Abstract
Reuptake and clearance of released serotonin (5-HT) are critical in serotonergic neurotransmission. Serotonin transporter (SERT) is mainly responsible for clearing the extracellular 5-HT. Controlled trafficking, phosphorylation, and protein stability have been attributed to robust SERT activity. H3 histamine receptors (H3Rs) act in conjunction and regulate 5-HT release. H3Rs are expressed in the nervous system and located at the serotonergic terminals, where they act as heteroreceptors. Although histaminergic and serotonergic neurotransmissions are thought to be two separate events, whether H3Rs influence SERT in the CNS to control 5-HT reuptake has never been addressed. With a priori knowledge gained from our studies, we explored the possibility using rat hippocampal synaptosomal preparations. We found that treatment with H3R/H4R-agonists immepip and (R)-(−)-α-methyl-histamine indeed resulted in a time and concentration-dependent decrease in 5-HT transport. On the other hand, treatment with H3R/H4R-inverse agonist thioperamide caused a moderate increase in 5-HT uptake while blocking the inhibitory effect of H3R/H4R agonists. When investigated further, immepip treatment reduced the level of SERT on the plasma membrane and its phosphorylation. Likewise, CaMKII inhibitor KN93 or calcineurin inhibitor cyclosporine A also inhibited SERT function; however, an additive effect with immepip was not seen. High-speed in vivo chronoamperometry demonstrated that immepip delayed 5-HT clearance while thioperamide accelerated 5-HT clearance from the extracellular space. Immepip selectively inhibited SERT activity in the hippocampus, cortex, but not in the striatum, midbrain, and brain stem. Thus, we report here a novel mechanism of regulating SERT activity by H3R-mediated CaMKII/calcineurin pathway in a brain region-specific manner, and perhaps synaptic 5-HT in the CNS that controls 5-HT clearance.
Keywords: Histamine, histamine H3 Receptor, serotonin, serotonin transporter, clearance, depolarization
INTRODUCTION
Serotonin (5-HT) transporter (SERT) proteins are expressed in the serotonergic neurons.1 The main function of SERT is to ensure synaptic availability of 5-HT via reuptake of released 5-HT.2, 3 Polymorphisms in SERT gene promoter, introns, and rare functional coding single nucleotide polymorphisms in exons have been implicated in depression, anxiety, obsessive-compulsive disorder (OCD) and autism. (Reviewed in 4, 5) Furthermore, commonly used therapeutic agents to treat depression and OCD primarily block serotonin transporter (SERT) activity 3, 6, 7 and modulate serotoninergic signals in the CNS. 2, 8 SERT activity, and kinetics of 5-HT transport are affected by many cellular events such as phosphorylation, protein-protein interaction, trafficking, surface expression, and protein stability. Several protein kinase(s) and phosphatase(s) play crucial roles in these pathways and control SERT activity. Furthermore, homo and heteroreceptors expressed in the 5-HT neurons regulate SERT function by modulating signaling cascades. (Reviewed in 9, 10) Histamine (HA) modulates sleep, alertness, memory, feeding behavior as well as affects the endocrine system.11, 12 HA has been implicated in many neuronal diseases such as Gilles de la Tourette syndrome, tic disorder, Parkinson’s disease and schizophrenia.11, 13-15 Histaminergic neurons originate from the tuberomammillary nucleus and project into several brain areas, including the hippocampus, cerebral cortex, amygdala, substantia nigra and striatum 11 HA has four G-protein coupled receptors (GPCR), namely H1, H2, H3, and H4.16 All four HA-receptors are expressed in the central nervous system (CNS).12, 17 Among the four receptors, H3R has gained significant attention because it is expressed in several brain regions and plays a role as a presynaptic receptor that inhibits neurotransmitter release, including HA, 5-HT, dopamine (DA), noradrenaline, GABA and acetylcholine.11, 18 H3R also acts postsynaptically in the striatum and modulates striatal function.13, 19 Of particular interest is that they are present and active in serotonergic (dorsal raphe) neurons.20 It has been shown that activation of H3R prevents 5-HT release in the cortex, hippocampus, midbrain-dorsal raphe, and substantia nigra.21-23 In addition, H3R, coupled with Gi/o, inhibits adenyl cyclase and G-protein-mediated inhibition of presynaptic calcium channels.24, 25 Furthermore, activated H3R regulates the level of intracellular Ca2+ and modulates the activity of protein kinase A (PKA), MAPK, ERK2, Akt, GSK3ß, and CaMKII.11, 26-30
Similar to SERT antagonists, H3R antagonists also cause anxiolytic and antidepressant-like effects with improved memory and cognition.12, 31, 32 Because both H3R and SERT are expressed in the serotonergic neurons, it is conceivable that HA could regulate 5-HT physiology through blocking its release and SERT-mediated 5-HT uptake. Moreover, intracellular signaling that activates H3R could also modulate SERT trafficking, phosphorylation and activity. However, whether activated H3R regulates SERT-mediated 5-HT uptake in the CNS is currently not known, and the present study investigates this veritable possibility using rat brain hippocampal synaptosomes. Our findings uncover that HA indeed regulates CNS serotonergic neurotransmission by modulating SERT-mediated 5-HT clearance via H3R. We further delineate the underlying mechanism using H3R/H4R agonists and an inverse agonist and studying their effects on SERT transporter.
RESULTS AND DISCUSSION
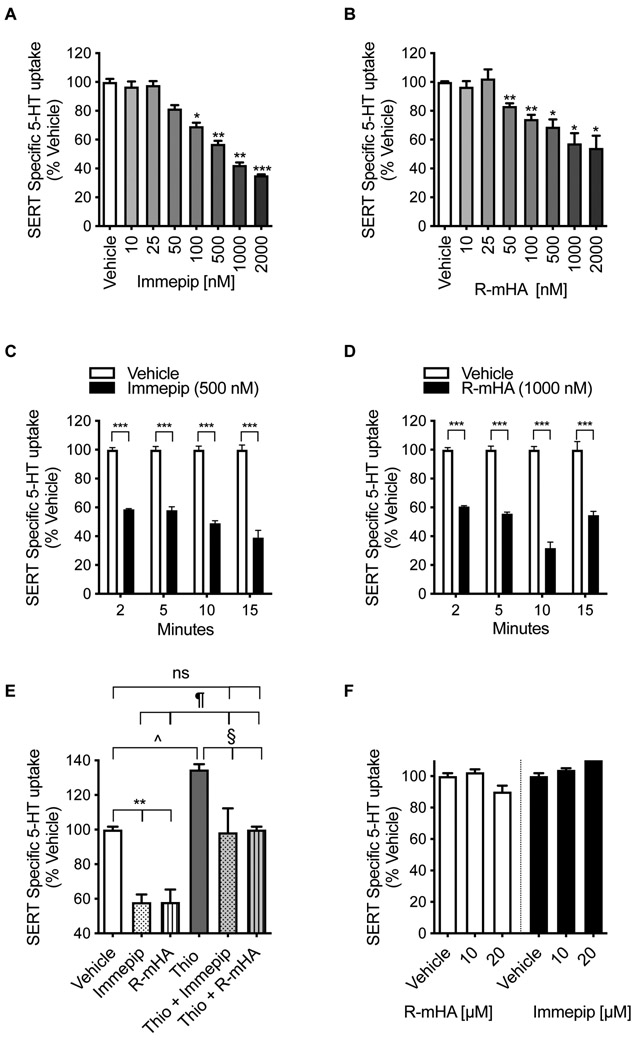
H3R/H4R Agonists Reduce SERT Activity.
Activation of H3R reduces 5-HT neuronal firing and 5-HT release providing unmistakable clues for a histamine-mediated regulation of serotonergic neurotransmission.20-23 Transcriptomic analysis of serotonergic (dorsal raphe) neurons also unambiguously detected H3R transcripts and its functional expression.20 It has been speculated that H3R expressed at serotonergic terminals acts as an inhibitory heteroreceptor.21-23 Since SERT activity is one of the critical factors in the regulation of 5-HT functions in CNS,2 we sought to determine whether SERT-mediated 5-HT uptake is affected by H3R activation. A pharmacological agent specific for H3R is lacking, and, therefore, we used the H3R/H4R agonists (R)-(−)-α-methyl-histamine (R-mHA) and immepip, and inverse agonist thioperamide similar to the studies that have used these agents before to activate H3R 33. The activity of SERT was measured by incubating with [3H]5-HT for 5 min. In a rat hippocampal synaptosome model, both R-mHA and immepip treatment caused a concentration and time-dependent reduction in SERT-mediated 5-HT transport (Figure 1A,B). Exposure of synaptosomes to immepip produced a dose (F(3,12)=152; P <0.0001) and time (F(7,22)=53; P <0.0001)-dependent decrease in SERT activity (Figure 1A,C). The effect of immepip was rapid with the maximum effect seen within 2 min of treatment (Figure 1C). Immepip did not alter SERT activity any further when we increased the treatment time up to 60 min (not shown). We then fixed the immepip pretreatment time to 5 min and varied the concentration. 5-HT uptake was significantly reduced at 100 nM (Bonferroni’s post hoc: 30.6 ± 2.3%, P <0.01) and more inhibition was observed at higher concentrations (Figure 1A). Another H3R/H4R agonist R-mHA also decreased SERT activity in a dose (F(2,11)=24; P <0.0001) and time (F(7,16)=78; P <0.0001)-dependent manner (Figure 1B,D). A significant effect of R-mHA was observed after 2 min treatment (Figure 1D). When the treatment time was fixed at 5 min, 50 nM R-mHA significantly reduced 5-HT uptake (Bonferroni’s post hoc: 25.7 ± 2.9%, P <0.05) and continued to affect at higher concentrations up to 2 μM (Figure 1B).
Figure 1: Regulation of 5-HT uptake by H3R/H4R ligands.
