Abstract
Conjunctival goblet cells (CGCs) are specialized cells that produce and secrete soluble mucins to the tear film that bathes the ocular surface. CGC numbers and functions are affected in various ocular surface diseases including dry eye disease with diverse etiologies. In this review we will (i) summarize the important functions of CGCs in ocular surface health, (ii) describe the ocular surface diseases that affect CGC numbers and function, (iii) provide an update on recent research outcomes that elucidate CGC differentiation, gene expression and functions, and (iv) present evidence in support of the prediction that restoring CGC numbers and/or functions is a viable strategy for alleviating ocular surface disorders that impact the CGCs.
Keywords: Conjunctiva, Goblet cells, Ocular surface, Cornea, Tear film
1. Introduction.
The ocular surface consists of a continuous epithelial tissue with regional specializations that covers the transparent cornea, bulbar and palpebral (also called tarsal) conjunctiva, meibomian glands, lacrimal and accessory lacrimal glands. The ocular surface serves as a protective barrier for the rest of the eye, with the cornea providing the transparent and refractive functions that enable proper focusing of light on retinal photoreceptors. The entire anterior surface of the ocular surface is covered by the tear film that consists of components secreted from specialized regions of the ocular surface. The ocular surface functional unit is integrated by neural, endocrine, vascular, and immune systems, which work together to promote a healthy cornea, that is essential for sight (Fig. 1a) 1, 2. Ocular surface disorders account for the bulk of the primary eye care services in the U.S.A., where dry eye alone affects about 6 million women and 3 million men with moderate to severe symptoms and an additional 20 to 30 million people with mild symptoms 3–5. It is necessary to fill the persistent knowledge gap in our understanding of the ocular surface development, maturation and maintenance to lower the burden of ocular surface disorders on our healthcare system.
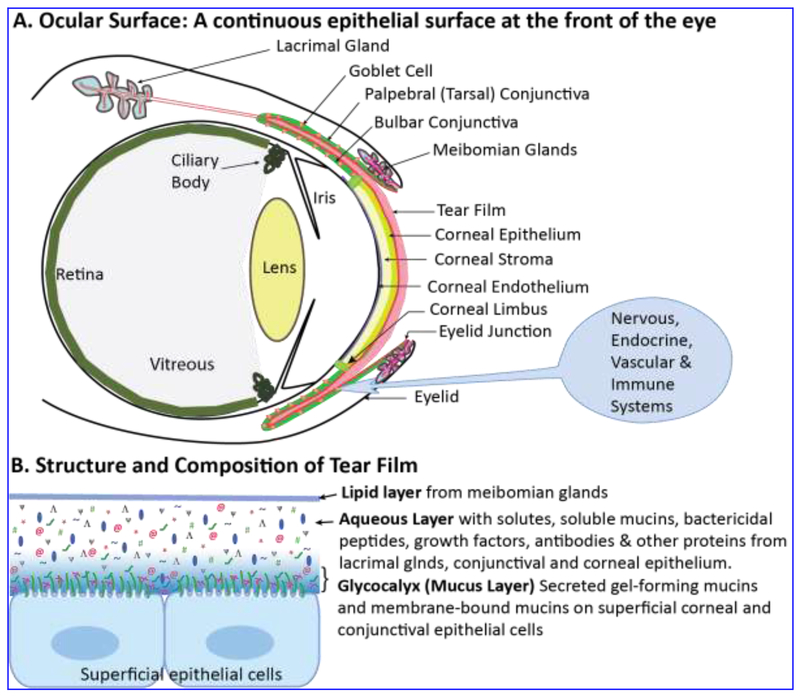
Figure 1. Tear film lubricates, nourishes and protects the ocular surface.
A. The ocular surface (red) is a continuous epithelial surface that covers the cornea, conjunctiva and the ductal surfaces of the meibomian and lacrimal glands. Despite being comprised of different cell types, the ocular surface is a single functional unit whose functions are integrated by nervous, vascular, endocrine and immune systems. B. Structure and composition of tear film that covers the ocular surface. The superficial lipid layer derived from the meibomian glands, central aqueous layer derived mostly from the lacrimal glands with soluble mucins from CGCs, and the basal glycocalyx (mucus layer) of secreted gel-forming mucins (red squiggly lines) and membrane-bound mucins (green squiggly lines connected to the superficial epithelial cells) are shown. We believe that the glycocalyx layer gradually transitions into the aqueous layer with no distinct boundary in between. Thus, the gel-forming mucins may also be present in lower concentrations (in non-gel form) in the aqueous layer.
Conjunctival goblet cells (CGCs) are specialized cells that produce and secrete soluble mucins for the tear film, facilitating its lubricant and protective functions. CGC numbers and functions are affected in various ocular surface diseases including dry eye disease with diverse etiologies. In this review, we summarize our current understanding of the (i) structure and functions of CGCs, (ii) ocular surface diseases that affect GC numbers and function, and (iii) signaling and gene regulatory networks that regulate GC differentiation, gene expression and functions, and present evidence in support of our prediction that reviving CGC numbers and/or functions is a viable strategy for alleviating ocular surface disorders that impact the GCs.
2. Structure and function of the tear film.
The tear film that lubricates and keeps the ocular surface moist, nourishes and protects it from microbial infections is a complex multi-layered fluid. It is comprised of a superficial lipid layer derived from the meibomian glands, central aqueous layer derived mostly from the lacrimal gland (with residual amounts derived from the conjunctival epithelium) and a basal glycocalyx comprised of mucins (Fig. 1b). The tear fluid has a dynamic structure that responds to environmental conditions and is renewed upon every blink 6. The superficial lipid layer prevents excessive evaporative loss and ensures a uniform surface that minimizes scatter and enables accurate refraction of light 7. The aqueous component derived largely from the lacrimal glands is supplemented by CGC-derived soluble mucins 2, 8, trefoil factors 9 and defensins, and solutes, growth factors, antibodies and other proteins from the serum 10. The basal glycocalyx comprised of the transmembrane- and secreted-mucins converts the hydrophobic lipid membranes of the ocular surface epithelial cells to a hydrophilic surface that is capable of retaining the tear film efficiently 2. We predict that the glycocalyx to gradually transition into the aqueous layer with no distinct boundary in between. Thus, secreted mucins may also be found in the aqueous compartment in monomeric, non-gel form (Fig. 1b).
Tear fluid composition reflects the health of different components of the ocular surface from which it is derived: the meibomian and lacrimal glands, conjunctival and corneal epithelial cells, and the CGCs. Large scale proteomics studies have identified hundreds of proteins in varying amounts within the tear fluid collected from healthy or diseased ocular surface, setting the stage for developing novel diagnostic markers and therapeutic targets for ocular surface pathologies 11–16. Though powerful, these large-scale proteomics studies tend to miss small peptides such as SLURP1 that serves important immunomodulatory function in the ocular surface 17–22. Additionally, tear biomarkers have been sought for systemic disorders such as Parkinson’s disease 23, Alzheimer’s disease 24 and multiple sclerosis 25. Thus, the tear fluid is a useful and accessible source for evaluating ocular surface health and several systemic diseases, and their response to treatment. Among tear fluid components, CGC-secreted mucins take a prominent spot as they serve important functions and are decreased in several disorders of the ocular surface including dry eye disease 8, 26, 27.
Mucins, members of a family of high molecular weight, heavily O-glycosylated proteins derived from CGC, have long been recognized as integral components of the tear fluid that help protect the ocular surface against dryness, pathogens and trauma 1, 2, 8, 27–31. There are two classes of mucins at the ocular surface: secreted (e.g., Muc2 and Muc5Ac) and membrane-associated (e.g., Muc1, Muc4 and Muc16). The secreted gel-forming mucins derived from the CGCs can homo-multimerize through D domains to form viscous mucin gel 1, 29, 32. The corneal and conjunctival epithelial cell membrane-associated mucins that form the base of the glycocalyx layer of the tear film have a single membrane-spanning domain and a short cytoplasmic tail. Their extracellular domains are constitutively shed into the tear film. When present in optimal amounts, CGC-produced soluble and secreted mucins together improve tear fluid stability, ocular surface hydration and lubrication, and clearance of pathogenic bacteria as well as particulate contaminants, providing a smooth and refractive corneal surface that minimizes scatter and facilitates clear sight29.
