Abstract
Purpose
To review our institutional experience of treating cholangiocarcinoma using stereotactic body radiation therapy (SBRT).
Methods and Materials
A total of 40 patients with intrahepatic (n = 25) or perihilar (n = 15) cholangiocarcinoma treated with SBRT were retrospectively reviewed. SBRT was delivered in 1 to 5 fractions with median dose of 40 Gy. Competing risk analysis was used to estimate cumulative incidence of local in-field, local out-of-field, regional, and distant failure. Kaplan-Meier and log-rank tests were used to calculate overall survival (OS). Toxicity was scored using Common Terminology Criteria for Adverse Events, version 4.0.
Results
The median follow-up time was 18 months. The 1-year incidence of local in-field, local out-of-field, regional, and distant failure was 8%, 23%, 13%, and 22%, respectively. Median OS was 23 months and 1- and 2-year OS rates were 69% and 39%, respectively. Patients with perihilar tumors had a 1-year incidence of regional failure of 24% and worse OS (P = .013). Patients with regional failure were more likely to develop distant metastases, 32% versus 19% at 1 year (P = .11). Acute grade 3 + hepatobiliary toxicity developed in 15 patients (36%).
Conclusions
In this series of cholangiocarcinoma patients treated with definitive SBRT, patterns of failure reveal that regional failures are not insignificant, particularly for perihilar tumors. Elective nodal irradiation of regional lymphatics should be considered when using SBRT. A prospective study of elective nodal irradiation in patients with perihilar tumors would further clarify whether this approach improves outcomes without increasing hepatobiliary toxicity.
Introduction
Cholangiocarcinoma is a rare and aggressive malignancy that accounts for approximately 3% of all gastrointestinal cancers in the United States.1 It is the second most common cancer of the liver after hepatocellular carcinoma, and in recent years, the incidence in the United States has been increasing.2 Tumors can arise from either intrahepatic or extrahepatic bile ducts and most commonly in the perihilar region, referred to as Klatskin tumors. Surgical resection offers the best chance of cure; however, the majority of patients present with inoperable disease.3 Even with surgical resection, as many as 50% of patients will have a local recurrence. In addition, regional and distant metastases remain a significant problem, especially in the setting of limited systemic treatment options.4 As such, exploration of the efficacy of other locoregional treatment modalities is warranted.
Radiation therapy has been successfully used for the definitive treatment of unresectable cholangiocarcinoma, typically in combination with systemic therapy. Data from MD Anderson show local control rates of 78% at 3 years with a biologic equivalent dose (BED) of >80.5 Gy using conventional or hypofractionation and chemotherapy.5 Recently, Sandler et al6 reported on 31 patients with unresectable cholangiocarcinoma who underwent stereotactic body radiation therapy (SBRT) at University of California, Los Angeles with a median survival of 15.7 months and 1-year local control rate of 78%; however, regional and distant failure rates were not reported. In this study, we have compiled one of the largest single-institution experiences of unresectable cholangiocarcinoma treated with SBRT. We specifically evaluated rates of regional and distant recurrence to propose elective nodal irradiation (ENI) as a novel treatment approach for patients with perihilar disease.
Methods and Materials
Patients
We retrospectively reviewed the records of 40 patients treated with definitive SBRT for unresectable cholangiocarcinoma at a single university center between 2003 and 2017. This study was approved by our institutional review board. All patients underwent a full staging workup, including history, physical examination, and cross-sectional imaging with either computed (CT) or positron emission tomography (PET) to evaluate for metastatic disease. Biopsy or brushings confirmed the diagnosis of cholangiocarcinoma in all patients. Patients were treated using either a robotic linear accelerator (n = 9, 2003–2007) or a standard linear accelerator (n = 33, 2008–2017). Detailed clinical and pathologic information was obtained for each patient using our electronic medical system. Patient characteristics, including stage at diagnosis, the presence of underlying liver disease, Child-Pugh scores, prior liver-directed therapies, and radiation and chemotherapy details, were recorded.
Follow-up information was obtained from patient records. Local, regional, and distant failure along with available toxicity data were recorded. Local failure (LF) was defined as failure within the liver within the irradiated field (in-field) or failure within the liver outside of the irradiated field (out-of-field hepatic). Regional failure (RF) was defined as lymph node metastases in regional draining nodal basins, such as the aortocaval, gastrohepatic, celiac, porta hepatis, and para-aortic regions. Distant failure (DF) was defined as failure outside of the liver and biliary system. Individual sites of DF, including peritoneum, lung, bone, brain, and soft tissue, were recorded. Date of death was determined by evaluation of patient records, the Social Security Death Index, and online published obituaries. Toxicity was defined as per Common Terminology Criteria for Adverse Events, version 4.0 criteria. Patients were followed every 3 months with physical examination and abdominal imaging in the form of either CT or PET.