(A, B) Dose-dependent effect of H3R/H4R-agonists on SERT activity. Synaptosomes prepared from rat hippocampus were preincubated with vehicle or various concentrations of R-mHA or immepip (nM: 10 to 2000) for 15 min at 37°C before performing the 5-HT uptake assays. 5-HT uptake was initiated by adding [3H]labeled 5-HT (50 nM) and continued for 5 min at 37°C, and then SERT specific 5-HT uptake was determined as described in the Methods. The results are presented as a percentage change from corresponding vehicle-treated controls. Values are expressed as mean ± S.E.M. *P< 0.05, **P< 0.01, ***P< 0.0001 compared with vehicle (One-Way ANOVA with Bonferroni multiple comparison tests). (C, D) Time course of the effect of H3R/H4R-agonists on SERT activity. Synaptosomes were preincubated with R-mHA (1000 nM) or immepip (500 nM) for the time indicated followed by [3H]5-HT (50 nM) uptake for 5 min at 37°C. The results are shown as a percentage of specific SERT-mediated uptake value relative to that of corresponding vehicle-treated control at each time point. ***P< 0.0001 compared with vehicle (One-Way ANOVA with Bonferroni multiple comparison tests). (E) Effect of H3R/H4R inverse agonist thioperamide on Immepip or R-mHA-mediated downregulation of SERT function. The synaptosomes were preincubated for 10 min with thioperamide (1000 nM) prior to the addition of vehicle or immepip (100 nM) or R-mHA (100 nM). Following additional 5 min incubation with [3H]5-HT (50 nM), SERT activity was measured and compared with vehicle control treatment. **P< 0.009 compared with control vehicle; ^P< 0.03 versus control vehicle; §P< 0.02 compared with thioperamide alone; ¶P< 0.01 compared with immepip or R-mHA alone (One-Way ANOVA with Bonferroni multiple comparison tests). (F) Effect of H3R/H4R agonists R-mHA and immepip on SERT activity in HEK-293 cells. To express SERT, HEK-293 cells were transiently transfected with hSERT as previously described34. After 24 hrs, cells were treated with vehicle and R-mHA or immepip (10, 20 μM) for 15 min at 37°C followed by 10 min 5-HT uptake assays. R-mHA: (R)-(−)-α-methyl-histamine, Thio: thioperamide, ns: nonsignificant.
Next, we used an H3R/H4R inverse agonist, thioperamide, to determine whether R-mHA and immepip-mediated inhibition of SERT activity was attributable to H3R/H4R activation. The hippocampal synaptosomes were preincubated with thioperamide (1000 nM) for 10 min prior to the addition of immepip (100 nM), R-mHA (100 nM), or vehicle (Figure 1E). We saw a significant effect on SERT activity (F(5,12)=18; P <0.0001). Post hoc analyses (Bonferroni’s test) revealed that immepip and R-mHA reduced SERT activity (% vehicle: immepip 58.1 ± 4.5; R-mHA 58.1 ± 7.2; P <0.01) in the absence of thioperamide, but in its presence, both did not affect (% vehicle: thioperamide + immepip 98.8 ± 13.9, thioperamide + R-mHA 100.0 ± 1.7, P =0.99). Interestingly, thioperamide treatment alone produced a noticeable increase above basal SERT activity (% vehicle: 134.8 ± 3.0, P <0.03) (Figure.1E). SERT activity was higher when treated with immepip or R-mHA in the presence of thioperamide when compared to that of immepip alone (% vehicle: thioperamide + immepip 98.8 ± 13.9; immepip 58.1 ± 4.5; P <0.01) or R-mHA alone (% vehicle: thioperamide + R-mHA 100.0 ± 1.7; R-mHA 58.1 ± 7.2; P <0.001). The stimulatory effect of thioperamide marginally declined with co-treatment of immepip or R-mHA (% vehicle: thioperamide 134.8 ± 3.0; thioperamide + immepip 98.8 ± 13.9 (P <0.02), thioperamide + R-mHA 100.0 ± 1.7 P <0.03). To rule out the possibility that the effect seen with immepip and R-mHA could be due to a direct interaction/binding with SERT, they were tested in HEK-293 cells lacking H3R but expressing SERT34. Both immepip and R-mHA had no significant effect on SERT activity even at high concentrations (10 and 20 μM, 5 min) (F(1, 2)=9; P =0.09) (Figure 1F). Collectively, these observations indicate that H3R/H4R agonists affect SERT activity, and their inverse agonist blocks such inhibitory effect while stimulating SERT activity further demonstrating that histaminergic system, indeed, influences serotonergic neurotransmission. Immepip (0.05 to 5 μM), R-mHA (0.025 to 1 μM), and thioperamide (0.1 to 1 μM) were used in a published study examining H3R-mediated inhibition of 5-HT release from brain slice preparations 21. Based on the observations (Figure 1A-D), in subsequent experiments, we treated hippocampal synaptosomes with 500 nM immepip for 15 min.
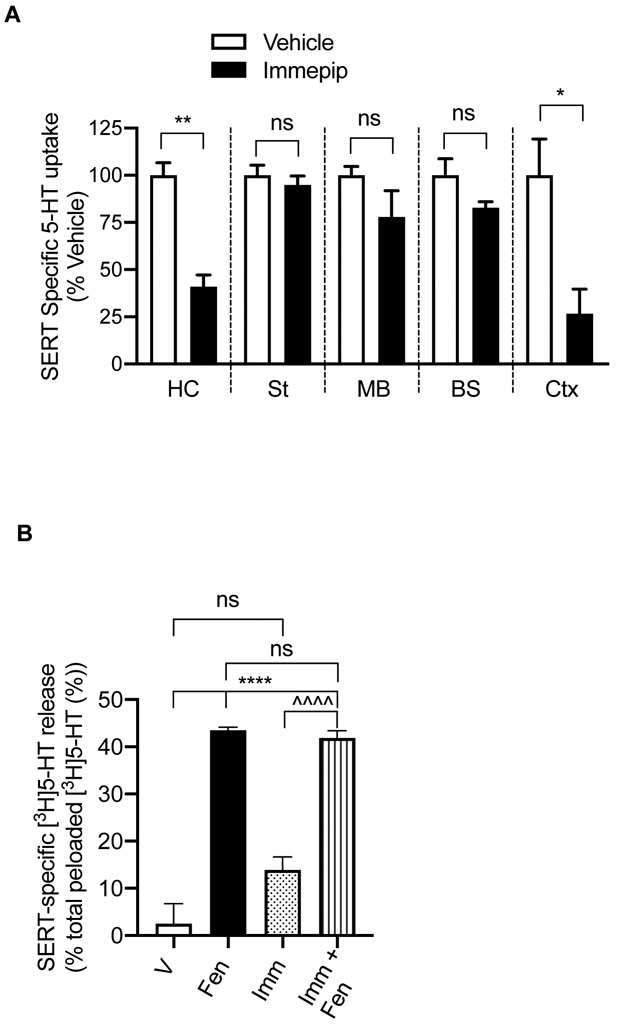
H3R/H4R Agonist Immepip Alters SERT Activity in a Brain Region-Specific manner.
To determine whether H3R/H4R agonist-mediated SERT regulation follows a particular pattern in the brain or varies in different regions of the brain, we obtained synaptosomes from hippocampus, cortex, striatum, midbrain and brain stem and examined the effect of immepip (500 nM, 15 min) on SERT activity (Figure 2A). Immepip treatment significantly reduced SERT-mediated 5-HT uptake in the hippocampus (t= 6.49, df=4; P <0.003) and cortex (t= 3.16, df=4; P <0.03); however, there was no statistically significant effect in the striatum (t= 0.72, df=4; P =0.51), midbrain (t= 1.50, df=4; P =0.21), and brainstem (t= 1.85, df=4; P =0.14). It’s worth noting that striatum, midbrain, and brain stem, where an effect is lacking, do not have H3R or H4R on serotonergic terminals. However, some studies have reported that activation of H3R inhibited 5-HT release in the midbrain-dorsal raphe and substantia nigra. 21-23 Further studies are needed to corroborate functional expression of HA-receptors and their downstream signaling in 5-HT terminals located in different regions. Regardless, it is clear from our findings that H3R/H4R agonist immepip downregulates SERT activity in a brain region-specific manner, thus, indicating HA likely influences synaptic 5-HT levels differentially in serotonergic neurotransmission.
Figure 2: Regulation of 5-HT uptake by H3R/H4R agonist immepip in different regions of the rat brain, and the effect of immepip on SERT-mediated 5-HT efflux.
(A) Effect of immepip on SERT activity in different brain regions. Synaptosomes isolated from the hippocampus, striatum, midbrain, brainstem, and cortex were pretreated with the vehicle or immepip (500 nM) for 15 min at 37°C. SERT specific 5-HT uptake was measured as described in the Methods and Figure 1. **P< 0.003, *P< 0.03 compared to corresponding vehicle control, ns: nonsignificant (unpaired two-tailed Student’s t-test). (B) Effect of immepip on SERT-mediated [3H]5-HT efflux. Hippocampal synaptosomes were preloaded with [3H]5-HT and fluoxetine-sensitive [3H]5-HT efflux was measured as described in the Methods. [3H]5-HT-preloaded synaptosomes were treated with vehicle or D-fenfluramine (2 μM) or immepip (500 nM) or D-fenfluramine (2 μM) + immepip (500 nM) for 30 min at 37°C. ****P< 0.0001 compared with vehicle control, ^^^^P< 0.0001 compared with immepip, ns: nonsignificant (One-Way ANOVA with Bonferroni multiple comparison tests). HC: hippocampus, St: striatum, MB: midbrain, BS: brain stem, Ctx: cortex, V: vehicle, Fen: D-fenfluramine, Imm: immepip.
H3R/H4R Agonist Immepip Does Not Affect SERT-Mediated 5-HT Efflux.
