Abstract
Sleep disturbance is a symptom of and a well-known risk factor for depression. Further, atypical functioning of the HPA axis has been linked to the pathogenesis of depression. The purpose of this study was to examine the role of adolescent HPA axis functioning in the link between adolescent sleep problems and later depressive symptoms.
Methods:
A sample of 157 17–18 year old adolescents (61.8% female) completed the Pittsburgh Sleep Quality Inventory (PSQI) and provided salivary cortisol samples throughout the day for three consecutive days. Two years later, adolescents reported their depressive symptoms via the Center for Epidemiological Studies Depression Scale (CES-D).
Results:
Individuals (age 17–18) with greater sleep disturbance reported greater depressive symptoms two years later (age 19–20). This association occurred through the indirect effect of sleep disturbance on the cortisol awakening response (CAR) (indirect effect = 0.14, 95%CI [.02-.39]).
Conclusions:
One pathway through which sleep problems may lead to depressive symptoms is by up-regulating components of the body’s physiological stress response system that can be measured through the cortisol awakening response. Behavioral interventions that target sleep disturbance in adolescents may mitigate this neurobiological pathway to depression during this high-risk developmental phase.
Keywords: adolescence, cortisol awakening response, depression, HPA axis, sleep
Depression accounts for nearly half of the global burden of disease associated with psychiatric disorders (Whiteford et al., 2013) and is among the most costly chronic health conditions to society (Merikangas et al., 2011). Risk for depression is low in childhood, rises dramatically during adolescence (Merikangas et al., 2010; Rohde, Lewinsohn, Klein, Seeley, & Gau, 2012), and peaks in early adulthood (ages 18–24; Rohde et al., 2012). The long-term consequences of depression during phases of important social and occupational development, such as adolescence, can be significant. Moreover, interventions targeting depressive symptoms during late adolescence are limited (e.g., TADS Team, 2007). Thus, identifying prospective risk factors for depression may help us to identify intervention targets that can mitigate risk during this key developmental phase. Sleep disturbance has been identified as one of these prospective risk factors. Yet, little is known about the psychophysiological processes through which sleep disturbances presage depression. One possibility is that persistent sleep disturbance alters the functioning of the hypothalamic–pituitary–adrenal (HPA) axis, which has been implicated in the pathogenesis of depression (e.g., Baumeister, Lightman, & Pariante, 2014). The purpose of this study was to determine whether daily functioning of the HPA axis mediated the well-established link between sleep and depression over time.
Sleep disturbance is pervasive in depression, with 50%–90% of depressed patients reporting problems with sleep (Casper et al., 1985; Lewinsohn, Rohde, & Seeley, 1998a; Soehner, Kaplan, & Harvey, 2014). However, sleep disturbance not only is a symptom of depression but also can act as a robust predictor (Baglioni et al., 2011; Cho et al., 2008; Irwin et al., 2018; Lee et al., 2013). Several studies in pediatric samples have shown that sleep problems prospectively predict depressive symptoms (Gregory et al., 2005; Raniti et al., 2017; Roberts & Duong, 2014). Adolescents who regularly sleep 8.5 hr per night are at the lowest risk for depression and anxiety (Ojio, Nishida, Shimodera, Togo, & Sasaki, 2016), and sleep disorders (e.g., pediatric sleep apnea) are prospectively associated with increased depression risk (Chang, Chen, & Liu, 2017). Further, experimental sleep deprivation has a negative effect on mood and emotion regulation (Palmer & Alfano, 2017). In contrast, effective treatment of sleep problems ameliorates depressive symptoms in adolescents (Blake, Sheeber, Youssef, Raniti, & Allen, 2017) and adults (Ashworth et al., 2015; Ye et al., 2015). Yet, the neurobiological processes through which the well-established association between sleep disturbance and depression occur remain unclear.
One pathway through which sleep disturbance may increase risk for depression is through alterations to the functioning of the HPA axis (Buckley & Schatzberg, 2005). In healthy individuals, cortisol is highest in the morning, rises dramatically within the 30–45 min after waking, and declines throughout the remainder of the day. The initial rise in cortisol is referred to as the cortisol awakening response (CAR), and the decline across the day is referred to as the diurnal cortisol slope. Individuals reporting persistent sleep problems exhibit a general upregulation in cortisol throughout the day, which is the final secretory product of the axis. Total sleep time is negatively correlated with circulating cortisol across the day (Chrousos, Vgontzas, & Kritikou, 2016). Experimental sleep disruption in the form of repeated arousals throughout the night also induce elevated cortisol the subsequent day (Chrousos et al., 2016), and individuals reporting greater sleep disturbances demonstrate flatter cortisol slopes across the day (Kumari et al., 2009; Zeiders, Doane, & Adam, 2011) and a larger CAR (Kumari et al., 2009). This may occur in part through the exaggerating effect that sleep deprivation has on receptors for adrenocorticotropic hormone (ACTH) on the adrenal cortex, which trigger the secretion of cortisol (Meerlo, Sgoifo, & Suchecki, 2008; Suchecki, Lobo, Hipólide, & Tufik, 1998). In turn, having a larger CAR has been identified as a prospective predictor of depressive symptoms (Hsiao et al., 2013; Kuhlman et al., 2017), depressive episode onset 1 (Adam et al., 2010) and 2.5 years later (Vrshek-Schallhorn et al., 2013), and depression recurrence (Hardeveld et al., 2014). It is important to note, however, that some studies have failed to observe this association (Carnegie et al., 2014; LeMoult, Ordaz, Kircanski, Singh, & Gotlib, 2015) while others have found the prospective association between CAR and depressive symptoms varies by stress exposure (Schuler et al., 2017). To our knowledge, only one study has tested HPA axis functioning as a mediator between sleep disturbances and depressive symptoms, finding that in prostate cancer survivors, diurnal cortisol slope and total diurnal cortisol exposure indirectly linked sleep problems with depressive symptoms over 4 months (Hoyt, Bower, Irwin, Weierich, & Stanton, 2016). However, we are unaware of any studies examining these pathways in adolescents.
The purpose of this study was to determine whether CAR and diurnal cortisol slope contribute to the association between sleep disturbances in late adolescence and depressive symptoms in early adulthood, when risk for depression is highest. In this study, we focus on two indices of HPA axis functioning: CAR and diurnal slope. CAR has been repeatedly identified as a prospective predictor of depression (Adam et al., 2010; Kuhlman et al., 2017; Vrshek-Schallhorn et al., 2013), and diurnal cortisol slope was selected given that basal cortisol is consistently elevated in depressed children and adolescents compared to controls (Lopez-Duran, Kovacs, & George, 2009). We hypothesized that having more sleep disturbance would be associated with higher depressive symptoms 2 years later, and that having a larger CAR and less decline in cortisol from waking to bedtime would mediate this prospective association.
