Abstract
Objective:
To compare the long-term survival of patients undergoing minimally invasive vs. open gastrectomy for gastric adenocarcinoma (GA) in the United States and China.
Methods:
Data on patients with GA who underwent gastrectomy without neoadjuvant therapy were retrieved from prospectively maintained databases at Memorial Sloan Kettering Cancer Center (MSKCC) and Fujian Medical University Union Hospital (FMUUH). Using propensity score matching (PSM), equally sized cohorts of patients with similar clinical and pathological characteristics who underwent minimally invasive vs. open gastrectomy were selected. The primary endpoint of the study was 5-year overall survival (OS).
Results:
We identified 479 patients who underwent gastrectomy at MSKCC between 2000 and 2012 and 2935 at FMUUH treated between 2006 and 2014. Of the total 3432 patients, 1355 underwent minimally invasive and 2059 underwent open gastrectomy. All patients had at least 5 years of potential follow-up. Before PSM, most patient characteristics differed significantly between patients undergoing the two types of surgery. After PSM each cohort included 889 -matched patients, and actual 5-year OS did not differ significantly between cohorts: 54.0% after minimally invasive and 50.4% after open gastrectomy, respectively (p = 0.205). Subgroup analysis confirmed that survival was similar between surgical cohorts among patients for each stage of GA and for those undergoing distal vs. total/proximal gastrectomy. On multivariable analysis, surgical approach was not an independent prognostic factor.
Conclusions:
Following PSM of US and Chinese patients with GA undergoing gastrectomy, long-term survival does not significantly differ between patients undergoing minimally invasive vs. open gastrectomy.
Introduction
Although patients with gastric adenocarcinoma (GA) have traditionally undergone surgical resection via an open approach, minimally invasive (laparoscopic or robotic-assisted) gastrectomy is being increasingly used [1–4]. The potential benefits of minimally invasive gastrectomy for GA include decreased postoperative pain, decreased length of stay, decreased blood loss, and better cosmetic results [1–3, 5], while its drawbacks include a long learning curve and potentially worse long-term survival if negative margins are not achieved [4, 6].
Prospective clinical trials have demonstrated that laparoscopic distal [5, 7] and total gastrectomy [8] have similar oncologic outcomes compared to open surgery for patients with early gastric cancer. Laparoscopic distal gastrectomy has even been recommended for clinical stage I GA according to the latest Japanese gastric cancer treatment guidelines [9]. More recently, several multicenter randomized controlled trials have found that laparoscopic gastrectomy is also safe and feasible for advanced gastric cancer in terms of short-term outcomes [2, 10–12] and 3-year survival [13].
Several studies have revealed that robotic gastrectomy is as safe and effective as laparoscopic gastrectomy in treating both early and advanced GA [4, 14, 15], yielding similar short-term surgical [1] and long-term oncological outcomes [16]; this evidence includes a meta-analysis of data on 4576 patients [17].
Nonetheless, more studies are needed to ensure that long-term outcomes are not being compromised with the use of minimally invasive gastrectomy, especially for advanced GA. In addition, there is very limited evidence regarding the survival outcomes after minimally invasive gastrectomy for both Western and Eastern patients with GA. In this study, we compared 5-year overall survival (OS) between patients undergoing curative-intent gastrectomy for GA by either minimally invasive (laparoscopic or robotic-assisted) vs. open approaches at two high-volume institutions in the United States and China for whom 5 years of follow-up data were available. The two surgical cohorts were matched for clinical and tumor characteristics to eliminate potential bias caused by selection for either approach.
Patients and methods
Patients
We queried the databases of Memorial Sloan Kettering Cancer Center (MSKCC, New York, USA) and Fujian Medical University Union Hospital (FMUUH, Fuzhou, China) for GA patients who underwent curative-intent minimally invasive or open gastrectomy without neoadjuvant therapy between January 2000 and January 2012 (for MSKCC) or between January 2006 to January 2014 (for FMUUH). Eligible patients met the following criteria: histologically confirmed diagnosis of GA; tumor located in the gastric or gastroesophageal junction (Siewert type II or III); no other malignancy; no distant metastasis or invasion of adjacent organs; no preoperative therapy (neoadjuvant chemotherapy or chemoradiotherapy); no D3 lymphadenectomy; R0 resection; and complete clinical and follow-up data available. This search identified 3414 patients, of which 479 were treated at MSKCC and 2935 at FMUUH. Of the total, 2059 underwent laparoscopic or robotic gastrectomy and 1355 underwent open gastrectomy.
All surgeries were performed by highly experienced surgeons. The extent of resection (distal or proximal/total gastrectomy) was decided according to the tumor location. The extent of lymph node dissection was performed according to the Japanese Gastric Cancer Association definitions in the second English Edition (1998) [18] and the third English edition (2010) [19]. The surgical approach (laparoscopic versus open) was agreed upon by the patient and surgeon after thorough discussion [6, 20–22]. Written informed consent was obtained from all the patients prior to surgery. Differentiated types included papillary and tubular adenocarcinomas; undifferentiated types included poorly differentiated adenocarcinoma, signet ring cell carcinoma, and mucinous adenocarcinoma [23]. Tumor stage was assigned according to the 8th edition of Union for International Cancer Control (UICC)/American Joint Committee on Cancer (AJCC) staging system of gastric cancer [24], or the Japanese Gastric Cancer Association [19, 25]. Patients with stage II or more advanced cancer were routinely recommended to receive adjuvant chemotherapy with 5-fluororacil-containing regimens for 4–6 months.