We previously reported that activation of α-adrenoreceptor reduced SERT-mediated 5-HT uptake and efflux.35 Here, we investigated whether H3R/H4R agonist immepip affects SERT-mediated 5-HT release in the hippocampal synaptosomes. These synaptosomes were pre-loaded with [3H]5-HT in the presence of vesicular monoamine transporter-inhibitor reserpine to block vesicular [3H]5-HT accumulation, nisoxetine, and GBR12935 (inhibitors of norepinephrine transporter (NET) and DA transporter (DAT), respectively) to avoid [3H]5-HT accumulation in DA and NE terminals. In addition, nisoxetine and GBR12935 were included during the release assay to block any [3H]5-HT release and reuptake through NET and DAT. As in our previous study, we used D-fenfluramine to trigger SERT-mediated 5-HT efflux in the hippocampus synaptosomes. 35 A significant treatment effect on SERT-mediated [3H]5-HT was observed (F(3,17)=; P <0.0001), (Figure 2B). Post hoc analyses (Bonferroni’s test) revealed that D- fenfluramine (3 μM, 5 min) treatment of [3H]5-HT-preloaded hippocampus synaptosomes resulted in an elevated efflux of [3H]5-HT sensitive to SERT-specific blocker fluoxetine (43.5 ± 0.66 % of [3H]5-HT efflux from the total [3H]5-HT preloaded, P <0.0001). Though immepip treatment showed a trend towards increased fluoxetine-sensitive [3H]5-HTefflux, it did not reach a statistical significance (13.89 ± 2.77 %, P =0.062). Coapplication of immepip with D-fenfluramine did not significantly affect [3H]5-HT efflux when compared to D- fenfluramine treatment alone (D- fenfluramine: 43.5 ± 0.66 %; immepip plus D- fenfluramine: 41.86 ± 1.47%, P <0.0001). Our previous findings showed that α-adrenoreceptor inhibited both SERT mediated 5-HT uptake and efflux; 35 however, this study shows HA-receptor(s) affect only the SERT-mediated 5-HT uptake but not the efflux. Thus, it appears that the heteroreceptors expressing at the 5-HT terminals modulate SERT activity differentially and selectively.
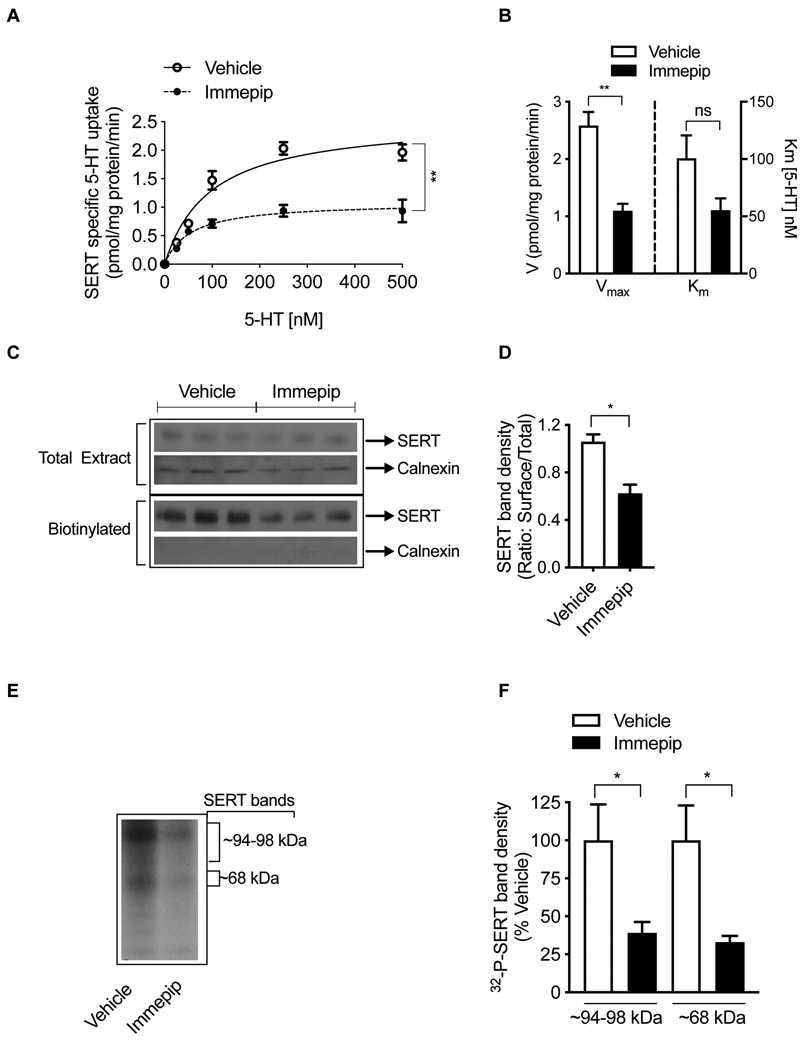
H3R/H4R Agonist Immepip Alters SERT Kinetics, Surface SERT Density, and SERT Phosphorylation.
The kinetics of SERT activity is another crucial factor in the regulation of 5-HT neurotransmission. 2 A significant reduction (~57%) in the maximal velocity (Vmax) was observed following immepip (500 nM, 15 min) treatment of hippocampal synaptosomes with no noticeable change in the affinity for 5-HT (Figure 3A,B). Vmax was decreased from 2.59 ± 0.24 pmol/mg protein/min (vehicle) to 1.10 ± 0.12 pmol/mg protein/min (immepip) (t= 5.57, df=4; P <0.006). Km for 5-HT was 100.60 ± 20.09 nM (vehicle) and 55.42 ± 10.24 nM (immepip) (t= 2.01, df=4; P =0.12). Kinetic parameters shown in Figure 3A,B reveal a significant decrease in Vmax suggesting that immepip treatment regulates the availability of functional surface SERTs. When we measured the level of surface SERT using biotinylation and western blot analysis, immepip treatment (500 nM, 15 min) decreased cell surface expression of SERT (Figure 3C). Quantitated immunoblot surface SERT density showed a significant reduction (~41 %; t=4.58, df=4; P <0.01) caused by immepip treatment compared to that of vehicle control (Figure 3D). Interestingly, the total level of SERT did not change with immepip (Figure 3C). Immepip or vehicle exposure did not alter the expression of the endoplasmic reticulum protein calnexin. Less than 0.3% of total calnexin was detected in avidin-bound fractions (Figure 3C). The absence of calnexin in the surface biotinylated fraction indicates that the synaptosomes were intact and that the intracellular SERT and other proteins were not biotinylated during the surface-biotinylation procedure. Consistent with the inhibitory effect of immepip on SERT activity (Figure 1A,C), the results from surface biotinylation studies indicate that immepip reduces surface expression of SERT, hinting that this might be the mechanism by which immepip decreases 5-HT transport. We have previously shown that controlled internalization and plasma membrane insertion of SERT attributes to plasma membrane expression of SERT.34, 36-39 However, whether regulated SERT internalization or plasma membrane delivery or both engaged in HA-receptor(s)-mediated reduction in surface SERT remains to be studied.
Figure 3: Regulation of SERT kinetics, surface SERT expression, and SERT phosphorylation by H3R/H4R agonist immepip.
(A, B) Effect of immepip on SERT kinetics. Hippocampal synaptosomes were pretreated with the vehicle or immepip (500 nM) for 15 min at 37°C. 5-HT uptake was measured over a concentration range of 25-500 nM using 5 min uptake period and SERT-specific activity was determined as described in the Methods. Using the Michaelis-Menten equation (Prism), Km (substrate (5-HT) affinity) and Vmax (maximal velocity) were determined. Values are shown from three animals as mean ± SEM. **P< 0.005 compared with vehicle control, ns: nonsignificant (unpaired two-tailed Student’s t-test). (C, D) Effect of immepip on surface expression of SERT. Synaptosomes were treated with vehicle or immepip (500 nM) for 15 min and surface biotinylation, immunoblotting and quantification were performed as described in the Methods. Aliquots (20 μl) of total extract (750 μl) and entire biotinylated fractions were subjected to SDS-PAGE. (C) Representative SERT immunoblots show total, biotinylated (surface) SERT (~96-100 kDa), and calnexin (~90 kDa). (D) Relative band densities of biotinylated SERT that were normalized to total SERT from three samples are presented as mean ± S.E.M. *P <0.01 compared with vehicle control (unpaired two-tailed Student’s t-test). (E, F) Effect of immepip on SERT phosphorylation. Metabolically-labeled hippocampal synaptosomes with [32P]orthophosphate were treated with vehicle or immepip (500 nM) for 15 min. Immunoprecipitation, SDS-PAGE and autoradiography were performed as described in the Methods. A representative autoradiogram of three independent experiments with 32P-labelled SERT bands (~94-98 kDa) and (~68 kDa) is shown (E). A bar graph (F) shows the percent average of 32P-labelled SERT band (~94-98 kDa) and (~68 kDa) density from vehicle-treated control. *P < 0.05, compared to vehicle control (unpaired two-tailed Student’s t-test).
From our previous studies, we have established that SERT is a phosphoprotein and that the phospho-status of SERT is regulated by many kinases. (Reviewed in 9, 10) To verify whether the H3R/H4R agonist immepip also affects the phosphorylation status of SERT, we used metabolically-32P-labelled hippocampal synaptosomes and examined SERT. Consistent with our previous studies,34, 37 immunoprecipitation (IP) analysis using SERT-specific antibody revealed that SERT was indeed phosphorylated (~94-98 kDa and 68 kDa) (Figure 3E). Treatment with immepip (500 nM, 15 min) significantly reduced the density of phosphorylated SERT (~94-98 kDa: 60.86 ± 7.18 %; t=2.47, df=6; P <0.05; 68 kDa: 66.97 ± 4.13 % t=2.88, df=4; P <0.05) (Figure 3F) revealing a role for HA-receptor(s)-signaling in SERT phosphorylation, which may contribute to the inhibitory effect.