Method
Participants
Participants in the current study were from a longitudinal study designed to understand how psychosocial factors, family processes, and daily experiences contribute to early risk for poor health (Chiang et al., 2017; Guan et al., 2016). Enrollment for the larger study (n = 316) began in 10th and 11th grades and included follow-up assessments 2 and 4 years after enrollment. The current study focused on participants during the transition from late adolescence to early adulthood (e.g., the second and third assessments of the larger study), which spanned from 12th grade to 3 years after high school. The inclusion criteria for the present study were having at least 2 complete days of data for diurnal HPA axis functioning, sleep at the second assessment, and depressive symptoms at the third and final assessment. Cases without complete data on any of these variables were listwise deleted. Of note, no participants were excluded for missing saliva samples, and all of the 157 participants included had complete data for all 3 days of diurnal HPA axis regulation. At the second assessment, 53.5% of participants were enrolled in high school, 36.9% were in college, and 9.6% were not in school. Participants were from diverse backgrounds: 46.5% of the sample was Latino, 14.0% was Asian, 31.2% was European, and 8.3% reported “other” for ethnicity. This demographic distribution is similar to that of the Los Angeles metropolitan area (US Census Bureau, 2009). Adolescents in this sample had parents with a wide range of educational backgrounds: 14.8% had parents who did not complete high school, 15.6% had parents with a high school diploma, 46.6% had parents with some college including vocational training, and 23.0% had parents with a bachelor’s degree or higher. There were no differences in household income, p = .34, parent education, p = .16, body mass index (BMI), p = .42, or depressive symptoms, p = .73, between the sample in the present analyses and the 316 participants initially enrolled in the larger study. However, the present analytic sample had a higher proportion of female participants, χ2 = 7.33, p = .007, and a nonsignificantly lower proportion of Asian participants than the initially enrolled sample, χ2 = 6.78, p = .079.
Procedures
Participants were recruited from four Los Angeles high schools that were selected based on racial and ethnic diversity as well as the willingness of the school to allow our team to recruit from their student population. Recruitment involved in-class presentations, distribution of study flyers and recruitment forms during presentations, and postal mailings to students’ homes. Study visits occurred in participants’ homes between October and August.1 Adolescents completed all questionnaires using an electronic tablet. Upon completion of the questionnaires, participants were instructed to collect five saliva samples each day for the next 3 consecutive days (see detail below). Study staff returned to participants’ homes to collect completed materials, and adolescents were compensated for their time. Data for the present analyses focused on self-reported sleep disturbance, depressive symptoms, and salivary cortisol assessed when participants were 17–18 years of age, and depressive symptoms assessed 2 years later, when participants were 19–20 years old. See Figure 1 for a study procedures timeline.
Figure 1.
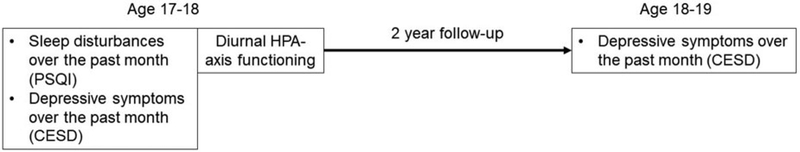
Timeline of study procedures.
Measures
Sleep disturbance
Participants completed the 19-item Pittsburgh Sleep Quality Index (PSQI) about their sleep over the past month (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). The PSQI returns a global sleep disturbance score that can range from 0 to 21; higher scores indicate worse sleep disturbance, and scores greater than 5 indicate clinically meaningful problems with sleep (Dietch et al., 2016). The PSQI global scores were computed from seven component scores that can range from 0 to 3. These sleep components include subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. In the present sample, the internal reliability of the seven components of the PSQI was α = .62. It should be noted that the internal reliability of the PSQI in adolescent and young adult samples tends to be lower than in the original validation sample (e.g., Lund, Reider, Whiting, & Prichard, 2010). In this sample, the reliability was partly driven by Component 6, use of sleep medication, which was rare (14%) in our sample even in participants with the poorest sleep. Due to the instability of individual PSQI component scores (Cole et al., 2006; Mollayeva et al., 2016), no analyses were conducted with individual component scores.
Depressive symptoms
Depressive symptoms in the past week were assessed via the Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977). The CES-D is a 20-question, self-report instrument with excellent reliability and validity (Radloff, 1977). Scores on the CES-D can range from 0 to 60, and a score greater than 15 suggests clinically significant symptoms of depression (Radloff, 1977). The CES-D has demonstrated good sensitivity and specificity, with major depressive episodes determined via semistructured clinical interview (Mulrow et al., 1995; Stockings et al., 2015). This measure also demonstrated excellent internal reliability in the current sample, age 17–18 α = .91 and age 19–20 α = .92.
HPA axis functioning
Participants provided saliva samples using Salivettes (Sarstedt, Inc.) 5 times per day across 3 consecutive days: waking, 15 and 30 min after waking, before dinner, and at bedtime. They were instructed to collect saliva prior to eating, drinking, or brushing their teeth, to avoid tobacco and caffeinated products half an hour prior to collection, and to store samples in their refrigerators until study staff returned to collect the completed materials. To encourage compliance, reminders of when to provide saliva samples were sent via text throughout the day. Participants used electronic time stampers (Dymo Corporation, Stamford, CT) and stamping booklets to record time and completion of each saliva sample. The electronic time stampers were preset with a security code that precluded participants from tampering with the date and time. Returned saliva samples were stored at −80 °C until they were shipped for assay. Saliva samples were assayed for cortisol concentrations (nmol/L) by the Laboratory of Biological Psychology at the Technical University of Dresden, Germany, using high-sensitivity chemiluminescence-immunoassays (IBL International, Hamburg, Germany). All saliva samples for an individual were assayed on the same plate in duplicate, and coefficients of variation were <8%.
We computed two indices of diurnal HPA axis functioning: CAR and diurnal slope. Both indices were averages across the 3 sampling days to reduce the influence of day-to-day withinperson variability on the indices (Adam & Kumari, 2009). CAR was computed by subtracting the concentration of salivary cortisol at waking from the concentration at 30 min postwaking to reflect reactivity of the system following waking (see Stalder et al., 2016, for CAR expert consensus guidelines). On average, the +30 min sample was collected 32 min (SD = 10) after waking. There were 12 participants whose 30-min postwaking sample was taken more than 15 min early or late, and 48 participants whose 30-min postwaking sample was taken more than 5 min early or late. There were no significant differences in CAR magnitude between participants with sample timings more than 15 min or 5 min late or early, F (1, 155) = 1.28, p = .26 and F (1, 155) = 0.01, p = .92, respectively. The pattern of results was not different when the number of minutes between the waking and 30-min postwaking sample was included as a covariate in the models. Consistent with previous studies using the CAR index (Kuhlman, Geiss, Vargas, & Lopez-Duran, 2015; Kuhlman et al., 2017; Kuhlman, Repetti, Reynolds, & Robles, 2016), negative CAR values were recoded as 0 to reduce the influence of a delay between actual waking and the waking saliva sample on CAR magnitude (Okun et al., 2010). We also computed the slope of diurnal cortisol decline throughout the day. Consistent with previous studies (Kuhlman et al., 2016, 2017), we subtracted cortisol concentrations at waking from cortisol concentrations at bedtime, and divided that value by the number of hours between the waking and bedtime samples.