Follow-up
The primary outcome of 5-year OS was calculated from the date of surgery to the date of death from any cause or last follow-up (July 2017 at MSKCC and January 2019 at FMUUH). Patients were followed every 3 months during the first 2 years after surgery and every 6 months for the following 3 years. The median follow-up time was 60.2 months (range, 0.2 −138.8 months). The Institutional Review Boards of the participating hospitals approved this study.
Statistical analysis
The Chi-square test was used to compare categorical variables between the two groups, and the independent sample t-test was used to compare continuous variables. To minimize bias in this retrospective study, the cohorts of patients undergoing minimally invasive or open gastrectomy were propensity score-matched at a 1:1 ratio as previously reported [26]. Propensity scores were based on age, sex, tumor differentiation, tumor location, pathological T stage, and pathological N stage. The two cohorts were matched using a greedy approach with a caliper width of 0.1 standard deviations of the logit of the propensity score. OS was estimated using the Kaplan-Meier method and analyzed by the log-rank test. Factors that were deemed of potential importance on the univariate analysis were included in the multivariate analysis, which employed a Cox proportional hazards model. Hazard ratios (HRs) are presented with 95% confidence intervals (CIs). The HRs associated with minimally invasive surgery after refitting separate propensity-score–weighted survival models for each subgroup were analyzed and illustrated by forest plot [27]. All p values are two-tailed; those < 0.05 were considered significant. All statistical analyses were performed in SPSS version 22.0 (IBM, Chicago, IL, USA) and R version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
Clinicopathologic characteristics of patients from MSKCC and FMUUH are shown in Supplementary Table 1. The differences between these patients are consistent with previous reports of patients with GA in the US and China [28–30]. Using combined data (n=3432), patients were stratified into cohorts based on whether they underwent minimally invasive (n=1355) or open gastrectomy (n=2059) (Supplementary Fig. 1). There are 412 and 67 patients at MSKCC underwent open and minimally invasive gastrectomy before matching, respectively. And, there are 290 and 55 patients at MSKCC underwent open and minimally invasive gastrectomy after matching, respectively.
