Bilateral Mastectomy Rates Among Neoadjuvant Chemotherapy Recipients in California From 1998 to 2012. Ann Surg 2017. August;266(2):353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong SM, Freedman RA, Sagara Y, Aydogan F, Barry WT, Golshan M. Growing Use of Contralateral Prophylactic Mastectomy Despite no Improvement in Long-term Survival for Invasive Breast Cancer. Ann Surg 2017. March;265(3):581–9. [DOI] [PubMed] [Google Scholar]
- 11.Hunt KK, Euhus DM, Boughey JC, et al. Society of Surgical Oncology Breast Disease Working Group Statement on Prophylactic (Risk-Reducing) Mastectomy. Ann Surg Oncol 2017. February;24(2):375–97. [DOI] [PubMed] [Google Scholar]
- 12.Portschy PR, Kuntz KM, Tuttle TM. Survival outcomes after contralateral prophylactic mastectomy: a decision analysis. J Natl Cancer Inst 2014. August;106(8). [DOI] [PubMed] [Google Scholar]
- 13.Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol 2007. November 20;25(33):5203–9. [DOI] [PubMed] [Google Scholar]
- 14.Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol 2009. March 20;27(9):1362–7. [DOI] [PubMed] [Google Scholar]
- 15.Gao X, Fisher SG, Emami B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: a population-based study. Int J Radiat Oncol Biol Phys 2003. July 15;56(4):1038–45. [DOI] [PubMed] [Google Scholar]
- 16.King TA, Sakr R, Patil S, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol 2011. June 1;29(16):2158–64. [DOI] [PubMed] [Google Scholar]
- 17.Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol 1996. October;14(10):2738–46. [DOI] [PubMed] [Google Scholar]
- 18.Basu NN, Ross GL, Evans DG, Barr L. The Manchester guidelines for contralateral risk-reducing mastectomy. World J Surg Oncol 2015. August 7;13:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bedrosian I, Hu CY, Chang GJ. Population-based study of contralateral prophylactic mastectomy and survival outcomes of breast cancer patients. J Natl Cancer Inst 2010. March 17;102(6):401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peralta EA, Ellenhorn JD, Wagman LD, Dagis A, Andersen JS, Chu DZ. Contralateral prophylactic mastectomy improves the outcome of selected patients undergoing mastectomy for breast cancer. Am J Surg 2000. December;180(6):439–45. [DOI] [PubMed] [Google Scholar]
- 21.Jagsi R, Hawley ST, Griffith KA, et al. Contralateral Prophylactic Mastectomy Decisions in a Population-Based Sample of Patients With Early-Stage Breast Cancer. JAMA Surg 2017. March 1;152(3):274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mougalian SS, Soulos PR, Killelea BK, et al. Use of neoadjuvant chemotherapy for patients with stage I to III breast cancer in the United States. Cancer 2015. August 1;121(15):2544–52. [DOI] [PubMed] [Google Scholar]
- 23.Pilewskie M, Zabor EC, Mamtani A, Barrio AV, Stempel M, Morrow M. The Optimal Treatment Plan to Avoid Axillary Lymph Node Dissection in Early-Stage Breast Cancer Patients Differs by Surgical Strategy and Tumor Subtype. Ann Surg Oncol 2017. November;24(12):3527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boughey JC, Attai DJ, Chen SL, et al. Contralateral Prophylactic Mastectomy (CPM) Consensus Statement from the American Society of Breast Surgeons: Data on CPM Outcomes and Risks. Ann Surg Oncol 2016. October;23(10):3100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Institutes of Health. NIH consensus conference. Treatment of early-stage breast cancer. Jama 1991. January 16;265(3):391–5. [PubMed] [Google Scholar]
- 26.Cassidy MR, Zabor EC, Stempel M, Mehrara B, Gemignani ML. Does response to neoadjuvant chemotherapy impact breast reconstruction? Breast J 2018. July;24(4):567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]