PLATELET TRANSFUSION IN TRAUMA
Transfusion of whole blood or its components (platelets [PLTs], red blood cells [RBCs], plasma) at controlled ratios can significantly mitigate coagulopathies and improve survival in trauma.1 However, donated PLTs normally have a shelf life of approximately 5 days at room temperature, which poses substantial logistic challenges in availability and timely transfusion. Research is currently ongoing to enhance PLT shelf life and availability via utilization of pathogen reduction technologies as well as reduced-temperature storage (cooling, lyophilization, etc.). In parallel, other alternatives to donor-derived PLTs are being investigated, including bioreactor-based in vitro production of PLTs from progenitor or stem cells2 as well as manufacturing of biosynthetic or artificial PLTs.3 Here we briefly review the design approaches of ‘artificial PLTs’ and our contribution to this exciting area of transfusion medicine.
BIOINSPIRED DESIGN OF ARTIFICIAL PLTS
Platelets are the major players in stanching bleeding at the site of injury and vascular breach. They do this by adhering, activating, and aggregating at the bleeding site to form the primary hemostatic plug. PLTs also amplify coagulation outputs (thrombin generation and thus fibrin formation from fibrinogen) by presenting negatively charged phospholipid-rich membrane (e.g., phosphatidylserine) on activated PLT surface to promote colocalization of coagulation factor complexes and Ca2+.4 PLT adhesion at the bleeding site is mediated by PLT GPIbα binding to von Willebrand Factor (VWF) and PLT GPVI and GPIa/IIIa binding to collagen. PLT aggregation is driven by fibrinogen-mediated bridging of activated PLT surface integrin GPIIb-IIIa. Thus, the design of biosynthetic or artificial PLTs has focused on emulating these functions on synthetic particle platforms. Early examples are found in designs like the “plateletsome” that involved incorporating PLT-derived glycoproteins into liposomal membrane.5 Evolved variants of this design are found in technologies like the infusible PLT membrane (IPM, Cypress Bioscience)6 and Thrombosomes (CellPhire),7 where entire membranes of outdated PLTs are extracted, sterilized, lyophilized, and reconstituted into vesicles. Thrombosomes have shown significant preclinical promise and are currently in clinical evaluation. Other approaches have involved decorating albumin microparticles with fibrinogen (Synthocytes)8 and conjugating PLT-relevant recombinant glycoproteins (e.g., GPIbα, GPIa-IIa) onto synthetic particles.9 Microparticle and nanoparticle surface decoration with proteins poses stability challenges due to steric interference between the big protein molecules. Therefore, strategies have evolved toward using small peptides to render PLT-mimetic functionalities. Examples of these are seen in designs involving surface decoration of RBCs or synthetic polymeric particles with fibrinogenmimetic arginine-glycine-aspartic acid (RGD) and alanineglycine-aspartic acid (AGD) peptides that can bind to active PLT integrin GPIIb-IIIa.10–13 These designs essentially recapitulate only the fibrinogen-mediated aggregation mechanism of active PLTs, and they have shown promising hemostatic capacity in animal models. An important aspect to consider for successful clinical translation of these approaches is the binding specificity of the peptides. For example, the RGD peptides used in some designs12,13 are highly ubiquitous, and this may pose cross-reactivity challenges in vivo. Also, ubiquitous RGD peptides are known to trigger activation of resting circulating PLTs;14 this may pose systemic prothrombotic risks in vivo.