Before matching, the cohort undergoing minimally invasive gastrectomy was significantly younger (mean age 61.1 vs. 62.3 years, p = 0.003), included more male patients (74.6% vs. 70.6%, p = 0.010), and included more patients with well- or moderately-differentiated tumors (43.1% vs. 33.3%, p < 0.001) compared with those undergoing open gastrectomy (Table 1). Furthermore, patients undergoing open surgery had more upper third tumors (30.9% vs. 24.8%, p < 0.001) and more tumors of pT4 stage (44.5% vs. 35.2%, p < 0.001). However, there were no significant differences in tumor size, type of gastrectomy, number of metastatic lymph nodes, number of harvested lymph nodes, pN stage, or pTNM stage between the two groups.
Table 1.
Characteristics of cohorts defined by surgical approach before and after propensity score matching. Categorical data are presented as n (%) and continuous data as mean ± SD.
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| Open (n =1355) | Ml (n =2059) | p | Open (n =889) | Ml (n =889) | p | |
| Age, years | 62.3 ± 12.1 | 61.1 ± 11.4 | 0.003 | 60.6 ± 11.1 | 60.3 ± 11.2 | 0.812 |
| Gender | 0.010 | 0.382 | ||||
| Male | 956 (70.6) | 1535 (74.6) | 657 (73.9) | 674 (75.8) | ||
| Female | 399 (29.4) | 524 (25.4) | 232 (26.1) | 215 (24.2) | ||
| Tumor size (cm) | 4.8 ± 2.9 | 4.6 ± 2.7 | 0.259 | 5.2 ± 2.8 | 5.1 ± 2.7 | 0.230 |
| Differentiation type | <0.001 | 0.474 | ||||
| Differentiated | 451 (33.3) | 887 (43.1) | 398 (44.8) | 383 (43.1) | ||
| Undifferentiated/unknown | 904 (66.7) | 1172 (56.9) | 491 (55.2) | 506 (56.9) | ||
| Tumor location | <0.001 | 0.053 | ||||
| Lower third | 579 (42.7) | 877 (42.5) | 393 (44.2) | 346 (38.9) | ||
| Middle third | 230 (17.0) | 415 (20.2) | 125 (14.1) | 178 (20.0) | ||
| Upper third or GE junction | 418 (30.9) | 510 (24.8) | 263 (29.6) | 243 (27.4) | ||
| Distributed throughout | 128 (9.4) | 257 (12.5) | 108 (12.1) | 122 (13.7) | ||
| Resection extent | 0.574 | 0.388 | ||||
| Distal | 619 (45.7) | 938 (45.6) | 386 (43.4) | 367 (41.3) | ||
| Proximal/total | 736 (54.3) | 1121 (54.4) | 503 (56.6) | 522 (58.7) | ||
| Number of positive LNs | 6.0 ± 9.0 | 6.3 ± 12.5 | 0.420 | 7.5 ± 10.1 | 7.3 ± 9.1 | 0.122 |
| Number of LNs examined | 26.1 ± 13.2 | 32.8 ± 13.4 | 0.409 | 28.2 ± 14.0 | 34.2 ± 13.3 | 0.897 |
| pT stage | <0.001 | 0.123 | ||||
| T1 | 331 (24.5) | 536 (26.0) | 154 (17.3) | 144 (16.2) | ||
| T2 | 148 (10.9) | 234 (11.4) | 98 (11.1) | 85 (9.6) | ||
| T3 | 272 (20.1) | 565 (27.4) | 170 (19.1) | 210 (23.6) | ||
| T4 | 604 (44.5) | 724 (35.2) | 467 (52.5) | 450 (50.6) | ||
| pN stage | 0.652 | 0.741 | ||||
| N0 | 511 (37.8) | 798 (38.8) | 267 (30.0) | 250 (28.1) | ||
| N1 | 205 (15.1) | 294 (14.2) | 131 (14.7) | 127 (14.3) | ||
| N2 | 205 (15.1) | 325 (15.8) | 144 (16.3) | 159 (17.9) | ||
| N3a | 241 (17.8) | 354(17.2) | 176 (19.8) | 189 (21.3) | ||
| N3b | 193 (14.2) | 288 (14.0) | 171 (19.2) | 164 (18.4) | ||
| pTNM stage | 0.138 | 0.471 | ||||
| I | 379 (28.0) | 632 (30.7) | 190 (21.4) | 171 (19.2) | ||
| II | 286 (21.1) | 446 (21.7) | 172 (19.3) | 185 (20.8) | ||
| III | 690 (50.9) | 981 (47.6) | 527 (59.3) | 533 (60.0) | ||
| Postop Chemotherapy | 0.516 | 0.739 | ||||