3. Conjunctival biology and the role of GCs in ocular surface health.
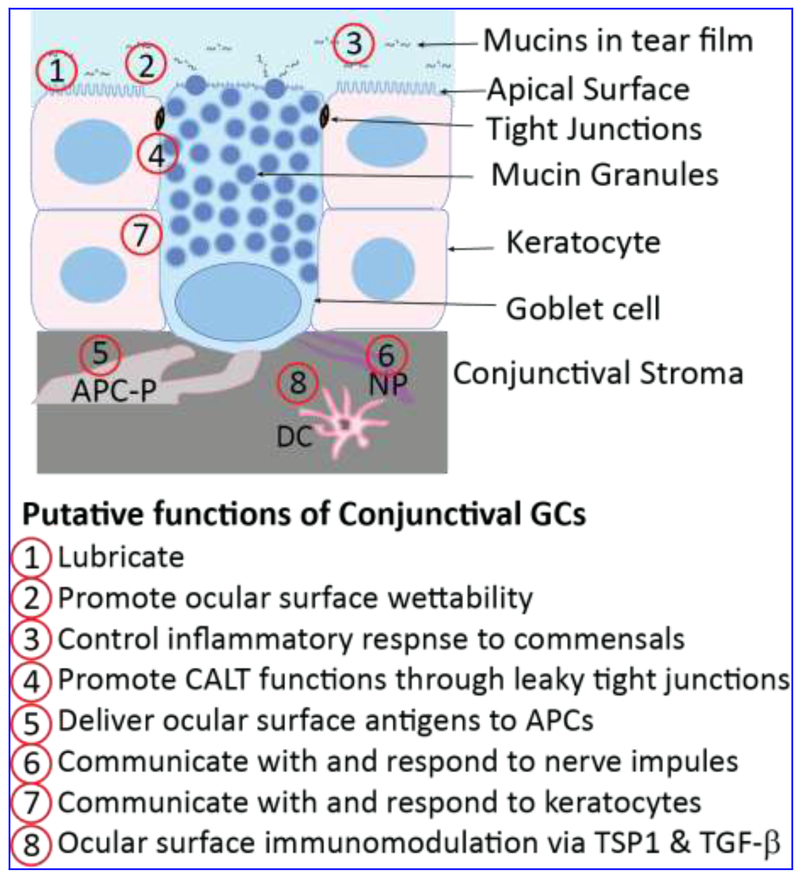
The conjunctiva is a non-keratinizing stratified epithelium with interspersed GCs that covers the inner eyelids and the outer eyeball extending to the limbus that borders the cornea (Fig. 2). Unlike the 5-8 cell-layered corneal epithelium built on a clear basement membrane, the conjunctival epithelium is 2-3 cell-layered and lacks a well-organized basement layer. CGC distribution is species-specific. Mouse GCs occur in clusters enriched in the conjunctival fornix 33, while they are clustered in the nasal region of the human conjunctiva, with few individual cells in the bulbar28, and the lid wiper region 34. Human CGCs secrete MUC5AC, while mouse CGCs secrete both Muc5ac and Muc5b 1, 2. In some animals including mice and rats, CGCs span the length of the 2-3 cell layered stratified epithelium, that allows them to easily convey information between the external environment and the stroma (Fig. 2). In humans though, CGCs are mostly restricted to the apical half of the conjunctiva. The GC nucleus is pushed towards the basal side, with the mucin granules filling much of the superficial side. On the stromal side, CGCs interact with antigen presenting cells (APCs), suggesting that GC can deliver antigens to APCs 35. Tight junctions between GCs and the neighboring epithelial cells involve pore-forming claudins-2 and -10 that regulate paracellular transport in “leaky” epithelia 36, 37. Though P2Y(2) agonists stimulate both conjunctival stratified squamous cells and CGCs, sympathetic nerves are known to stimulate stratified squamous cells, with their effect on CGCs unknown 1. In contrast, parasympathetic nerves stimulate secretion from GCs, without influencing neighboring stratified squamous cells (Fig. 2). Though growth factors, acetylcholine, histamine and prostaglandins stimulate GC-derived mucin secretion 38–42, their effects on conjunctival stratified squamous epithelial cells are not well studied. Thus, the presence of different cell types with highly specialized functions within the conjunctiva presents both valuable opportunities and difficult challenges to those interested in elucidating ocular surface biology.
Figure 2. Structure and functions of CGCs.
GCs are present in clusters or interspersed among stratified keratocytes and form leaky tight junctions with the neighboring cells. In the mouse conjunctiva, GCs unlike keratocytes, extend from the surface to the underlying extracellular matrix, where they interact with antigen presenting cell processes. Human GCs also are elongated and span the apical half of the conjunctiva. These cells are packed with multiple mucin granules which push the nucleus towards the base. Putative functions of CGCs are listed below, with the sites of these functions indicated in the schematic. GC-derived TGF-β2 drives dendritic cells toward an immature tolerogenic state. APC-P, Antigen presenting cell processes; NP, nerve processes; CALT, conjunctiva-associated lymphoid tissue; DC, dendritic cell; TSP1, thrombospondin-1; TGF-β, transforming growth factor-β.
Definitive identity and location of CGC precursors remains to be established. Pioneering studies from a couple of groups revealed that (i) the conjunctival cell lineage is distinct from that of the corneal epithelium 43, 44, (ii) CGCs are slow-cycling cells with proliferative capabilities 43, 45, (iii) CGCs and keratinocytes share common bipotent progenitors in the forniceal region 46, and (iv) a stem cell population is widely distributed but enriched in the medial canthal and inferior forniceal region in humans 47. The suggestion that the corneal and conjunctival epithelia are equipotent with similar oligopotent stem cells in the corneal, limbal and conjunctival epithelia 48 is debated as inconsistent with the known properties of the ocular surface epithelial cells 49. Compound niches with variable numbers of proliferating cells, slow-cycling corneal progenitor cells, and post-mitotic GCs were found to migrate from the limbal area into the cornea with limbal stem cell deficiency, facilitating formation of more GCs 50. Collectively, these studies highlight the need for further identification of bona fide markers of conjunctival stem cells, and the developmental cues that guide them towards a GC fate.
4. Ocular surface functions of GCs.
Historically, ocular surface functions of GC were linked to the properties of mucins that they secrete: lubrication, surface wetting, and preventing microbial infection by maintaining mucosal barrier integrity (Fig. 2) 8, 51, 52. Such a connection was supported by the reduction of CGCs observed in severe cicatrizing ocular surface diseases that often ended in corneal keratinization and opacity. Lack of overt ocular surface phenotype and the absence of excessive microbial infection in Spdef−/−, Muc5ac−/− or Muc5b−/− conjunctiva suggested that these GC functions are dispensable for normal ocular surface health in the absence of challenges 53–57. This notion however was dispelled by the gene expression studies that argue in favor of a protective function for CGCs 53. The expression of proinflammatory Il1-α, Il-1β, Tnf-α, and epithelial cell keratinization-associated Sprr2h and Tgm1 which are usually up-regulated in dry eye disease, was also up-regulated in Spdef-null conjunctiva that lacked GCs 53. Taken together, these results suggest a protective role for CGCs in the ocular surface.