SBRT simulation and treatment
SBRT treatment planning and delivery details have been previously described.7 Briefly, patients were simulated in supine position with arms up in an Alpha cradle (Smithers Medical Products, Inc, Canton, OH) and wing board. A CT with contrast was obtained for all patients and 32 patients also underwent a treatment planning PET scan. Four-dimensional (4D) respiratory monitoring was used for tumor motion verification. Fiducials (n = 21) or a biliary stent (n = 17) were placed in 38 patients. Surrogate structures used for motion management included fiducials in 21 patients, biliary stent in 17 patients, and diaphragm in 2 patients.
Normal tissue and tumor contouring was performed using the MultiPlan (Accuray, Sunnyvale, CA; n = 9) or Eclipse (Varian Medical Systems Inc, Palo Alto, CA; n = 32) workstations. In all cases, treatment planning scans were fused with diagnostic imaging to help aid in the delineation of the gross tumor volume (GTV). The GTV was defined on contrast-enhanced CT or PET-CT as the primary tumor. In cases where the primary tumor was not visible on cross-sectional imaging, a clinical target volume was constructed in its place using details from endoscopic retrograde cholangiopancreatography reports. Lymph nodes that were grossly involved by tumor at the time of treatment planning, defined as increased radiotracer uptake on treatment planning PET, or those >1 cm in short axis on CT, were included in the GTV. The internal target volume (ITV) was generated from the 30% to 70% scan phases using the 4-dimensional CT, although all phases were evaluated to determine which had the maximal diaphragmatic excursion so as to be excluded from the ITV. A 2- to 3-mm expansion was automatically generated from the ITV to create the planning target volume.
Prescribed doses were determined at the attending's discretion and ranged from 26 to 50 Gy (median dose 40 Gy) in 1 to 5 fractions (median 5 fractions). Dose constraints included: sparing ≥700 mL of normal liver from receiving ≥15 Gy, keeping the mean dose to the liver ≤15 Gy, keeping the V33 of the duodenum <1 mL, and the duodenal Dmax <40 Gy. The mean dose to the central hepatobiliary tract (cHBT) was restricted to <14 Gy, and V72 <21 mL.8
Statistical analysis
Statistical analysis was conducted using Statistical Analysis Software (version 9.4, SAS Institute Inc, Cary, NC). Time to recurrence or death was calculated from the date of diagnosis the date of local, regional, and distant failure or death, respectively. Competing risks analysis was performed using the Fine and Gray method to analyze cumulative incidence of local, regional, and distant failure, with death as a competing risk. The relationship between dosimetric and clinical parameters and grade 2 or higher toxicity was analyzed using a univariable logistic regression model. Overall survival was calculated using Kaplan-Meier and log-rank tests with patients censored at date of last follow-up, loss to follow-up, or death. The binary relationship between OS and several clinical parameters including regional failure, patient age at diagnosis, Karnofsky performance status, tumor size, total radiation dose, BED, tumor location, Child-Pugh score, and pretreatment Child-Pugh class was analyzed using a univariable logistic regression model. Statistical significance was determined as a 2-sided P value of < .05.
Results
Patients
A total of 40 patients treated with SBRT were included in the final analysis. The baseline patient characteristics are presented in Table 1. The median age was 71 years (range, 45-89 years). The majority of patients had intrahepatic (n = 25, 62.5%) cholangiocarcinoma, and only 6 patients (15%) had involved lymph nodes at the time of diagnosis. Child-Pugh score was available for 33 patients at initial consultation–most patients had Child-Pugh A (n = 23, 57.5%) or B (n = 8, 20%) liver function. Ten patients (25%) received chemotherapy before radiation treatment, with all except for one patient completing chemotherapy >1 month before radiation treatment start. The type of neoadjuvant chemotherapy received was gemcitabine in 10 patients, cisplatin in 6 patients, and 5-fluorouracil in 1 patient. A total of 17 (42.5%) patients received chemotherapy after SBRT, most commonly gemcitabine plus cisplatin (n = 9) and gemcitabine alone (n = 4). One patient received adjuvant FOLFOX, and chemotherapy type was unknown for 2 patients. One patient received both neoadjuvant and adjuvant chemotherapy. Only 1 patient underwent orthotopic liver transplant. The median radiation dose was 40 Gy (range, 26-50 Gy) given over a median of 5 fractions (range, 1-5 fractions).