| Yes | 646 (47.7) | 1005 (48.8) | 463 (52.1) | 470 (52.9) | ||
| No | 709 (52.3) | 1054 (51.2) | 426 (47.9) | 419 (47.1) | ||
| Postop Radiation | <0.001 | 0.247 | ||||
| Yes | 51 (3.8) | 8 (0.4) | 8 (0.9) | 4 (0.4) | ||
| No | 1304 (96.2) | 2051 (99.6) | 881 (99.1) | 885 (99.6) | ||
MI, minimally invasive; GE, gastroesophageal; LNs, lymph nodes; Postop, postoperative.
Propensity score matching narrowed the cohorts to 889 patients each. As shown in Table 1, all clinical and pathological variables of the matched samples were not significantly different.
Survival outcomes
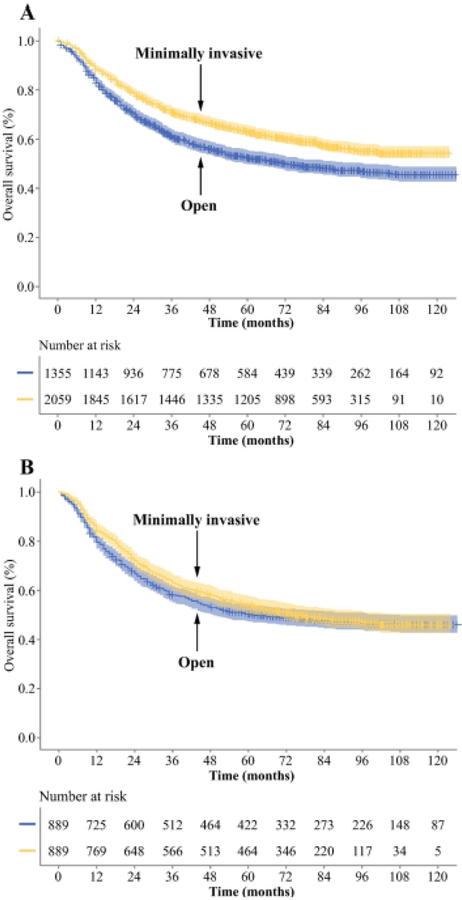
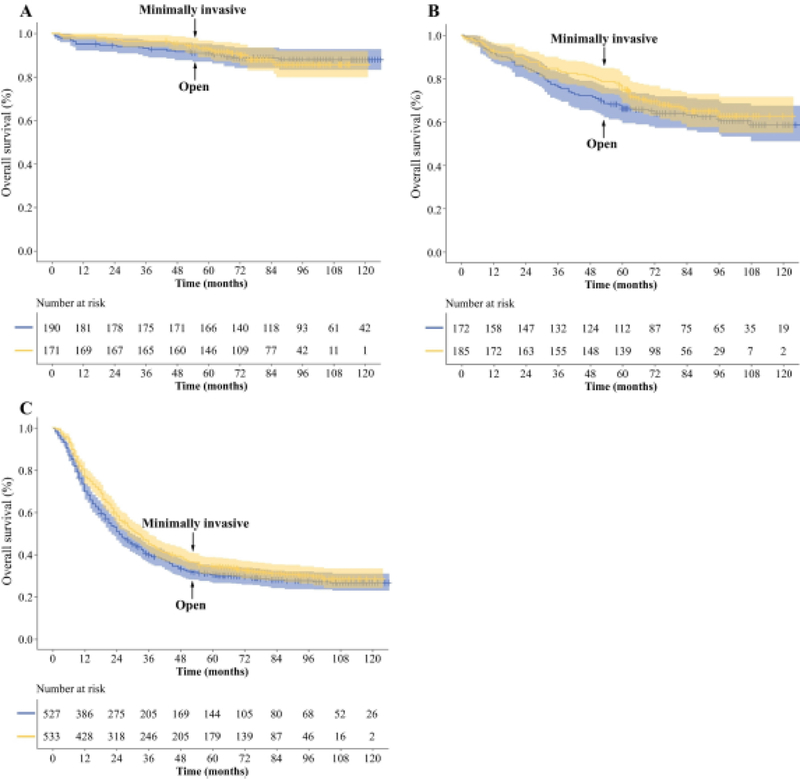
Before matching, 5-year OS for the cohort who underwent minimally invasive gastrectomy was significantly longer than for those undergoing open gastrectomy by Kaplan-Meier survival analysis (p < 0.001, Fig. 1A). After PSM, there was no significant difference between patients undergoing minimally invasive vs. open gastrectomy (p = 0.205, Fig. 1B). Five-year OS similarly did not differ between the matched cohorts of patients undergoing gastrectomy by minimally invasive vs. open gastrectomy within stage-specific groups as defined by the UICC/AJCC (stage I, p = 0.893; stage II, p = 0.352; stage III, p = 0.054, Fig. 2) or the Japanese Gastric Cancer Association (early GA, p = 0.848; advanced GA, p = 0.745; Supplementary Fig. 2). OS also did not differ between surgical approaches within groups of patients undergoing distal or total gastrectomy (Supplementary Fig. 3).
Figure 1. Comparison of overall survival between patients undergoing minimally invasive or open gastrectomy before (A) and after (B) propensity score matching.
Graphs display the number of patients at risk at different time points. Shaded areas represent 95% CI.
Figure 2. Comparison of overall survival between patients undergoing minimally invasive or open gastrectomy according to pTNM stage in the propensity-matched cohort.
(A) Stage I, (B) stage II, (C) stage III. Graphs display the number of patients at risk. Shaded areas represent 95% CI.
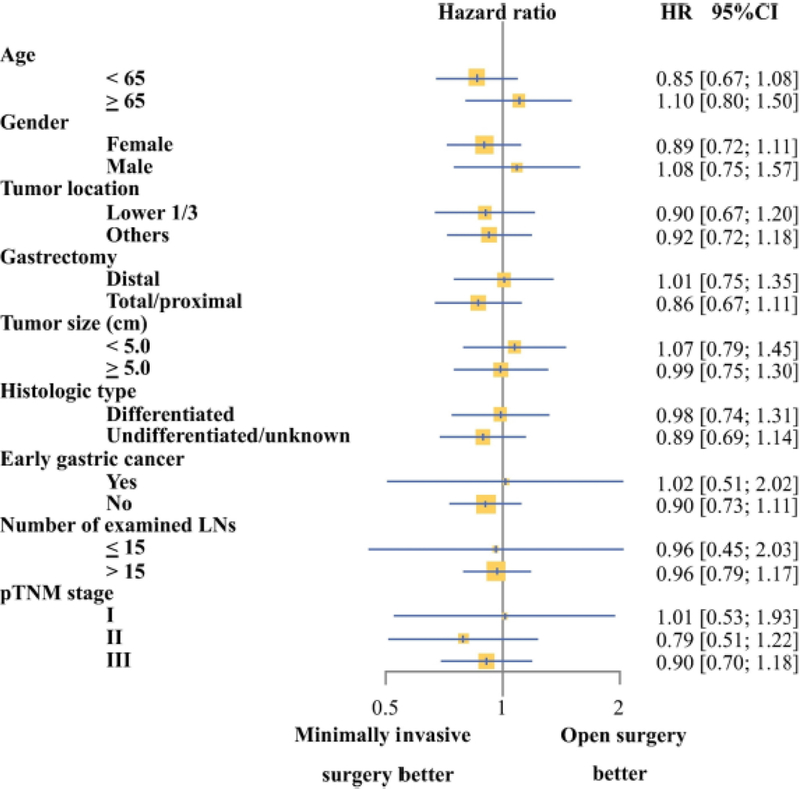
We further examined whether risk of death differed between patients undergoing minimally invasive vs. open gastrectomy within subgroups divided by mean age (< 65 years and ≥ 65 years), gender, tumor size (< 5.0 cm and ≥ 5.0 cm), type of gastrectomy (distal gastrectomy and total or proximal gastrectomy), histologic type (undifferentiated and undifferentiated or unknown), and number of examined lymph nodes (≤ 15 and > 15). The two types of surgery were associated with comparable risk of death in all subgroups (Fig. 3).
Figure 3. Forest plot of hazard ratios (HRs) comparing minimally invasive with open gastrectomy in cohort subsets.
HRs less than 1.0 favor combined-modality therapy. p values are from the subset test of interaction.
