Abstract
Background
Acute pulmonary embolism (PE) is a common cause of death, accounting for 50,000 to 200,000 deaths annually. It is the third most common cause of mortality among the cardiovascular diseases, after coronary artery disease and stroke. The advent of multi‐detector computed tomographic pulmonary angiography (CTPA) has allowed better assessment of PE regarding visualisation of the peripheral pulmonary arteries, increasing its rate of diagnosis. More cases of peripheral PEs, such as isolated subsegmental PE (SSPE) and incidental PE, have thereby been identified. These two conditions are usually found in patients with few or none of the classic PE symptoms such as haemoptysis or pleuritic pain, acute dyspnoea or circulatory collapse. However, in patients with reduced cardiopulmonary reserve, classic PE symptoms can be found with isolated SSPEs. Incidental SSPE is found casually in asymptomatic patients, usually by diagnostic imaging performed for other reasons (for example routine CT for cancer staging in oncology patients). Traditionally, all PEs are anticoagulated in a similar manner independent of their location, or number and size of the thrombi. It has been suggested that many patients with SSPE may be treated without benefit, increasing adverse events by a possible unnecessary use of anticoagulants. Patients with isolated SSPE, or incidental PE, may have a more benign clinical presentation compared to those with proximal PEs. However, the clinical significance in patients, and their prognosis, needs to be studied to evaluate whether anticoagulation therapy is required. This is the second update of the Cochrane systematic review published in 2014.
Objectives
To assess the effectiveness and safety of anticoagulation therapy versus control in patients with isolated subsegmental pulmonary embolism (SSPE) or incidental SSPE.
Search methods
The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase, CINAHL and AMED databases and World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials registers to 26 November 2019. We also undertook reference checking to identify additional studies.
Selection criteria
We included randomised controlled trials of anticoagulation therapy versus control in patients with SSPE or incidental SSPE.
Data collection and analysis
Two review authors inspected all citations identified to ensure reliable assessment. If relevant studies were identified, we planned for two review authors to independently extract data and to assess the methodological quality of identified trials using the criteria recommended in the Cochrane Handbook for Systematic Reviews of Interventions.
Main results
We did not identify any studies that met the inclusion criteria.
Authors' conclusions
There is no evidence from randomised controlled trials to assess the effectiveness and safety of anticoagulation therapy versus control in patients with isolated subsegmental pulmonary embolism (SSPE) or incidental SSPE. Well‐conducted research is required before informed practice decisions can be made.
Plain language summary
Anticoagulant treatment for subsegmental pulmonary embolism
Background
Acute pulmonary embolism (PE) is a common cause of death, accounting for 50,000 to 200,000 deaths annually. It is the third most common cause of mortality among the cardiovascular diseases, after coronary artery disease and stroke. The advent of multi‐detector computed tomographic pulmonary angiography (CTPA) has allowed better assessment of PE regarding visualisation of the peripheral pulmonary arteries, increasing its rate of diagnosis. More cases of peripheral PEs, such as isolated subsegmental PE (SSPE) and incidental PE, have thereby been identified. These two conditions are usually found in patients with few or no classic symptoms such as coughing (including coughing up blood), chest or upper back pain, acute shortness of breath, or general or specific failure of the circulation that is either cardiac or peripheral in nature. However, in patients with an impaired cardiac and pulmonary condition the classic PE symptoms can be found with isolated SSPEs. Incidental SSPE is found casually in asymptomatic patients, usually by diagnostic imaging performed for other reasons (for example routine computed tomography (CT) for cancer staging in oncologic patients). Patients with isolated SSPE or incidental PE may have a more benign clinical presentation compared to those with proximal PEs. However, the clinical significance and prognosis in these patients has to be studied to evaluate whether anticoagulation therapy is required.
Review question
What is the effectiveness and safety of anticoagulation therapy versus control in patients with subsegmental pulmonary embolism (SSPE) or incidental SSPE?
Study characteristics
We did not identify any studies that met the inclusion criteria.
Key results
There is no evidence from randomised controlled trials (current to 26 November 2019) on the effectiveness and safety of anticoagulation therapy versus control in patients with isolated subsegmental pulmonary embolism (SSPE) or incidental SSPE. We cannot draw any conclusions. Well‐conducted research is required before informed practice decisions can be made.
Quality of evidence
It is not possible to review methodological quality in the absence of studies eligible for inclusion in the review.
Background
Description of the condition
Acute pulmonary embolism (PE) is a common cause of death, accounting for 50,000 to 200,000 deaths annually (Torbicki 2008), with a reported 30‐day mortality rate of approximately 10% (den Exter 2014). As many as 95% of patients who die, do so prior to diagnosis, with the majority of deaths occurring in untreated patients (Dalen 2002; Jiménez 2007).
In the early 1990s, helical computed tomography (CT) was introduced for the diagnosis of PE (Remy‐Jardin 1992). It could reliably detect a central PE but was limited in excluding a small PE (Mullins 2000; Rathbun 2000). Estimates of the incidence of pulmonary embolism (PE) in the general population increased following the introduction of D‐dimer testing and computed tomographic pulmonary angiography (CTPA) in the 1990s (Alotaibi 2016; Huang 2014). The use of multi‐detector row CT (MDCT) improved visualisation up to the levels of the segmental and subsegmental pulmonary arteries (Le Gal 2006), thereby enhancing more confident diagnosis of smaller PEs, with one observational study reporting that 15% of patients with symptomatic PE have SSPE (Goy 2015). In many institutions MDCT has replaced scintigraphy as the imaging modality of choice for the detection of PE (Schoepf 2001). Furthermore, in 2007, MDCT angiography fulfilled the conditions to replace pulmonary angiography as the reference standard for the diagnosis of an acute PE (Nazaroglu 2009). With advances of this imaging modality and its widespread use, the incidental finding of PE in patients undergoing MDCT for reasons other than PE increased (Storto 2005). This points to the possibility of over‐diagnosis since along with an 81% increase in the rate of PE diagnosis in United States, there was no substantial reduction in the mortality rate (Ruiz 2003; Wiener 2011). The proportion of subsegmental pulmonary embolism (SSPE) detected ranges from 4.7% with single‐detector row CT (SDCT) to 9.4% with MDCT. In spite of this difference, the three‐month rate of VTE in untreated patients was 0.9% in SDCT and 1.1% in MDCT. It is likely that many patients considered as PE negative according to SDCT may have had SSPE. Even with the higher proportion of SSPE detected by MDCT compared with single‐detector CT, no considerably higher thromboembolic recurrences were prevented (Carrier 2010).
Patients with a small PE have been found to experience less dyspnoea and proximal deep venous thrombosis (DVT) and are less frequently classified as having a high clinical probability of PE when compared to those with segmental or more proximal PE. Furthermore, patients with SSPE and incidental SSPE tend to present with lower plasma levels of biomarkers and fewer echocardiographic alterations of right ventricular dysfunction (Peiman 2016). Therefore, the diagnosis is very difficult and is sometimes incidental. Approximately half of incidental PEs can involve the lobar or more proximal pulmonary arteries, whereas the other half are more distal (Dentali 2010; O'Connell 2011a).
Clinicians who are interested in small PEs, particularly those limited to the subsegmental arteries and isolated subsegmental PE (ISSPE), are beginning to question whether every small embolus that is discovered at MDCT is clinically important and requires anticoagulation (Le Gal 2006).
Description of the intervention
The clinical significance of SSPE, ISSPE and incidental PE is uncertain, particularly in patients with few or no symptoms of PE. Nevertheless, in most of these cases patients are anticoagulated for long periods of time after the diagnosis because even patients with a proven diagnosis of small PE are believed to have an increased risk of recurrence and mortality in the near future (Eyer 2005). However, it is not known whether these emboli in fact represent a risk factor for future thromboembolic events, and there is no consistent evidence that patients with SSPE benefit from short‐ and long‐term anticoagulation therapy.