CGCs represent a major cellular component of the innate immune system, producing and secreting gel-forming soluble mucins, trefoil factors, defensins and other anti-microbial agents to the tear film to provide a barrier that prevents encroachment by the external microbiota and limits exposure to commensals, which in turn averts chronic inflammatory responses 1, 2, 9, 58. Though airway GCs are known to secrete cytokines and chemokines, induce Th2 responses and promote tissue restoration 59, it was not clear if the CGCs serve a similar function in the ocular surface. However, the absence of CGCs or decreased mucin secretion results in inflammatory responses, consistent with a role for GCs in maintaining immune quiescence and tolerance 60, 61. A recent study identified CGCs as a cellular source of active TGF-β2 in ocular mucosa, implicating these cells in ocular surface immunomodulation 35. Moreover, CGCs facilitate tolerogenic host immune responses towards commensal microflora and clearance of pathogens by promoting adaptive immune responses against them. Collectively, these reports suggest a role for CGCs in ocular surface immune homeostasis (Fig. 2).
Additional putative functions of GCs include communicating with and responding to the connected nerves and antigen presenting cells on the matrix side, and facilitating conjunctiva-associated lymphoid tissue (CALT) functions via leaky tight junctions with neighboring keratocytes (Fig. 2) 31. In the small intestine and the colon, GC-associated antigen passages (GAPs) have been found to facilitate luminal antigen presentation to underlying dendritic cells, aiding in development of immune tolerance 62, 63 (Fig. 2). The presence of CALT-well-organized lymphoid tissue comprised of intraepithelial lymphocytes, subepithelial lymphoid follicles, lymphatics and blood vessels-suggested the presence of similar GAPs within the conjunctiva 58, 64. Consistent with this prediction, GAPs were recently found on the mouse conjunctiva, providing evidence that CGCs also contribute to ocular surface immune tolerance by modulating antigen distribution and antigen specific immune response 65, 66.
5. Gene expression during CGC differentiation.
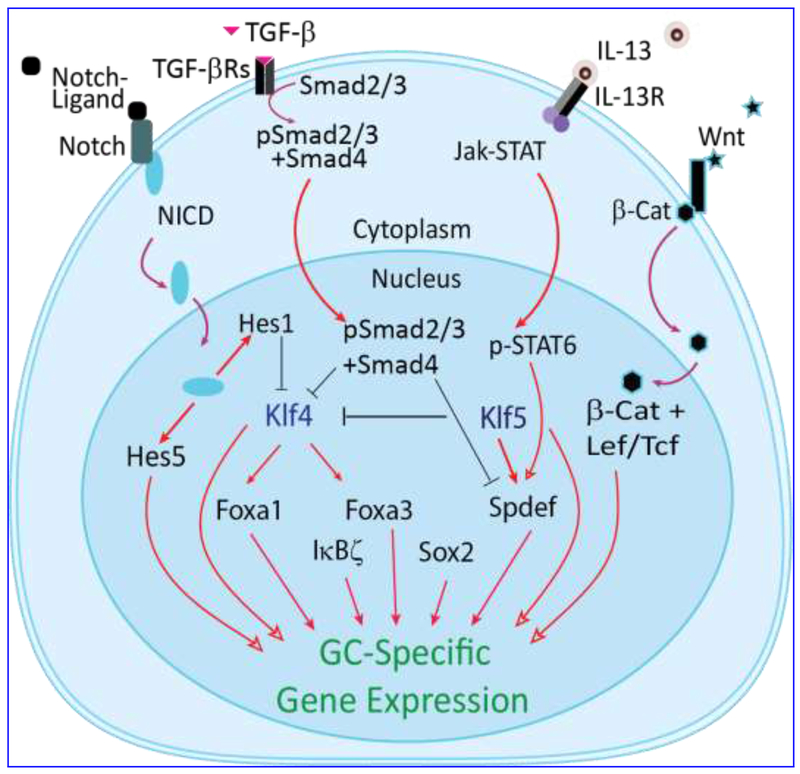
Although CGCs have long been recognized as a major source of the soluble mucins such as Muc2 and Muc5ac in the tear, molecular mechanisms that regulate CGC differentiation and function remain poorly studied. A unified picture emerging from the studies involving differentiation of CGCs, as well as gut and lung GC establishes the involvement of Notch and Wnt pathways (Fig. 3). Conditional inhibition of canonical Notch signaling by over-expression of dominant negative mastermind-like 1 (Maml1) affected CGC differentiation, induced epithelial hyperplasia and aberrant desquamation 67. Ablation of Notch-regulated zinc finger transcription factors Klf4 or Klf5 resulted in loss of GCs in the conjunctiva 68–73 and the gut 74, 75. Additional defects were noted in the Klf4- and Klf5-deficient ocular surface, suggesting that the influence of KLF4 and KLF5 is not limited to GCs, and that they regulate the whole ocular surface epithelial differentiation 68, 70, 71, 76–81.
Figure 3. Gene regulatory network in GC differentiation.
Schematic shows key signal transduction pathways and genes known to influence CGC differentiation. The influence of IL-13, Notch, and Wnt signaling pathways on Spdef functions are inferred from studies on the lung and intestine GC fate determination. In pro-inflammatory conditions, TGF-β signaling suppresses Klf4 and Spdef affecting GC functions. NICD, Notch intracellular domain; Jak, Janus kinase; STAT, signal transducer and activator of transcription; pSTAT6, Phospho-STAT6; SPDEF, SAM-pointed domain ETS factor; IL-13, Interleukin-13; IL-13R, IL-13 receptor; β-Cat, β-catenin; KLF4, Krüppel-like factor-4; Lef/Tcf, Lymphoid enhancer factor/T-cell factor.
We catalogued the changes in gene expression during GC development by comparing the conjunctival forniceal transcriptome at post-natal day- 9 (PN9), PN14 and PN20, when CGCs are absent, developing and present, respectively 82. Prominent among the transcription factors whose expression is significantly affected in the mouse conjunctiva during early postnatal development are the members of the forkhead box (Fox), SRY-related HMG box (Sox), Ets and Krüppel-like family members (Table 1). We also compared gene expression patterns in corresponding regions of the Klf4-deficient conjunctiva, to identify the Klf4-target genes that play essential roles in CGC differentiation and function 82 (Fig. 3). Concurrent with GC differentiation, pathways related to glycoprotein biosynthesis, mesenchymal-epithelial transition, mucosal immunity, endocytic and neural regulation were increased 82.
Table 1.
Transcription factors with altered expression during CGC development (From Gupta et al., 2011 82)
| Transcription factor family | Upregulated during CGC differentiation | Downregulated during CGC differentiation |
|---|---|---|
| Krüppel-like factors | Klf4, Klf6, Klf9 | Klf13, Klf7, Klf10, Gli2 |
| Epithelial-specific Ets (ESE)factors | Spdef, Ehf, Elf3, Elf5 | Ets1 |
| Forkhead box family proteins | Foxa3, Foxa1, Foxk1, Foxo3, Foxp1 | Foxp2 |
| High mobility group proteins | Hmgb1, Hmgb2 | Hmg20b |
| Sox family members | Sox21 | Sox4, Sox11, Sox12, |
| Interferon regulatory factors | Irf1 | Irf3 |
Sterile alpha motif-pointed domain ETS factor (SPDEF), a Wnt responsive transcription factor54, is a key regulator of GC differentiation 53, 83. A subtractive microarray analysis comparing wild type and Spdef−/− conjunctival epithelial gene expression identified Wnt pathway genes Frzb, Dixdc1, Wnt5b and Wnt11 as downregulated in the Spdef−/− conjunctiva lacking GCs 53. Consistently, GC numbers in Frzb−/− mice are significantly reduced 53. Transient transfection studies established that KLF4 and KLF5 trans-activated the Spdef promoter activity, placing them upstream in the heirarchical network of transcription factors regulating GC differentiation (Fig. 3). Conditional deletion of TGF-β in Krt14-positive conjunctival cells upregulated Spdef expression and induced epithelial and GC hyperplasia suggesting that TGF-β suppresses Spdef promoter activity 83. Consistently, Smad3 was demonstrated to bind Spdef promotor and prevent Spdef transcription 83 (Fig. 3). The forkhead box protein A3 (Foxa3) also influences GC differentiation. Spdef and Foxa3 reciprocally regulate each other In the airway epithelia 56,57, and the conjunctiva 83. Foxa3 induces GC metaplasia in airway epithelia 84, and is downregulated in Spdef null lung 56 and conjunctiva 53 suggesting that Foxa3 collaborates with Spdef in regulation of GC differentiation (Fig. 3). While these studies have begun to identify GC-enriched transcription factors with a role in their differentiation, additional studies involving trans-differentiation approach are essential to define the minimal set of transcription factors required to establish the CGC lineage, as in other systems 85–93.