Univariate and multivariate survival analyses of prognostic factors
Univariate analysis revealed that age ≥ 65 years, non-distal tumor location, tumor size ≥ 5 cm, undifferentiated type, proximal or total resection, and pTNM stage II or III were significantly associated with patients’ OS (Table 2). In addition, postoperative chemotherapy and postoperative radiation were not significantly related to the OS in univariate analysis. Multivariate analysis narrowed the list of independent prognostic factors for OS to older age (OR 1.283; 95% CI, 1.124–1.463; p = 0.001), non-distal location (OR 1.437; 95% CI, 1.101–1.875; p = 0.008), large tumor size (OR 1.693; 95% CI, 1.439–1.993; p = 0.001), proximal or total resection (OR 1.792; 95% CI, 1.363–2.355; p = 0.001), stage II (OR 2.896; 95% CI, 2.036–4.118; p < 0.001), and stage III (OR 6.976; 95% CI, 5.030–9.675; p < 0.001). Minimally invasive vs. open approach was not a significant prognostic variable on univariate and multivariate analysis.
Table 2.
Univariable and multivariable analysis of clinical and pathological factors associated with overall survival in matched cohort. OS data are percentages.
| Variables | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| OS at 5 years | p | Odds ratio | p | |
| Age | < 0.001 | 0.001 | ||
| < 65 | 56.2 | 1.00 (reference) | ||
| ≥ 65 | 46.1 | 1.283 (1.124–1.463) | ||
| Gender | 0.953 | - | ||
| Male | 52.3 | - | ||
| Female | 51.8 | - | ||
| Location | < 0.001 | 0.008 | ||
| Distal | 59.8 | 1.00 (reference) | ||
| Others | 47.2 | 1.437 (1.101–1.875) | ||
| Tumor size (cm) | < 0.001 | 0.001 | ||
| < 5.0 | 74.5 | 1.00 (reference) | ||
| ≥ 5.0 | 33.4 | 1.693 (1.439–1.993) | ||
| Differentiation type | < 0.001 | 0.233 | ||
| Differentiated | 60.4 | 1.00 (reference) | ||
| Undifferentiated/unknown | 45.8 | 1.087 (0.948–1.247) | ||
| Number of examined | 0.446 | - | ||
| LNs | ||||
| > 15 | 53.8 | - | ||
| ≤ 15 | 51.6 | - | ||
| Resection extent | < 0.001 | 0.001 | ||
| Distal | 63.7 | 1.00 (reference) | ||
| Proximal/total | 43.7 | 1.792 (1.363–2.355) | ||
| TNM stage | < 0.001 | < 0.001 | ||
| I | 91.8 | 1.00 (reference) | ||
| II | 65.7 | 2.896 (2.036–4.118) | ||
| III | 32.4 | 6.976 (5.030–9.675) | ||
| Surgical approach | 0.205 | 0.115 | ||
| Open | 50.4 | 1.00 (reference) | ||
| Minimally invasive | 54.0 | 0.900 (0.790–1.026) | ||
| Postop | 0.142 | |||
| Chemotherapy | ||||
| Yes | 53.6 | |||
| No | 51.3 | |||
| Postop Radiation | 0.854 | |||
| Yes | 53.2 | |||
| No | 51.9 | |||
Postop, postoperative.
Discussion
In this retrospective study of prospectively collected data from two high-volume units for gastric cancer surgery in the US and China, 5-year OS following minimally invasive gastrectomy was similar to that following open gastrectomy after propensity score matching. To our knowledge, this is the first study to compare long-term survival between patients with GA who underwent gastrectomy by the two approaches in a combined Western and Eastern cohort. Despite differences in patient demographics and perioperative treatment between the East and the West, a “real-world study” was indeed necessary to fully assess the oncologic efficacy of minimally invasive gastrectomy, which was one of the advantages of this study. Another major advantage of this study is that all patients had at least 5 years of potential follow-up and thus actual 5-year OS is reported rather than actuarial 5-year OS.
Laparoscopic and robotic surgeries were considered as a single group in the current study on the basis of prior studies showing them to have equivalent outcomes. A prospective, multicenter comparative study showed that they have similar perioperative surgical outcomes [1], and retrospective studies have found them to have similar short-term recovery and long-term oncologic outcomes [14].
We did not compare the short-term outcomes of minimally invasive and open gastrectomy, as many studies, including randomized clinical trials, have clearly shown them to have similarly good short-term outcomes. The feasibility and safety of laparoscopic distal and total gastrectomy for stage I GA were confirmed by the KLASS-01 [31] and KLASS-03 [8] trials, and for advanced GA by the CLASS01 [11], LSSG0901 [12], KLASS-02 [2], and COACT 1001 [10] studies. Robotic gastrectomy was shown to be as safe as laparoscopic gastrectomy in a prospective, multicenter comparative study [1], and a subgroup analysis found that the two approaches have similar surgical outcomes for obese patients [32]. Finally, minimally invasive gastrectomy (both robotic and laparoscopic approaches) was found to have equivalent oncologic outcomes to those of open gastrectomy in a retrospective study using data from the US National Cancer Data Base (NCDB) [33].
Our investigation addresses the need for further evidence to recommend the minimally invasive approaches to gastrectomy for GA. While several trials indicate that laparoscopy has equivalent 3- to 5-year survival outcomes to those of open gastrectomy in both stage I [7] and advanced GA [13], 3 more multicenter randomized controlled trials are ongoing [8, 12, 31]. No prospective studies have yet evaluated robotic surgery, nor has survival been analyzed for each stage.