Our published studies demonstrate that differential SERT phosphorylations via specific kinases mediate SERT regulation. For example, activation of PKC,39, 40 kappa-opioid receptor,36 and PP2a inhibition40, 41 stimulate SERT phosphorylation with concomitant decrease in SERT activity. However, inhibition of Akt37 and p38 MAPK34 decreases both SERT phosphorylation and SERT activity. We have also shown that Src-mediated tyrosine phosphorylation of SERT on Tyr47, Tyr142, Tyr350, Tyr358 residues regulates SERT protein stability and function.38 Stimulation of Thr276 phosphorylation in SERT is necessary for protein kinase G (PKG) to induce SERT activity. 42 Interestingly, SERT substrates and inhibitors34, 43 and disease-linked human SERT-coding variants44 modulate the phosphorylation status of SERT via specific kinases/phosphatases. Given the complexity of the role of SERT phosphorylation in the regulation of SERT, whether SERT phosphorylation is a requisite for HA-receptor(s)-mediated SERT regulation needs additional investigations including identification of SERT phosphorylation site(s)/motif(s) and the mechanisms by which SERT phosphorylation regulate SERT functional expression in response to HA-receptor(s)-modulation.
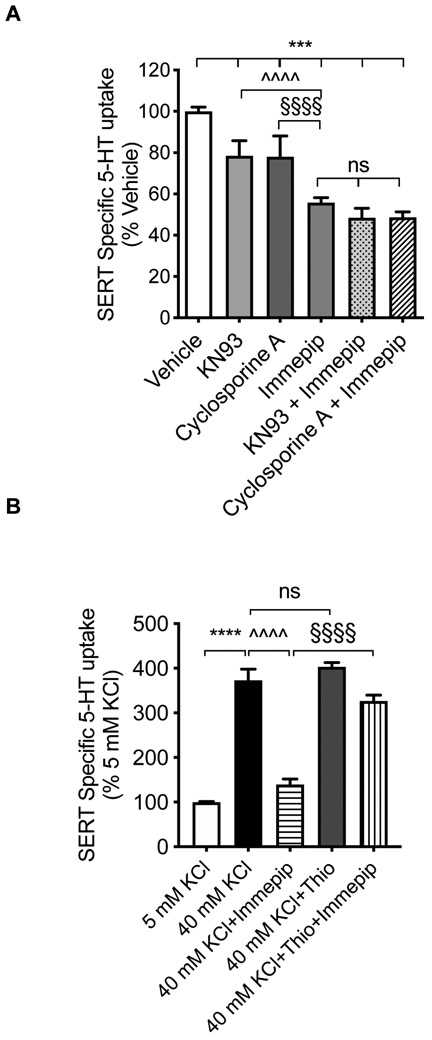
The Effect of Immepip on SERT Activity Is Dependent on CaMKII and Calcineurin Pathways.
It has been shown that H3R activation in nerve terminals inhibits Ca2+ entry.25, 28 Studies including ours have demonstrated that CaMKII or calcineurin reduces SERT activity when Ca2+ is removed,45,34, 46 On the contrary, in the presence of calcium, their Ca2+-dependent kinase and phosphatase activity stimulates SERT activity. 36, 45-47 Therefore, the most likely mechanism by which H3R-agonist such as immepip could inhibit SERT is by repressing Ca2+ inflow that would render CaMKII /or calcineurin inactive. To investigate this possibility, hippocampal synaptosomes were incubated with maximal effective concentration of CaMKII inhibitor KN93 (2.5μM) 36 or calcineurin inhibitor cyclosporine A (5.0 μM) or immepip (1 μM) alone, or immepip together with KN93 or cyclosporine A. A significant treatment effect on SERT-mediated [3H]5-HT was observed (F(5, 25)=59.3; P <0.0001) (Figure 4A). Post hoc analyses (Bonferroni’s test) revealed that KN93, cyclosporine A, and immepip independently inhibited SERT activity compared to vehicle control (inhibition % of vehicle: KN93: 21.49 ± 3.66; cyclosporine A: 22.00 ± 4.15; immepip: 44.18 ± 1.09; P <0.0001). Immepip induced more inhibition of SERT activity than KN93 or cyclosporine A alone (P <0.0001). However, when immepip was added to synaptosomes preincubated with KN93 or cyclosporine A, inhibition of SERT activity was similar to that elicited by immepip individually with no additive inhibitory effect (% inhibition: immepip: 44.18 ± 1.09, KN93, + immepip: 51.56 ± 1.73, P =0.09; cyclosporine A + immepip: 51.32 ± 1.21, P =0.13) (Figure 4A). Cytosolic Ca2+ governs the activities of CaMKII and calcineurin.48 Thus, Ca2+-CaMKII and calcineurin signaling axis may engage H3R-mediated SERT regulation. Consistent with previous reports, these results show inhibiting CaMKII and calcineurin reduces SERT activity. However, our data show a non-additive effect of immepip on CaMKII and calcineurin-dependent SERT inhibition, which might be because of convergence of H3R and CaMKII-calcineurin signaling pathways and that the mechanism is one and the same. While H3R triggered downstream signaling needs further investigation, H3R linked CaMKII-calcineurin signaling may affect SERT regulation at different levels. It has been shown that Ca2+-calcineurin activity promotes direct interaction of calcineurin with SERT and results in higher SERT activity and surface expression.47 Thus, it is possible that reduced Ca2+-calcineurin activity following H3R activation may result in decreased calcineurin activity and its interaction with-SERT. Such an effect can lead to reduced surface SERT expression and function as seen in the present study. Additionally, a previous report demonstrated that Ca2+-CaMKII promotes SERT-syntaxin 1A interaction in a SERT phosphorylation-dependent manner, and controls the transport stoichiometry of SERT. 49 Thus, it is likely that altered Ca2+- CaMKII-calcineurin following H3R activation may influence SERT-mediated 5-HT uptake at multiple levels including regulated SERT trafficking, phosphorylation, and protein-protein interactions. Further studies are warranted to address these possible mechanisms.
Figure 4: Effect of inhibitors of CaMKII and calcineurin A on H3R/H4R agonist-mediated SERT regulation, and attenuation of depolarization by H3R/H4R agonist.
(A) Effect of CaMKII and calcineurin inhibition on H3R-mediated SERT regulation. Hippocampal synaptosomes were pre-incubated with vehicle or CaMKII inhibitor KN93 (2.5 μM) or calcineurin inhibitor cyclosporine A (5.0 μM) as indicated for 45 min, and then vehicle or immepip (1 μM) was added and continued for 15 min. SERT mediated 5-HT uptake was determined as described in the legend to Figure 1. ^^^^P< 0.0001 compared between immepip and KN93 alone; §§§§P< 0.0001 compared between immepip and cyclosporine A alone, nsP= 0.1 compared with immepip alone (One-Way ANOVA with Bonferroni multiple comparison tests). (B) Effect of H3R on depolarization-induced SERT activity. SERT specific 5-HT uptake by synaptosomes was performed with a modified KRH buffer containing two concentrations of K+ (5 mM KCl or 40 mM KCl). As indicated, H3R/H4R inverse agonist thioperamide was preincubated for 10 min (1000 nM) prior to the addition of vehicle or immepip (500 nM). Incubation was continued for 15 min at 37°C, and SERT-specific 5-HT uptake was measured. ****P< 0.0001 compared with 5 mM KCl ; ^^^^P < 0.0001) compared with 40 mM KCl, §§§§P< 0.0001compared with 40 mM KCl + immepip, nsP= 0.99 compared with 40 mM KCl (One-Way ANOVA with Bonferroni multiple comparison test).
H3R/H4R-Agonist Immepip Attenuates Depolarization-Dependent SERT Upregulation.
Since R-mHA inhibits calcium entry during depolarization25 and H3R controls depolarization-linked activation of histamine synthesis by inhibiting Ca2+-CaMKII and adenylate cyclase pathways,28 we examined whether immepip-mediated SERT inhibition is sensitive to depolarization. KRH assay buffer containing 40 mM KCl (for K+) was used to stimulate depolarization.50 Figure 4B shows a significant treatment effect on SERT-mediated [3H]5-HT uptake (F(4, 37)=82.25; P <0.0001). Post hoc analyses (Bonferroni’s test) revealed that 40 mM KCl significantly increased SERT activity when compared to 5 mM KCl (% of 5 mM KCl: 373.1 ± 25.01; P <0.0001). Immepip exposure near completely abolished the K+ depolarization (40 mM KCl)-induced enhancement of SERT activity (% stimulation: 40 mM KCl: 373.1 ± 25.01; P <0.0001; Immepip + KCl: 139.4 ±12.5; P <0.0001). H3R-inverse agonist thioperamide prevented that effect of immepip (% stimulation: Immepip + KCl: 139.4 ±12.5; thioperamide + Immepip + KCl: 326.6 ±13.31; P <0.0001) (Figure 4B). As shown above in Figure 1E, thioperamide treatment increased SERT activity; however, it did not show further enhancement with 40 mM KCl, suggesting that the stimulatory effect of depolarization on SERT activity reached the maximal threshold. Together, these results show that K+ triggered depolarization increases SERT activity and immepip abolishes that activity. It is possible that the release of 5-HT from synaptosomes following depolarization may influence SERT regulation as we reported,43 and also the released 5-HT could activate 5-HT1B autoreceptor-mediated signaling that could stimulate SERT activity as has been shown before.51, 52 Further investigations are needed to determine how immepip-mediated signaling interferes with depolarization-triggered SERT activity. Nevertheless, our findings thus far indicate a novel mechanism of HA-receptor(s) acting as heteroreceptors suppress depolarization-induced SERT activity in hippocampal synaptosomes.
H3R/H4R-Inverse Agonist Increases SERT Function While H3R/H4R Agonist Prolongs in vivo 5-HT Clearance in the Hippocampus.