Table 1.
Patient and treatment characteristics
| Characteristic | n = 41 (%) |
|---|---|
| Age at diagnosis | |
| Median | 71 |
| Range | 45-89 |
| Sex | |
| Male | 22 (54) |
| Female | 19 (46) |
| Tumor location | |
| Intrahepatic | 26 (63) |
| Perihilar | 15 (37) |
| Tumor size (cm) | |
| Median | 4.2 |
| Range | 1.0-12.5 |
| Lymph node status | |
| Positive | 6 (15) |
| Negative | 35 (85) |
| ECOG performance status | |
| 1 | 26 (63) |
| 2 | 15 (37) |
| Child-Pugh class | |
| A | 23 (56) |
| B | 9 (22) |
| C | 2 (5) |
| Not assessable | 7 (17) |
| Prior chemotherapy | |
| Yes | 10 (24) |
| No | 31 (76) |
| Unknown | 0 (0) |
| Type of prior chemotherapy (n = 10) | |
| 5-FU | 1 (10) |
| Cisplatin | 6 (60) |
| Gemcitabine | 10 (100) |
| Adjuvant chemotherapy | |
| Yes | 17 (41) |
| No | 21 (51) |
| Unknown | 3 (8) |
| Radiation dose to primary tumor (Gy) | |
| Median | 40 |
| Range | 26-50 |
Abbreviation: 5-FU = 5-fluorouracil.
Eight patients (20%) had prior liver resection, 3 (7.5%) prior radiofrequency ablation (RFA), and 5 (12.5%) prior transarterial chemoembolization. No patients had prior transarterial radioembolization. Patients were treated with SBRT in 1 to 5 fractions (n = 39) except for 1 patient who was treated in 10 fractions but was included in this analysis owing to undergoing a hypofractionated course with SBRT-like treatment planning and delivery. Of the 6 patients with lymph node (LN) + disease at the time of SBRT, 5 had the grossly involved lymph nodes included in the GTV. The 1 patient who did not have LNs included in the GTV had resolution of a previously enlarged porta hepatis after neoadjuvant gemcitabine.
Recurrence and overall survival
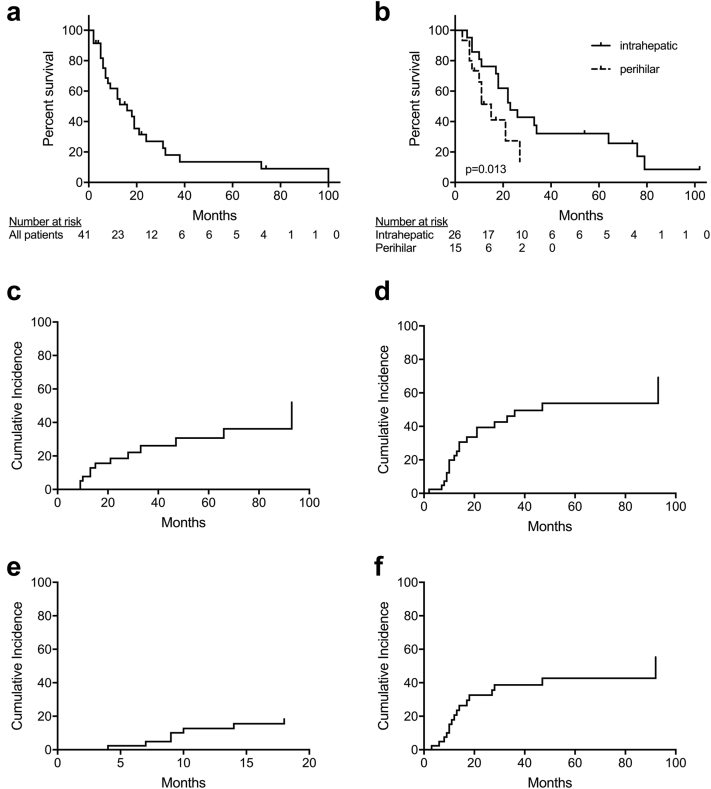
The median follow-up time was 18 months (range, 1-100 months). A total of 27 patients (67.5%) died. The median overall survival (OS) for the entire cohort was 23 months (95% confidence interval [CI], 15-35 months) with 1- and 2-year OS of 66% (95% CI, 52%-81%) and 39% (95% CI, 23%-55%), respectively (Fig 1a). Patients with perihilar tumors had a median OS of 10 months versus 23 months for patients with intrahepatic disease, (P = .018; Fig 1b). On univariable analysis, there was no correlation with OS and patient age (P = .12), performance status (P = .27), tumor size (P = .78), radiation dose (P = .63), BED (P = .82), or pretreatment Child-Pugh score (P = .72).