Surprisingly, differences in 5-year OS between patients who underwent minimally invasive vs. open surgery increased with tumor TNM stage. Although no difference was statistically significant in any of the stage subgroups, we unexpectedly found that 5-year survival after minimally invasive gastrectomy tended to be higher than that following open gastrectomy, especially in patients with stage III GA. There may be several reasons for this phenomenon. Minimally invasive surgery causes less systemic trauma, which has been shown experimentally to reduce tumor recurrence [34], and to induce lower stress responses and better preserve immune function [35], whereas conventional open surgery increases serum levels of markers of inflammation such as CRP and IL-6 [34]. This difference in stress and immune impact is likely more important in stage III patients. Second, because faster recovery allows more patients to receive adjuvant systemic chemotherapy [36], patients with stage III tumors may obtain more survival benefit from earlier postoperative therapy than those with stage II. The small differences could also be explained by selection bias that is not eliminated by PSM.
It’s worth noting that our report has several differences from prior publications. First, to our knowledge is the only such large scale comparison between minimally invasive and open surgery in US and Chinese GA patients, although a number of studies have successfully demonstrated the differences of patient demographics, treatment policies, and treatment outcomes between US and Asia, which is fundamental different from the present study [28–30, 37, 38]. Second, compared with previous studies focus on long-term survival after minimally invasive surgery and open surgery for GA [13, 39–41], the median follow-up time of this study was longer. Thus, this study provides valuable information that may be used to design future international prospective studies.
There are several limitations to the present study. First, our study is limited by its retrospective nature and the attendant biases including selection bias. As examples, advanced tumors were less often managed with minimally invasive surgery, and advanced tumors at MSKCC were usually treated with neoadjuvant chemotherapy leading to exclusion of these patients from this study. Additional confounding issues include: (1) patients at the two institutions received different perioperative therapy and had differing durations of follow-up and (2) patients at the two institution had likely had different patient preferences, socioeconomic status, or other patient characteristics. Second, we did not monitor mid- or long-term complications, nutrition status, quality of life, or daily activities. Third, disease-free survival was not investigated in this study; however, a large number of studies have demonstrated that OS is a reliable measure of the prognosis of cancer patients [42–44]. Still, it should be noted that our conclusions have not been externally validated, calling for well-designed multicenter randomized trials to definitively compare the long-term outcomes of minimally invasive gastrectomy (including robotic-assisted surgery) to open gastrectomy in GA patients. Our findings may support the broader use of minimally invasive gastrectomy by other institutions or in other regions, and provides reference data for potential future randomized trials.
Conclusion
In conclusion, our study suggests that minimally invasive gastrectomy is an oncologically safe procedure for both Western and Eastern patients with GA in terms of long-term survival.
Supplementary Material
Synopsis:
In a combined cohort of patients with gastric adenocarcinoma treated at high-volume cancer centers in the Unites States and China, we compared 5-year overall survival between patients treated with minimally invasive vs. open gastrectomy. Survival did not differ between equally sized cohorts of patients matched for clinical and tumor characteristics.
Acknowledgements
This research was funded in part by a National Institutes of Health/National Cancer Institute Cancer Center Support Grant (P30 CA008748); National Nature Science Foundation of China (No. 81871899); the Construction Project of Fujian Province Minimally Invasive Medical Centre (No. [2017] 171); and the Natural Science Foundation of Fujian Province (2019J01155).
The authors thank Jessica Moore, senior editor at MSKCC, for editing this manuscript.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Disclosures: The authors declare they have no commercial interests related to this study.
References:
- 1.Kim HI, Han SU, Yang HK et al. Multicenter Prospective Comparative Study of Robotic Versus Laparoscopic Gastrectomy for Gastric Adenocarcinoma. Ann Surg. 2016; 1: 103–9. [DOI] [PubMed] [Google Scholar]