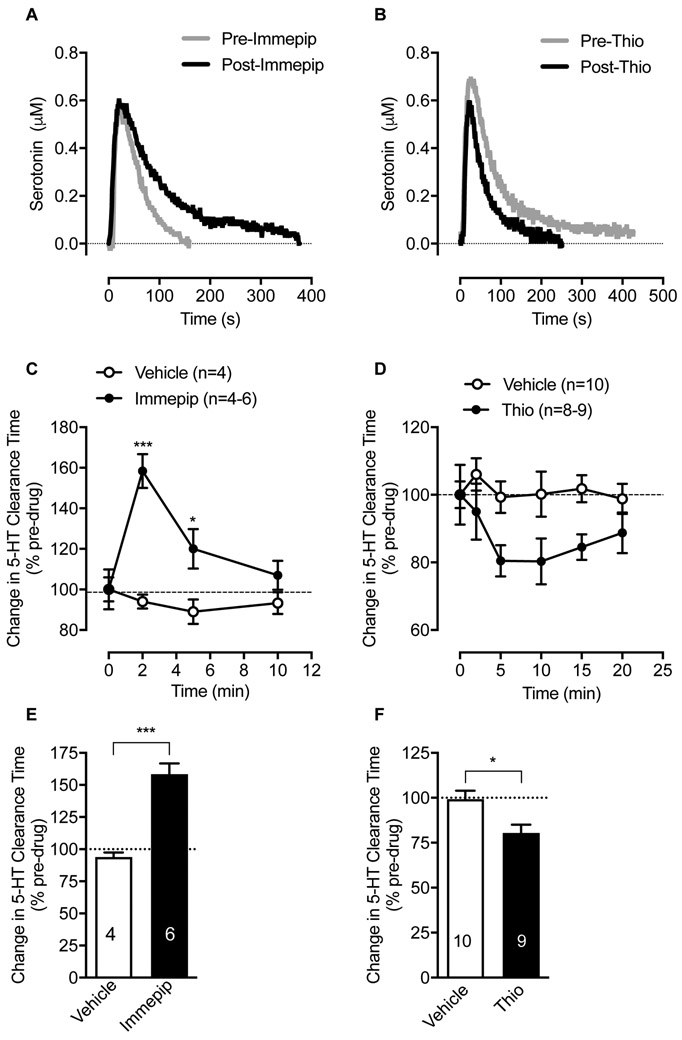
It is known that H3R is constitutively active in the CNS53. Given our results that H3R/H4R-inverse agonist thioperamide alone produced a significant elevation of SERT activity in ex vivo hippocampal synaptosomal preparations (Figure 1E), we next determined whether in vivo administration of thioperamide increases SERT activity. The upregulation of SERT by H3R/H4R-inverse agonist is noteworthy, and it suggests that basal HA-mediated signaling regulates SERT functional expression and synaptic 5-HT availability in vivo as well. To test whether in vivo modulation of HA receptor(s) regulates SERT-mediated 5-HT clearance, we assessed hippocampal 5-HT clearance in anesthetized rats using in vivo high-speed chronoamperometry. Figure 5 A and B shows the oxidation current produced by pressure-ejection of 5-HT into hippocampus either before or after locally applying immepip or thioperamide. Clearance of 5-HT by 80% (T80) was quantitated and plotted as a function of time after drug or saline delivery (Figure 5C, D). In these studies, intrahippocampal injection of immepip (20 pmol) delayed 5-HT clearance (prolonged T80) in comparison with saline (F(3, 28)=5.8; P <0.003), (Figure 5C, E). The effect of immepip reached maximum 2 min post-injection (t= 6.0, df=8; P <0.0003) (Figure 5C) and returned to baseline by 10 min (Figure 5C). The relationship between time and effect of immepip on 5-HT clearance was significant (F(3, 28)=7.3; P <0.001). In contrast, ejection of thioperamide (20 pmol) resulted in faster 5-HT clearance (reduced T80) 5 min post-delivery compared with control saline (t= 2.8, df=17; P <0.01) (Figure 5F) with significant main treatment effect (F(1, 99)=15.1; P <0.0002) (Figure 5D) on 5-HT clearance. Ejection of saline (vehicle) had no significant effect on 5-HT clearance time (F(3, 12)=0.5; P=0.7), (Figure 5C,D). Corroborating the inhibitory effects of immepip on SERT activity in hippocampal synaptosomes (Figure 1), in vivo chronoamperometry revealed decreased clearance of exogenously applied 5-HT following local hippocampal infusion of immepip and increased 5HT clearance with thioperamide infusion.
Figure 5: Effect of local hippocampal administration of H3R/H4R ligands on the clearance of exogenously supplied 5-HT.
in-vivo 5-HT clearance in the hippocampus of anesthetized rats was monitored by chronoamperometry as described in the Methods. (A) in-vivo effect of H3R/H4R agonist immepip on 5-HT clearance. Once reproducible 5-HT signals were obtained, immepip (20 pmol) was pressure ejected into the hippocampus, and 2 min later followed with 5-HT. Shown are representative oxidation currents (using in vitro calibration factor, current was converted to a micromolar value) produced by pressure-ejection of 5-HT into hippocampus either before (grey trace) or after (black trace) locally applying immepip. Traces are overlaid for ease of comparison. (C) 5-HT clearance T80 (time to clear 5-HT by 80%) as a function of time elapsed after local application of immepip. Data are expressed as percentage change from pre-drug/vehicle baseline. ***p< 0.001, *p< 0.05 compared with vehicle baseline (Two-Way ANOVA with Bonferroni multiple comparison tests). (E) The maximum effect of immepip on T80 2 min post-application. (***p< 0.0003, Two-tailed Student’s t-test, n is shown within the bars). (B) in vivo effect of H3R/ H3R inverse agonist thioperamide on 5-HT clearance. Following reproducible 5-HT signals, thioperamide (20 pmol) was pressure ejected into hippocampus, 2 min later followed with 5-HT. Shown are representative oxidation currents produced by pressure-ejection of 5-HT in hippocampus either before (grey trace) or after (black trace) locally applying thioperamide. (D) 5-HT clearance T80 (time to clear 5-HT by 80%) as a function of time elapsed after local application of thioperamide. Data are expressed as percentage change from pre-drug/vehicle baseline. There was a main effect of treatment (***p<0.0002 (Two-Way ANOVA with Bonferroni multiple comparison tests). (F) The maximum effect of thioperamide on T80, 5 min post-application. (*p < 0.01, Two-tailed Student’s t-test, n is shown within the bars). Thio: thioperamide.
R-mHA and immepip interact with both H3R and H4R. Thioperamide is an inverse agonist for both receptors. Both H3R and H4R are coupled to Gi/o and modulate similar signaling cascades (Reviewed in and see references therein: 11, 16, 33). Because our conclusions are based on the results from the use of these compounds, it is difficult to discriminate between H3R or H4R. Nonetheless, it should be noted that functional expression of either of them in 5-HT neurons is necessary to be able to modulate SERT. While the expression of H3R is evident in 5-HT neurons,20 to our knowledge, expression of H4R in 5-HT neurons has not been reported. With the available information, we conclude that H3R activation regulates SERT and affects serotonergic neurotransmission, while further studies on H4R expression in 5-HT neurons is necessary to reach a conclusion.
In conclusion, our findings demonstrate that H3R/H4R agonists downregulate SERT function in a brain region-specific manner in this ex vivo model. We show that in the rat hippocampal synaptosomes, H3R/H4R agonists reduce 5-HT uptake, surface SERT level, and its phosphorylation. We also present evidence for a role of calcineurin/CaMKII in mediating the acute effects of H3R/H4R agonists. Moreover, these agonists suppress K+ depolarization to alter SERT function. Concordantly, the ex vivo observations are replicable in vivo as well in the hippocampus of anesthetized rats where H3R/H4R agonists reduce 5-HT clearance while H3R inverse agonist facilitates such clearance. Collectively, these results shed light on a novel mechanism where histaminergic connection underlies and influences the serotonergic neurotransmission. Our findings bear serious implications given the fact that both serotonergic and histaminergic signaling coordinately regulate several crucial physiological processes and behaviors. For example, both HA and 5-HT are known to play important roles in sleep, memory, and mood.31, 54-56 Similar to SERT blockers (SSRIs), H3R agonists show anxiolytic effects while H3R antagonists have antidepressant effects.32 Also, altered SERT expression modulates retention of fear extinction memory, a predisposition to anxiety disorders, and depression.57, 58 Interestingly, H3R activation in hippocampus improves memory consolidation after contextual fear conditioning.27 Striatal postsynaptic H3R has been implicated in the repetitive behavioral pathology of tic disorder, a comorbidity found in OCD and autism patients.59, 60 A rare SERT coding mutant Ile425Val and altered SERT functions have been associated with OCD, autism, and Tourette’s syndrome.61 Moreover, SSRIs are first-choice treatments for patients suffering from depression and OCD.62 A modulatory role of HA on 5-HT neuron firing in awake and sleeping phases has been reported.44, 63 Given the fact that H3Rs act as heteroreceptors in 5-HT neurons and modulate serotonergic function,21-23 and since HA and 5-HT elicited behaviors converge, we put forward a new paradigm where the physiological effects of 5-HT are regulated by H3R through its actions on both 5-HT release and clearance. Unraveling the complexities that ultimately impact SERT function will resolve many HA and 5-HT-based pathophysiological conditions observed in brain disorders. Certainly, several questions remain to be answered. Nonetheless, our findings highlight the fact that targeting this pathway could be a promising avenue in developing new treatment modalities for brain disorders.
METHODS
Reagents and Antibodies.
4-(1H-Imidazol-4-ylmethyl)piperidine dihydrobromide (Immepip dihydrobromide), S-[2-(Imidazol-4-yl)ethyl]isothiourea dihydrobromide (Imetit dihydrobromide) and (R)(−)-α-Methyl-1H-imidazole-4-ethanamine dihydrochloride ((R)-(−)-α-methyl-histamine) were obtained from Sigma-Aldrich (St. Louis, MO). 5-Hydroxy-[3H]tryptamine trifluoroacetate ([3H]5-HT), 32PO4 carrier-free orthophosphate and Optiphase Supermix were purchased from PerkinElmer (Waltham, MA). Protein A-Sepharose was obtained from Amersham Biosciences (Piscataway, NJ). ECL reagents, Sulfosuccinimidyl-2-(biotinamido)ethyl-1,3-dithiopropionate (EZ link NHS-Sulfo-SS-biotin) and NeutrAvidin Agarose were from Pierce (Rockford, IL). HRP-conjugated secondary antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). Reagents for SDS-polyacrylamide gel electrophoresis and Bradford Protein Assay were purchased from Bio-Rad (Hercules, CA). Anti-calnexin was from Enzo Life Sciences, Inc (Farmingdale, NY). Our laboratory-generated SR-12 SERT antibody and its characterization of specificity have been published previously.34, 38, 39 All other chemicals were from either Sigma-Aldrich or Thermo Fisher Scientific (Pittsburgh, PA) unless otherwise indicated.