Figure 1.
(a-f) Overall survival for all cholangiocarcinoma patients (n = 42) treated with stereotactic body radiation therapy (a) and for patients with intrahepatic (n = 26) versus perihilar (n = 16) cholangiocarcinoma, respectively (b). Panels c to f show disease recurrence in patients with cholangiocarcinoma treated with stereotactic body radiation therapy. The image shows cumulative incidence of (c) local in-field, (d) local out-of-field, (e) regional, and (f) distant failure.
A total of 12 patients (30%) experienced in-field local failure (LF) and 17 (42.5%) experienced out-of-field hepatic LF. Only 1 patient experienced isolated in-field LF and 4 patients experienced isolated out-of-field hepatic LF. The 1- and 2-year cumulative incidence of in-field LF was 8% and 19%, respectively (Fig 1c). The 1- and 2-year cumulative incidence of out-of-field hepatic LF was 23% and 39%, respectively (Fig 1d). There was no correlation between in-field LF and tumor size (P = .63), radiation dose (P = .74), biliary stent use (P = .17), or BED (P = .34). Patients with intrahepatic tumors had higher in-field LF compared with those with perihilar tumors, but this difference did not reach statistical significance (40% vs 13%, P = .09).
Seven patients (17%) experienced RF. There were 3 porta hepatis failures, 4 para-aortic failures, 4 aortocaval failures, 4 gastrohepatic failures, and 1 retrocrural failure. The 1- and 2-year incidence of RF was 13% and 18%, respectively (Fig 1e). There was no difference in incidence of RF based on initial lymph node positivity (P = .26). There was no significant difference in RF based on tumor location (P = .25); however, the 1- and 2-year cumulative incidence of RF for patients with intrahepatic cholangiocarcinoma was 8% compared with 24% for patients with perihilar cholangiocarcinoma. Furthermore, patients with RF had worse OS, 42% versus 72% at 1 year, but this difference did not reach statistical significance (P = .069). Regional failure details are outlined in Table 2.
Table 2.
Toxicity
| Type of toxicity | No. patients |
|---|---|
| Acute nonhepatobiliary toxicity (n = 19) | |
| grade 1-2 | 18 |
| grade 3-4 | 1 |
| grade 5 | 0 |
| Acute hepatobiliary toxicity (n = 19) | |
| grade 1-2 | 2 |
| grade 3-4 | 16 |
| grade 5 | 1 |
| Late nonhepatobiliary toxicity (n = 9) | |
| grade 1-2 | 8 |
| grade 3-4 | 1 |
| grade 5 | 0 |
| Late hepatobiliary toxicity (n = 18) | |
| grade 1-2 | 1 |
| grade 3-4 | 17 |
| grade 5 | 0 |
There were 15 (37.5%) DF. In order of decreasing frequency, sites of DF included the lungs (n = 7), peritoneum (n = 6), soft tissue (n = 3), and bone (n = 1). The 1- and 2-year cumulative incidence of DF was 22% and 31%, respectively (Fig 1f). Patients with RF had a 1-year cumulative incidence of DF of 39% versus 19% in patients without RF (P = .15).
Toxicity
The treatment was well tolerated overall. A total of 20 patients (50%) experienced acute grade 1 to 2 toxicity and 9 patients (22.5%) experienced late grade 1 to 2 toxicity as evaluated by Common Terminology Criteria for Adverse Events, version 4.0. The most common grade 1 to 2 toxicities were fatigue, nausea, and abdominal pain. No patients experienced acute or late gastrointestinal (GI) fistula, perforation, or stenosis. One patient experienced a late grade 2 GI ulceration and one late grade 2 GI obstruction (Table 3). Only 1 patient experienced acute grade 3 + non-HB toxicity in the form of severe abdominal pain. Fifteen patients (37.5%) experienced late grade 3 + HB toxicity, the most common of which was HB infection requiring intravenous antibiotics. Causes for late grade 3 + HB toxicity included biliary instrumentation (n = 3), biliary stent obstruction with debris or sludge (n = 1), biliary stricture (n = 4), tumor progression (n = 3), liver abscess resulting in sepsis (n = 2), and acute cholecystitis (n = 1). In one case the underlying cause of late HB toxicity was attributed to elevated liver enzymes.