- 2.Lee HJ, Hyung WJ, Yang HK et al. Short-Term Outcomes of a Multicenter Randomized Controlled Trial Comparing Laparoscopic Distal Gastrectomy with D2 Lymphadenectomy to Open Distal Gastrectomy for Locally Advanced Gastric Cancer (KLASS-02-RCT). Ann Surg. 2019. [DOI] [PubMed] [Google Scholar]
- 3.Herrera-Almario G, Strong VE. Minimally Invasive Gastric Surgery. Ann Surg Oncol. 2016; 12: 3792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang KK, Park DJ, Yoon SS. Laparoscopic Versus Open Surgery for Gastric Adenocarcinoma: Innovation Continues to Challenge Tradition. Ann Surg. 2016; 2: 223–5. [DOI] [PubMed] [Google Scholar]
- 5.Honda M, Hiki N, Kinoshita T et al. Long-Term Outcomes of Laparoscopic Versus Open Surgery for Clinical Stage I Gastric Cancer: The LOC-1 Study. Ann Surg. 2016; 2: 214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang SY, Roh KH, Kim YN et al. Surgical Outcomes After Open, Laparoscopic, and Robotic Gastrectomy for Gastric Cancer. Ann Surg Oncol. 2017; 7: 1770–7. [DOI] [PubMed] [Google Scholar]
- 7.Kim HH, Han SU, Kim MC et al. Effect of Laparoscopic Distal Gastrectomy vs Open Distal Gastrectomy on Long-Term Survival Among Patients with Stage I Gastric Cancer: The KLASS-01 Randomized Clinical Trial. Jama Oncol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyung WJ, Yang HK, Han SU et al. A Feasibility Study of Laparoscopic Total Gastrectomy for Clinical Stage I Gastric Cancer: A Prospective Multi-Center Phase II Clinical Trial, KLASS 03. Gastric Cancer. 2019; 1: 214–22. [DOI] [PubMed] [Google Scholar]
- 9.Japanese Gastric Cancer Treatment Guidelines 2014 (Ver. 4). Gastric Cancer. 2017; 1: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park YK, Yoon HM, Kim YW et al. Laparoscopy-Assisted versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: Results From a Randomized Phase II Multicenter Clinical Trial (COACT 1001). Ann Surg. 2018; 4: 638–45. [DOI] [PubMed] [Google Scholar]
- 11.Hu Y, Huang C, Sun Y et al. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol. 2016; 12: 1350–7. [DOI] [PubMed] [Google Scholar]
- 12.Inaki N, Etoh T, Ohyama T et al. A Multi-Institutional, Prospective, Phase II Feasibility Study of Laparoscopy-Assisted Distal Gastrectomy with D2 Lymph Node Dissection for Locally Advanced Gastric Cancer (JLSSG0901). World J Surg. 2015; 11: 2734–41. [DOI] [PubMed] [Google Scholar]
- 13.Yu J, Huang C, Sun Y et al. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients with Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA. 2019; 20: 1983–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao Y, Xi H, Qiao Z et al. Comparison of Robotic- and Laparoscopic-Assisted Gastrectomy in Advanced Gastric Cancer: Updated Short- and Long-Term Results. Surg Endosc. 2019; 2: 528–34. [DOI] [PubMed] [Google Scholar]
- 15.Lu J, Zheng HL, Li P et al. A Propensity Score-Matched Comparison of Robotic Versus Laparoscopic Gastrectomy for Gastric Cancer: Oncological, Cost, and Surgical Stress Analysis. J Gastrointest Surg. 2018; 7: 1152–62. [DOI] [PubMed] [Google Scholar]
- 16.Obama K, Kim YM, Kang DR et al. Long-Term Oncologic Outcomes of Robotic Gastrectomy for Gastric Cancer Compared with Laparoscopic Gastrectomy. Gastric Cancer. 2018; 2: 285–95. [DOI] [PubMed] [Google Scholar]
- 17.Bobo Z, Xin W, Jiang L et al. Robotic Gastrectomy Versus Laparoscopic Gastrectomy for Gastric Cancer: Meta-Analysis and Trial Sequential Analysis of Prospective Observational Studies. Surg Endosc. 2019; 4: 1033–48. [DOI] [PubMed] [Google Scholar]
- 18.Japanese GCA. Japanese Classification of Gastric Carcinoma - 2Nd English Edition −. Gastric Cancer. 1998; 1: 10–24. [DOI] [PubMed] [Google Scholar]
- 19.Japanese Classification of Gastric Carcinoma: 3Rd English Edition. Gastric Cancer. 2011; 2: 101–12. [DOI] [PubMed] [Google Scholar]
- 20.Li P, Lin JX, Tu RH et al. Early Unplanned Reoperations After Gastrectomy for Gastric Cancer are Different Between Laparoscopic Surgery and Open Surgery. Surg Endosc. 2019; 12: 4133–42. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y, Hua J, Li J et al. Long-Term Outcomes of Laparoscopic Versus Open Gastrectomy for Advanced Gastric Cancer: A Large Cohort Study. Am J Surg. 2019; 4: 750–6. [DOI] [PubMed] [Google Scholar]
- 22.Xu BB, Lu J, Zheng ZF et al. Comparison of Short-Term and Long-Term Efficacy of Laparoscopic and Open Gastrectomy in High-Risk Patients with Gastric Cancer: A Propensity Score-Matching Analysis. Surg Endosc. 2019; 1: 58–70. [DOI] [PubMed] [Google Scholar]
- 23.Kano Y, Ohashi M, Ida S et al. Oncological Feasibility of Laparoscopic Subtotal Gastrectomy Compared with Laparoscopic Proximal Or Total Gastrectomy for cT1N0M0 Gastric Cancer in the Upper Gastric Body. Gastric Cancer. 2019. [DOI] [PubMed] [Google Scholar]