Anticoagulation treatment should be administered immediately in all patients with a confirmed diagnosis of PE and in patients with a high clinical suspicion of acute PE who are awaiting the outcome of diagnostic tests provided there are no absolute contraindications such as active bleeding, haemorrhagic disease, severe uncontrolled hypertension and recent surgery (Kearon 2008). Prompt anticoagulation can only be achieved with parenteral anticoagulants such as unfractionated heparin (UFH), low molecular weight heparin (LMWH), or the pentasaccharide fondaparinux as a bridge to oral vitamin K antagonists (VKA) (Ageno 2012). The majority of patients receive intravenous UFH administered as a bolus followed by a continuous infusion titrated to a target activated partial thromboplastin time of two to three times the upper limit of normal. Weight‐based nomograms may achieve therapeutic levels of anticoagulation more quickly (Piazza 2006).
In some situations intravenous UFH is the preferred modality of initial anticoagulation, for 1) patients with severe renal insufficiency (creatinine clearance < 30 mL/min); 2) patients at high risk of bleeding; 3) high‐risk hypotensive patients; and, as a rule, 4) extremely overweight, underweight, or older patients (≥ 80 years) (Raschke 1993). With the exception of the above circumstances, UFH has largely been replaced by LMWH given subcutaneously in weight‐adjusted doses.
Long‐term treatment of PE with an oral VKA, such as warfarin, remains the standard therapy of choice for the majority of patients with PE. When PE is provoked by a temporary risk factor the recommended duration of treatment is at least three months. In cases of unprovoked PE, or PE provoked by a permanent risk factor (for example malignancy, thrombophilia, recurrent venous thromboembolism), the treatment should be extended (Kearon 2012).
Systematic reviews of previous trials indicate that LMWH has been shown to be as safe and effective as intravenous UFH for the initial treatment of patients with PE (Segal 2007). For acute PE treatment, the limitations of UFH include an unpredictable anticoagulant response with the need for frequent monitoring, a relatively narrow therapeutic window, and the potential for severe toxicity, especially heparin‐induced thrombocytopenia (HIT) in ≤ 3% of patients (Spyropoulos 2007). The three LMWH preparations currently approved for use in the United States are enoxaparin, dalteparin and tinzaparin. Compared with UFH, LMWHs have a longer plasma half‐life, a more predictable dose response relationship, and lower inter‐individual variability in the anticoagulant response to fixed doses. As a result of their pharmacokinetic properties, a desirable anticoagulant effect is achieved when the LMWH is administered subcutaneously either once or twice daily, for both prophylaxis and treatment, and it usually does not require dose adjustments or laboratory monitoring (Dinwoodey 2006). However, some unsolved issues need to be addressed in specific trials before LMWHs can definitively replace UFH in the treatment of all forms of PE. The therapeutic role of LMWH in patients with massive PE who are haemodynamically unstable remains undetermined.
Fondaparinux is one of a new class of antithrombotic agents and is based on the pentasaccharide region of the heparin molecule, which is specific for antithrombin binding. Fondaparinux selectively inhibits factor Xa by binding to antithrombin (Dinwoodey 2006). In haemodynamically and clinically stable patients with acute PE, fondaparinux is as safe and effective as intravenous UFH when it is administered in a fixed dose once daily by subcutaneous injection, and it does not require laboratory monitoring. Fondaparinux, given that it is excreted via the kidneys, is contraindicated in patients with severe renal insufficiency (creatinine clearance < 30 ml/min). In contrast to heparin compounds, fondaparinux does not cause HIT (Piazza 2006).
Non‐vitamin K antagonist oral anticoagulants (NOACs), known as factor Xa inhibitors (rivaroxaban, apixaban and edoxaban) and direct thrombin inhibitors (dabigatran), are currently available for the treatment of venous thromboembolism (VTE). In patients with PE who are haemodynamically stable (PE without hypotension), NOACs are the recommended form of anticoagulant treatment (Konstantinides 2019). However, the 10th edition of the Antithrombotic Therapy for VTE Disease guideline recommends that "initial parenteral anticoagulation is given before dabigatran and edoxaban; is not given before rivaroxaban and apixaban; and is overlapped with VKA therapy" (Kearon 2016).
How the intervention might work
Anticoagulants such as UFH, LMWH, fondaparinux and NOACs have no significant thrombolytic action on clot burden. These drugs act by altering the dynamic balance between inherent thrombogenic and fibrinolytic processes, so reducing blood clotting. They can, therefore, prevent PE.
The outcome of patients with acute PE, as in several diseases, depends on rapid diagnosis and appropriate treatment. For patients with diagnosed PE, anticoagulation clearly improves survival and as many as 95% of patients who die do so prior to diagnosis, with the majority of deaths occurring in untreated patients (Torbicki 2008). For patients who receive appropriate treatment, the 14‐ and 90‐day mortality rates are nearly 10% and 20%, respectively (Dalen 1975). However, there is still controversy regarding anticoagulation in patients with SSPE who are clinically stable or without symptoms of PE, or both, and little is known about the clinical significance of SSPE.
The epidemiologic patterns of PE have changed since CTPA was introduced in 1998. Compared with the pre‐CTPA era, the PE incidence has risen by 81%, from 62.1 per 100,000 to 112.3 per 100,000, between 1998 and 2006, mortality changed little, and case‐fatality decreased from 12.1% to 7.8%, suggesting that the additionally diagnosed PE cases could be less lethal (Wiener 2011). This fact has created an excess of positive tests resulting in lower thresholds for diagnosis, which could be deemed over‐diagnosis. Probably in this new scenario much of the increased incidence in PE consists of cases that are clinically unimportant, that is cases that would not have been fatal even if left undiagnosed and untreated (Wiener 2011).
PE is incidentally found in 1% to 6% of all patients undergoing CTPA of the thorax for indications other than PE. At autopsy, 50% to 90% of patients show recent or old PE when the examination of the pulmonary arteries is carefully performed (Ryu 1998). Gurney 1993 suggested that small emboli are common and that a healthy lung acts as a filter to protect the systematic circulation, suggesting that small PE can often be asymptomatic and resolve unnoticed. It is possible that incidental SSPE can represent a more benign subset of disease.
Why it is important to do this review
Currently there is no straightforward recommendation based on systematic reviews of trials for the treatment of SSPE, and there is no consistent evidence that patients with SSPE benefit from short‐ and long‐term anticoagulation therapy. Despite this, it appears that patients with SSPE are generally treated, mainly for ethical reasons. This is the second update of the original Cochrane Review published in 2014 (Yoo 2014; Yoo 2016).
Objectives
To assess the effectiveness and safety of anticoagulation therapy versus control in patients with isolated subsegmental pulmonary embolism (SSPE) or incidental SSPE.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised controlled trials (RCTs), quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods), and controlled clinical trials (CCTs) in this systematic review. This was in anticipation of not finding many RCTs in this area.
Types of participants
We planned to include adults (> 18 years old) presenting in a stable clinical condition, as defined by the included studies, and diagnosed with asymptomatic or symptomatic isolated SSPE and incidental SSPE by one of the following: computed tomography pulmonary angiography (CTPA); multi‐detector computed tomography (MDCT); pulmonary digital angiography; or pulmonary arteriograms.
We defined SSPE as small peripheral clots located beyond the fifth‐order pulmonary arteries, which are now frequently detected by the new generation of CTPA or MDCT (Donato 2010). SSPE typically presents with pleuritic chest pain caused by infarction, however often it is found in patients with minor symptoms or patients who are asymptomatic.