Table 3.
Characteristics of patients with regional lymph node metastases after stereotactic body radiation therapy for cholangiocarcinoma
| Patient | Age/sex | Stage at diagnosis | Primary tumor location | Chemotherapy | SBRT details | Time to LN failure | Location(s) of LN failure |
|---|---|---|---|---|---|---|---|
| 1 | 88/Male | T1N0 | Intrahepatic | None | 45 Gy in 5 fractions | 6 mo | Gastrohepatic, porta hepatis, para-aortic, retrocrural |
| 2 | 80/Male | T2bN1 | Intrahepatic | None | 35 Gy in 5 fractions | 3 mo | Gastrohepatic, para-aortic |
| 3 | 73/Female | T1N0 | Intrahepatic | None | 40 Gy in 5 fractions | 2 mo | Gastrohepatic |
| 4 | 54/Male | T4N1 | Intrahepatic | Adjuvant cisplatin + gemcitabine; cisplatin held due to low blood counts | 42.5 Gy in 5 fractions | 10 mo | Aortocaval, porta hepatis |
| 5 | 86/Male | T4N0 | Extrahepatic | None | 40 Gy in 5 fractions | 5 mo | Aortocaval |
| 6 | 75/Female | T3N0 | Extrahepatic | Adjuvant gemcitabine | 30 Gy in 1 fraction (CyberKnife) | 4 mo | Porta hepatis |
| 7 | 82/Male | T3N1 | Extrahepatic | Adjuvant gemcitabine | 40 Gy in 10 fractions | 7 mo | Gastrohepatic, para-aortic, portal caval |
Abbreviations: LN = lymph node; SBRT = stereotactic body radiation therapy.
Discussion
Cholangiocarcinoma is a rare and aggressive malignancy for which surgical treatment is the only option for cure. Throughout the past 2 decades, radiation treatment dose-escalation has emerged as a viable alternative option for patients who are not surgical candidates; however, median survival still lags behind surgical series.9, 10, 11 SBRT is a sophisticated method of radiation treatment delivery that allows for the ablation of tumors adjacent to critical structures. Given the proximity of these tumors to the liver, duodenum, or stomach, and their origin in the biliary system, SBRT is particularly suited to treating this patient population.
Our study details the outcomes and toxicity of one of the largest cohorts of cholangiocarcinoma patients treated with definitive SBRT to date. Our results compare favorably to previously published series12, 13, 14 with a median OS of 22 months and 1-year OS of 69% (Table 4). Although previous radiation studies have focused on local recurrence, few have reported regional recurrence rates.9 In our cohort, only one patient had an isolated local recurrence, and the majority of LF cases were accompanied by regional and distant metastases. The incidence of RF differed by tumor location with rates of 1-year RF of 22% in patients with perihilar cholangiocarcinoma versus 8% in patients with intrahepatic disease. In addition, patients with RF were more likely to experience DF and to have worse OS; however, these numbers did not reach statistical significance likely owing to the small patient population in this analysis. Nevertheless, given that lymph node involvement is a gateway to distant metastases,15 it is reasonable to hypothesize that eradicating micrometastatic disease in the lymph nodes would result in improved outcomes. As radiation is a local-regional treatment modality, it is important to consider extending our treatment fields to encompass sites of possible regional failure in this high-risk patient population.
Table 4.