Animals
Male Sprague-Dawley rats of 200-280 g body weight were housed in a temperature and humidity-controlled facility with a 12-h light/dark cycle at an ambient temperature of 19–22°C. Food and water were provided ad libitum. Experiments were conducted during the light phase. All animal procedures were carried out in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University.
Synaptosome Preparation
Synaptosomes were prepared as described previously.34 Briefly, rats were rapidly decapitated, the brain regions were dissected on ice, homogenized in ice-cold 0.32 M sucrose, and centrifuged at 1000 x g for 15 min at 4°C. The synaptosomal fractions were obtained by centrifuging the supernatant at 12,000 x g for 20 min. The pellet was resuspended in 0.32 M sucrose and the protein concentration was determined using the DC protein assay kit (BioRad).
5-HT-Uptake Assay (SERT Activity)
SERT activity was measured as described previously.34 As indicated in the Figure legends, chemical agents or appropriate vehicle were incubated with synaptosomes (30 to 50 μg) at 37°C in 0.5 ml of Krebs-Ringer buffer (25 mM Na2HCO3, 124 mM NaCl, 5 mM KCl, 5 mM MgSO4, 1.5 mM CaCl2, 10 mM glucose, 0.1 mM pargyline and 0.1 mM ascorbic acid pH 7.3, saturated with 95% O2, 5% CO2). [3H]5-HT uptake was measured using 50 nM of [3H]5-HT at 37°C for 5 min with or without SERT blocker 0.1 μM fluoxetine. Total [3H]5-HT accumulation in the absence of fluoxetine minus in its presence was defined as SERT specific [3H]5-HT uptake. For SERT kinetic analysis (Km, Vmax), [3H]5-HT was mixed with unlabeled 5-HT from 10 nM to 500 nM. At the end of the assay, the samples were subjected to rapid filtration using Brandel Cell Harvester with 0.3% polyethyleneimine coated GF-B filters and washed three times with 5 ml cold PBS. The radioactivity retained on the filter was quantitated in a liquid scintillation counter.
5-HT Efflux Assay in vitro.
Release assay was performed as we described previously with a few modifications.35 Briefly, the hippocampal synaptosomes were incubated with 5 nM [3H]5-HT in Krebs-Ringer buffer for 1 h at 37°C with 1μM reserpine (inhibitor for vesicular monoamine transporter (VMAT), 50 nM GBR12935 (inhibitor for DAT) and 10 nM nisoxetine (inhibitor for NET) followed by centrifugation at 12,000 x g twice to remove excess [3H] 5-HT. The resulting pellet was suspended in ice-cold KRH buffer. Reserpine, GBR12935, and nisoxetine were added to all buffers to block [3H]5-HT accumulation in synaptic vesicles and NE and DA terminals. 50 μg of preloaded synaptosomes were then added to 0.5 ml total volume of KRH buffer containing D-fenfluramine (2 μM) or immepip (500 nM) or D-fenfluramine (2 μM) + immepip (500 nM) or vehicle with or without fluoxetine (1 μM) as indicated in the Figure legends. After 30 min incubation, the efflux assay was terminated by rapid filtration as described above under “5-HT uptake assays.” SERT blocker fluoxetine (1 μM) was used to confirm SERT-specific [3H]5-HT efflux. SERT specific [3H]5-HT efflux was determined by subtracting [3H]5-HT efflux in the presence of fluoxetine from that in the absence of fluoxetine. The [3H]5-HT efflux was calculated by subtracting the radioactivity retained on the filter from that in the total preloaded [3H]5-HT in 50 μg synaptosomes.
Surface Biotinylation and Immunoblotting.
As we described previously,34 hippocampal synaptosomes (250-300 μg) were pretreated with modulators or vehicle at 37°C for a specified time as in the Figure legends. Following centrifugation (12,000 x g for 20 min, 4°C) of the sample, the pellet was suspended and biotinylated with EZ link NHS-Sulfo-SS-biotin (1 mg/1 mg protein) for 30 min at 4°C in cold Krebs-bicarbonate buffer. Excess NHS-Sulfo-SS-biotin was quenched with the same buffer containing 100 mM glycine. The biotinylated hippocampal synaptosomes were solubilized in radioimmunoprecipitation assay (RIPA) lysis buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% SDS, and 1% sodium deoxycholate) supplemented with protease inhibitors (1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μM pepstatin, and 250 μM phenylmethylsulfonyl fluoride) and phosphatase inhibitors (10 mM sodium fluoride, 50 mM sodium pyrophosphate, 5 mM sodium orthovanadate, and 1 μM okadaic acid). Samples were centrifuged at 25,000 x g for 30 min at 4°C. NeutrAvidin Agarose resin was then added to the clear supernatant to bind with the biotinylated proteins. After washing NeutrAvidin Agarose resins three times with RIPA buffer, bound biotinylated proteins were eluted with 50 μl Laemmli sample buffer (62.5 mM Tris-HCl pH 6.8, 20 % glycerol, 2 % SDS and 5 % ß-mercaptoethanol). Proteins were separated on a 10% SDS-PAGE, transferred to a PVDF membrane, probed with a SERT-specific SR-12 antibody, and SERT proteins were detected using ECL or ECL plus reagent. The same blots were stripped and re-probed for the level of intracellular endoplasmic reticulum protein calnexin using anti-calnexin antibody so as to validate the surface biotinylation of plasma membrane proteins and normalize loading/membrane transfer protein levels. Immunoreactive band densities within a linear range in the same blot was quantitated using NIH ImageJ (version 1.52a, NIH) software. The relative intensity of biotinylated SERT (representing the surface pool) was normalized to the total SERT protein.
SERT Phosphorylation in Synaptosomes.
Synaptosomes were isolated from the hippocampus and suspended in saturated Krebs-bicarbonate buffer (25 mM Na2HCO3, 124 mM NaCl, 5 mM KCl, 1.5 mM CaCl2, 5 mM MgSO4, 10 mM glucose, pH 7.3). Synaptosome preparations (250 to 300 μg) were incubated with 5.0 mCi 32P carrier-free orthophosphate (1 mg protein/1 ml) while continuously shaking at 37°C for 45 min. Immepip (500 nM) or vehicle was added and incubated for another 15 min at 37°C. The sample was centrifuged, and the pellet was resuspended in RIPA buffer containing protease and phosphatase inhibitors as above and then passed through a 25-gauge needle 10 times followed by shaking for 1 hour at 4°C. The sample was then centrifuged at 25,000 x g for 40 min at 4°C, and the clear supernatant was subjected to immunoprecipitation with SR-12 antibody. The specificity of the SR-12 antibody for SERT has been well characterized in our previous studies.34, 39 Protein A sepharose beads were used to isolate the SR-12 antibody-bound proteins, and the beads were washed with RIPA buffer followed by the addition of 50 μL Laemmli sample buffer and incubating for 30 min at 22°C. The eluted samples were subjected to 10% SDS-PAGE, and the 32P radiolabeled SERT proteins were detected with autoradiographic technique. The autoradiographic images from multiple exposures were then scanned and digitized. The 32P-labeled SERT band density was analyzed and quantitated using NIH ImageJ software (version 1.52a, NIH).
High-Speed Chronoamperometry Studies in vivo.
High-speed chronoamperometric recordings were performed using the FAST-12 system (Quainton, Lexington, KY), as described previously.64 Electrochemical recording units comprised a Nafion-coated, single carbon fiber electrode (30-μm diameter tip) attached to a multi-barreled micropipette (tip diameters between 10 and 15 μm). Nafion (5% solution; Aldrich Chemical Co., Milwaukee, WI) prevents interference from anionic substances in extracellular fluid. The electrode and micropipette tips were separated by 300 ± 20 μm. Intraperitoneal injection of chloralose (85 mg/kg) and urethane (850 mg/kg) was administered to anesthetized rats. To facilitate breathing, a tube was inserted into the trachea. Body temperature was kept at 37 ±1°C by a water-circulated heating pad (Seabrook, Cincinnati, OH). An electrochemical recording assembly was lowered into CA3 region of dorsal hippocampus (anterior-posterior, −4.1; midline, +3.5; dorsoventral, −3.6) (Paxinos and Watson, 2005) using a stereotaxic frame. The sensitivity of each electrode to the 5-HT metabolite, 5-HIAA (250 μM) was examined, and then electrodes were calibrated to increasing concentrations of 5-HT (0.5 to 3.0 μM). Only electrodes that displayed a selectivity ratio for 5-HT over 5-HIAA of >1000:1 and a linear response (r2 ≥ 0.997) to 5-HT were used. The detection limit for 5-HT was defined as the concentration that produced an oxidation current with a signal-to-noise ratio of 3. Micropipette barrels were filled with 5-HT (200 μM), H3R/H4R agonist immepip (150 μM) or H3R/H4R inverse agonist thioperamide (150 μM), or vehicle. All drugs were dissolved in 0.1 M phosphate-buffered saline containing 100 μM ascorbic acid as an antioxidant. 5-HT was pressure-ejected (5-25 p.s.i. for 0.25-3 s) at 3 to 10-min intervals until signals were reproducible. 5-HT (10 ± 1 picomoles) was delivered in a volume of 50 ± 3 nl. After reproducible 5-HT signals were obtained, vehicle, immepip or thioperamide was delivered into CA3 region of the hippocampus. These solutions were pressure-ejected over 10 to 20 s to minimize disturbances to the baseline electrochemical signal. Immepip or thioperamide was ejected in a volume of 136 nl to deliver 20 pmols. An equivalent volume of vehicle was delivered as a control. A 60 to 90 s delay was given before the next application of 5-HT to allow sufficient time for diffusion of drug to areas around the recording electrode. After delivery, the time taken to clear 5-HT by 80% (T80) was quantitated and plotted as a function of time.