Isolated SSPE can be unique (one subsegmental vessel involved) or multiple (two or more subsegmental vessels involved) (Le Gal 2006).
Incidental SSPE is found casually in asymptomatic patients, usually by diagnostic imaging performed for other reasons (for example routine CT for cancer staging), while symptomatic SSPE is found in patients presenting with pleuritic pain or acute dyspnoea, or both.
Asymptomatic SSPE may occur in patients with distinct risk factors including hypercoagulation state, trauma or neoplasm, and in patients with deep venous thrombosis (DVT), both above and below the knee. Since modern MDCT is performed frequently in these patients, for various clinical indications, the incidental detection of SSPE on routine CTPA or MDCT is not uncommon.
We planned to exclude segmental PE since its treatment is mandatory in all cases.
Types of interventions
Intervention group: anticoagulation therapy
Control group: no intervention or placebo
We planned to consider anticoagulation therapy administered subcutaneously, intravenously, or orally.
Types of outcome measures
Primary outcomes
Three‐month thromboembolic risk (recurrence defined as a new SSPE or a new event with minimal segmental defects, or both, diagnosed by CTPA)
Major bleeding defined as fatal or clinically overt bleeding resulting in a fall in haemoglobin levels by 2 g/L or more or bleeding into critical anatomical sites (retroperitoneal, intraocular, pericardial, atraumatic intra‐articular, subdural haematoma, intraspinal haemorrhage) or leading to transfusion of ≥ 2 U of blood or red cells (White 2008)
All‐cause mortality
Secondary outcomes
Six‐month thromboembolic risk (recurrence defined as a new SSPE or a new event with minimal segmental defects, or both, diagnosed by CTPA)
Minor bleeding defined as bleeding requiring intervention but not qualifying as a major bleed, including bleeding precipitating treatment cessation (White 2008)
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for randomised controlled trials and controlled clinical trials without language, publication year or publication status restrictions:
the Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web searched on 26 November 2019);
the Cochrane Central Register of Controlled Trials (CENTRAL) Cochrane Register of Studies Online (CRSO 2019, Issue 10);
MEDLINE (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) (searched from 1 January 2017 to 26 November 2019);
Embase Ovid (searched from 1 January 2017 to 26 November 2019);
CINAHL Ebsco (searched from 1 January 2017 to 26 November 2019);
AMED Ovid (searched from 1 January 2017 to 26 November 2019).
The Information Specialist modelled search strategies for other databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6, Lefebvre 2011). Search strategies for major databases are provided in Appendix 1.
The Information Specialist searched the following trials registries on 26 November 2019:
the World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch);
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
We checked the reference lists of the identified studies for additional citations.
Data collection and analysis
Selection of studies
Two review authors (HHBY, VSNN) independently screened the trials identified by the literature search. We resolved disagreements by consulting with the third review author (PJFVB) and consulted with him for quality assurance of the processes.
Data extraction and management
We planned for two review authors (HHBY, VSNN) to independently extract data. We planned to resolve any discrepancies by discussion. We planned to use a standard data extraction form to extract the following information: characteristics of the study (design, method of randomisation); participants; interventions; outcomes (types of outcome measures, adverse events). We then planned to check for errors before entering the data into Review Manager.
Assessment of risk of bias in included studies
For the assessment of study quality, we planned to use the risk of bias approach for Cochrane reviews (Higgins 2011). We would use the following six criteria.
Random sequence generation
Is the allocation sequence adequately generated, for example with random number tables, computer‐generated random numbers? We planned to record this as 'low risk of bias' (the method used is either adequate or unlikely to introduce confounding), 'uncertain risk of bias' (there is insufficient information to assess whether the method used is likely to introduce confounding), or 'high risk of bias' (the method used, for example a quasi‐randomised trial, is likely to introduce confounding).
Allocation concealment
Is allocation adequately concealed in a way that would not allow either the investigators or the participants to know or influence allocation to an intervention group before an eligible participant was entered into the study (for example using central randomisation or sequentially numbered, opaque, sealed envelopes held by a third party)? We planned to record this as 'low risk of bias' (the method used, for example central allocation, is unlikely to introduce bias in the final observed effect), 'uncertain risk of bias' (there is insufficient information to assess whether the method used is likely to introduce bias in the estimate of effect), or 'high risk of bias' (the method used, for example an open random allocation schedule, is likely to introduce bias in the final observed effect).
Blinding
Are the study participants and personnel blinded from knowledge of which intervention a participant received? We planned to note where there has been partial blinding (for example where it has not been possible to blind participants but where outcome assessment was carried out without knowledge of group assignment). We planned to record this as 'low risk of bias' (blinding was performed adequately, or the outcome measurement is not likely to be influenced by lack of blinding), 'uncertain risk of bias' (there is insufficient information to assess whether the type of blinding used is likely to introduce bias in the estimate of effect), or 'high risk of bias' (no blinding or incomplete blinding, and the outcome or the outcome measurement is likely to be influenced by lack of blinding).
Incomplete outcome data
Are incomplete outcome data adequately addressed? Incomplete outcome data essentially include attrition, exclusions, and missing data. If any withdrawals occurred, were they described and reported by treatment group with the reasons given? We planned to record whether or not there were clear explanations for withdrawals and dropouts in the treatment groups. An example of an adequate method to address incomplete outcome data is the use of an intention‐to‐treat analysis (ITT). This item was planned to be recorded as 'low risk of bias' (the underlying reasons for missing data are unlikely to make treatment effects depart from plausible values, or proper methods have been employed to handle missing data), 'uncertain risk of bias' (there is insufficient information to assess whether the missing data mechanism in combination with the method used to handle missing data is likely to introduce bias in the estimate of effect), or 'high risk of bias' (the crude estimate of effects, for example a complete case estimate, will clearly be biased due to the underlying reasons for missing data and the methods used to handle missing data are unsatisfactory).
Selective reporting
Are reports of the study free from any suggestion of selective outcome reporting? We planned to interpret this as evidence that statistically non‐significant results might have been selectively withheld from publication, for example selective under‐reporting of data or selective reporting of a subset of data. We planned to record this as 'low risk of bias' (the trial protocol is available and all of the trial’s pre‐specified outcomes that are of interest in the review have been reported, or similar), 'uncertain risk of bias' (there is insufficient information to assess whether the magnitude and direction of the observed effect are related to selective outcome reporting), or 'high risk of bias' (not all of the trial’s pre‐specified primary outcomes have been reported, or similar).
Other bias
As a first step, we planned to copy information relevant to making a judgment on this criterion from the original publication into an assessment table. If additional information was available from the study authors, we planned to also enter this in the table along with an indication that it was unpublished information. Two review authors (HHBY and VSNN) planned to independently make a judgment as to whether the risk of bias for each criterion was considered to be 'low', 'uncertain', or 'high'. We would resolve disagreements by discussion.
We planned to consider trials which were classified as low risk of bias in sequence generation, allocation concealment, blinding, incomplete data, and selective outcome reporting as low bias‐risk trials.
Measures of treatment effect
We planned to use the risk ratio (RR) as the effect measure with 95% confidence intervals (CI) for dichotomous data.
We planned to present the results as mean differences (MD) with 95% CIs for continuous data. When pooling data across studies we would estimate the MD if the outcomes were measured in the same way between trials. We planned to use the standardised mean difference (SMD) to combine trials that measured the same outcome but used different methods.
Unit of analysis issues
The unit of analysis was planned to be each patient recruited into the trials.