Studies on stereotactic body radiation therapy for inoperable extrahepatic cholangiocarcinoma
| Study | Study design | No. of lesions | No. of EHCC (%) | No. of IHC (%) | Median dose, Gy | Median no. of fractions | LC at 1 y | OS at 1 y |
|---|---|---|---|---|---|---|---|---|
| Gkika et al9 | Retrospective | 43 | 26 (60%) | 17 (40%) | 45 | NR | 78% | 56% |
| Polistina et al12 | Retrospective | 10 | 10 (100%) | 0 | 30 | 3 | 80% | NR |
| Barney et al16 | Retrospective | 10 | 4 (40%) | 6 (60%) | 55 | 5 | 100% | 73% |
| Momm et al13 | Retrospective | 13 | 13 (100%) | 0 | 48 | 12 | 78% | NR |
| Mahadevan et al10 | Retrospective | 42 | 11 (26%) | 31 (74%) | 30 | 3 | 88% | 58% |
| Sandler et al6 | Retrospective | 31 | 25 (81%) | 6 (19%) | 40 | 5 | 78% | 59% |
| Jung et al11 | Retrospective | 58 | 25 (43%) | 33 (57%) | 45 | 3 | 85% | 45% |
| Kopek et al14 | Retrospective | 27 | 26 (96%) | 1 (4%) | 45 | 3 | NR | NR |
| Tao et al5 | Retrospective | 69 | 0 | 69 (100%) | 58 | 15 | 81% | 87% |
| This analysis | Retrospective | 41 | 15 (37%) | 26 (63%) | 40 | 5 | 92% | 69% |
Abbreviations: EHCC = extrahepatic cholangiocarcioma; IHC = intrahepatic cholangiocarcinoma; LC = local control; OS = overall survival.
Surgical series corroborate this hypothesis.17, 18, 19, 20 Gil et al19 found tumor size ≥4 cm and lymph node metastases as the most significant predictors of recurrence and survival among 153 patients treated with primary surgery for cholangiocarcinoma. The authors proposed adjuvant RT fields cover the resection bed and the perihilar, periduodenal, peripancreatic, celiac, caval, and para-aortic lymph node areas in light of high (64.5%) locoregional failure rates in their patient population. Similarly, Choi et al20 recently mapped sites of regional lymph node metastases in 93 patients with extrahepatic biliary carcinoma treated with surgical resection. A total of 38 patients experienced regional lymph node failure, most commonly at the celiac, superior mesenteric, para-aortic, and common hepatic locations. The authors proposed adjuvant radiation treatment encompassing these areas as a means to decrease regional failure rates with superior-inferior radiation treatment volumes extending from the T10/11 interspace to the L1/2 interspace and laterally 1 cm on either side of the vertebral bodies.
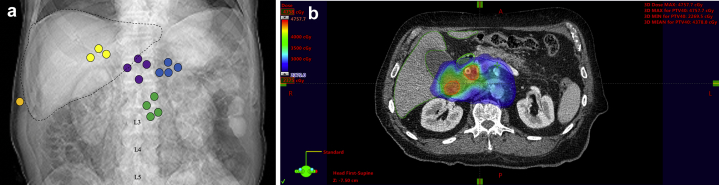
At our institution, we have recently incorporated a novel approach of ENI for the treatment of patients with perihilar cholangiocarcinoma because these patients were more likely to have RF at 1 year compared with those with intrahepatic disease. We now include a larger area of regional at risk lymph nodes that encompasses the sites of most frequent nodal failure in our patient population, including the gastrohepatic, aortocaval, porta hepatis, and para-aortic lymph nodes (Fig 2a). We do not routinely include the retrocrural lymph nodes in our treatment field because the 1 retrocrural failure in our cohort occurred in isolation. Our dose and fractionation is based on the ease and feasibility of short-course radiation for rectal cancer.21 The primary tumor and involved lymph nodes are treated to ~40 to 45 Gy in 5 fractions whereas the at-risk lymph nodes mentioned are treated to 25 Gy in 5 fractions. A representative axial slice of an ENI SBRT treatment plan is depicted in Figure 2b. So far, 2 patients with perihilar tumors and positive lymph nodes have been treated with this approach. On follow-up, one patient had a favorable response to treatment with eradication of nodal metastases and stable size of his primary tumor 9 months after SBRT. The second patient was found to have new intrahepatic metastases and growth of a single para-aortic lymph node 7 months after SBRT.
Figure 2.
(a) Representative schematic showing regions of nodal failure in cholangiocarcinoma patients treated with stereotactic body radiation therapy. Nodal regions are as follows: yellow = porta hepatis, purple = aortocaval, blue = gastrohepatic, green = para-aortic, orange = retrocrural. (b) Representative cross-sectional image of an elective nodal irradiation stereotactic body radiation therapy treatment plan. The image is in colorwash, 23.75 Gy isodose cloud showing coverage of the planning target volume (red), elective nodal region (blue), liver (dark green), and duodenum (light green).