6. Effect of ocular surface disorders on GCs.
Inflammatory diseases of the ocular surface such as keratoconjunctivitis sicca (KCS; dry eye), blepharitis, Stevens Johnson Syndrome (SJS), Sjogren’s Syndrome, Ocular Cicatricial Pemphigoid (OCP), and Graft Versus Host Disease (GVHD) result in decreased GC numbers with concurrent decrease in Muc5ac and other GC products in the tear film (Fig. 4) 26, 32, 94. In general, the number of GCs is negatively correlated with the extent of inflammation in these diseases. Though mouse models of dry eye have been useful for studying the influence of inflammation on GC numbers 95, underlying molecular mechanisms that drive the reduction in GC numbers in these inflammatory diseases remain elusive. In a rabbit model of dry eye induced by topical administration of commonly used preservative benzalkonium chloride (BAC) for 5 weeks, decreased GC density and other KCS symptoms persisted for 2 weeks after BAC removal 96. Inflammatory cytokines such as TNF-α, IFN-γ mediate the decrease in GC numbers and their secretory function in Sjögren’s syndrome-associated ocular surface inflammation 38. Elevated expression of TNF-α and IFN-γ in the conjunctiva of Tsp1 deficient mouse model of Sjögren’s syndrome affects GC functions 38, 4. These reports demonstrate that CGC survival and function is negatively influenced by the ocular surface inflammatory disorders with diverse etiologies (Fig. 4).
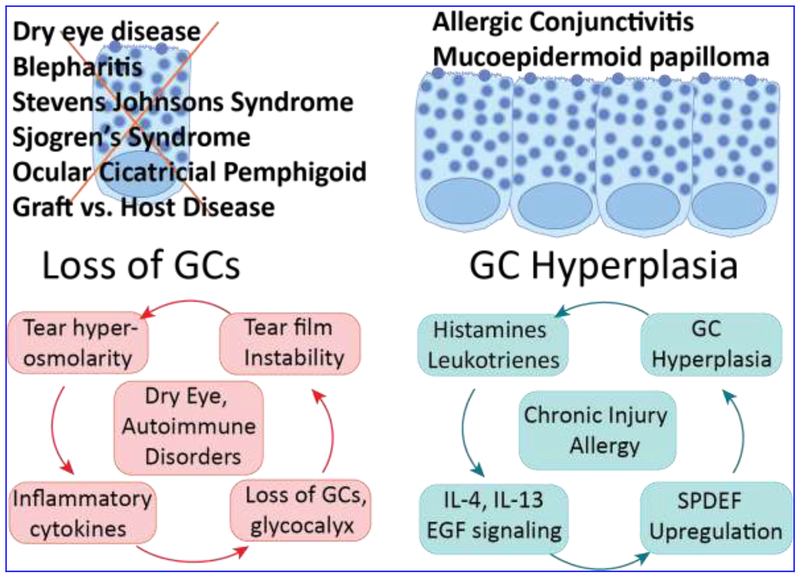
Figure 4. Effect of ocular surface disorders on GCs.
Loss of GCs is associated with dry eye disease and certain autoimmune disorders that affect the eye. In contrast, GC hyperplasia and mucin hypersecretion are reported in allergic conjunctivitis, chronic injuries and conjunctival papilloma. Cyclical nature of these events and the known pathways that influence them are indicated in the lower panel. While the numerous inputs that lead to the ongoing damage are not shown, what is central is the role of GC mucins that lubricate and protect the ocular surface. The GCs can be damaged by cytokines and toxins (such as mitomycin-C during trabeculectomies), and the tear film can be further destabilized in a dry environment. We predict that a better understanding of the molecular mechanisms that regulate normal CGC differentiation in early postnatal stages, their functions in later stages, and how they are altered in diverse disease conditions will facilitate development of novel approaches for therapeutic modulation of GC numbers and functions in the near future.
In contrast, exposure to allergic conditions stimulates CGC survival, proliferation, and secretory function, suggesting that GC numbers and functions are tightly regulated in response to external cues. Allergic conjunctivitis and inverted mucoepidermoid papilloma result in increased GC numbers and hypersecretion of mucins to the tear film. Histamines, leukotrienes, prostaglandins and other allergic mediators stimulate mucin secretion 31. Histamine receptors transactivate EGFR, which leads to phosphorylation of AKT and increased intracellular Ca2+ and stimulate mucin secretion (Fig. 4). Chronic injury or allergy causes lung GC hyperplasia and mucus hypersecretion, mediated by interleukins IL-4, IL-13, and EGF signaling 56. In the airway epithelium, GC metaplasia that accompanies recurrent viral infections depends on IL-13-mediated elevated activity of the transcription factor Foxa3 84. The natural killer T cell-derived IL-13 stimulates GC numbers via upregulation of transcription factor SPDEF, that induces GC differentiation 57, 97. Collectively, these reports suggest that CGC numbers and functions are fine-tuned in response to the specific nature of the external stimuli (Fig. 4).
The reports summarized above demonstrate that GC numbers and functions are altered in response to (i) ocular surface genetic disorders, (ii) dysregulated immune responses as in GVHD, (iii) external stimuli in the form of environmental allergens and (iv) chronic injury due to dry eye or other cicatrizing disorders. In view of the importance of GC functions in ocular surface health, it is predictable that a better understanding of the molecular mechanisms that regulate GC differentiation and functions, and how they are altered in diverse disease conditions, will facilitate development of novel approaches for therapeutic modulation of GC numbers and functions. Below, we discuss some of the current efforts in this direction.
7. Tissue- and cell-based approaches for restoration of CGC numbers and functions.
As described above, the conjunctiva becomes damaged in a variety of ocular surface disorders. In these situations, although a healthy conjunctival autograft is ideal for regeneration of conjunctival epithelium, it is not always feasible prompting consideration of other mucosal epithelia as alternatives. As oral mucosa does not contain GCs it does not supplement the tear film. Nasal mucosal grafts contain GCs and may be useful in extreme dry eye situations 98. Thin and translucent amniotic membrane possesses anti-inflammatory and antiangiogenic properties, and is widely used in ocular surface reconstruction as it supports conjunctival re-epithelialization when conjunctival stem cells remain in the recipient and repopulate during the respite 99.
Conjunctival cells have been cultivated ex vivo on scaffolds paving the way for obtaining conjunctival grafts for transplants in patients with conjunctival diseases 100–104. Co-culturing subconjunctival fibroblasts with conjunctival epithelial cells supported the progenitor cells, providing a useful approach to expand conjunctival progenitor cells for potential clinical use 105. In cases where limbal stem cell deficiency also leads to significant CGC deficiency, a combined conjunctival limbal autograft and living-related conjunctival limbal allograft was found to maximize the amount of healthy limbal stem cells while also minimizing the antigenic burden 106. In a mouse model of inflammation-mediated dry eye, periorbital administration of mesenchymal stem cells decreased the ocular surface expression of inflammatory cytokines and infiltration of CD4(+) T cells while increasing tear production and the number of GCs 107. However, progenitor cell-derived epidermal sheets cultured on amniotic membranes failed to regenerate GCs when used for ocular surface reconstruction in monkeys, highlighting the need to further refine these cell and tissue-based approaches for CGC regeneration 108.