Dealing with missing data
We planned to perform analysis on an ITT basis whenever possible (Newell 1992). An ITT analysis is an analysis in which all the participants in a trial are analysed according to the intervention to which they were allocated, whether they received the intervention or not. We would assume that participants who dropped out were non‐responders. For each trial we planned to report whether or not the investigators stated if the analysis was performed according to the ITT principle. If participants were excluded after allocation, we would report any details provided in full. Otherwise, we planned to adopt an available‐case analysis.
Assessment of heterogeneity
We planned to quantify inconsistency among the pooled estimates using the I2 statistic. This illustrates the percentage of the variability in effect estimates resulting from heterogeneity rather than sampling error (Higgins 2003; Higgins 2011). I2 = [(Q ‐ df)/Q] x 100%, where Q is the Chi2 statistic and df its degrees of freedom. We would assess heterogeneity between the trials by visual examination of the forest plot to check for overlapping CIs, the Chi2 test for homogeneity with a 10% level of significance, and the I2 statistic. We planned to use an I2 statistic value of less than 25% to denote low heterogeneity, 50% or greater significant heterogeneity, and 75% or greater substantial heterogeneity.
Assessment of reporting biases
We planned to assess the likelihood of potential publication bias using funnel plots provided that there were at least ten trials. This would have been in addition to assessing the risk of selective outcome reporting, considered under assessment of risk of bias in included studies. When small studies in a meta‐analysis tend to show larger treatment effects, we would consider other causes including selection biases, poor methodological quality, heterogeneity, artefact and chance.
Data synthesis
We planned to use the fixed‐effect model to analyse the data. If significant heterogeneity (for example I2 greater than 50%) was identified, we would compute pooled estimates of the treatment effect for each outcome using a random‐effects model (with two or more studies). We planned to undertake quantitative analyses of outcomes on an ITT basis.
Summary of findings and assessment of the certainty of the evidence
We planned to present the overall certainty of the evidence for each outcome according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which takes into account issues not only related to internal validity (risk of bias), but also evaluates the directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias (Atkins 2004). We planned to present a summary of the evidence in a 'Summary of findings' table, which provides key information about the best estimate of the magnitude of the effect, in relative terms and as absolute differences, numbers of participants, and studies addressing each important outcome and the rating of the overall confidence in effect estimates for each outcome. We planned to present results for the outcomes as described in Types of outcome measures. If meta‐analysis was not possible, we planned to present the results in a narrative 'Summary of findings' table.
Subgroup analysis and investigation of heterogeneity
In the case of significant clinical heterogeneity (I2 > 50%), we planned to use subgroup analysis. Subgroup analyses are secondary analyses in which the participants are divided into groups according to shared characteristics and the outcome analyses are conducted to determine if any significant treatment effect occurs according to that characteristic. If data permit, we intend to carry out the following subgroup analyses:
different types of anticoagulant therapy (e.g. low molecular weight heparin (LMWH), unfractionated heparin (UFH));
different doses for the same anticoagulant therapy (e.g. 1 mg/kg of fractionated heparin, 2 mg/kg of fractionated heparin);
different routes for UFH (e.g. subcutaneous, intravenous, oral);
short‐term (up to 30 days) versus long‐term follow‐up (> 30 days);
different co‐morbidities (e.g. chronic obstructive pulmonary disease (COPD), cardiac insufficiency);
single SSPE versus multiple SSPE.
We would perform the Chi2 test for subgroup differences set at a P value of 0.05 should sufficient data be available.
Sensitivity analysis
If there were an adequate number of studies, we intended to perform a sensitivity analysis to explore causes of heterogeneity and the robustness of the results. We planned to include the following factors in the sensitivity analysis, separating studies according to:
type of study design (RCTs versus CCTs);
trials with low risk of bias versus those with high risk of bias;
rates of withdrawal for each outcome (< 20% versus ≥ 20%).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies.
Results of the search
See Figure 1.
1.
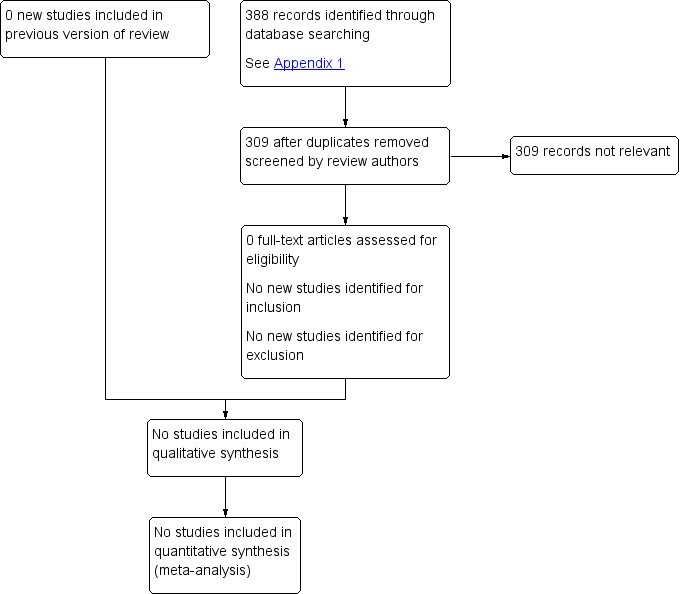
Study flow diagram.
Included studies
We identified no studies that met the inclusion criteria for this update or previous versions.
Excluded studies
No new studies were excluded for this update. A total of three studies were excluded from the previous versions (Donato 2010; Eyer 2005; Stein 1995). Donato 2010 was excluded because it was a cohort study, Eyer 2005 because it was a retrospective study and Stein 1995 because it was a case series.
Risk of bias in included studies
It was not possible to review methodological quality in the absence of studies eligible for inclusion in the review.
Effects of interventions
No published or unpublished studies were identified that assessed the effectiveness and safety of anticoagulation therapy versus control in patients with isolated subsegmental pulmonary embolism (SSPE) or incidental SSPE.
Discussion
Since the 1930s and the inception of anticoagulation, the PE mortality rate has been reduced from 30% to less than 3% (Meaney 1997). Traditionally, all patients with PEs are anticoagulated in a similar manner independent of the location, number and size of the thrombus, which can range from saddle to isolated subsegmental. This can explain the unchanged PE mortality rates over recent years despite all the advances in the diagnosis and treatment of the disease (Wiener 2011). Prasad 2012 suggests that many patients with subsegmental PE (SSPE) may be treated without benefit and indeed with increasing adverse events because of possible unnecessary use of anticoagulants. However, for ethical reasons and because of uncertain outcomes, all patients diagnosed with PE are still anticoagulated, regardless of clinical presentation. In a retrospective study, Raslan 2018 showed that 87% of patients with SSPE were systemically anticoagulated and this was followed by a high rate (34%) of clinically meaningful bleeding. This highlights that over‐treatment of SSPE is common, and associated with the most fearful complication of the anticoagulant therapy.
Computed tomographic pulmonary angiography (CTPA) changed the assessment of PE, improving its sensitivity for diagnosis, resulting in an increase in incidence from 62.1 to 112 per 100,000 adults in the Unites States, and, as a consequence, increasing the proportion of patients who receive anticoagulation therapy. Despite this, the concomitant reduction in mortality that was expected in diagnosed patients has not been observed (3% reduction, from 12.3 to 11.9 per 100,000 adults (Carrier 2010; Wiener 2011). It is possible that the additional thrombi identified by CTPA are not massive or segmental PEs but, on the contrary, could be subsegmental or incidental PE, which usually show few or no symptoms (Wiener 2011). Furthermore, many diagnoses of subsegmental PEs are more likely to be false‐positive interpretations than more proximal PEs (Pena 2012; Stein 2006).