Approximately one-third of patients experienced grade 3 + HB toxicity. Of importance, although the absolute number of patients with grade 3 + HB toxicity was high (n = 15), the reasons for the toxicity varied from tumor progression to biliary instrumentation. The likelihood that SBRT would increase stent-related complications is unknown, but it is possible that SBRT influenced rates of biliary stricture in this patient population as prior studies have demonstrated rates of stent replacement on the order of 5% to 15% at 3 months.7 Whether the addition of ENI would increase HB toxicity is unknown, and additional study is required to assess whether our proposed regimen of 25 Gy in 5 fractions delivered to the abdominal area is safe and effective.
In this population of patients with tumors arising from the biliary tree, dose to the central hepatobiliary structures will be high, and this group is more susceptible to this type of toxicity than others. We have previously reported on our experience with SBRT and HB toxicity.8 The analysis revealed that grade 3 + HB toxicity was strongly associated with dose to the central hepatobiliary tree. In our analysis, 50% of patients with cholangiocarcinoma experienced grade 3 + HB toxicity compared with 17.5% of patients with hepatic cholangiocarcioma, and 11.7% of patients with liver metastases. The strongest dose predictors for G3 + HB toxicity were VBED1040 ≥ 37 mL and VBED1030 ≥ 45 mL, but only for patients with hepatic cholangiocarcioma. Dose to hepatobiliary structures was not associated with toxicity in patients with cholangiocarcinoma, suggesting that there are other contributors to this process such as tumor growth, stent clogging, and biliary instrumentation.
Limitations of our study include its small sample size, retrospective nature, and potential for selection bias. Our patient cohort represents patients who were either unfit for surgery or progressed after prior liver-directed therapies. Therefore, there may be lead-time bias in their outcomes. Distant failure remained a significant problem, likely as a result of limitations in administering aggressive chemotherapy in older patients with multiple comorbidities. In fact, only 60% of patients received systemic treatment, and there was significant variation in the chemotherapeutic agents administered. In addition, a large portion of our patients experienced grade 3 + HB toxicity, but it remains difficult to determine whether this was due to the disease or treatment. Finally, there was heterogeneity with respect to the radiation dose delivered over this 10-year period, and it is possible that rates of regional lymph node recurrence are underestimated in this study due to limitations in sensitivity of currently available imaging modalities.22
The strengths of this study include a comprehensive assessment of patterns of failure and real-world outcomes of cholangiocarcinoma patients treated with SBRT. Our findings compare favorably to previously published series. Despite small patient numbers, it seems as though individuals with perihilar cholangiocarcinoma exhibit higher rates of regional failure, and for these patients we propose a novel approach of ENI with the goal of eradicating microscopic disease possibly contributing to high rates of distant failure and potentially poorer survival in this group.
In conclusion, patients with perihilar cholangiocarcinoma had a 22% rate of regional lymph node failure after definitive SBRT. We would propose adding an elective nodal volume to cover the regional lymphatics. A prospective study of ENI in patients with perihilar tumors would further shed light on if this approach improves outcomes without subsequent increases in hepatobiliary toxicity.
Footnotes
Sources of support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures: Dr Chang receives research support from Varian Medical Systems, Inc and holds stock in ViewRay, Inc.