8. Small molecule therapeutics for improving CGC functions.
The pathologies that relate to loss of GCs and their mucin production can be ameliorated by enhancing the secretion from the fewer remaining GCs, and/or sustaining GC survival or stimulating their genesis.
8.1. Targeting Resolvins.
Resolvins are metabolic byproducts of omega-3 fatty acids that belong to a class of polyunsaturated fatty acid (PUFA) metabolites termed specialized pro-resolving mediators (SPMs). Resolvins are grouped into sub-classes based on the straight chain PUFA from which they are formed. Resolvins actively terminate inflammation that is a part of the etiology of dry eye disease among other means by regulating GC mucin secretion, and therefore are attractive as a novel treatment option for sustaining ocular surface homeostasis in dry eye disease 109. Resolvin-D1 preserves the mucous layer and maintains homeostasis by stimulating GC secretion via phospholipase-A2, -C, -D, ERK1/2 and Ca2+/CamK 110. Resolvin-D2 on the other hand elevates cAMP to increase intracellular [Ca2+] and stimulate GC secretion 111. Chronic inflammatory diseases such as allergic conjunctivitis cause GC hyperplasia and increased mucin secretion. Resolvins-D1 and -E1 controlled inflammatory leukotriene-stimulated mucin secretion by preventing the increase in intracellular [Ca2+] and activation of ERK1/2 112. These results demonstrate that resolvins hold the promise to be effective modulators of CGC function and deserve additional attention.
8.2. Targeting Notch signaling.
The means to increasing GC numbers could focus on directing resident stem cells toward GC differentiation; this could occur by directing the molecular signals of cell fate. Notch signaling plays a major role in decisions leading to the formation of either secretory or absorptive cells in the intestinal epithelium 113. Blocking the Notch cascade, which is dependent on cleavage of the intracellular domain to transit to the nucleus, with a γ - secretase inhibitor resulted in conversion of proliferative crypt cells into post-mitotic GCs in the small intestine 114, 115. Intraperitoneal injection of Dibenzazepine, a γ-secretase inhibitor resulted in GC hyperplasia in the gut114. GCs fail to develop upon conditional ablation of Klf4, a Notch-regulated transcription factor in the intestine 75 and conjunctiva 70. In the Klf4-conditional null cornea, Notch1 expression is upregulated roughly 2-fold 69. Conversely, inhibition of Notch signaling increases Klf4 expression and GC differentiation, and reduces proliferation and tumor formation suggesting reciprocal regulation between Notch signaling and KLF4 116. Consistently, cell cycle arrest and accumulation of GCs in the gut upon γ–secretase inhibitor treatment was mediated by upregulation of KLF4, a negative regulator of cell cycle 115. Based on these studies, it is predicted that suppressing the Notch pathway in the conjunctival epithelial cells will promote the formation of GCs, providing a new avenue for alleviating the discomfort of ocular surface disorders associated with GC loss. Cautionary to this approach, the finding that tissue-specific inducible and irreversible inactivation of Notch-1 in adult corneal epithelium resulted in hyperkeratosis and hyperplasia, leading to the formation of a hyperproliferative, vascularized and opaque plaque also calls for an abundance of caution in such attempts 117. Thus, this avenue of increasing GC numbers via directed differentiation will require further study, determining whether Notch inhibition limited in extent or duration can avoid these confounding side effects.
8.3. Targeting CXCR3-Ligands.
Very recently it has been reported that activation of the CXCR3 chemokine receptor improves GC numbers after conjunctiva damage during trabeculectomy, when compared to diluent alone 118. Subconjunctival injection of an activating chemokine resulted in more GCs in the rabbit eye after the surgical procedure. The mechanism for this increase is not described but may involve either direct effects on GC precursors, or indirectly by modulating the inflammatory environment. Suppression of fibrosis by these chemokines 119–121 would be consistent with allowing a regenerative healing with GC repopulation. As the cycle of injury and chronic inflammation leading to fibrosis contributes to DED, disrupting this should limit the extent or chronicity of disease. This is supported by the finding that suppression of inflammation may rescue GCs after cataract surgery 122. Further, the CXCR3 ligand CXCL10 is produced by M1-type macrophages 123, suggesting that this may be part of the physiologic response to wounding that protects precursor or stem cells in the face of death ligands or early inflammation. Even though quite preliminary and speculative at present, this report suggests that modulation of conjunctival inflammation may provide relief from DED via rescue of GCs.
9. Conclusions.
Ocular surface mucosal epithelium serves a vital barrier function against external assaults. This protective function is aided by CGCs that produce and secrete mucins, trefoil factor, and defensins. CGC-secreted mucins play a key role in maintaining the ocular surface mucosal epithelial homeostasis. These mucins not only promote tear film integrity to maintain a moist and appropriately refractory film but also serve as anti-microbial and anti-inflammatory substances. When inflammatory ocular surface disorders affect CGC numbers and/or functions, the resulting decrease in secreted mucins leads to a dysregulated feed-forward cycle that further drives the pathology increasing the risk of vision loss.
Despite the obvious importance of CGCs for ocular surface health, several gaps remain in our understanding of CGC biology, impeding with the development of new therapeutic strategies for devastating ocular surface diseases. Molecular mechanisms that regulate the mucin production and secretion by CGCs in healthy and disease-ravaged ocular surface remain incompletely understood despite recent efforts to elucidate the network of genes involved in CGC differentiation and function. Though CGC-enriched transcription factors and their roles in GC differentiation have been identified, mucin gene regulation itself remains poorly studied. Another understudied area relates to the events leading up to decreased CGC numbers and functions in cicatrizing ocular surface diseases. Further studies elucidating the molecular mechanisms involved in inflammatory cytokine-mediated destruction, and similar studies defining mechanisms of survival or expansion of CGCs are essential to fully realize the potential of promising leads for modulating CGC numbers and functions in disease conditions.
Acknowledgements:
This work was supported by NIH grants: R01 EY026533 (SKS), R01 EY022898 (SKS), R01 GM063569 (AW), R01 GM069668 (AW), P30 EY08098; by unrestricted grants from Research to Prevent Blindness and the Eye and Ear Foundation of Pittsburgh. We thank Dr. Anil Tiwari and Ms. Sudha Swamynathan for critical comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: AW is a founder and holder of equity in Ocugenix, a privately held biotechnology start up that is developing activators of CXCR3 for the treatment of ocular pathologies. AW is a named inventor on patents held by the University of Pittsburgh on these potential therapeutics.