Data analysis
All continuous variables were examined for normality and found to be normally distributed. To test the hypothesized associations and mediating processes between late adolescent sleep problems, CAR, and later depressive symptoms, we fit a mediator model with bootstrap resampling for assessing indirect effects (Hayes, 2013; Preacher & Hayes, 2008). There are several methods of statistically testing mediation (see MacKinnon, Fairchild, & Fritz, 2007, for a thorough review and comparison of statistical approaches to mediation). The most traditional has been the causal steps approach. This approach requires that first there be a significant effect of the independent variable (IV) on the dependent variable (DV), a significant effect of the IV on the mediator (M), and a significant effect of M on the DV. Theoretically, the difference in the effect of the IV on the DV with and without M can then be used to infer mediation (MacKinnon et al., 2007). However, the requirement of these three significant pathways in any given data set substantially reduces the power to detect mediation, contributing to Type II errors. The currently recommended method for testing mediation instead uses bootstrap resampling, which does not require a significant effect of the IV on the DV to test for mediation (although a theoretical association is critical for study design and interpretation of the results). Instead, the product of the effect of the IV on M and M on the DV when accounting for the IV can be used to infer mediation. The relative mathematical equivalence of these (difference vs. product) approaches has been well established, despite their differences in assumptions, power, and utility (MacKinnon et al., 2007).
In the present analyses, we employed the PROCESS macro for SPSS developed by Preacher and Hayes (2008). All analyses generated 95% bias-corrected and accelerated bootstrap confidence intervals (CIs) for the indirect effects using 5,000 bootstrap samples. In this model, the independent variable (IV: sleep problems) affects the dependent variable (DV: depressive symptoms) through the mediator (CAR or diurnal slope). The indirect effect of the mediator is its unique effect on the outcome (Preacher & Hayes, 2008). This approach uses ordinary least squares regression to establish the association between (a) the IV (sleep problems) and the mediator (CAR), (b) the mediator and the DV (depressive symptoms) while accounting for the IV, (c) the IV and the DV, and (d) ab, the cross-product of a and b. If the upper and lower bounds of the bootstrap CI for ab do not contain zero, the mediated effect is considered statistically significant (Hayes, 2013).
We also explored the impact of several potential covariates on the key variables in our models using bivariate χ2, Pearson correlations, or analysis of variance depending on variable structure. These included sociodemographic (sex, parent education, household income, and ethnicity), theoretical (depressive symptoms at the time of the sleep and HPA axis assessment and BMI), and methodological variables (time between the sleep/HPA axis and final depressive symptoms assessment, waking time on saliva collection days, and minutes between the waking and 30 min postwaking saliva samples) that are known to be associated with the development of depression or HPA axis functioning. Waking time was determined via self-report of waking time on the saliva collection diary, which is a reliable measure of waking time when compared to polysomnography (Okun et al., 2010). All models were first tested unadjusted for covariates. Then, any covariates that significantly influenced independent, mediator, or dependent variables were then included in our mediation model to determine whether hypotheses were supported above and beyond the role of these covariates.
Results
Participants in this study were, on average, 18.23 (SD = 0.75) years old at time of the sleep disturbance assessment and diurnal cortisol sampling. At this assessment, participants reported a wide range of sleep disturbance, with 49.7% exceeding the threshold for clinically meaningful sleep problems. Participants exhibited a typical diurnal pattern of cortisol regulation. Upon waking, the majority (65.6%) of participants exhibited an increase in cortisol, and almost all adolescents (97.4%) exhibited a decline in cortisol from waking to the last sample of the day. Finally, participants reported a wide range of depressive symptoms at both assessments with 42.7% and 32.6% of the sample exceeding the clinical cutoff at the first (age 17–18) and second assessments (age 19–20), respectively. See Table 1 for descriptive statistics for all key continuous variables in the analyses and the bivariate correlations between them.
Table 1.
Descriptive statistics and bivariate correlations between key study variables
| M (SD) | Min-Max | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | |
|---|---|---|---|---|---|---|---|---|---|---|
| Late adolescent assessment (ages 17–18) | ||||||||||
| 1. Age | 18.23 (0.75) | 14.5–20 | 1.00 | |||||||
| 2. Sleep disturbance | 5.68 (2.88) | 0–15 | ….012 | 1.00 | ||||||
| 3. Depressive symptoms | 15.19 (10.20) | 0–46 | −.067 | .500** | 1.00 | |||||
| 4. Cortisol awakening response (nmol/L) | 7.69 (9.61) | 0–46.31 | −.120 | .204* | .154+ | 1.00 | ||||
| 5. Diurnal cortisol slope | −1.25 (0.64) | −3.37–0.30 | .106 | .096 | .092 | .194* | 1.00 | |||
| 6. Waking time on saliva sampling days | 8:32 (1:40) | 4:10–12:41 | .252** | .105 | .106 | −.043 | .128 | 1.00 | ||
| 7. Hours awake on saliva sampling days | 15.21 (2.96) | 10.57–23.00 | −.046 | .037 | −.128 | .049 | .087 | −.079 | 1.00 | |
| Early adulthood assessment (ages 19–20) | ||||||||||
| 8. Time elapsed between assessments (years) | 1.98 (0.22) | 1.46–2.64 | −.143+ | .029 | .024 | −.036 | −.119 | −.127 | −.038 | 1.00 |
| 9. Depressive symptoms | 15.27 (10.42) | 0–50 | −.051 | .316** | .508** | .197* | .029 | .053 | −.066 | .023 |
p < .01.
p < .05.
p < .10.
Family household income, parent education, time between assessments, waking time on saliva sampling days, and minutes between waking and 30-min postwaking sample were not associated with any of the key variables of interest, ps > .11. Female participants reported higher depressive symptoms at age 19–20 than male participants, p = .045, Asian participants exhibited a larger CAR than any other ethnic group, p = .018, participants with a higher BMI exhibited a smaller CAR, p = .032, and participants reporting more depressive symptoms at age 17–18 also had nonsignificantly larger CARs, p = .055, more sleep disturbances,p < .001, and greater depressive symptoms at age 19–20, p < .001. Thus, we tested our models with and without sex, ethnic background, BMI, and depressive symptoms at age 17–18.
Sleep as a prospective predictor of depressive symptoms
Individuals who reported more sleep disturbance also reported higher depressive symptoms 2 years later, b =1.15, SE = 0.28, p < .001, and reports of sleep disturbance accounted for 10% of the variance in later depressive symptoms, R2 = .10, F (1, 155) = 17.24, p < .001. The prospective association between sleep and depressive symptoms 2 years later remained significant after accounting for sex, Asian ethnic background, and BMI, b = 0.88, SE = 0.31, p = .005. However, sleep disturbances at age 18 were not a significant predictor of later depressive symptoms when accounting for concurrent depressive symptoms, b = 0.14, SE = 0.32, p = .67.2
Sleep disturbance and diurnal indices of HPA axis functioning
Greater sleep disturbance was associated with having a larger CAR, b = 0.68, SE = 0.26, p = .01, and sleep disturbance accounted for 20% of variability in CAR, R2 = .20, F (1, 155) = 6.76, p = .010. This pattern did not change when adjusting for covariates (age 17–18 depressive symptoms, sex, ethnic background, and BMI), b = 0.75, SE = 0.32, p = .02. Sleep disturbance was not associated with the slope of diurnal cortisol decline, b = 0.02, SE = 0.02, p = .24. This pattern did not change when adjusting for covariates, b = 0.02, SE = 0.02, p = .31. See Table 2 for the results of these models.
Table 2.