SSPEs have been more prevalent in patients with low or intermediate probability ventilation/perfusion scans (Stein 1995; Stein 1997), and Perrier 1996 and Perrier 1999 showed that these patients can be safely monitored and managed with serial lower‐extremity ultrasonography without traditional anticoagulant therapy. Some authors have considered that one of the functions of the pulmonary circulation is to prevent small clots from entering the systemic circulation by acting as a filter and believe that such distal clots may occur even in healthy people, thus causing little clinical consequences (Gurney 1993; Schoepf 2004). Therefore, in theory, anticoagulation in these cases would be unnecessary.
The clinical outcomes for asymptomatic and symptomatic patients with isolated SSPE that have been missed by traditional diagnostic techniques remain an enigma. A cohort study conducted by Donato 2010 found that in patients diagnosed with SSPE and who were not anticoagulated, the recurrence and mortality were 0% at three months follow‐up, which is similar to a meta‐analysis of the findings for patients with a negative CTPA for PE showing the recurrence and fatal PE to be 1.4% and 0.5%, respectively (Moores 2004). In some exceptional conditions anticoagulant treatment has been discussed as unnecessary for: a) asymptomatic or symptomatic isolated SSPE with no deep venous thrombosis (DVT), suitable cardiopulmonary reserve and self‐limited risk factors; b) isolated SSPE or an indeterminate MDCT result, no DVT and anticoagulation treatment contraindicated (Eyer 2005; Goodman 2005).
It is also possible that isolated SSPE or incidental PE found by CTPA represents a more benign subset of PE, which reinforces the importance of a study on the clinical significance of peripheral and small clots and, therefore, the actual necessity of anticoagulating these patients. Not doing so would avoid the risks of this type of treatment. However, when the isolated SSPE or incidental PE is symptomatic among cancer patients, this is associated with poorer survival compared with patients without PE (O'Connell 2011b). In patients with cancer, the risk of recurrent venous thromboembolism is relevant even with anticoagulant therapy. Patients with SSPE also seem to have a higher risk of recurrent venous thromboembolism then patients with proximal PE (Kraaijpoel 2019).
Some physicians advocate that patients with any PE (including SSPE or incidental SSPE) on CTPA should be treated, especially if they have cancer and proximal thrombi, but conclusive evidence in support of this recommendation is still insufficient (Dobler 2019; Konstantinides 2014).
The European Society of Cardiology recommends a more individualised approach for patients with SSPE and suggests that there may be a role for lower limb ultrasonography to rule out a DVT (which requires treatment), and that in a patient with isolated SSPE and negative leg ultrasonography, an individualised decision about anticoagulant therapy needs to be based on the risk/benefit ratio of anticoagulation and the presence of lower limb DVT (Konstantinides 2014). However, in patients with cancer, even in an incidental PE, whether it involves segmental or more proximal branches, multiple subsegmental vessels, or a single subsegmental vessel in association with DVT the recommended anticoagulant treatment should use the same approach as for symptomatic PE (Konstantinides 2019).
An isolated SSPE without cancer may not require anticoagulation therapy, and case by case decision making is therefore recommended taking into account the patient's situation and preference (Dobler 2019).
The 10th edition of the Antithrombotic Therapy for VTE Disease guideline suggests clinical surveillance over anticoagulation in patients with SSPE under specific conditions such as DVT of the legs and in other locations are excluded by ultrasonography imaging; high risk of bleeding; no high risk factors for recurrent or progressive VTE (such as hospitalised patients, reduced mobility and active cancer) and good cardiopulmonary reserve (Kearon 2016).
Summary of main results
There is currently no evidence from RCTs to assess the effectiveness and safety of anticoagulation therapy versus control in patients with isolated subsegmental pulmonary embolism (SSPE) or incidental SSPE.
Overall completeness and applicability of evidence
There is currently no evidence from RCTs.
Quality of the evidence
It was not possible to review certainty of the evidence in the absence of studies eligible for inclusion in the review.
Potential biases in the review process
We have tried to minimise potential biases in our review process by performing extensive literature searches.
Agreements and disagreements with other studies or reviews
There are few well‐conducted studies comparing the clinical outcomes among treated and untreated patients with SSPE. During the search for this review we identified three articles, which were excluded from this systematic review because of the observational design. A case series by Stein 1995 demonstrated that untreated patients had mild PE, characterised by pulmonary angiograms and a ventilation/perfusion scan to involve smaller vessels and result in fewer perfusion defects, which was different from the observed PE in treated patients. In the same study, death or fatal recurrent PE occurred in one of 20 (5%) untreated patients and in nine of 376 (2.4%) of those who were treated (P = NS). A retrospective study by Eyer 2005 in which 25 of 67 (37%) patients with isolated SSPE were not treated showed that no recurrent PE or adverse effects were reported during follow‐up, similar to the observed finding in the treated group (0% recurrent PE and 3% mortality from other diseases). In a cohort study Donato 2010 reviewed 10,453 consecutive CTPAs over a 74‐month period and 93 patients with isolated SSPE were selected for study: 71 (76%) were treated for PE and 22 (23.6%) were observed only. After a three‐month follow‐up, the treated group showed two non‐PE‐related deaths (2.8%), eight bleeding events (11.3%) and one PE recurrence (1.4%), while no deaths, no PE recurrence or bleeding events were reported in the untreated group. The authors concluded that the short‐term prognosis for recurrent PE may be lower than the risk of adverse events with anticoagulation in patients at high risk of haemorrhage. Comparing the results of Donato 2010 with the clinical course of Carson 1992, it can be observed that the three‐month outcomes of patients with isolated SSPE, including PE recurrence and PE‐related mortality, were significantly better (1.05% recurrence, 0% mortality) than the outcomes reported in treated classic PE (8% recurrence, 1.7% mortality). Although the studies reported above are not interventional, their results reinforce the theory that isolated SSPE could represent a select group of patients with favourable prognosis even if left untreated.
A systematic review and meta‐analysis by Carrier 2010 showed that CTPA has increased the rate of patients diagnosed with SSPE, with 9.4% who potentially benefit from anticoagulant treatment. However, the expected reduction of the three‐month risk of thromboembolism has not occurred, therefore suggesting that SSPE may not be clinically relevant.
A review by Morgan 2015, identified six observational studies and suggested that clinical outcomes are comparable in this subgroup of patients. However, the observational nature of the studies rules out definitive conclusions from these results.
A cohort study conducted by den Exter 2013 analysed 3728 consecutive patients with clinically suspected PE. Isolated symptomatic SSPE was found in 116 of 748 (15.5%) patients with confirmed PE and who were treated. During three months of follow‐up, the respective non‐significant cumulative risks for recurrent venous thromboembolism (VTE) were 3.6% for SSPE and 2.5% for proximal PE (hazard ratio (HR) 1.6, 95% CI 0.5 to 4.8); and for mortality were 10.7% and 6.5% (HR 1.5, 95% CI 0.8 to 2.6). In the group in which PE had been ruled out, 25 patients (0.8%) developed VTE during follow‐up with a cumulative risk of 1.1%. There were 156 deaths out of 2980 (5.2%) patients with PE ruled out and their cumulative risk (5.4%) was significantly lower compared with the risk for patients with SSPE (P = 0.01). In contrast to the hypothesis that SSPE may represent a more benign subset of PE, this study showed that for patients with symptomatic SSPE, even treated, the incidence of recurrent VTE and mortality were significantly higher than for patients without PE.
The systematic review and meta‐analysis by Bariteau 2018, emphasised the lack of studies making a coherent assumption about any benefit or damage following anticoagulation for SSPE. Comparison of pooled data from uncontrolled outcome studies showed no increase in VTE recurrence or death rates for patients who were not treated. However, again, this is limited by few numbers and the scarcity of controlled clinical trials.
A prospective observational study assessing the safety of withholding anticoagulation in patients with isolated symptomatic SSPE without DVT is in progress and is currently recruiting participants (NCT01455818).