References
- 1.Dartt DA. Regulation of mucin and fluid secretion by conjunctival epithelial cells. Prog Retin Eye Res 2002;21:555–576. [DOI] [PubMed] [Google Scholar]
- 2.Gipson IK. The ocular surface: the challenge to enable and protect vision: the Friedenwald lecture. Invest Ophthalmol Vis Sci 2007;48:4390; 4391–4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrand KF, Fridman M, Stillman IO, Schaumberg DA. Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. Am J Ophthalmol 2017;182:90–98. [DOI] [PubMed] [Google Scholar]
- 4.Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf 2017;15:334–365. [DOI] [PubMed] [Google Scholar]
- 5.Bradley JL, Ozer Stillman I, Pivneva I, Guerin A, Evans AM, Dana R. Dry eye disease ranking among common reasons for seeking eye care in a large US claims database. Clin Ophthalmol 2019;13:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szczesna-Iskander DH. Post-blink tear film dynamics in healthy and dry eyes during spontaneous blinking. Ocul Surf 2018;16:93–100. [DOI] [PubMed] [Google Scholar]
- 7.Bron AJ, Tiffany JM. The meibomian glands and tear film lipids. Structure, function, and control. Adv Exp Med Biol 1998;438:281–295. [DOI] [PubMed] [Google Scholar]
- 8.Gipson IK, Argueso P. Role of mucins in the function of the corneal and conjunctival epithelia. International review of cytology 2003;231:1–49. [DOI] [PubMed] [Google Scholar]
- 9.Langer G, Jagla W, Behrens-Baumann W, Walter S, Hoffmann W. Secretory peptides TFF1 and TFF3 synthesized in human conjunctival goblet cells. Invest Ophthalmol Vis Sci 1999;40:2220–2224. [PubMed] [Google Scholar]
- 10.Nees DW, Fariss RN, Piatigorsky J. Serum albumin in mammalian cornea: implications for clinical application. Invest Ophthalmol Vis Sci 2003;44:3339–3345. [DOI] [PubMed] [Google Scholar]
- 11.Ananthi S, Santhosh RS, Nila MV, Prajna NV, Lalitha P, Dharmalingam K. Comparative proteomics of human male and female tears by two-dimensional electrophoresis. Exp Eye Res 2011;92:454–463. [DOI] [PubMed] [Google Scholar]
- 12.Azkargorta M, Soria J, Ojeda C, et al. Human Basal Tear Peptidome Characterization by CID, HCD, and ETD Followed by in Silico and in Vitro Analyses for Antimicrobial Peptide Identification. Journal of proteome research 2015. [DOI] [PubMed] [Google Scholar]
- 13.Lema I, Brea D, Rodriguez-Gonzalez R, Diez-Feijoo E, Sobrino T. Proteomic analysis of the tear film in patients with keratoconus. Mol Vis 2010;16:2055–2061. [PMC free article] [PubMed] [Google Scholar]
- 14.Li B, Sheng M, Xie L, et al. Tear proteomic analysis of patients with type 2 diabetes and dry eye syndrome by two-dimensional nano-liquid chromatography coupled with tandem mass spectrometry. Invest Ophthalmol Vis Sci 2014;55:177–186. [DOI] [PubMed] [Google Scholar]
- 15.Tong L, Zhou XY, Jylha A, et al. Quantitation of 47 human tear proteins using high resolution multiple reaction monitoring (HR-MRM) based-mass spectrometry. Journal of proteomics 2015;115:36–48. [DOI] [PubMed] [Google Scholar]
- 16.Zhou L, Beuerman RW. Tear analysis in ocular surface diseases. Progress in Retinal and Eye Research 2012;31:527–550. [DOI] [PubMed] [Google Scholar]
- 17.Campbell G, Swamynathan S, Tiwari A, Swamynathan SK. The secreted Ly-6/uPAR related protein-1 (SLURP1) stabilizes epithelial cell junctions and suppresses TNF-alpha-induced cytokine production. Biochem Biophys Res Commun 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swamynathan S, Buela KA, Kinchington P, et al. Klf4 regulates the expression of Slurp1, which functions as an immunomodulatory peptide in the mouse cornea. Invest Ophthalmol Vis Sci 2012;53:8433–8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swamynathan S, Delp EE, Harvey SA, Loughner CL, Raju L, Swamynathan SK. Corneal Expression of SLURP-1 by Age, Sex, Genetic Strain, and Ocular Surface Health. Invest Ophthalmol Vis Sci 2015;56:7888–7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swamynathan S, Loughner CL, Swamynathan SK. Inhibition of HUVEC tube formation via suppression of NFkappaB suggests an anti-angiogenic role for SLURP1 in the transparent cornea. Exp Eye Res 2017;164:118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swamynathan S, Swamynathan SK. SLURP-1 modulates corneal homeostasis by serving as a soluble scavenger of urokinase-type plasminogen activator. Invest Ophthalmol Vis Sci 2014;55:6251–6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swamynathan S, Tiwari A, Loughner CL, et al. The secreted Ly6/uPAR-related protein-1 suppresses neutrophil binding, chemotaxis, and transmigration through human umbilical vein endothelial cells. Scientific reports 2019;9:5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boerger M, Funke S, Leha A, et al. Proteomic analysis of tear fluid reveals disease-specific patterns in patients with Parkinson’s disease - A pilot study. Parkinsonism Relat Disord 2019;63:3–9. [DOI] [PubMed] [Google Scholar]
- 24.Kallo G, Emri M, Varga Z, et al. Changes in the Chemical Barrier Composition of Tears in Alzheimer’s Disease Reveal Potential Tear Diagnostic Biomarkers. PLoS One 2016;11:e0158000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cicalini I, Rossi C, Pieragostino D, et al. Integrated Lipidomics and Metabolomics Analysis of Tears in Multiple Sclerosis: An Insight into Diagnostic Potential of Lacrimal Fluid. International journal of molecular sciences 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Argueso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren syndrome. Invest Ophthalmol Vis Sci 2002;43:1004–1011. [PubMed] [Google Scholar]
- 27.Argueso P, Gipson IK. Epithelial mucins of the ocular surface: structure, biosynthesis and function. Exp Eye Res 2001;73:281–289. [DOI] [PubMed] [Google Scholar]
- 28.Kessing SV. Mucous gland system of the conjunctiva. A quantitative normal anatomical study. Acta ophthalmologica 1968;Suppl 95:91.+. [PubMed] [Google Scholar]
- 29.Mantelli F, Argueso P. Functions of ocular surface mucins in health and disease. Curr Opin Allergy Clin Immunol 2008;8:477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiffany JM. The normal tear film. Developments in ophthalmology 2008;41:1–20. [DOI] [PubMed] [Google Scholar]
- 31.Dartt DA, Masli S. Conjunctival epithelial and goblet cell function in chronic inflammation and ocular allergic inflammation. Curr Opin Allergy Clin Immunol 2014;14:464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinoshita S, Kiorpes TC, Friend J, Thoft RA. Goblet cell density in ocular surface disease. A better indicator than tear mucin. Arch Ophthalmol 1983;101:1284–1287. [DOI] [PubMed] [Google Scholar]
- 33.Gipson IK, Tisdale AS. Visualization of conjunctival goblet cell actin cytoskeleton and mucin content in tissue whole mounts. Exp Eye Res 1997;65:407–415. [DOI] [PubMed] [Google Scholar]
- 34.Knop N, Korb DR, Blackie CA, Knop E. The lid wiper contains goblet cells and goblet cell crypts for ocular surface lubrication during the blink. Cornea 2012;31:668–679. [DOI] [PubMed] [Google Scholar]
- 35.Contreras-Ruiz L, Masli S. Immunomodulatory cross-talk between conjunctival goblet cells and dendritic cells. PLoS One 2015;10:e0120284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gunzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiological reviews 2013;93:525–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochimica et biophysica acta 2008;1778:631–645. [DOI] [PubMed] [Google Scholar]
- 38.Contreras-Ruiz L, Ghosh-Mitra A, Shatos MA, Dartt DA, Masli S. Modulation of conjunctival goblet cell function by inflammatory cytokines. Mediators Inflamm 2013;2013:636812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu J, Chen L, Shatos MA, et al. Presence of EGF growth factor ligands and their effects on cultured rat conjunctival goblet cell proliferation. Exp Eye Res 2008;86:322–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He M, Lippestad M, Li D, Hodges RR, Utheim TP, Dartt DA. Activation of the EGF Receptor by Histamine Receptor Subtypes Stimulates Mucin Secretion in Conjunctival Goblet Cells. Invest Ophthalmol Vis Sci 2018;59:3543–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanno H, Horikawa Y, Hodges RR, et al. Cholinergic agonists transactivate EGFR and stimulate MAPK to induce goblet cell secretion. Am J Physiol Cell Physiol 2003;284:C988–998. [DOI] [PubMed] [Google Scholar]