Cell Culture, Transfections, and 5-HT Uptake.
Human embryonic kidney cells (HEK-293) were seeded on 24-well (75,000 cells per well) plates in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. Cells were grown in an atmosphere of 5% CO2 and 95% humidity atmosphere at 37°C. After 24 h, cells in each well were transfected with 0.25 μg of hSERT cDNA or pcDNA 3.0 using Lipofectamine™ according to the manufacturer’s instructions. Cells were preincubated with vehicle, immepip or R-mHA at 10 and 20 μM for 5 min. [3H]5-HT uptake was measured by incubating the cells with 50 nM of [3H]5-HT at 37°C for 10 min with or without SERT blocker 0.1 μM fluoxetine. Uptake was terminated by removing the radiolabeled uptake buffer followed by rapidly washing the cells with KRH buffer. Cells were lysed with Optiphase Supermix (400 μl), and the radioactivity was measured using a liquid scintillation counter. SERT specific [3H]5-HT transport was calculated by subtracting [3H]5-HT radioactivity counts measured in the presence of fluoxetine from that measured in the absence of fluoxetine.
Statistical Analyses
All values are expressed as mean ± SEM. As described in Figure legends, one-way or two-way analysis of variance was used followed by post hoc testing (Bonferroni) for multiple comparisons. For two-sample comparisons, Student’s t-test (unpaired) was performed. A value of P < 0.05 was considered statistically significant. GraphPad Prism 8 (GraphPad, San Diego, CA) was used for statistical analyses and to create charts and graphs.
Acknowledgment
This work was supported by the National Institutes of Mental Health Grants MH062612 (S.R) and MH112731 (S.R).
Abbreviations
- 5-HT
5-hydorxytryptamine (serotonin)
- Akt/PKB
(protein kinase B)
- ANOVA
analysis of variance
- CaMKII
Calcium/calmodulin dependent protein kinase II
- CNS
central nervous system
- GPCRs
G-protein coupled receptors
- GSK3α/ß
glycogen synthase kinase-3α/ß
- H3R
histamine 3 receptor
- H4R
histamine 4 receptor
- HA
histamine
- p38 MAPK
p38 mitogen-activated protein kinase
- PKA
protein kinase A
- PKC
protein kinase C
- PKC
protein kinase G
- PP2A
protein phosphatase 2A catalytic subunit
- R-mHA
(R)-(−)-α-methyl-histamine
- SERT
serotonin transporter
- SSRI
selective serotonin-reuptake inhibitor
- Thio
thioperamide
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Hensler JG, Ferry RC, Labow DM, Kovachich GB, and Frazer A (1994) Quantitative autoradiography of the serotonin transporter to assess the distribution of serotonergic projections from the dorsal raphe nucleus, Synapse 17, 1–15. [DOI] [PubMed] [Google Scholar]
- 2.Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, and Lesch KP (1998) Altered brain serotonin homeostasis and locomotor insensitivity to 3,4-methylenedioxymetamphetamine (“ecstasy”) in serotonin transporter-deficient mice, Mole Pharmacol 53, 649–655. [DOI] [PubMed] [Google Scholar]
- 3.Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, Ganapathy V, and Blakely RD (1993) Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization, Proc Natl Acad Sci U S A 90, 2542–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hahn MK, and Blakely RD (2007) The functional impact of SLC6 transporter genetic variation, Annu Rev Pharmacol Toxicol 47, 401–441. [DOI] [PubMed] [Google Scholar]
- 5.Murphy DL, and Moya PR (2011) Human serotonin transporter gene (SLC6A4) variants: their contributions to understanding pharmacogenomic and other functional GxG and GxE differences in health and disease, Curr Opin Pharmacol 11, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blakely RD, Ramamoorthy S, Qian Y, Schroeter S, and Bradley C Regulation of antidepressant-sensitive serotonin transporters In Neurotransmitter Transporters: Structure, Function, and Regulation, Reith MEA, Eds.; Second ed., Humana Press, NJ, 1997; 29–72. [Google Scholar]
- 7.Barker EL, and Blakely RD Norepinephrine and serotonin transporters: Molecular targets of antidepressant drugs In Psychopharmacology: The Fourth Generation of Progress, Bloom FE, Kupfer DJ, Eds.; Raven Press, New York, 1995; 321–333. [Google Scholar]
- 8.Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, and Andrews AM (2004) Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression, J Neurosci Methods 140, 169–181. [DOI] [PubMed] [Google Scholar]
- 9.Ramamoorthy S, Shippenberg TS, and Jayanthi LD (2011) Regulation of monoamine transporters: Role of transporter phosphorylation, Pharmacol Ther 129, 220–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bermingham DP, and Blakely RD (2016) Kinase-dependent Regulation of Monoamine Neurotransmitter Transporters, Pharmacol Rev 68, 888–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas HL, Sergeeva OA, and Selbach O (2008) Histamine in the nervous system, Physiol Rev 88, 1183–1241. [DOI] [PubMed] [Google Scholar]
- 12.Kohler CA, da Silva WC, Benetti F, and Bonini JS (2011) Histaminergic mechanisms for modulation of memory systems, Neural Plast 2011, 328602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nuutinen S, and Panula P (2010) Histamine in neurotransmission and brain diseases, Adv Exp Med Biol 709, 95–107. [DOI] [PubMed] [Google Scholar]
- 14.Rapanelli M, and Pittenger C (2016) Histamine and histamine receptors in Tourette syndrome and other neuropsychiatric conditions, Neuropharmacology 106, 85–90. [DOI] [PubMed] [Google Scholar]
- 15.Schneider EH, Neumann D, and Seifert R (2014) Modulation of behavior by the histaminergic system: lessons from HDC-, H3R- and H4R-deficient mice, Neurosci Biobehav Rev 47, 101–121. [DOI] [PubMed] [Google Scholar]
- 16.Panula P, Chazot PL, Cowart M, Gutzmer R, Leurs R, Liu WL, Stark H, Thurmond RL, and Haas HL (2015) International Union of Basic and Clinical Pharmacology. XCVIII. Histamine Receptors, Pharmacol Rev 67, 601–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas H, and Panula P (2003) The role of histamine and the tuberomamillary nucleus in the nervous system, Nat Rev Neurosci 4, 121–130. [DOI] [PubMed] [Google Scholar]
- 18.Pillot C, Heron A, Cochois V, Tardivel-Lacombe J, Ligneau X, Schwartz JC, and Arrang JM (2002) A detailed mapping of the histamine H(3) receptor and its gene transcripts in rat brain, Neuroscience 114, 173–193. [DOI] [PubMed] [Google Scholar]
- 19.Ferrada C, Ferre S, Casado V, Cortes A, Justinova Z, Barnes C, Canela EI, Goldberg SR, Leurs R, Lluis C, and Franco R (2008) Interactions between histamine H3 and dopamine D2 receptors and the implications for striatal function, Neuropharmacology 55, 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spaethling JM, Piel D, Dueck H, Buckley PT, Morris JF, Fisher SA, Lee J, Sul JY, Kim J, Bartfai T, Beck SG, and Eberwine JH (2014) Serotonergic neuron regulation informed by in vivo single-cell transcriptomics, FASEB J 28, 771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Threlfell S, Cragg SJ, Kallo I, Turi GF, Coen CW, and Greenfield SA (2004) Histamine H3 receptors inhibit serotonin release in substantia nigra pars reticulata, J Neurosci 24, 8704–8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fink K, Schlicker E, Neise A, and Gothert M (1990) Involvement of presynaptic H3 receptors in the inhibitory effect of histamine on serotonin release in the rat brain cortex, Naunyn Schmiedebergs Arch Pharmacol 342, 513–519. [DOI] [PubMed] [Google Scholar]
- 23.Schlicker E, Betz R, and Gothert M (1988) Histamine H3 receptor-mediated inhibition of serotonin release in the rat brain cortex, Naunyn Schmiedebergs Arch Pharmacol 337, 588–590. [DOI] [PubMed] [Google Scholar]
- 24.Lovenberg TW, Roland BL, Wilson SJ, Jiang X, Pyati J, Huvar A, Jackson MR, and Erlander MG (1999) Cloning and functional expression of the human histamine H3 receptor, Mol Pharmacol 55, 1101–1107. [PubMed] [Google Scholar]
- 25.Takeshita Y, Watanabe T, Sakata T, Munakata M, Ishibashi H, and Akaike N (1998) Histamine modulates high-voltage-activated calcium channels in neurons dissociated from the rat tuberomammillary nucleus, Neuroscience 87, 797–805. [DOI] [PubMed] [Google Scholar]
- 26.Drutel G, Peitsaro N, Karlstedt K, Wieland K, Smit MJ, Timmerman H, Panula P, and Leurs R (2001) Identification of rat H3 receptor isoforms with different brain expression and signaling properties, Mol Pharmacol 59, 1–8. [PubMed] [Google Scholar]
- 27.Giovannini MG, Efoudebe M, Passani MB, Baldi E, Bucherelli C, Giachi F, Corradetti R, and Blandina P (2003) Improvement in fear memory by histamine-elicited ERK2 activation in hippocampal CA3 cells, J Neurosci 23, 9016–9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torrent A, Moreno-Delgado D, Gomez-Ramirez J, Rodriguez-Agudo D, Rodriguez-Caso C, Sanchez-Jimenez F, Blanco I, and Ortiz J (2005) H3 autoreceptors modulate histamine synthesis through calcium/calmodulin- and cAMP-dependent protein kinase pathways, Mol Pharmacol 67, 195–203. [DOI] [PubMed] [Google Scholar]
- 29.Bongers G, Sallmen T, Passani MB, Mariottini C, Wendelin D, Lozada A, Marle A, Navis M, Blandina P, Bakker RA, Panula P, and Leurs R (2007) The Akt/GSK-3beta axis as a new signaling pathway of the histamine H(3) receptor, J Neurochem 103, 248–258. [DOI] [PubMed] [Google Scholar]