Authors' conclusions
Implications for practice.
There is no evidence from randomised controlled trials to assess the effectiveness and safety of anticoagulation therapy versus control in patients with isolated subsegmental pulmonary embolism (SSPE) or incidental SSPE. We are unable to draw any conclusions regarding implications for practice.
Implications for research.
This review highlights the need for continued research into the selection of appropriate treatment for acute PE. The low statistical power of the available non‐RCT evidence argues strongly in favour of designing new RCTs for further evaluation of the risk‐benefit ratio of anticoagulant therapy in isolated subsegmental or incidental PE.
This review therefore intends to encourage researchers to perform RCTs to answer the clinical question under study. We believe that these studies can be feasible and safe in carefully selected groups of SSPE patients (more specifically in patients with isolated subsegmental pulmonary embolism (SSPE) or incidental SSPE) and this knowledge will bring both clinical and economic advantages.
What's new
| Date | Event | Description |
|---|---|---|
| 5 December 2019 | New search has been performed | New search run. No new studies included. No new studies excluded. |
| 5 December 2019 | New citation required but conclusions have not changed | New search run. No new studies included. No new studies excluded. Text updated to reflect current Cochrane standards. No change to conclusions. |
History
Protocol first published: Issue 11, 2012 Review first published: Issue 4, 2014
| Date | Event | Description |
|---|---|---|
| 4 January 2016 | New search has been performed | New search run and review updated. No new studies identified |
| 4 January 2016 | New citation required but conclusions have not changed | New search run and review updated. No new studies identified. Minor changes to the text. No change to conclusions |
Acknowledgements
We express our gratitude to Regina El Dib and Thais Queluz for their important contribution to the previous versions of this review. We would like to thank the Cochrane Vascular editorial base for their help during the preparation of this review.
Appendices
Appendix 1. Database searches
| Source | Search strategy | Hits retrieved |
| CENTRAL via CRSO | #1 MESH DESCRIPTOR Pulmonary Embolism EXPLODE ALL TREES 914 #2 MESH DESCRIPTOR Thromboembolism EXPLODE ALL TREES 1863 #3 emboli*:TI,AB,KY 9779 #4 microemboli*:TI,AB,KY 284 #5 micro‐emboli*:TI,AB,KY 47 #6 (PE or VTE):TI,AB,KY 6230 #7 (pulmonary near3 clot):TI,AB,KY 10 #8 (lung near3 clot):TI,AB,KY 0 #9 SSPE:TI,AB,KY 9 #10 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 15708 #11 subsegment*:TI,AB,KY 82 #12 #10 AND #11 23 |
26 March 2018: 6 26 Nov 2019: 23 |
| Clinicaltrials.gov | subsegmental | 26 March 2018: 6 26 Nov 2019: 9 |
| ICTRP Search Portal | subsegmental | 26 March 2018: 0 26 Nov 2019: 0 |
| MEDLINE | 1 exp Pulmonary Embolism/ 35920 2 exp Thromboembolism/ 50963 3 (PE or SSPE).ti,ab. 33828 4 vte.ti,ab. 8480 5 emboli$.ti,ab. 110498 6 microemboli$.ti,ab. 3095 7 (pulmonary adj4 clot$).ti,ab. 269 8 (lung adj4 clot$).ti,ab. 62 9 or/1‐8 192737 10 subsegment$.ti,ab. 1750 11 9 and 10 480 |
26 March 2018: 78 26 Nov 2019: 43 |
| EMBASE | 1 exp lung embolism/ 66865 2 thromboembolism/ 49292 3 venous thromboembolism/ 30143 4 embolis*.ti,ab. 61015 5 microembolis*.ti,ab. 648 6 (pulmonary adj4 clot$).ti,ab. 323 7 vte.ti,ab. 16062 8 (PE or SSPE).ti,ab. 45946 9 (lung adj4 clot$).ti,ab. 76 10 or/1‐9 190671 11 subsegment*.ti,ab. 2032 12 10 and 11 610 13 randomized controlled trial/ 446235 14 controlled clinical trial/ 411625 15 random$.ti,ab. 1147104 16 randomization/ 69127 17 intermethod comparison/ 222434 18 placebo.ti,ab. 219134 19 (compare or compared or comparison).ti. 329390 20 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 1585695 21 (open adj label).ti,ab. 61445 22 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 155643 23 double blind procedure/ 121111 24 parallel group$1.ti,ab. 19247 25 (crossover or cross over).ti,ab. 71025 26 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 244589 27 (assigned or allocated).ti,ab. 285412 28 (assigned or allocated).ti,ab. 285412 29 (controlled adj7 (study or design or trial)).ti,ab. 256876 30 (volunteer or volunteers).ti,ab. 169739 31 trial.ti. 210016 32 or/13‐31 3414033 33 12 and 32 191 |
26 March 2018: 168 26 Nov 2019: 31 |
| CINAHL | S11 S9 AND S10 S10 TX subsegment* S9 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 S8 TX SSPE S7 TX pulmonary N3 clot S6 TX VTE S5 TX PE S4 TX micro‐emboli* S3 TX microemboli* S2 TX emboli* S1 MH "Thromboembolism+" |
26 March 2018: 9 26 Nov 2019: 21 |
| AMED | 1 exp Pulmonary embolism/ 53 2 exp Thromboembolism/ 72 3 emboli*.ti,ab. 205 4 (PE or SSPE).ti,ab. 159 5 vte.ti,ab. 33 6 microemboli*.ti,ab. 5 7 (pulmonary adj4 clot*).ti,ab. 0 8 (lung adj4 clot*).ti,ab. 0 9 or/1‐8 418 10 subsegment*.ti,ab. 1 11 9 and 10 1 |
26 March 2018: 0 26 Nov 2019: 0 |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Donato 2010 | Cohort study |
| Eyer 2005 | Retrospective study |
| Stein 1995 | Case series |
Differences between protocol and review
In the previous version of the review, our control group was defined as 'no intervention' However, as a randomised study in SSPE with a no active treatment as control would be possible, we included both 'placebo' and 'no intervention' in the definition of control. We reclassified 'all‐cause mortality' as a primary outcome, and removed 'dropouts and losses to follow‐up' from secondary outcomes as the use of dropouts as a surrogate marker for safety or tolerability is subject to bias (Higgins 2011).