Unstandardized coefficient estimates predicting age 17–18 CAR and diurnal slope from sleep disturbances over the past month and key covariates
| CAR |
Diurnal slope |
|||||
|---|---|---|---|---|---|---|
| Predictor | R2 | b (SE) | p | R2 | b (SE) | p |
| .16 | .0003 | .04 | .43 | |||
| Intercept | 9.38 (4.32) | .03 | −1.44 (0.30) | <.001 | ||
| Sleep disturbance | 0.75 (0.32) | .02 | 0.02 (0.02) | .31 | ||
| Sex | 0.70 (1.67) | .68 | −0.06 (0.12) | .62 | ||
| Ethnicity | 5.98 (2.19) | .007 | −0.10 (0.16) | .51 | ||
| BMI | −0.34 (0.16) | .03 | 0.001 (0.01) | .95 | ||
| Depressive symptoms | 0.07 (0.09) | .48 | 0.01 (0.01) | .28 | ||
HPA axis functioning and depressive symptoms
We then examined the association between diurnal indices of HPA axis functioning and depressive symptoms 2 years later. Having a larger CAR was associated with having more depressive symptoms 2 years later, b = 0.15, SE = 0.07, p = .023. This observation remained when covariates were added to the model, b = 0.19, SE = 0.09, p = .030. Overall, the covariate adjusted model of sleep problems and CAR accounted for 27% of variance in depressive symptoms 2 years later, R2 = .27, F = 7.98, p < .001. See Table 3 for results of the direct and total models predicting age 19–20 depressive symptoms.
Table 3.
Unstandardized coefficient estimates predicting age 19–20 depressive symptoms from age 17–18 sleep disturbances, CAR, and key covariates
| Direct model |
Total model |
|||||
|---|---|---|---|---|---|---|
| R2 | b (SE) | p | R2 | b (SE) | p | |
| .24 | <.001 | .27 | <.001 | |||
| Intercept | −0.14 (4.26) | .97 | −1.90 (4.28) | .66 | ||
| Sleep disturbance | 0.14 (0.32) | .67 | −0.06 (0.32) | .99 | ||
| Sex | 2.38 (1.65) | .15 | 2.25 (1.63) | .17 | ||
| Ethnicity | 0.93 (2.16) | .67 | −0.20 (2.19) | .93 | ||
| BMI | 0.25 (0.15) | .11 | 0.31 (0.15) | .05 | ||
| Depressive symptoms | 0.47 (0.09) | <.001 | 0.46 (0.09) | <.001 | ||
| CAR | – | – | 0.19 (0.09) | .030 | ||
Diurnal cortisol slope was not associated with depressive symptoms 2 years later, b = −0.03, SE =1.25, p = .98, and this pattern did not change when adjusting for covariates, b = −0.05, SE =1.24, p = .97.
Cortisol awakening response mediates the association between sleep and depressive symptoms
Finally, we tested whether CAR mediated the link between sleep disturbances and later depressive symptoms. In the unadjusted model, there was a significant total effect of sleep disturbance and CAR on depressive symptoms 2 years later, c =1.15, SE = 0.28, 95% CI [0.60, 1.69]. This total effect was composed of a significant direct effect of sleep disturbance on later depressive symptoms, c1 = 1.04, SE = 0.28, 95% CI [0.49, 1.60], and a significant indirect effect of sleep disturbance on later depressive symptoms through CAR, ab = 0.10, SE = 0.07, 95% CI [0.005, 0.31]. See Figure 2 for the results of the adjusted model depicting the significant indirect effect of sleep on later depressive symptoms via CAR at the time of the sleep assessment. The indirect effect of sleep disturbances on later depressive symptoms via a larger CAR remained significant after including previous depressive symptoms, sex, BMI, and ethnicity in the model, adjusted ab = 0.14, SE = 0.09, 95% CI [0.02, 0.39]. The fully unstandardized and adjusted indirect effect was small, d = 0.04, SE = 0.02, 95% CI [0.0001, 0.09].3 This suggests that sleep disturbance is associated with depressive symptoms 2 years later through the impact on CAR, independent of concurrent depressive symptoms at the time of the sleep assessment, though there are likely other mediators contributing to this phenomenon.
Figure 2.
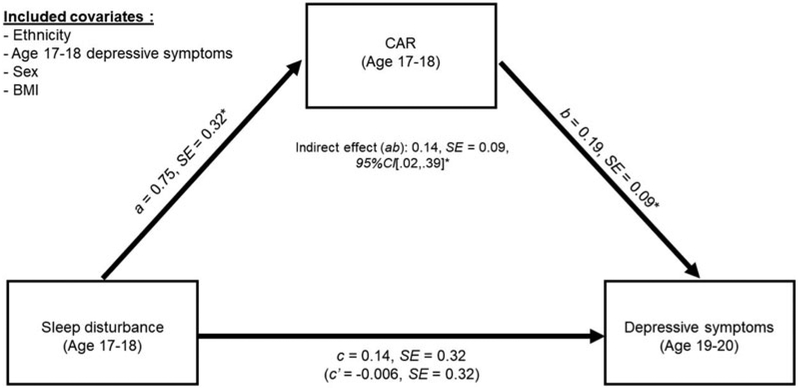
Cortisol awakening response (CAR) mediates the association between sleep disturbance in late adolescence and depressive symptoms in early adulthood.
*p < .05.
Discussion
Sleep disturbance among adolescents is rampant with farreaching health consequences (Shochat, Cohen-Zion, & Tzischinsky, 2014), including increased risk for depression and depressive symptoms. Reports of both sleep disturbance and depressive symptoms across the transition from late adolescence to early adulthood were high in our sample, although consistent with that observed in other adolescent samples (Doane, Gress-Smith, & Breitenstein, 2015). Yet, not all adolescents who experience sleep disturbances will later develop depression. We found that greater symptoms of sleep disturbance were associated with depressive symptoms 2 years later through the effects of sleep disturbance on CAR. These results extend previous observations that both sleep disturbance and CAR prospectively predict depressive symptoms while also extending these findings to show that the effect of sleep disturbance on functioning of the HPA axis may contribute to the pathogenesis of depression over time.
There are several possibilities for how sleep disturbance may be contributing to larger CAR. First, sleep deprivation may operate as a psychobiological stressor to the body. CAR increases in the context of daily stressors as a way of mobilizing resources to meet the demands of the day (Fries, Dettenborn, & Kirschbaum, 2009). Further, experimental sleep deprivation causes individuals to mount larger cortisol responses to stress (Minkel et al., 2014; van Dalfsen & Markus, 2018). Second, sleep deprivation may alter adrenal sensitivity to ACTH. A combination of animal and human research has argued that the magnitude of cortisol increase in response to waking is a measure of adrenal sensitivity to ACTH (Clow, Hucklebridge, Stalder, Evans, & Thorn, 2010). Briefly, ACTH receptors in the adrenal gland are inactive during sleep, thus inhibiting the synthesis and secretion of cortisol. Upon waking, the immediate rise in glucocorticoids represents the sensitivity of adrenal ACTH receptors. There is strong experimental evidence from animal models that sleep deprivation increases adrenal sensitivity to ACTH (Meerlo et al., 2008; Suchecki et al., 1998), as evidenced by less secretion of ACTH necessary to stimulate a robust glucocorticoid response following stress. These two mechanisms are not mutually exclusive, and both may be at play in this sample. This may be especially true during adolescence when sleep disturbance can largely be attributed to the distress and time constraints associated with social and academic demands. The amount of sleep necessary to achieve academically is 1 hr shorter in adolescence than that needed for optimal mental health (Fuligni, Arruda, Krull, & Gonzales, 2018).