In consideration of these design challenges and recognizing that PLT’s injury site–targeted hemostatic capability involves a combination of adhesion and aggregation via highly specific binding interactions (Fig. 1), our laboratory has engineered a novel artificial PLT system. Our approach involves decorating nanoparticle surfaces with a combination of high-specificity/high-affinity VWF-binding peptides, collagen-binding peptides, and active GPIIb-IIIa–binding fibrinogen-mimetic peptides.15 Although we have demonstrated this design predominantly in liposomal nanoparticles, the “heteromultivalent surface decoration” approach remains amenable to be adapted to other biomedical nanoparticle or microparticle platforms, e.g., albumin nanoparticles.16 For the liposome-based design, which is named SynthoPlate, the VWF-binding peptides, collagen-binding peptides, and fibrinogen-mimetic peptides were conjugated to polyethylene glycol (PEG)-terminated to distearyl phosphatidylethanolamine (DSPE-PEG), and the resultant DSPE-PEG-peptide conjugates were combined at controlled ratios with distearyl phosphatidylcholine and cholesterol to form PLT-mimetic liposomal vesicles approximately 200 nm in diameter. We have extensively evaluated this technology in vitro to establish its stability, sterilizability, and long (6- to 9-month) shelf life.17 We have further evaluated SynthoPlate in several small and large animal models of thrombocytopenia and trauma, with very promising results in terms of reducing blood loss, stabilizing blood pressure, and improving survival.17–19 Ongoing evaluation of SynthoPlate includes pharmacology/toxicology analysis, as well as studying its field deployability as a lyophilized powder for point-of-injury and en-route hemorrhage control. Early transfusion of PLTs (e.g., within 1–3 hours post-injury) can significantly improve survival in trauma, and hence such “artificial PLT” technologies provide a promising option for transfusion management of trauma in prehospital settings where natural PLTs are of zero or limited availability. Since the design is made of fully synthetic components, there is no need for type matching (i.e., universally applicable), and these agents can be potentially used in veterinary transfusion as well (e.g., in canine and equine trauma). Additionally, such designs can be utilized as a delivery platform for adjunctive therapeutic agents to augment hemostatic outputs, as we have recently demonstrated by packaging tranexamic acid (TXA) within PLT-targeted liposomal nanoparticles.20 Altogether, our findings and ongoing studies demonstrate significant potential of the bioinspired artificial PLT designs for transfusion applications in trauma and other clinical contexts where donor PLT transfusions may have logistic limitations.
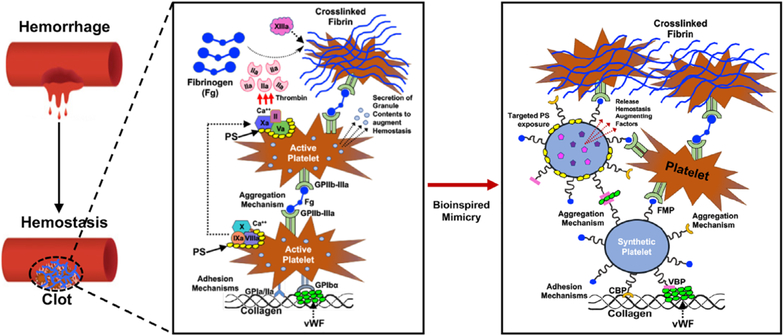
Fig. 1.
Schematic showing PLT-mediated mechanisms of hemostasis and the design of artificial PLTs inspired by them; for example, SynthoPlate is a bioinspired “artificial PLT” design using a liposomal template that recapitulates the hemostatically relevant adhesion and aggregation mechanisms of PLTs and can further allow targeted exposure of procoagulant lipids (e.g., phosphatidylserine [PS]) as well as delivery of adjunctive therapeutic molecules to further augment hemostatic outputs.
KEY IDEAS.
Among blood components, platelets (PLTs) present the toughest logistic challenges in transfusion due to limited availability, difficult portability and storage, high contamination risks, and very short shelf life (approx.5 days).
Robust research efforts are being directed to develop biologic PLTs in vitro as well as design biosynthetic and artificial PLT technologies that can potentially resolve these challenges to allow adequate availability and timely transfusion to improve survival in trauma.
Acknowledgments
The authors acknowledge NIH Grant HL121212.
Footnotes
CONFLICT OF INTEREST
Anirban Sen Gupta is a co-inventor on patent US 9107845 regarding ‘synthetic platelet’ technology. Anirban Sen Gupta is a co-founder of Haima Therapeutics, a biotech company focused on clinical translation of artificial platelet technologies. Aditya Girish and Ujjal Sekhon have disclosed no conflicts of interest.