Contributions of authors
HHBY: conceiving the review, undertaking manual searches, screening search results, organizing retrieval of papers, screening retrieved papers against inclusion criteria, appraising quality of papers, abstracting data from papers, writing to authors of papers for additional information, obtaining additional data about papers, obtaining and screening data on unpublished studies, data management for the review, entering data into Review Manager (RevMan 5), data entry, interpretation of data, statistical inferences, writing the review, responsible for reading and checking review before submission VSNN: screening search results, screening retrieved papers against inclusion criteria, appraising quality of papers, abstracting data from papers, data management for the review, entering data into Review Manager (RevMan 5), RevMan statistical data analysis, other statistical analysis not using RevMan, data entry, interpretation of data, statistical inferences, writing the review, responsible for reading and checking review before submission PJFVB: obtaining and screening data on unpublished studies, data management for the review, interpretation of data, statistical inferences, writing the review, responsible for reading and checking review before submission
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
HHBY: none known VSNN: none known PJFVB: none known
New search for studies and content updated (no change to conclusions)
References
References to studies excluded from this review
Donato 2010 {published data only}
- Donato AA, Khoche S, Santora J, Wagner B. Clinical outcomes in patients with isolated subsegmental pulmonary emboli diagnosed by multi‐detector CT pulmonary angiography. Thrombosis Research 2010;126(4):e266‐70. [DOI] [PubMed] [Google Scholar]
Eyer 2005 {published data only}
- Eyer BA, Goodman LR, Washington L. Clinicians' response to radiologists' reports of isolated subsegmental pulmonary embolism or inconclusive interpretation of pulmonary embolism using MDCT. American Journal of Roentgenology 2005;184(2):623‐8. [DOI] [PubMed] [Google Scholar]
Stein 1995 {published data only}
- Stein PD, Henry JW, Relyea B. Untreated patients with pulmonary embolism. Outcome, clinical, and laboratory assessment. Chest 1995;107(4):931‐5. [DOI] [PubMed] [Google Scholar]
Additional references
Ageno 2012
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G, American College of Chest Physicians. Oral anticoagulant therapy. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2012;141(2 Suppl):e44s–88s. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alotaibi 2016
- Alotaibi GS, Wu C, Senthilselvan A, McMurtry MS. Secular trends in incidence and mortality of acute venous thromboembolism: The AB‐VTE population‐based study. American Journal of Medicine 2016;129(8:879):e:19‐26. [DOI: 10.1016/j.amjmed.2016.01.041] [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. British Medical Journal 2004;328(7454):1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bariteau 2018
- Bariteau A, Stewart LK, Emmett TW, Kline JA. Systematic review and meta‐analysis of outcomes of patients with subsegmental pulmonary embolism with and without anticoagulation treatment. Academic Emergency Medicine 2018;25(7):828‐35. [DOI] [PubMed] [Google Scholar]
Carrier 2010
- Carrier M, Righini M, Wells PS, Perrier A, Anderson DR, Rodger MA, et al. Subsegmental pulmonary embolism diagnosed by computed tomography: incidence and clinical implications. A systematic review and meta‐analysis of the management outcome studies. Journal of Thrombosis and Haemostasis 2010;8(8):1716‐22. [DOI] [PubMed] [Google Scholar]
Carson 1992
- Carson JL, Kelley MA, Duff A, Weg JG, Fulkerson WJ, Palevsky HI, et al. The clinical course of pulmonary embolism. New England Journal of Medicine 1992;326(19):1240–5. [DOI] [PubMed] [Google Scholar]
Dalen 1975
- Dalen JE, Alpert JS. Natural history of pulmonary embolism. Progress in Cardiovascular Diseases 1975;17(4):259‐70. [DOI] [PubMed] [Google Scholar]
Dalen 2002
- Dalen JE. Pulmonary embolism: what have we learned since Virchow?: treatment and prevention. Chest 2002;122(5):1801‐17. [DOI] [PubMed] [Google Scholar]
den Exter 2013
- Exter PL, Es J, Klok FA, Kroft LJ, Kruip MJHA, Kamphuisen PW, et al. Risk profile and clinical outcome of symptomatic subsegmental acute pulmonary embolism. Blood 2013;122(7):1144‐9. [DOI] [PubMed] [Google Scholar]
den Exter 2014
- Exter PL, Hulle TV, Klok FA, Huisman MV. Advances in the diagnosis and management of acute pulmonary embolism. Thrombosis Research 2014;133:S10‐S16. [DOI] [PubMed] [Google Scholar]
Dentali 2010
- Dentali F, Ageno W, Becattini C, Galli L, Gianni M, Riva N, et al. Prevalence and clinical history of incidental, asymptomatic pulmonary embolism: a meta‐analysis. Thrombosis Research 2010;125(6):518‐22. [DOI] [PubMed] [Google Scholar]
Dinwoodey 2006
- Dinwoodey DL, Ansell JE. Heparins, low‐molecular‐weight heparins, and pentasaccharides. Clinics in Geriatric Medicine 2006;22(1):1‐15, vii. [DOI] [PubMed] [Google Scholar]
Dobler 2019
- Dobler CC. Overdiagnosis of pulmonary embolism: definition, causes and implications. Breathe 2019;15(1):45‐52. [DOI: 10.1183/20734735.0339‐2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodman 2005
- Goodman LR. Small pulmonary emboli: what do we know?. Radiology 2005;234:654‐8. [DOI] [PubMed] [Google Scholar]
Goy 2015
- Goy J, Lee J, Levine O, Chaudhry S, Crowther M. Sub‐segmental pulmonary embolism in three academic teaching hospitals: a review of management and outcomes. Journal of Thrombosis and Haemostasis 2015;13(2):214‐8. [DOI: 10.1111/jth.12803] [DOI] [PubMed] [Google Scholar]
Gurney 1993
- Gurney JW. No fooling around: direct visualization of pulmonary embolism. Radiology 1993;188:618‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Huang 2014
- Huang W, Goldberg RJ, Anderson FA, Kiefe CI, Spencer FA. Secular trends in occurrence of acute venous thromboembolism: the Worcester VTE study (1985‐2009). American Journal of Medicine 2014;127(9):829‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jiménez 2007
- Jiménez Castro D, Sueiro A, Díaz G, Escobar C, García‐Rull S, Picher J, et al. Prognostic significance of delays in diagnosis of pulmonary of pulmonary embolism. Thrombosis Research 2007;121(2):153‐8. [DOI] [PubMed] [Google Scholar]
Kearon 2008
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ, American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians evidence‐based clinical practice guidelines (8th edition). Chest 2008;133(6 Suppl):S454–S545. [DOI] [PubMed] [Google Scholar]
Kearon 2012
- Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis. 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):e419–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kearon 2016
- Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic Therapy for VTE Disease CHEST Guideline and Expert Panel Report. Chest 2016;149(2):315‐52. [DOI] [PubMed] [Google Scholar]
Konstantinides 2014
- Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). European Heart Journal 2014;35(43):3033–69k. [DOI] [PubMed] [Google Scholar]
Konstantinides 2019
- Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). European Respiratory Journal 2019;54:1901647. [DOI: ] [DOI] [PubMed] [Google Scholar]
Kraaijpoel 2019
- Kraaijpoel N, Bleker SM, Meyer G, Mahe I, Munoz A, et al. Treatment and long‐term clinical outcomes of incidental pulmonary embolism in patients with cancer: An international prospective cohort study. Journal of Clinical Oncology 2019;37(20):1713‐20. [DOI] [PubMed] [Google Scholar]
Le Gal 2006
- Gal G, Righini M, Parent F, Strijen M, Couturaud F. Diagnosis and management of sub‐segmental pulmonary embolism. Journal of Thrombosis and Haemostasis 2006;4(4):724–31. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Meaney 1997
- Meaney JFM, Weg JG, Chenevert TL, Stafford‐Johnson D, Hamilton BH, Prince MR. Diagnosis of pulmonary embolism with magnetic resonance angiography. New England Journal of Medicine 1997;336(20):1422‐7. [DOI] [PubMed] [Google Scholar]
Moores 2004
- Moores LK, Jackson WL Jr, Shorr AF, Jackson JL. Meta‐analysis: outcomes in patients with suspected pulmonary embolism managed with computed tomographic pulmonary angiography. Annals of Internal Medicine 2004;141(11):866‐74. [DOI] [PubMed] [Google Scholar]
Morgan 2015
- Morgan C, Choi H. Do patients with a clinically suspected subsegmental pulmonary embolism need anticoagulation therapy?. Emergency Medicine Journal 2015;32:744‐7. [DOI] [PubMed] [Google Scholar]
Mullins 2000
- Mullins MD, Becker DM, Hagspiel KD, Philbrick JT. The role of spiral volumetric computed tomography in the diagnosis of pulmonary embolism. Archives of Internal Medicine 2000;160(3):293–8. [DOI] [PubMed] [Google Scholar]
Nazaroglu 2009
- Nazaroglu H, Ozmen CA, Akay HO, Kilinc I, Bilici A. 64‐MDCT pulmonary angiography and CT venography in the diagnosis of thromboembolic disease. American Journal of Roentgenology 2009;192(3):654–61. [DOI] [PubMed] [Google Scholar]
NCT01455818
- NCT01455818. A study to evaluate the safety of withholding anticoagulation in patients with subsegmental PE who have a negative serial bilateral lower extremity ultrasound (SSPE). clinicaltrials.gov/ct2/show/NCT01455818 (first received 20 October 2011).