Consistent with several previous studies (Adam et al., 2010; Kuhlman et al., 2017; Vrshek-Schallhorn et al., 2013), a larger CAR was associated with greater depressive symptoms 2 years later in this sample. Enhanced adrenal sensitivity to ACTH is one of the earliest HPA axis dysregulations observed in depressed patients (Ehlert, Gaab, & Heinrichs, 2001). One of the implications of enhanced adrenal sensitivity to ACTH is increased exposure to circulating glucocorticoids. Glucocorticoids increase attention to negatively valenced information and impede retrieval of affective memory, which may play a direct role in effective emotion regulation, coping with stressful life events, and the pathogenesis of depression (Erickson, Drevets, & Schulkin, 2003; Lupien, McEwen, Gunnar, & Heim, 2009). In addition, increased adrenal sensitivity may play an indirect role in the pathogenesis of depression through inflammation. Glucocorticoids play a central role in regulating inflammation (Baschant & Tuckermann, 2010). However, chronic exposure to glucocorticoids can induce glucocorticoid resistance within immune cells leaving inflammation to be poorly regulated, which has also been linked to the pathogenesis of depression (Miller, Maletic, & Raison, 2009). Here we provide evidence that sleep disturbance, a disrupted basic physiological process and source of psychological distress, may be an important behavioral factor that leads to depression through an index of HPA axis functioning, namely, CAR.
In contrast, we did not find that variability in diurnal cortisol slope prospectively predicted depression, nor that diurnal cortisol slope mediated the link between sleep problems and depressive symptoms over time. While CAR may indicate adrenal sensitivity to ACTH, diurnal cortisol regulation is largely controlled by the hypothalamus in an effort to maintain homeostasis throughout the body (Tsigos & Chrousos, 2002) and by clock genes in the adrenal cortex that maintain circadian regulation of cortisol release (Oster et al., 2006). The specificity of our results to CAR and not diurnal cortisol slope may underscore the utility of developing a better understanding of how different HPA axis indices represent underlying neurobiology in ways that increase our understanding of the pathogenesis of disease. Certainly, all HPA axis indices measured using cortisol represent possible dysregulations at any point in the hormonal cascade; our results suggest that increases in CAR magnitude may be a more sensitive measure of alterations to HPA axis function that have long-term implications for health in a fairly healthy community sample.
It is important to acknowledge that sleep disturbances were not directly related to depressive symptoms 2 years later after controlling for past depressive symptoms. This was true even when the sleep item in the depressive symptom inventory was removed from the scale. This observation in our data introduces the possibility that depressive symptoms are maintained over time, in part, through upregulations in CAR or other neuroendocrine indices. However, this does not appear to be the case in our sample. Both the bivariate and adjusted associations between age 17–18 depressive symptoms and CAR were nonsignificant (see Tables 1 and 2). This may indicate that independent of whether an individual is currently experiencing clinically significant depressive symptoms, persistent sleep disturbances have the potential to upregulate CAR and may presage depressive symptoms over time. Alternatively, it may suggest that among depressed adolescents, it is the sleep disturbance symptoms that have implications for HPA axis function, which presage broader symptom maintenance. This is critical for clinical scientists to better understand because sleep disturbance may also be an indicator of depression severity. For example, depressed adolescents with sleep disturbances report more severe depressive symptoms (McGlinchey, Reyes-Portillo, Turner, & Mufson, 2017), and measures of sleep disturbance prospectively predict suicidal ideation in young adults above and beyond depressive symptoms (Bernert, Hom, Iwata, & Joiner, 2017). Future investigations may consider testing other social, behavioral, and biological factors that mediate the link between specific depressive symptoms and the emergence of depression over time.
The results of this study should be considered within the context of its limitations. First, despite the longitudinal nature of the study design, these results are correlational, and causal associations cannot be inferred. While the period of sleep disturbances assessed by the PSQI in this sample entirely preceded saliva collection days, these occurred in close proximity to one another. Thus, it is likely that the sleep disturbances reported on the PSQI were sustained during the CAR collection period. However, average sleep time on the nights preceding saliva collection did not differ between adolescents with and without clinically significant sleep problems, p = .53. That being said, CAR is a relatively unstable neuroendocrine index in part due to day-to-day variability in sleep, diet, mood, and other factors (Kuhlman, Robles, Dickenson, Reynolds, & Repetti, 2019; Ross, Murphy, Adam, Chen, & Miller, 2014). The results of our models do not change when accounting for sleep duration on the nights preceding each day of saliva sampling. A more comprehensive assessment of the distal and proximal characteristics of poor sleep as predictors of CAR and other measures of neuroendocrine functioning will be important considerations in studies aiming to replicate and extend these results. There is a bidirectional association between sleep problems and depressive symptoms in both adolescents (Roberts & Duong, 2014) and adults (Bouwmans, Conradi, Bos, Oldehinkel, & de Jonge, 2017). Thus, only experimental studies of sleep deprivation or interventions could truly inform the causal associations between sleep disturbance, CAR, and depression. Further, there are likely a number of important behavioral, cognitive, and biological mediators that explain the link between sleep problems and depressive symptoms that need to be identified and interrogated as mechanisms of action in effective depression prevention trials that target sleep. Sleep disturbance and depression in this study were operationalized using a continuous measure of symptoms assessed via a self-report questionnaire. Thus, no confirmation of a depressive episode or insomnia was made, and exploration of how these processes may differ when sleep is measured objectively would contribute further to our understanding. However, the data supporting the external validity of the CES-D (Stockings et al., 2015) as well as the consistency of our results with previous studies looking at both sleep (Alvaro, Roberts, & Harris, 2013) and CAR (Adam et al., 2010; Vrshek-Schallhorn et al., 2013) as prospective predictors of diagnosed episodes of depression inspire confidence. Further, selfreport of sleep problems, often via the PSQI, remains the most common method of assessing insomnia in clinical settings, thus underscoring the potential translational utility of our findings to clinical scientists and practitioners. That being said, the internal reliability of the PSQI in our sample was lower than previously published samples, suggesting that the sleep problems reported in our sample may be more reflective of the adolescence-specific constraints on sleep (e.g., homework, school start time, and socializing) than insomnia, for which this measure was validated. Finally, our measure of CAR in this study was computed using the difference between cortisol at waking and 30 min after waking. Our measures of CAR are largely consistent with best practices in the measurement of CAR (Stalder et al., 2016), however differ somewhat from the most updated best practices, which suggest examining the area under the curve across three or more morning samples and recommend using track cap compliance devices (e.g., MEMS caps) for sample collection. As CAR magnitude becomes established as a prospective predictor of depression in more samples, it will be important to further characterize the most sensitive measures of CAR that capture this underlying neurobiological process. In particular, 34.4% of our participants did not exhibit a CAR. This is similar to the rates of CAR nonresponse observed in other samples (Kuhlman et al., 2016, 2017), although the factors underlying the absence of CAR still need to be characterized.