REFERENCE
- 1.Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets and red blood cells in 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma. The PROPPR randomized clinical trial. JAMA 2015;313:471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strassel C, Gachet C, Lanza F. On the way to in vitro platelet production. Front Med (Lausanne) 2018;5:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modery-Pawlowski CL, Tian LL, Pan V, et al. Approaches to synthetic platelet analogs. Biomaterials 2013;34:526–41. [DOI] [PubMed] [Google Scholar]
- 4.Versteeg HH, Heemskerk JW, Levi M, et al. New fundamentals in hemostasis. Physiol Rev 2013;93:327–58. [DOI] [PubMed] [Google Scholar]
- 5.Rybak ME. Renzulli LA. A liposome based platelet substitute, the plateletsome, with hemostatic efficacy. Biomat Artif Cell Immobilization. Biotechnol 1993;21:101–18. [DOI] [PubMed] [Google Scholar]
- 6.Nasiri S Infusible platelet membrane as a platelet substitute for platelet transfusion: an overview. Blood Transfus 2013;11:337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barroso J, Osborne B, Teramura G, et al. Safety evaluation of a lyophilized platelet-derived hemostatic product. Transfusion 2018;58:2969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levi M, Friederich PW, Middleton S, et al. Fibrinogen-coated albumin microcapsules reduce bleeding in severely thrombocytopenic rabbits. Nat Med 1999;5:107–11. [DOI] [PubMed] [Google Scholar]
- 9.Takeoka S, Teramura Y, Okamura Y, et al. Rolling properties of rGPIba-conjugated phospholipid vesicles with different membrane flexibilities on vWf surface under flow conditions. Biochem Biophys Res Commun 2002;296:765–70. [DOI] [PubMed] [Google Scholar]
- 10.Coller BS, Springer KT, Beer JH, et al. Thromboerythrocytes. in vitro studies of a potential autologous semi-artificial alternative to platelet transfusions. J Clin Invest 1992;89:546–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeoka S, Okamura Y, Teramura Y, et al. Function of fibrinogen gamma-chain dodecapeptide-conjugated latex beads under flow. Biochem Biophys Res Commun 2003;312:773–9. [DOI] [PubMed] [Google Scholar]
- 12.Bertram JP, Williams CA, Robinson R, et al. Intravenous hemostat: nanotechnology to halt bleeding. Sci Transl Med 2009;1: 11ra22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gkikas M, Peponis T, Mesar T, et al. Systemically administered hemostatic nanoparticles for identification and treatment of internal bleeding. ACS Biomater Sci Eng 2019;5:2563–76. [DOI] [PubMed] [Google Scholar]
- 14.Du XP, Plow EF, Frelinger AL 3rd, et al. Ligands activate integrin alpha IIb beta 3 (platelet GPIIb-IIIa). Cell 1991;65: 409–16. [DOI] [PubMed] [Google Scholar]
- 15.Modery-Pawlowski CL, Tian LL, Ravikumar M, et al. In vitro and in vivo hemostatic capabilities of a functionally integrated platelet-mimetic liposomal nanoconstruct. Biomaterials 2013; 34:3031–41. [DOI] [PubMed] [Google Scholar]
- 16.Anselmo AC, Modery-Pawlowski CL, Menegatti S, et al. Platelet-like nanoparticles (PLNs): engineering shape, flexibility and surface chemistry of nanocarriers to target vascular injuries. ACS Nano 2014;8:11243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hickman DA, Pawlowski CL, Shevitz A, et al. Intravenous synthetic platelet (SynthoPlate) nanoconstructs reduce bleeding and improve ‘golden hour’ survival in a porcine model of traumatic arterial hemorrhage. Sci Rep 2018;8:3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shukla M, Sekhon UD, Betapudi V, et al. In vitro characterization of SynthoPlate™ (synthetic platelet) technology and its in vivo evaluation in severely thrombocy topenic mice. J Thromb Haemost 2017;15:375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dyer MR, Hickman D, Luc N, et al. Intravenous administration of synthetic platelets (SynthoPlate) in a mouse liver injury model of uncontrolled hemorrhage improves hemostasis. J Trauma Acute Care Surg 2018;84:917–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girish A, Hickman DA, Banerjee A, et al. Trauma-targeted delivery of tranexamic acid improves hemostasis and survival in rat liver hemorrhage model. J Thromb Haemost 2019;17: 1632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]