- 42.Halm DR, Halm ST. Secretagogue response of goblet cells and columnar cells in human colonic crypts. Am J Physiol Cell Physiol 2000;278:C212–233. [DOI] [PubMed] [Google Scholar]
- 43.Wei ZG, Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells are preferentially located in fornical epithelium: implications on conjunctival epithelial homeostasis. Invest Ophthalmol Vis Sci 1995;36:236–246. [PubMed] [Google Scholar]
- 44.Wei ZG, Lin T, Sun TT, Lavker RM. Clonal analysis of the in vivo differentiation potential of keratinocytes. Invest Ophthalmol Vis Sci 1997;38:753–761. [PubMed] [Google Scholar]
- 45.Pellegrini G, Golisano O, Paterna P, et al. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol 1999;145:769–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su L, Cui H, Xu C, Xie X, Chen Q, Gao X. Putative rabbit conjunctival epithelial stem/progenitor cells preferentially reside in palpebral conjunctiva. Curr Eye Res 2011;36:797–803. [DOI] [PubMed] [Google Scholar]
- 47.Stewart RM, Sheridan CM, Hiscott PS, Czanner G, Kaye SB. Human Conjunctival Stem Cells are Predominantly Located in the Medial Canthal and Inferior Forniceal Areas. Invest Ophthalmol Vis Sci 2015;56:2021–2030. [DOI] [PubMed] [Google Scholar]
- 48.Majo F, Rochat A, Nicolas M, Jaoude GA, Barrandon Y. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature 2008;456:250–254. [DOI] [PubMed] [Google Scholar]
- 49.Sun TT, Tseng SC, Lavker RM. Location of corneal epithelial stem cells. Nature 2010;463:E10–11; discussion E11. [DOI] [PubMed] [Google Scholar]
- 50.Pajoohesh-Ganji A, Pal-Ghosh S, Tadvalkar G, Stepp MA. Corneal goblet cells and their niche: implications for corneal stem cell deficiency. Stem Cells 2012;30:2032–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med 2010;363:2233–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roy MG, Livraghi-Butrico A, Fletcher AA, et al. Muc5b is required for airway defence. Nature 2014;505:412–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marko CK, Menon BB, Chen G, Whitsett JA, Clevers H, Gipson IK. Spdef null mice lack conjunctival goblet cells and provide a model of dry eye. Am J Pathol 2013;183:35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gregorieff A, Stange DE, Kujala P, et al. The ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology 2009;137:1333–1345 e1331–1333. [DOI] [PubMed] [Google Scholar]
- 55.Noah TK, Kazanjian A, Whitsett J, Shroyer NF. SAM pointed domain ETS factor (SPDEF) regulates terminal differentiation and maturation of intestinal goblet cells. Exp Cell Res 2010;316:452–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park KS, Korfhagen TR, Bruno MD, et al. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J Clin Invest 2007;117:978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen G, Korfhagen TR, Xu Y, et al. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest 2009;119:2914–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knoop KA, Newberry RD. Goblet cells: multifaceted players in immunity at mucosal surfaces. Mucosal immunology 2018;11:1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rajavelu P, Chen G, Xu Y, Kitzmiller JA, Korfhagen TR, Whitsett JA. Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J Clin Invest 2015;125:2021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dogru M, Matsumoto Y, Okada N, et al. Alterations of the ocular surface epithelial MUC16 and goblet cell MUC5AC in patients with atopic keratoconjunctivitis. Allergy 2008;63:1324–1334. [DOI] [PubMed] [Google Scholar]
- 61.Dogru M, Asano-Kato N, Tanaka M, et al. Ocular surface and MUC5AC alterations in atopic patients with corneal shield ulcers. Curr Eye Res 2005;30:897–908. [DOI] [PubMed] [Google Scholar]
- 62.Knoop KA, McDonald KG, McCrate S, McDole JR, Newberry RD. Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal Immunology 2015;8:198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McDole JR, Wheeler LW, McDonald KG, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 2012;483:345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knop N, Knop E. Conjunctiva-associated lymphoid tissue in the human eye. Invest Ophthalmol Vis Sci 2000;41:1270–1279. [PubMed] [Google Scholar]
- 65.Barbosa FL, Xiao Y, Bian F, et al. Goblet Cells Contribute to Ocular Surface Immune Tolerance-Implications for Dry Eye Disease. International journal of molecular sciences 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ko BY, Xiao Y, Barbosa FL, de Paiva CS, Pflugfelder SC. Goblet cell loss abrogates ocular surface immune tolerance. JCI insight 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y, Lam O, Nguyen MT, et al. Mastermind-like transcriptional co-activator-mediated Notch signaling is indispensable for maintaining conjunctival epithelial identity. Development 2013;140:594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Swamynathan S, Kenchegowda D, Piatigorsky J, Swamynathan SK. Regulation of Corneal Epithelial Barrier Function by Kruppel-like Transcription Factor 4. Invest Ophthalmol Vis Sci 2011;52:1762–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swamynathan SK, Davis J, Piatigorsky J. Identification of candidate Klf4 target genes reveals the molecular basis of the diverse regulatory roles of Klf4 in the mouse cornea. Invest Ophthalmol Vis Sci 2008;49:3360–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Swamynathan SK, Katz JP, Kaestner KH, Ashery-Padan R, Crawford MA, Piatigorsky J. Conditional deletion of the mouse Klf4 gene results in corneal epithelial fragility, stromal edema, and loss of conjunctival goblet cells. Mol Cell Biol 2007;27:182–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Young RD, Swamynathan SK, Boote C, et al. Stromal edema in klf4 conditional null mouse cornea is associated with altered collagen fibril organization and reduced proteoglycans. Invest Ophthalmol Vis Sci 2009;50:4155–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Swamynathan SK. Kruppel-like factors: three fingers in control. Human genomics 2010;4:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kenchegowda D, Swamynathan S, Gupta D, Wan H, Whitsett Ja, Swamynathan SK. Conditional Disruption of Mouse Klf5 Results in Defective Eyelids with Malformed Meibomian Glands, Abnormal Cornea and Loss of Conjunctival Goblet Cells Developmental Biology 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bell SM, Zhang L, Xu Y, et al. Kruppel-like factor 5 controls villus formation and initiation of cytodifferentiation in the embryonic intestinal epithelium. Dev Biol 2013;375:128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Katz JP, Perreault N, Goldstein BG, et al. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development 2002;129:2619–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Delp EE, Swamynathan S, Kao WW, Swamynathan SK. Spatiotemporally Regulated Ablation of Klf4 in Adult Mouse Corneal Epithelial Cells Results in Altered Epithelial Cell Identity and Disrupted Homeostasis. Invest Ophthalmol Vis Sci 2015;56:3549–3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kenchegowda D, Harvey SAK, Swamynathan S, Lathrop KL, Swamynathan SK. Critical Role of Klf5 in Regulating Gene Expression during Post-Eyelid Opening Maturation of Mouse Corneas. PLoS One 2012;7:e44771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kenchegowda D, Swamynathan S, Gupta D, Wan H, Whitsett J, Swamynathan SK. Conditional disruption of mouse Klf5 results in defective eyelids with malformed meibomian glands, abnormal cornea and loss of conjunctival goblet cells. Dev Biol 2011;356:5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Loughner CL, Tiwari A, Kenchegowda D, Swamynathan S, Swamynathan SK. Spatiotemporally Controlled Ablation of Klf5 Results in Dysregulated Epithelial Homeostasis in Adult Mouse Corneas. Invest Ophthalmol Vis Sci 2017;58:4683–4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tiwari A, Loughner CL, Swamynathan S, Swamynathan SK. KLF4 Plays an Essential Role in Corneal Epithelial Homeostasis by Promoting Epithelial Cell Fate and Suppressing Epithelial-Mesenchymal Transition. Invest Ophthalmol Vis Sci 2017;58:2785–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tiwari A, Swamynathan S, Alexander N, et al. KLF4 Regulates Corneal Epithelial Cell Cycle Progression by Suppressing Canonical TGF-beta Signaling and Upregulating CDK Inhibitors P16 and P27. Invest Ophthalmol Vis Sci 2019;60:731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gupta D, Harvey SA, Kaminski N, Swamynathan SK. Mouse conjunctival forniceal gene expression during postnatal development and its regulation by Kruppel-like factor 4. Invest Ophthalmol Vis Sci 2011;52:4951–4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McCauley HA, Liu CY, Attia AC, et al. TGFbeta signaling inhibits goblet cell differentiation via SPDEF in conjunctival epithelium. Development 2014;141:4628–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen G, Korfhagen TR, Karp CL, et al. Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity. American journal of respiratory and critical care medicine 2014;189:301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang P, He Z, Ji S, et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature 2011;475:386–389. [DOI] [PubMed] [Google Scholar]