Conclusions
The prevalence of sleep disturbance and depression are both high during adolescence. The best available interventions for adolescent depression only remit 37% of depressive episodes (e.g., TADS Team, 2007), and treating depression during adolescence does not yet appear to reduce the costs of adolescent depression to society, such as later health service utilization (Lewinsohn, Rohde, & Seeley, 1998b). In contrast, sleep problems can be effectively treated in adolescents (Blake et al., 2017), which appear to also confer reductions in depressive symptoms. Given that adolescent depression is associated with a more severe course of illness (Rohde et al., 2012), intervening in a preventative manner on a core risk factor such as sleep may be a lucrative area of further investigation. Here we identify a biological pathway through which sleep disturbance may lead to depression over time, namely, via the impact of sleep disturbance on CAR. This finding may afford greater precision in preventing adolescent depression. Furthermore, CAR is heritable and ecologically valid (Fries et al., 2009; Wüst, Federenko, Hellhammer, & Kirschbaum, 2000), making it a candidate biomarker for future studies evaluating the effectiveness of sleep interventions aimed at depression prevention.
Acknowledgments
Financial Support. The composition of this manuscript was made possible by the National Institute of Mental Health through a career development award that was awarded to Dr. Kuhlman (K08MH112773), and the collection of this data was made possible by the Eunice Kennedy Shriver Institute of Child Health and Human Development (Grants R01HD062547 and P2C-HD041022) and the National Institute on Aging (Grants P30-AG017265 and P30-AG028748).
Footnotes
When accounting for whether assessments occurred during the regular school year or during the summer, there were no changes to our results.
Given that the CES-D includes an item about sleep disturbance, “Your sleep was restless,” we also tested our results with this item removed. There were no differences in any of our findings when this item was removed. To this end and in order to make our sample descriptives optimally comparable with other published studies, we have only reported our results with the entire scale.
When we included PSQI scores at the final assessment in our full model predicting depressive symptoms at the final assessment, CAR remained a significant predictor of depressive symptoms across the follow-up, b = 0.01, SE = 0.004, p = .039, despite the strong association between concurrent PSQI scores and depressive symptoms, b = 0.06, SE = 0.02, p < .001, though the indirect effect was similar in magnitude but nonsignificant, b = 0.12, SE = 0.09, 95% CI [−0.02, 0.31].
References
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, & Griffith JW (2010). Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology, 35, 921–931. doi: 10.1016/j.psyneuen.2009.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, & Kumari M (2009). Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology, 34, 1423–1436. doi: 10.1016/j.psyneuen.2009.06.011 [DOI] [PubMed] [Google Scholar]
- Alvaro PK, Roberts RM, & Harris JK (2013). A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep, 36, 1059–1068. doi: 10.5665/sleep.2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth DK, Sletten TL, Junge M, Simpson K, Clarke D, Cunnington D, & Rajaratnam SMW (2015). A randomized controlled trial of cognitive behavioral therapy for insomnia: An effective treatment for comorbid insomnia and depression. Journal of Counseling Psychology, 62, 115–123. doi: 10.1037/cou0000059 [DOI] [PubMed] [Google Scholar]
- Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, … Riemann D (2011). Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. Journal of Affective Disorders, 135, 10–19. doi: 10.1016/j.jad.2011.01.011 [DOI] [PubMed] [Google Scholar]
- Baschant U, & Tuckermann J (2010). The role of the glucocorticoid receptor in inflammation and immunity. Journal of Steroid Biochemistry and Molecular Biology, 120, 69–75. doi: 10.1016/j.jsbmb.2010.03.058 [DOI] [PubMed] [Google Scholar]
- Baumeister D, Lightman SL, & Pariante CM (2014). The interface of stress and the HPA axis in behavioural phenotypes of mental illness In Pariante CM & Lapiz-Bluhm MD (Eds.), Behavioral neurobiology of stress-related disorders (pp. 13–24). Berlin: Springer. [DOI] [PubMed] [Google Scholar]
- Bernert RA, Hom MA, Iwata NG, & Joiner TE (2017). Objectively assessed sleep variability as an acute warning sign of suicidal ideation in a longitudinal evaluation of young adults at high suicide risk. Journal of Clinical Psychiatry, 78, e678–e687. doi: 10.4088/JCP.16m11193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake MJ, Sheeber LB, Youssef GJ, Raniti MB, & Allen NB (2017). Systematic review and meta-analysis of adolescent cognitive-behavioral sleep interventions. Clinical Child and Family Psychology Review, 20, 227–249. doi: 10.1007/s10567-017-0234-5 [DOI] [PubMed] [Google Scholar]
- Bouwmans MEJ, Conradi HJ, Bos EH, Oldehinkel AJ, & de Jonge P (2017). Bidirectionality between sleep symptoms and core depressive symptoms and their long-term course in major depression. Psychosomatic Medicine, 79, 336–344. doi: 10.1097/PSY.0000000000000407 [DOI] [PubMed] [Google Scholar]
- Buckley TM, & Schatzberg AF (2005). On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: Normal HPA axis activity and circadian rhythm, exemplary sleep disorders. Journal of Clinical Endocrinology & Metabolism, 90, 3106–3114. doi: 10.1210/jc.2004-1056 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF III, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Carnegie R, Araya R, Ben-Shlomo Y, Glover V, O’Connor TG, O’Donnell KJ, … Lewis G (2014). Cortisol awakening response and subsequent depression: Prospective longitudinal study. British Journal of Psychiatry, 204, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper RC, Redmond DE, Katz MM, Schaffer CB, Davis JM, & Koslow SH (1985). Somatic symptoms in primary affective disorder: Presence and relationship to the classification of depression. Archives of General Psychiatry, 42,1098–1104. doi: 10.1001/archpsyc.1985.01790340082012 [DOI] [PubMed] [Google Scholar]
- Chang C-H, Chen S-J, & Liu C-Y (2017). Pediatric sleep apnea and depressive disorders risk: A population-based 15-year retrospective cohort study. PLOS ONE, 12, e0181430. doi: 10.1371/journal.pone.0181430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang JJ, Kim JJ, Almeida DM, Bower JE, Dahl RE, Irwin MR,…Fuligni AJ (2017). Sleep efficiency modulates associations between family stress and adolescent depressive symptoms and negative affect. Journal of Adolescent Health, 61, 501–507. doi: 10.1016/j.jadohealth.2017.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Lavretsky H, Olmstead R, Levin MJ, Oxman MN, & Irwin MR (2008). Sleep disturbance and depression recurrence in community-dwelling older adults: A prospective study. American Journal of Psychiatry, 165, 1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos G, Vgontzas AN, & Kritikou I (2016). HPA axis and sleep In De Groot LJ, Chrousos G, & Dungan K (Eds.), Endotext (1st ed.). South Dartmouth, MA: MDText.com. [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, & Thorn L (2010). The cortisol awakening response: More than a measure of HPA axis function. Neuroscience & Biobehavioral Reviews, 35, 97–103. doi: 10.1016/j.neubiorev.2009.12.011 [DOI] [PubMed] [Google Scholar]