References
- 1.Blechacz B. Cholangiocarcinoma: Current knowledge and new developments. Gut Liver. 2017;11:13–26. doi: 10.5009/gnl15568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massarweh N.N., El-Serag H.B. Epidemiology of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Control J Moffitt Cancer Cent. 2017;24 doi: 10.1177/1073274817729245. 1073274817729245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirstein M.M., Vogel A. Epidemiology and risk factors of cholangiocarcinoma. Visc Med. 2016;32:395–400. doi: 10.1159/000453013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebata T., Ercolani G., Alvaro D., Ribero D., Tommaso L.D., Valle J.W. Current status on cholangiocarcinoma and gallbladder cancer. Liver Cancer. 2017;6:59–65. doi: 10.1159/000449493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tao R., Krishnan S., Bhosale P.R. Ablative radiotherapy doses lead to a substantial prolongation of survival in patients with inoperable intrahepatic cholangiocarcinoma: A retrospective dose response analysis. J Clin Oncol. 2016;34:219–226. doi: 10.1200/JCO.2015.61.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandler K.A., Veruttipong D., Agopian V.G. Stereotactic body radiotherapy (SBRT) for locally advanced extrahepatic and intrahepatic cholangiocarcinoma. Adv Radiat Oncol. 2016;1:237–243. doi: 10.1016/j.adro.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osmundson E.C., Wu Y., Luxton G., Bazan J.G., Koong A.C., Chang D.T. Predictors of toxicity associated with stereotactic body radiation therapy to the central hepatobiliary tract. Int J Radiat Oncol Biol Phys. 2015;91:986–994. doi: 10.1016/j.ijrobp.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 8.Toesca D.A.S., Osmundson E.C., Eyben R. von. Central liver toxicity after SBRT: An expanded analysis and predictive nomogram. Radiother Oncol. 2017;122:130–136. doi: 10.1016/j.radonc.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Gkika E., Hallauer L., Kirste S. Stereotactic body radiotherapy (SBRT) for locally advanced intrahepatic and extrahepatic cholangiocarcinoma. BMC Cancer. 2017;17:781. doi: 10.1186/s12885-017-3788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahadevan A., Dagoglu N., Mancias J. Stereotactic body radiotherapy (SBRT) for intrahepatic and hilar cholangiocarcinoma. J Cancer. 2015;6:1099–1104. doi: 10.7150/jca.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung D.H., Kim M.-S., Cho C.K. Outcomes of stereotactic body radiotherapy for unresectable primary or recurrent cholangiocarcinoma. Radiat Oncol J. 2014;32:163–169. doi: 10.3857/roj.2014.32.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polistina F.A., Guglielmi R., Baiocchi C. Chemoradiation treatment with gemcitabine plus stereotactic body radiotherapy for unresectable, non-metastatic, locally advanced hilar cholangiocarcinoma. Results of a five year experience. Radiother Oncol. 2011;99:120–123. doi: 10.1016/j.radonc.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Momm F., Schubert E., Henne K. Stereotactic fractionated radiotherapy for Klatskin tumours. Radiother Oncol J. 2010;95:99–102. doi: 10.1016/j.radonc.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Kopek N., Holt M.I., Hansen A.T., Høyer M. Stereotactic body radiotherapy for unresectable cholangiocarcinoma. Radiother Oncol. 2010;94:47–52. doi: 10.1016/j.radonc.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Jutric Z., Johnston W.C., Hoen H.M. Impact of lymph node status in patients with intrahepatic cholangiocarcinoma treated by major hepatectomy: A review of the National Cancer Database. HPB. 2016;18:79–87. doi: 10.1016/j.hpb.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barney BM, Olivier KR, Miller RC, Haddock MG. Clinical outcomes and toxicity using stereotactic body radiotherapy (SBRT) for advanced cholangiocarcinoma. Radiat Oncol. 2012;7:67. doi: 10.1186/1748-717X-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song S., Kim K., Chie E.K. Locoregional recurrence after curative intent resection for intrahepatic cholangiocarcinoma: Implications for adjuvant radiotherapy. Clin Transl Oncol. 2015;17:825–829. doi: 10.1007/s12094-015-1312-0. [DOI] [PubMed] [Google Scholar]
- 18.Ghiassi-Nejad Z., Tarchi P., Moshier E. Prognostic factors and patterns of locoregional failure after surgical resection in patients with cholangiocarcinoma without adjuvant radiation therapy: Optimal field design for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 2017;99:805–811. doi: 10.1016/j.ijrobp.2017.06.2467. [DOI] [PubMed] [Google Scholar]
- 19.Gil E., Joh J.-W., Park H.C., Yu J.I., Jung S.H., Kim J.M. Predictors and patterns of recurrence after curative liver resection in intrahepatic cholangiocarcinoma, for application of postoperative radiotherapy: A retrospective study. World J Surg Oncol. 2015;13:227. doi: 10.1186/s12957-015-0637-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi H.S., Kang K.M., Jeong B.K. Patterns of failure after resection of extrahepatic bile duct cancer: Implications for adjuvant radiotherapy indication and treatment volumes. Radiat Oncol Lond Engl. 2018;13:85. doi: 10.1186/s13014-018-1024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narang A.K., Meyer J. Neoadjuvant short-course radiation therapy for rectal cancer: Trends and controversies. Curr Oncol Rep. 2018;20:68. doi: 10.1007/s11912-018-0714-x. [DOI] [PubMed] [Google Scholar]
- 22.Mao Y. Radiologic assessment of lymph nodes in oncologic patients. Curr Radiol Rep. 2014;2:36. [Google Scholar]