- 86.Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature 2011;475:390–393. [DOI] [PubMed] [Google Scholar]
- 87.Shen CN, Slack JM, Tosh D. Molecular basis of transdifferentiation of pancreas to liver. Nat Cell Biol 2000;2:879–887. [DOI] [PubMed] [Google Scholar]
- 88.Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. Cell 2004;117:663–676. [DOI] [PubMed] [Google Scholar]
- 89.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 2008;455:627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feng R, Desbordes SC, Xie H, et al. PU.1 and C/EBPalpha/beta convert fibroblasts into macrophage-like cells. Proc Natl Acad Sci U S A 2008;105:6057–6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010;463:1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.leda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 2010;142:375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Szabo E, Rampalli S, Risueno RM, et al. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature 2010;468:521–526. [DOI] [PubMed] [Google Scholar]
- 94.Ralph RA. Conjunctival goblet cell density in normal subjects and in dry eye syndromes. Invest Ophthalmol 1975;14:299–302. [PubMed] [Google Scholar]
- 95.Pflugfelder SC, Corrales RM, de Paiva CS. T helper cytokines in dry eye disease. Exp Eye Res 2013;117:118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li C, Song Y, Luan S, et al. Research on the stability of a rabbit dry eye model induced by topical application of the preservative benzalkonium chloride. PLoS One 2012;7:e33688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.De Paiva CS, Raince JK, McClellan AJ, et al. Homeostatic control of conjunctival mucosal goblet cells by NKT-derived IL-13. Mucosal immunology 2011;4:397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Henderson HWA, Collin JRO. Mucous membrane grafting. Developments in ophthalmology 2008;41:230–242. [DOI] [PubMed] [Google Scholar]
- 99.Tseng SC. Amniotic membrane transplantation for ocular surface reconstruction. Biosci Rep 2001;21:481–489. [DOI] [PubMed] [Google Scholar]
- 100.Eidet JR, Dartt DA, Utheim TP. Concise Review: Comparison of Culture Membranes Used for Tissue Engineered Conjunctival Epithelial Equivalents. Journal of functional blomaterlals 2015;6:1064–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meller D, Dabul V, Tseng SC. Expansion of conjunctival epithelial progenitor cells on amniotic membrane. Exp Eye Res 2002;74:537–545. [DOI] [PubMed] [Google Scholar]
- 102.Rosellini A, Papini S, Giannarini C, Nardi M, Revoltella RP. Human conjunctival epithelial precursor cells and their progeny in 3D organotypic culture. The International journal of developmental biology 2007;51:739–743. [DOI] [PubMed] [Google Scholar]
- 103.Bertolin M, Breda C, Ferrari S, et al. Optimized Protocol for Regeneration of the Conjunctival Epithelium Using the Cell Suspension Technique. Cornea 2019;38:469–479. [DOI] [PubMed] [Google Scholar]
- 104.Ang LP, Tan DT, Seah CJ, Beuerman RW. The use of human serum in supporting the in vitro and in vivo proliferation of human conjunctival epithelial cells. Br J Ophthalmol 2005;89:748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schrader S, Notara M, Tuft SJ, Beaconsfield M, Geerling G, Daniels JT. Simulation of an in vitro niche environment that preserves conjunctival progenitor cells. Regenerative medicine 2010;5:877–889. [DOI] [PubMed] [Google Scholar]
- 106.Cheung AY, Sarnicola E, Govil A, Holland EJ. Combined Conjunctival Limbal Autografts and Living-Related Conjunctival Limbal Allografts for Severe Unilateral Ocular Surface Failure. Cornea 2017;36:1570–1575. [DOI] [PubMed] [Google Scholar]
- 107.Lee MJ, Ko AY, Ko JH, et al. Mesenchymal stem/stromal cells protect the ocular surface by suppressing inflammation in an experimental dry eye. Molecular therapy: the journal of the American Society of Gene Therapy 2015;23:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lu R, Zhang X, Huang D, et al. Conjunctival reconstruction with progenitor cell-derived autologous epidermal sheets in rhesus monkey. PLoS One 2011;6:e25713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.He J, Bazan HE. Omega-3 fatty acids in dry eye and corneal nerve regeneration after refractive surgery. Prostaglandins, leukotrienes, and essential fatty acids 2010;82:319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lippestad M, Hodges RR, Utheim TP, Serhan CN, Dartt DA. Resolvin D1 Increases Mucin Secretion in Cultured Rat Conjunctival Goblet Cells via Multiple Signaling Pathways. Invest Ophthalmol Vis Sci 2017;58:4530–4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Botten N, Hodges RR, Li D, et al. Resolvin D2 elevates cAMP to increase intracellular [Ca(2+)] and stimulate secretion from conjunctival goblet cells. FASEB J 2019;33:8468–8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dartt DA, Hodges RR, Li D, Shatos MA, Lashkari K, Serhan CN. Conjunctival goblet cell secretion stimulated by leukotrienes is reduced by resolvins D1 and E1 to promote resolution of inflammation. J Immunol 2011;186:4455–4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fre S, Bardin A, Robine S, Louvard D. Notch signaling in intestinal homeostasis across species: the cases of Drosophila, Zebrafish and the mouse. Exp Cell Res 2011;317:2740–2747. [DOI] [PubMed] [Google Scholar]
- 114.van Es JH, van Gijn ME, Riccio O, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 2005;435:959–963. [DOI] [PubMed] [Google Scholar]
- 115.Real PJ, Tosello V, Palomero T, et al. Gamma-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia. Nat Med 2009;15:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ghaleb AM, Aggarwal G, Bialkowska AB, Nandan MO, Yang VW. Notch inhibits expression of the Kruppel-like factor 4 tumor suppressor in the intestinal epithelium. Mol Cancer Res 2008;6:1920–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nicolas M, Wolfer A, Raj K, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet 2003;33:416–421. [DOI] [PubMed] [Google Scholar]
- 118.Happ C, Conner IP, Dong ZM, et al. A novel combination therapy reduces subconjunctival fibrosis after glaucoma filtration surgery in the rabbit model. 2019;submitted. [DOI] [PubMed] [Google Scholar]
- 119.Jiang D, Liang J, Hodge J, et al. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J Clin Invest 2004;114:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yates CC, Whaley D, Kulasekeran P, et al. Delayed and deficient dermal maturation in mice lacking the CXCR3 ELR-negative CXC chemokine receptor. Am J Pathol 2007;171:484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bodnar RJ, Satish L, Yates CC, Wells A. Pericytes: A newly recognized player in wound healing. Wound Repair and Regeneration 2016;24:201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kato K, Miyake K, Kondo N, et al. Conjunctival Goblet Cell Density Following Cataract Surgery With Diclofenac Versus Diclofenac and Rebamipide: A Randomized Trial. Am J Ophthalmol 2017;181:26–36. [DOI] [PubMed] [Google Scholar]
- 123.Kapellos TS, Iqbal AJ. Epigenetic Control of Macrophage Polarisation and Soluble Mediator Gene Expression during Inflammation. Mediators Inflamm 2016:2016:6591703. [DOI] [PMC free article] [PubMed] [Google Scholar]