- 30.Rapanelli M, Frick LR, Horn KD, Schwarcz RC, Pogorelov V, Nairn AC, and Pittenger C (2016) The Histamine H3 Receptor Differentially Modulates Mitogen-activated Protein Kinase (MAPK) and Akt Signaling in Striatonigral and Striatopallidal Neurons, J Biol Chem 291, 21042–21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo T, Wang Y, Qin J, Liu ZG, and Liu M (2017) Histamine H3 Receptor Antagonist Prevents Memory Deficits and Synaptic Plasticity Disruption Following Isoflurane Exposure, CNS Neurosci Ther 23, 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yokoyama F, Yamauchi M, Oyama M, Okuma K, Onozawa K, Nagayama T, Shinei R, Ishikawa M, Sato Y, and Kakui N (2009) Anxiolytic-like profiles of histamine H3 receptor agonists in animal models of anxiety: a comparative study with antidepressants and benzodiazepine anxiolytic, Psychopharmacology (Berl) 205, 177–187. [DOI] [PubMed] [Google Scholar]
- 33.Hu W, and Chen Z (2017) The roles of histamine and its receptor ligands in central nervous system disorders: An update, Pharmacol Ther 175, 116–132. [DOI] [PubMed] [Google Scholar]
- 34.Samuvel DJ, Jayanthi LD, Bhat NR, and Ramamoorthy S (2005) A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression, J Neurosci 25, 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ansah TA, Ramamoorthy S, Montanez S, Daws LC, and Blakely RD (2003) Calcium-dependent inhibition of synaptosomal serotonin transport by the alpha 2-adrenoceptor agonist 5-bromo-N-[4,5-dihydro-1H-imidazol-2-yl]-6-quinoxalinamine (UK14304), J Pharmacol Exp Ther 305, 956–965. [DOI] [PubMed] [Google Scholar]
- 36.Sundaramurthy S, Annamalai B, Samuvel DJ, Shippenberg TS, Jayanthi LD, and Ramamoorthy S (2017) Modulation of serotonin transporter function by kappa-opioid receptor ligands, Neuropharmacology 113, 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajamanickam J, Annamalai B, Rahbek-Clemmensen T, Sundaramurthy S, Gether U, Jayanthi LD, and Ramamoorthy S (2015) Akt-mediated regulation of antidepressant-sensitive serotonin transporter function, cell-surface expression and phosphorylation, Biochem J 468, 177–190. [DOI] [PubMed] [Google Scholar]
- 38.Annamalai B, Mannangatti P, Arapulisamy O, Shippenberg TS, Jayanthi LD, and Ramamoorthy S (2012) Tyrosine phosphorylation of the human serotonin transporter: a role in the transporter stability and function, Mol Pharmacol 81, 73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jayanthi LD, Samuvel DJ, Blakely RD, and Ramamoorthy S (2005) Evidence for biphasic effects of protein kinase C on serotonin transporter function, endocytosis, and phosphorylation, Mol Pharmacol 67, 2077–2087. [DOI] [PubMed] [Google Scholar]
- 40.Ramamoorthy S, Giovanetti E, Qian Y, and Blakely RD (1998) Phosphorylation and regulation of antidepressant-sensitive serotonin transporters, J Biol Chem 273, 2458–2466. [DOI] [PubMed] [Google Scholar]
- 41.Bauman AL, Apparsundaram S, Ramamoorthy S, Wadzinski BE, Vaughan RA, and Blakely RD (2000) Cocaine and antidepressant-sensitive biogenic amine transporters exist in regulated complexes with protein phosphatase 2A, J Neurosci 20, 7571–7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramamoorthy S, Samuvel DJ, Buck ER, Rudnick G, and Jayanthi LD (2007) Phosphorylation of threonine residue 276 is required for acute regulation of serotonin transporter by cyclic GMP, J Biol Chem 282, 11639–11647. [DOI] [PubMed] [Google Scholar]
- 43.Ramamoorthy S, and Blakely RD (1999) Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants, Science 285, 763–766. [DOI] [PubMed] [Google Scholar]
- 44.Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, Cohen J, Mannangatti P, Jessen T, Thompson BJ, Ye R, Kerr TM, Carneiro AM, Crawley JN, Sanders-Bush E, McMahon DG, Ramamoorthy S, Daws LC, Sutcliffe JS, and Blakely RD (2012) Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior, Proc Natl Acad Sci U S A 109, 5469–5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yura A, Kiuchi Y, Uchikawa T, Uchida J, Yamazaki Y, and Oguchi K (1996) Possible involvement of calmodulin-dependent kinases in Ca2+-dependent enhancement of [3H]5-hydroxytryptamine uptake in rat cortex, Brain Research 738, 96–102. [DOI] [PubMed] [Google Scholar]
- 46.Jayanthi LD, Ramamoorthy S, Mahesh VB, Leibach FH, and Ganapathy V (1994) Calmodulin-dependent regulation of the catalytic function of the human serotonin transporter in placental choriocarcinoma cells, J Biol Chem 269, 14424–14429. [PubMed] [Google Scholar]
- 47.Seimandi M, Seyer P, Park CS, Vandermoere F, Chanrion B, Bockaert J, Mansuy IM, and Marin P (2013) Calcineurin interacts with the serotonin transporter C-terminus to modulate its plasma membrane expression and serotonin uptake, J Neurosci 33, 16189–16199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sola C, Barron S, Tusell JM, and Serratosa J (2001) The Ca2+/calmodulin system in neuronal hyperexcitability, Int J Biochem Cell Biol 33, 439–455. [DOI] [PubMed] [Google Scholar]
- 49.Ciccone MA, Timmons M, Phillips A, and Quick MW (2008) Calcium/calmodulin-dependent kinase II regulates the interaction between the serotonin transporter and syntaxin 1A, Neuropharmacology 55, 763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rozniecki JJ, Letourneau R, Sugiultzoglu M, Spanos C, Gorbach J, and Theoharides TC (1999) Differential effect of histamine 3 receptor-active agents on brain, but not peritoneal, mast cell activation, J Pharmacol Exp Ther 290, 1427–1435. [PubMed] [Google Scholar]
- 51.Hagan CE, McDevitt RA, Liu Y, Furay AR, and Neumaier JF (2012) 5-HT(1B) autoreceptor regulation of serotonin transporter activity in synaptosomes, Synapse 66, 1024–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Montanez S, Munn JL, Owens WA, Horton RE, and Daws LC (2014) 5-HT1B receptor modulation of the serotonin transporter in vivo: studies using KO mice, Neurochem Int 73, 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morisset S, Rouleau A, Ligneau X, Gbahou F, Tardivel-Lacombe J, Stark H, Schunack W, Ganellin CR, Schwartz JC, and Arrang JM (2000) High constitutive activity of native H3 receptors regulates histamine neurons in brain, Nature 408, 860–864. [DOI] [PubMed] [Google Scholar]
- 54.Bernaerts P, Lamberty Y, and Tirelli E (2004) Histamine H3 antagonist thioperamide dose-dependently enhances memory consolidation and reverses amnesia induced by dizocilpine or scopolamine in a one-trial inhibitory avoidance task in mice, Behav Brain Res 154, 211–219. [DOI] [PubMed] [Google Scholar]
- 55.Jones BE (2005) From waking to sleeping: neuronal and chemical substrates, Trends Pharmacol Sci 26, 578–586. [DOI] [PubMed] [Google Scholar]
- 56.Parmentier R, Anaclet C, Guhennec C, Brousseau E, Bricout D, Giboulot T, Bozyczko-Coyne D, Spiegel K, Ohtsu H, Williams M, and Lin JS (2007) The brain H3-receptor as a novel therapeutic target for vigilance and sleep-wake disorders, Biochem Pharmacol 73, 1157–1171. [DOI] [PubMed] [Google Scholar]
- 57.Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, and Holmes A (2007) Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice, J Neurosci 27, 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hartley CA, McKenna MC, Salman R, Holmes A, Casey BJ, Phelps EA, and Glatt CE (2012) Serotonin transporter polyadenylation polymorphism modulates the retention of fear extinction memory, Proc Natl Acad Sci U S A 109, 5493–5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rapanelli M, Frick L, Pogorelov V, Ohtsu H, Bito H, and Pittenger C (2017) Histamine H3R receptor activation in the dorsal striatum triggers stereotypies in a mouse model of tic disorders, Transl Psychiatry 7, e1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pittenger C (2017) Histidine Decarboxylase Knockout Mice as a Model of the Pathophysiology of Tourette Syndrome and Related Conditions, Handb Exp Pharmacol 241, 189–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moya PR, Wendland JR, Rubenstein LM, Timpano KR, Heiman GA, Tischfield JA, King RA, Andrews AM, Ramamoorthy S, McMahon FJ, and Murphy DL (2013) Common and rare alleles of the serotonin transporter gene, SLC6A4, associated with Tourette’s disorder, Mov Disord 28, 1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swedo SE, and Snider LA The neurobiology and treatment of obssessive-compulsive disorder In Neurobiology of Mental Illness, Charney DS, and Nestler EJ, Eds.; Second ed., Oxford University Press, Inc, New York, 2004; 628–638 [Google Scholar]
- 63.Sakai K, and Crochet S (2000) Serotonergic dorsal raphe neurons cease firing by disfacilitation during paradoxical sleep, Neuroreport 11, 3237–3241. [DOI] [PubMed] [Google Scholar]
- 64.Daws LC, Owens WA, and Toney GM Chapter 4, High-speed chronoamperometry to measure biogenic amine release and uptake in vivo In Neurotransmitter Transporters - Investigative Methods, Neuromethods; Bonisch HH, Sitte H. Eds.; Humana Press, New York, 2016; 118, 53–81. [Google Scholar]