- Cole JC, Motivala SJ, Buysse DJ, Oxman MN, Levin MJ, & Irwin MR (2006). Validation of a 3-factor scoring model for the Pittsburgh Sleep Quality Index in older adults. Sleep, 29, 112. [DOI] [PubMed] [Google Scholar]
- Dietch JR, Taylor DJ, Sethi K, Kelly K, Bramoweth AD, & Roane BM (2016). Psychometric evaluation of the PSQI in U.S. college students. Journal of Clinical Sleep Medicine, 12, 1121–1129. doi: 10.5664/jcsm.6050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doane LD, Gress-Smith JL, & Breitenstein RS (2015). Multi-method assessments of sleep over the transition to college and the associations with depression and anxiety symptoms. Journal of Youth and Adolescence, 44, 389–404. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Gaab J, & Heinrichs M (2001). Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: The role of the hypothalamus–pituitary–adrenal axis. Biological Psychology, 57, 141–152. doi: 10.1016/S0301-0511(01)00092-8 [DOI] [PubMed] [Google Scholar]
- Erickson K, Drevets W, & Schulkin J (2003). Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neuroscience & Biobehavioral Reviews, 27, 233–246. doi: 10.1016/S0149-7634(03)00033-2 [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, & Kirschbaum C (2009). The cortisol awakening response (CAR): Facts and future directions. International Journal of Psychophysiology, 72, 67–73. doi: 10.1016/j.ijpsycho.2008.03.014 [DOI] [PubMed] [Google Scholar]
- Fuligni AJ, Arruda EH, Krull JL, & Gonzales NA (2018). Adolescent sleep duration, variability, and peak levels of achievement and mental health. Child Development, 89, e18–e28. 10.1111/cdev.12729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AM, Caspi A, Eley TC, Moffitt TE, Oconnor TG, & Poulton R (2005). Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. Journal of Abnormal Child Psychology, 33, 157–163. [DOI] [PubMed] [Google Scholar]
- Guan S-SA, Bower JE, Almeida DM, Cole SW, Dahl RE, Irwin MR, … Fuligni AJ (2016). Parental support buffers the association of depressive symptoms with cortisol and C-reactive protein during adolescence. Brain, Behavior, and Immunity, 57, 134–143. doi: 10.1016/j.bbi.2016.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardeveld F, Spijker J, Vreeburg SA, Graaf RD, Hendriks SM, Licht CMM,… Beekman ATF (2014). Increased cortisol awakening response was associated with time to recurrence of major depressive disorder. Psychoneuroendocrinology, 50, 62–71. doi: 10.1016/j.psyneuen.2014.07.027 [DOI] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York: Guilford Press. [Google Scholar]
- Hoyt MA, Bower JE, Irwin MR, Weierich MR, & Stanton AL (2016). Sleep quality and depressive symptoms after prostate cancer: The mechanistic role of cortisol. Behavioral Neuroscience, 130, 351–356. doi: 10.1037/bne0000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao F-H, Chang K-J, Kuo W-H, Huang C-S, Liu Y-F, Lai Y-M,… Chan CLW (2013). A longitudinal study of cortisol responses, sleep problems, and psychological well-being as the predictors of changes in depressive symptoms among breast cancer survivors. Psychoneuroendocrinology, 38, 356–366. doi: 10.1016/j.psyneuen.2012.06.010 [DOI] [PubMed] [Google Scholar]
- Irwin MR, Archer G, Olmstead R, Brown TT, Teplin LA, Patel SR,… Breen EC (2018). Increased risk of depression in non-depressed HIV infected men with sleep disturbance: Prospective findings from the Multicenter AIDS Cohort Study. EBioMedicine, 36, 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Geiss EG, Vargas I, & Lopez-Duran NL (2015). Differential associations between childhood trauma subtypes and adolescent HPA-axis functioning. Psychoneuroendocrinology, 54, 103–114. doi: 10.1016/j.psyneuen.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Irwin MR, Ganz PA, Crespi CM, Petersen L, Asher A, & Bower JE (2017). Cortisol awakening response as a prospective risk factor for depressive symptoms in women after treatment for breast cancer. Psychosomatic Medicine, 79, 763–769. doi: 10.1097/PSY.0000000000000499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Repetti RL, Reynolds BM, & Robles TF (2016). Change in parent-child conflict and the HPA-axis: Where should we be looking and for how long? Psychoneuroendocrinology, 68, 74–81. doi: 10.1016/j.psyneuen.2016.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Robles TF, Dickenson L, Reynolds B, & Repetti RL (2019). Stability of diurnal cortisol measures across days, weeks, and years during middle childhood and early adolescence: Exploring the role of age, pubertal development, and sex. Psychoneuroendocrinology, 100, 67–74. doi: 10.1016/j.psyneuen.2018.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M, Badrick E, Ferrie J, Perski A, Marmot M, & Chandola T (2009). Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II Study. Journal of Clinical Endocrinology & Metabolism, 94, 4801–4809. doi: 10.1210/jc.2009-0555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Cho HJ, Olmstead R, Levin MJ, Oxman MN, & Irwin MR (2013). Persistent sleep disturbance: A risk factor for recurrent depression in community-dwelling older adults. Sleep, 36, 1685. doi: 10.5665/sleep.3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMoult J, Ordaz SJ, Kircanski K, Singh MK, & Gotlib IH (2015). Predicting first onset of depression in young girls: Interaction of diurnal cortisol and negative life events. Journal of Abnormal Psychology, 124, 850–859. doi: 10.1037/abn0000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Rohde P, & Seeley JR (1998a). Major depressive disorder in older adolescents: Prevalence, risk factors, and clinical implications. Clinical Psychology Review, 18, 765–794. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Rohde P, & Seeley JR (1998b). Treatment of adolescent depression: Frequency of services and impact on functioning in young adulthood. Depression and Anxiety, 7, 47–52. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Kovacs M, & George CJ (2009). Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology, 34, 1272–1283. doi: 10.1016/j.psyneuen.2009.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund HG, Reider BD, Whiting AB,& Prichard JR (2010). Sleep patterns and predictors of disturbed sleep in a large population of college students. Journal ofAdolescentHealth, 46,124–132. doi: 10.1016/j.jadohealth.2009.06.016 [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10, 434–445. doi: 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, & Fritz MS (2007). Mediation analysis. Annual Review of Psychology,58,593–614.doi: 10.1146/annurev.psych.58.110405.085542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinchey EL, Reyes-Portillo JA, Turner JB, & Mufson L (2017). Innovations in practice: The relationship between sleep disturbances, depression, and interpersonal functioning in treatment for adolescent depression. Child and Adolescent Mental Health, 22, 96–99. doi: 10.1111/camh.12176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerlo P, Sgoifo A, & Suchecki D (2008). Restricted and disrupted sleep: Effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Medicine Reviews, 12, 197–210. doi: 10.1016/j.smrv.2007.07.007 [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Brody D, Fisher PW, Bourdon K, & Koretz DS (2010). Prevalence and treatment of mental disorders among US children in the 2001–2004 NHANES. Pediatrics, 125, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He J, Burstein M, Swendsen J, Avenevoli S, Case B,… Olfson M (2011). Service utilization for lifetime mental disorders in U.S. adolescents: Results of the National Comorbidity Survey—Adolescent Supplement (NCS-A). Journal of the American Academy of Child & Adolescent Psychiatry, 50, 32–45. doi: 10.1016/j.jaac.2010.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, & Raison CL (2009). Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biological Psychiatry, 65, 732–741. doi: 10.1016/j.biopsych.2008.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkel J, Moreta M, Muto J, Htaik O, Jones C, Basner M, & Dinges D (2014). Sleep deprivation potentiates HPA axis stress reactivity in healthy adults. Health Psychology, 33, 1430–1434. doi: 10.1037/a0034219 [DOI] [PubMed] [Google Scholar]
- Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, & Colantonio A (2016). The Pittsburgh Sleep Quality Index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Medicine Reviews, 25, 52–73. doi: 10.1016/j.smrv.2015.01.009 [DOI] [PubMed] [Google Scholar]
- Mulrow CD, Williams JW, Gerety MB, Ramirez G, Montiel OM, & Kerber C (1995). Case-finding instruments for depression in primary care settings. Annals of Internal Medicine, 122, 913–921. doi: 10.7326/0003-4819-122-12-199506150-00004 [DOI] [PubMed] [Google Scholar]
- Ojio Y, Nishida A, Shimodera S, Togo F, & Sasaki T (2016). Sleep duration associated with the lowest risk of depression/anxiety in adolescents. Sleep, 39, 1555–1562. doi: 10.5665/sleep.6020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Krafty RT, Buysse DJ, Monk TH, Reynolds CF III, Begley A, & Hall M (2010). What constitutes too long of a delay? Determining the cortisol awakening response (CAR) using self-report and PSG-assessed wake time. Psychoneuroendocrinology, 35, 460–468. doi: 10.1016/j.psyneuen.2009.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J,… Eichele G (2006). The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metabolism, 4, 163–173. doi: 10.1016/j.cmet.2006.07.002 [DOI] [PubMed] [Google Scholar]
- Palmer CA, & Alfano CA (2017). Sleep and emotion regulation: An organizing, integrative review. Sleep Medicine Reviews, 31, 6–16. doi: 10.1016/j.smrv.2015.12.006 [DOI] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40, 879. doi: 10.3758/BRM.40.3.879 [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1 , 385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- Raniti MB, Allen NB, Schwartz O, Waloszek JM, Byrne ML, Woods MJ, … Trinder J (2017). Sleep duration and sleep quality: Associations with depressive symptoms across adolescence. Behavioral Sleep Medicine, 15, 198. [DOI] [PubMed] [Google Scholar]
- Roberts RE, & Duong HT (2014). The prospective association between sleep deprivation and depression among adolescents. Sleep, 37, 239–244. doi: 10.5665/sleep.3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Klein DN, Seeley JR, & Gau JM (2012). Key characteristics of major depressive disorder occurring in childhood, adolescence, emerging adulthood, and adulthood. Clinical Psychological Science, 1, 41–53. doi: 10.1177/2167702612457599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross KM, Murphy ML, Adam EK, Chen E, & Miller GE (2014). How stable are diurnal cortisol activity indices in healthy individuals? Evidence from three multi-wave studies. Psychoneuroendocrinology, 39, 184–193. doi: 10.1016/j.psyneuen.2013.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler KL, Ruggero CJ, Goldstein BL, Perlman G, Klein DN, & Kotov R (2017). Diurnal cortisol interacts with stressful events to prospectively predict depressive symptoms in adolescent girls. Journal of Adolescent Health, 61, 767–772. doi: 10.1016/j.jadohealth.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shochat T, Cohen-Zion M, & Tzischinsky O (2014). Functional consequences of inadequate sleep in adolescents: A systematic review. Sleep Medicine Reviews, 18, 75–87. doi: 10.1016/j.smrv.2013.03.005 [DOI] [PubMed] [Google Scholar]
- Soehner AM, Kaplan KA, & Harvey AG (2014). Prevalence and clinical correlates of co-occurring insomnia and hypersomnia symptoms in depression. Journal of Affective Disorders, 167(Suppl. C), 93–97. doi: 10.1016/j.jad.2014.05.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Kudielka BM, Adam EK, Pruessner JC, Wüst S, … Hellhammer DH (2016). Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology, 63, 414–432. doi: 10.1016/j.psyneuen.2015.10.010 [DOI] [PubMed] [Google Scholar]
- Stockings E, Degenhardt L, Lee YY, Mihalopoulos C, Liu A, Hobbs M, & Patton G (2015). Symptom screening scales for detecting major depressive disorder in children and adolescents: A systematic review and metaanalysis of reliability, validity and diagnostic utility. Journal of Affective Disorders, 174(Suppl. C), 447–463. doi: 10.1016/j.jad.2014.11.061 [DOI] [PubMed] [Google Scholar]
- Suchecki D, Lobo L, Hipólide D, & Tufik S (1998). Increased ACTH and corticosterone secretion induced by different methods of paradoxical sleep deprivation. Journal of Sleep Research, 7, 276–281. doi: 10.1046/j.1365-2869.1998.00122.x [DOI] [PubMed] [Google Scholar]
- TADS Team. (2007). The Treatment for Adolescents with Depression Study (TADS): Long-term effectiveness and safety outcomes. Archives of General Psychiatry, 64, 1132–1143. doi: 10.1001/archpsyc.64.10.1132 [DOI] [PubMed] [Google Scholar]
- Tsigos C, & Chrousos GP (2002). Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research, 53, 865–871. doi: 10.1016/S0022-3999(02)00429-4 [DOI] [PubMed] [Google Scholar]
- US Census Bureau. (2009). American Community Survey: Los Angeles Demographics. Retrieved December 19, 2018, from https://factfinder.census.gov/faces/nav/jsf/pages/community_facts.xhtml?src=bkmk
- van Dalfsen JH, & Markus CR (2018). The influence of sleep on human hypothalamic-pituitary-adrenal (HPA) axis reactivity: A systematic review. Sleep Medicine Reviews, 39, 187–194. [DOI] [PubMed] [Google Scholar]
- Vrshek-Schallhorn S, Doane LD, Mineka S, Zinbarg RE, Craske MG, & Adam EK (2013). The cortisol awakening response predicts major depression: Predictive stability over a 4-year follow-up and effect of depression history. Psychological Medicine, 43, 483–493. doi: 10.1017/S0033291712001213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, … Vos T (2013). Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. Lancet, 382, 1575–1586. doi: 10.1016/S0140-6736(13)61611-6 [DOI] [PubMed] [Google Scholar]
- Wüst S, Federenko I, Hellhammer DH, & Kirschbaum C (2000). Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology, 25, 707. [DOI] [PubMed] [Google Scholar]
- Ye Y, Zhang Y, Chen J, Liu J, Li X, Liu Y, … Jiang X-J (2015). Internet-based cognitive behavioral therapy for insomnia (ICBT-i) improves comorbid anxiety and depression—A meta-analysis of randomized controlled trials. PLOS ONE, 10, e0142258. doi: 10.1371/journal.pone.0142258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiders KH, Doane LD, & Adam EK (2011). Reciprocal relations between objectively measured sleep patterns and diurnal cortisol rhythms in late adolescence. Journal of Adolescent Health, 48, 566–571. doi: 10.1016/j.jadohealth.2010.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]