- 24.Lu J, Zheng CH, Cao LL et al. The Effectiveness of the 8Th American Joint Committee On Cancer TNM Classification in the Prognosis Evaluation of Gastric Cancer Patients: A Comparative Study Between the 7Th and 8Th Editions. Eur J Surg Oncol. 2017; 12: 2349–56. [DOI] [PubMed] [Google Scholar]
- 25.Japanese Classification of Gastric Carcinoma−−2nd English Edition--Response Assessment of Chemotherapy and Radiotherapy for Gastric Carcinoma: Clinical Criteria. Gastric Cancer. 2001; 1: 1–8. [DOI] [PubMed] [Google Scholar]
- 26.Tian S, Zhang X, Jiang R et al. Survival Outcomes with Thoracic Radiotherapy in Extensive-Stage Small-Cell Lung Cancer: A Propensity Score-Matched Analysis of the National Cancer Database. Clin Lung Cancer. 2019. [DOI] [PubMed] [Google Scholar]
- 27.Melamed A, Margul DJ, Chen L et al. Survival after Minimally Invasive Radical Hysterectomy for Early-Stage Cervical Cancer. N Engl J Med. 2018; 20: 1905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strong VE, Russo A, Yoon SS et al. Comparison of Young Patients with Gastric Cancer in the United States and China. Ann Surg Oncol. 2017; 13: 3964–71. [DOI] [PubMed] [Google Scholar]
- 29.Strong VE, Wu AW, Selby LV et al. Differences in Gastric Cancer Survival Between the U.S. And China. J Surg Oncol. 2015; 1: 31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin JX, Yi BC, Yoon C et al. Comparison of Outcomes for Elderly Gastric Cancer Patients at Least 80 Years of Age Following Gastrectomy in the United States and China. Ann Surg Oncol. 2018; 12: 3629–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim W, Kim HH, Han SU et al. Decreased Morbidity of Laparoscopic Distal Gastrectomy Compared with Open Distal Gastrectomy for Stage I Gastric Cancer: Short-Term Outcomes From a Multicenter Randomized Controlled Trial (KLASS-01). Ann Surg. 2016; 1: 28–35. [DOI] [PubMed] [Google Scholar]
- 32.Park JM, Kim HI, Han SU et al. Who May Benefit From Robotic Gastrectomy?: A Subgroup Analysis of Multicenter Prospective Comparative Study Data On Robotic Versus Laparoscopic Gastrectomy. Eur J Surg Oncol. 2016; 12: 1944–9. [DOI] [PubMed] [Google Scholar]
- 33.Leung K, Sun Z, Nussbaum DP, Adam MA, Worni M, Blazer DR. Minimally Invasive Gastrectomy for Gastric Cancer: A National Perspective On Oncologic Outcomes and Overall Survival. Surg Oncol. 2017; 3: 324–30. [DOI] [PubMed] [Google Scholar]
- 34.Agalar F, Daphan C, Hayran M, Sayek I. Laparoscopic Surgery is Associated with Less Tumour Growth Stimulation than Conventional Surgery: An Experimental Study. Br J Surg. 1997; 10: 1480. [DOI] [PubMed] [Google Scholar]
- 35.Veenhof AA, Vlug MS, van der Pas MH et al. Surgical Stress Response and Postoperative Immune Function After Laparoscopy Or Open Surgery with Fast Track Or Standard Perioperative Care: A Randomized Trial. Ann Surg. 2012; 2: 216–21. [DOI] [PubMed] [Google Scholar]
- 36.Kelly KJ, Selby L, Chou JF et al. Laparoscopic Versus Open Gastrectomy for Gastric Adenocarcinoma in the West: A Case-Control Study. Ann Surg Oncol. 2015; 11: 3590–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shim JH, Song KY, Jeon HM et al. Is Gastric Cancer Different in Korea and the United States? Impact of Tumor Location On Prognosis. Ann Surg Oncol. 2014; 7: 2332–9. [DOI] [PubMed] [Google Scholar]
- 38.Noguchi Y, Yoshikawa T, Tsuburaya A, Motohashi H, Karpeh MS, Brennan MF. Is Gastric Carcinoma Different Between Japan and the United States? Cancer-Am Cancer Soc. 2000; 11: 2237–46. [PubMed] [Google Scholar]
- 39.Shi Y, Xu X, Zhao Y et al. Long-Term Oncologic Outcomes of a Randomized Controlled Trial Comparing Laparoscopic Versus Open Gastrectomy with D2 Lymph Node Dissection for Advanced Gastric Cancer. Surgery. 2019. [DOI] [PubMed] [Google Scholar]
- 40.Li Z, Liu Y, Hao Y, Bai B, Yu D, Zhao Q. Surgical and Long-Term Oncologic Outcomes of Laparoscopic and Open Gastrectomy for Serosa-Positive (pT4a) Gastric Cancer: A Propensity Score-Matched Analysis. Surg Oncol. 2019; 167–73. [DOI] [PubMed] [Google Scholar]
- 41.Solaini L, Bazzocchi F, Pellegrini S et al. Robotic Vs Open Gastrectomy for Gastric Cancer: A Propensity Score-Matched Analysis On Short- and Long-Term Outcomes. Int J Med Robot. 2019; 5: e2019. [DOI] [PubMed] [Google Scholar]
- 42.Hirabayashi S, Kosugi S, Isobe Y et al. Development and External Validation of a Nomogram for Overall Survival After Curative Resection in Serosa-Negative, Locally Advanced Gastric Cancer. Ann Oncol. 2014; 6: 1179–84. [DOI] [PubMed] [Google Scholar]
- 43.Han DS, Suh YS, Kong SH et al. Nomogram Predicting Long-Term Survival After D2 Gastrectomy for Gastric Cancer. J Clin Oncol. 2012; 31: 3834–40. [DOI] [PubMed] [Google Scholar]
- 44.Lu J, Zheng ZF, Zhou JF et al. A Novel Prognosis Prediction Model After Completion Gastrectomy for Remnant Gastric Cancer: Development and Validation Using International Multicenter Databases. Surgery. 2019;166(3):314–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.