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837–41. [DOI] [PubMed] [Google Scholar]
O'Connell 2011a
- O'Connell C, Razavi P, Ghalichi M, Boyle S, Vasan S, Mark L, et al. Unsuspected pulmonary emboli adversely impact survival in patients with cancer undergoing routine staging multi‐row detector computed tomography scanning. Journal of Thrombosis and Haemostasis 2011;9(2):305‐11. [DOI] [PubMed] [Google Scholar]
O'Connell 2011b
- O'Connell, Razavi P, Liebman H. Symptoms adversely impact survival among patients with cancer and unsuspected pulmonary embolism. Journal of Clinical Oncology 2011;29(31):4208‐9. [DOI] [PubMed] [Google Scholar]
Peiman 2016
- Peiman S, Abbasi M, Allameh SF, Gharabaghi MA, Abtahi H, Safavi E. Subsegmental pulmonary embolism: a narrative review. Thrombosis Research 2016;138:55‐60. [DOI] [PubMed] [Google Scholar]
Pena 2012
- Pena E, Kimpton M, Dennie C, Peterson R, Gal G, Carrier M. Difference in interpretation of computed tomography pulmonary angiography diagnosis of subsegmental thrombosis in patients with suspected pulmonary embolism. Journal of Thrombosis and Haemostasis 2012;10(3):496‐8. [DOI] [PubMed] [Google Scholar]
Perrier 1996
- Perrier A, Bounameaux H, Morabia A, Moerloose P, Slosman D, Didier D, et al. Diagnosis of pulmonary embolism by a decision analysis‐based strategy including clinical probability, D‐dimer levels, and ultrasonography: a management study. Archives of Internal Medicine 1996;156(5):531–6. [PubMed] [Google Scholar]
Perrier 1999
- Perrier A, Desmarais S, Miron MJ, Moerloose P, Lepage R, Slosman D, et al. Non‐invasive diagnosis of venous thromboembolism in outpatients. Lancet 1999;353(9148):190–5. [DOI] [PubMed] [Google Scholar]
Piazza 2006
- Piazza G, Goldhaber SZ. Acute pulmonary embolism part II: treatment and prophylaxis. Circulation 2006 ;114(3):e42‐7. [DOI] [PubMed] [Google Scholar]
Prasad 2012
- Prasad V, Rho J, Cifu A. The diagnosis and treatment of pulmonary embolism. A metaphor for medicine in the evidence‐based medicine era. Archives of Internal Medicine 2012;172(12):955‐8. [DOI] [PubMed] [Google Scholar]
Raschke 1993
- Raschke RA, Reilly BM, Guidry JR, Fontana JR, Srinivas S. The weight‐based heparin dosing nomogram compared with a standard care nomogram: a randomized controlled trial. Annals of Internal Medicine 1993;119(9):874‐81. [DOI] [PubMed] [Google Scholar]
Raslan 2018
- Raslan IA, Chong J, Gallix B, Lee TC, McDonald EG. Rates of over treatment and treatment‐related adverse effects among patients with subsegmental pulmonary embolism. JAMA Internal Medicine 2018;178(9):1272‐4. [DOI: 10.1001/jamainternmed.2018.2971] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rathbun 2000
- Rathbun SW, Raskob GE, Whitsett TL. Sensitivity and specificity of helical computed tomography in the diagnosis of pulmonary embolism: a systematic review. Annals of Internal Medicine 2000;132(3):227‐32. [DOI] [PubMed] [Google Scholar]
Remy‐Jardin 1992
- Remy‐Jardin M, Remy J, Wattinne L, Giraud F. Central pulmonary thromboembolism: diagnosis with spiral volumetric CT with the single‐breath‐hold technique‐comparison with pulmonary angiography. Radiology 1992;185:381‐7. [DOI] [PubMed] [Google Scholar]
Ruiz 2003
- Ruiz Y, Caballero P, Caniego J, Friera A, Olivera M, Tagarro D, et al. Prospective comparison of helical CT with angiography in pulmonary embolism: global and selective vascular territory analysis. Interobserver agreement. European Radiology 2003;13:823‐9. [DOI] [PubMed] [Google Scholar]
Ryu 1998
- Ryu JH, Olson EJ, Pellikka PA. Clinical recognition of pulmonary embolism: problem of unrecognized and asymptomatic cases. Mayo Clinic Proceedings 1998;73(9):873‐9. [DOI] [PubMed] [Google Scholar]
Schoepf 2001
- Schoepf UJ, Kessler MA, Rieger CT, Herzog P, Klotz E, Wiesgigl S, et al. Multislice CT imaging of pulmonary embolism. European Radiology 2001;11(11):2278‐86. [DOI] [PubMed] [Google Scholar]
Schoepf 2004
- Schoepf UJ, Costello P. CT angiography for diagnosis of pulmonary embolism: state of the art. Radiology 2004;230(2):329–37. [DOI] [PubMed] [Google Scholar]
Segal 2007
- Segal JB, Streiff MB, Hofmann LV, Thornton K, Bass EB. Management of venous thromboembolism: a systematic review for a practice guideline. Annals of Internal Medicine 2007;146(3):211‐22. [DOI] [PubMed] [Google Scholar]
Spyropoulos 2007
- Spyropoulos AC. Investigational treatments of venous thromboembolism. Expert Opinion on Investigational Drugs 2007;16(4):431‐40. [DOI] [PubMed] [Google Scholar]
Stein 1997
- Stein PD, Henry JW. Prevalence of acute pulmonary embolism in central and subsegmental pulmonary arteries and relation to probability interpretation of ventilation/perfusion lung scans. Chest 1997;111(5):1246–8. [DOI] [PubMed] [Google Scholar]
Stein 2006
- Stein PD, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, et al. Multidetector computed tomography for acute pulmonary embolism. New England Journal of Medicine 2006;354(22):2317‐27. [DOI] [PubMed] [Google Scholar]
Storto 2005
- Storto ML, Credico A, Guido F, Larici AR, Bonomo L. Incidental detection of pulmonary emboli on routine MDCT of the chest. American Journal of Roentgenology 2005;184:264‐7. [DOI] [PubMed] [Google Scholar]
Torbicki 2008
- Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galiè N, Pruszczyk P, et al. ESC Committee for Practice Guidelines (CPG). Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). European Heart Journal 2008;29(18):2276‐315. [DOI] [PubMed] [Google Scholar]
White 2008
- White RH, Zhou H, Murin S. Death due to recurrent thromboembolism among younger healthier individuals hospitalized for idiopathic pulmonary embolism. Thrombosis and Haemostasis 2008;99(4):683‐90. [DOI] [PubMed] [Google Scholar]
Wiener 2011
- Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Archives of Internal Medicine 2011;171(9):831‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Yoo 2012
- Yoo HHB, Queluz THAT, Dib RP. Anticoagulant treatment for subsegmental pulmonary embolism. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD010222] [DOI] [Google Scholar]
Yoo 2014
- Yoo HHB, Queluz THAT, Dib R. Anticoagulant treatment for subsegmental pulmonary embolism. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD010222.pub2] [DOI] [PubMed] [Google Scholar]
Yoo 2016
- Yoo HHB, Queluz THAT, Dib RP. Anticoagulant treatment for subsegmental pulmonary embolism. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD010222.pub3] [DOI] [PubMed] [Google Scholar]


